1. Introduction
Esophageal cancer, an incurable gastrointestinal
cancer, is considered the sixth leading cause of cancer-related
deaths and the eighth most common type of cancer globally (1). Esophageal squamous cell carcinoma
(ESCC) is the predominant type of esophageal carcinoma worldwide,
especially in the Asian belt (2).
Esophageal cancer is prone to lymph node metastasis in the early
stages of the disease because the lymphatic system is
well-developed in the esophagus. Duan et al (3) reported that the lymph node metastasis
rate was 17.5% (25/143) even in pathological T1 esophageal cancer.
Furthermore, poor outcomes in patients with esophageal cancer are
also related to the propensity for metastases, even when tumors are
superficial (2). Due to the nature
of esophageal cancer, a variety of treatment strategies are
required, and multidisciplinary treatments, such as surgery,
chemotherapy, and radiation therapy, are not sufficiently effective
(4). Thus, new treatment methods
are required to improve the clinical outcomes.
Recently, the antitumor effect of metformin, an
antidiabetic drug, has been reported in many cancers (5). Due to its safety, metformin is widely
used to treat diabetes mellitus (DM). Lactic acidosis is thought to
be a significant side effect of the drug. However, it has been
reported that metformin does not increase the risk of lactic
acidosis in patients with type 2 diabetes who do not have heart,
renal, or liver failure (6). Since
it is a well-established DM management drug, metformin can be
anticipated to exhibit beneficial effects as a repurposed cancer
drug.
A wide range of therapeutic strategies, including
surgery, chemotherapy, radiation therapy, and immunotherapy, have
been selected to treat refractory ESCC. These strategies are often
used in combination to improve therapeutic efficacy further.
Metformin is expected to enhance the therapeutic efficacy of many
treatment strategies for ESCC because of its broad antitumor
effects and to exert synergistic effects in these combined
treatments. Therefore, it is very important to know more about the
antitumor effects of metformin; however, owing to the diversity of
the impact of metformin, a comprehensive understanding of its
mechanism is complex.
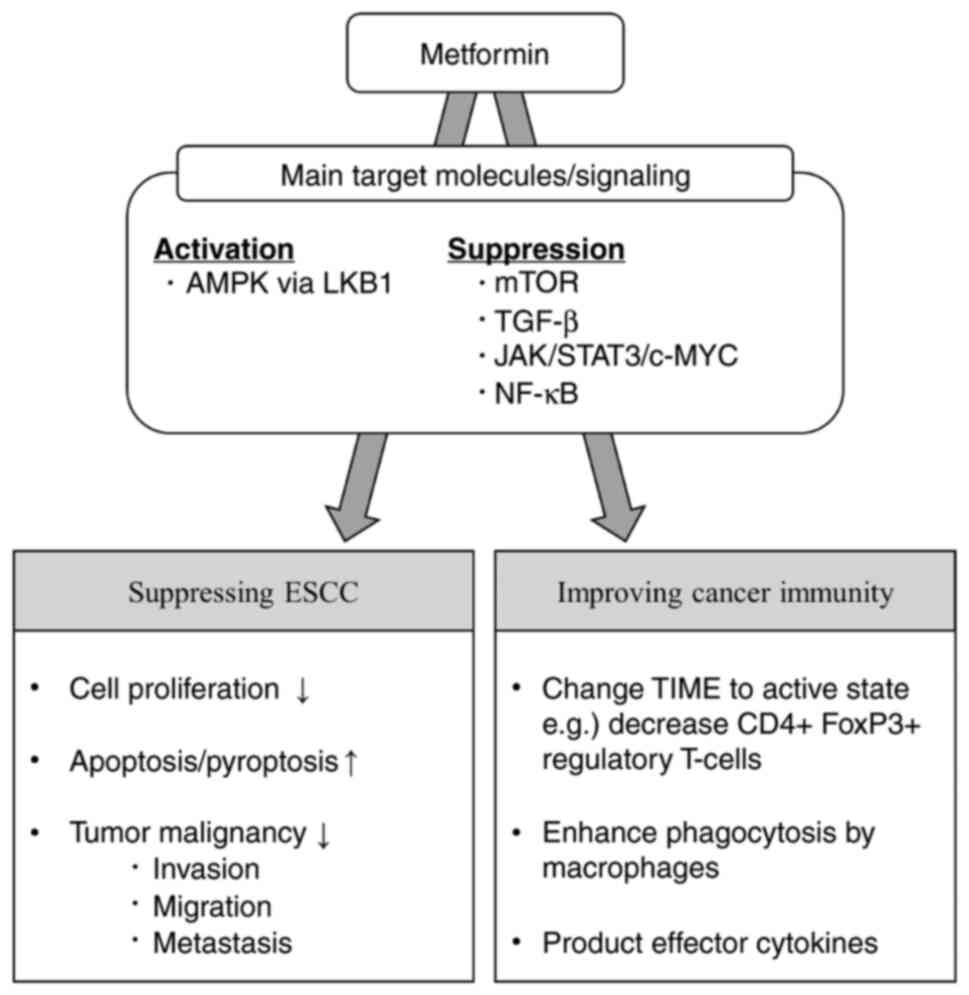
In this review, we described the molecular targets
of metformin in ESCC and then categorized and organized the
antitumor effects of metformin in cancer prevention, cancer
suppression, host immunity, and cancer metabolism (Fig. 1). Finally, we discussed the
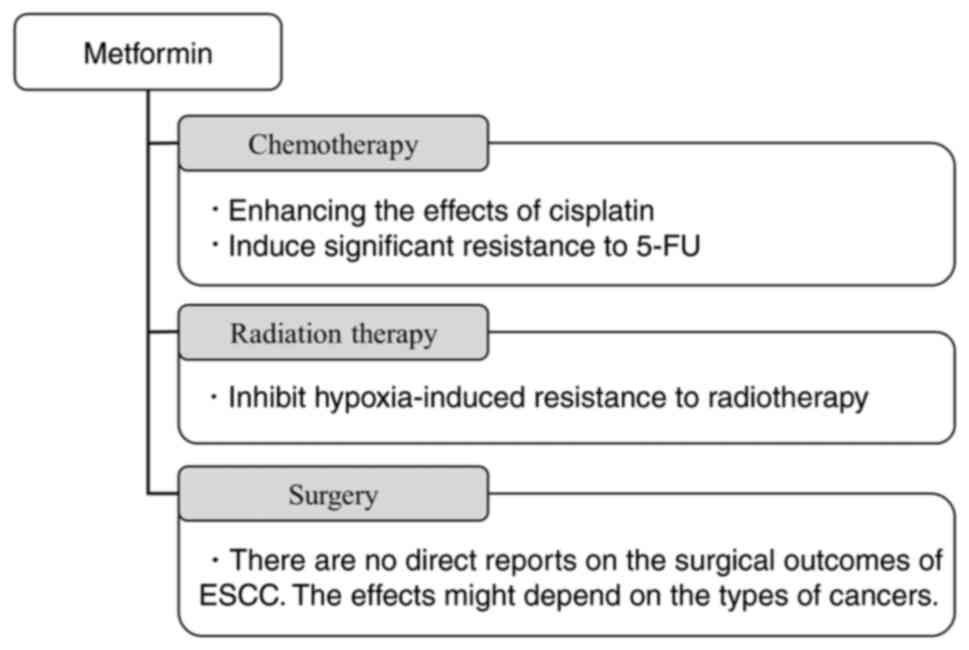
combined effects of metformin and other therapies (Fig. 2), and then we provided a
perspective on the potential benefits of metformin in the future
treatment of ESCC.
2. Target molecules of metformin
Many target proteins of metformin have been
reported, and their associated effects are diverse. The critical
target of metformin is the 5'-adenosine monophosphate-activated
protein kinase (AMPK) (7,8). A large body of evidence suggests that
AMPK functions as a tumor suppressor and that its expression is
downregulated in many cancers (9).
Metformin activates AMPK via liver kinase B1 (LKB1) (7). As a result, the expression of the
p-mammalian target of rapamycin (mTOR), p-p70S6K, and cyclin D1, as
well as the expression of the two mTOR-related genes, 4EBP1 and
S6K1, are suppressed, resulting in the suppression of downstream
molecular signaling and consequently ESCC carcinogenesis (10,11).
Another molecular target for metformin is the
transforming growth factor (TGF)-β signaling pathway. Nakayama
et al (12) reported that
metformin inhibits epithelial-mesenchymal transition (EMT) by
suppressing the Smad phosphorylation pathway and part of the
non-Smad pathway downstream of TGF-β that induces EMT.
Recently, it was reported that metformin inhibits
signal transducer and activator of transcription 3 (STAT3) and
nuclear factor-kappa B (NF-κB) signaling, which are essential
proteins in the inflammatory response of ESCC. STAT3 is an
inflammation-related molecule often correlated with metformin
(8,13,14).
Smoking is one of the risk factors for ESCC, and nicotine ingested
through smoking interacts with the choline receptor nicotinic
alpha7 subunit (CHRNA7), which induces cancer stem cells (CSCs) and
cancer-initiating cells (CICs), and subsequently activates the
Janus Kinase (JAK2)/STAT3/Sox2 signaling pathway (13). Metformin suppresses CHRNA7
expression via hypermethylation of CHRNA7 promoter DNA (13). This inactivation of the STAT3-Bcl-2
pathway by metformin contributes to the inhibition of ESCC growth
through crosstalk between apoptosis and autophagy (14). NF-κB is also a master regulator of
inflammation and immunity. It has been reported that metformin
decreases the nuclear translocation of NF-κB, thus inhibiting its
activation (15) and inhibiting
the phosphorylation of AKT, an upstream regulator of NF-κB
(16). Since metformin has a wide
range of effects, its antitumor effects are intertwined and
diverse.
3. ESCC prevention effects
Metformin effectively reduces the risk of tumor
development in many types of cancer, including ESCC (17). A prospective cohort study conducted
in Sweden between 2005 and 2015 showed that metformin reduces the
risk of ESCC (HR 0.68, 95% CI 0.54-0.85). The incidence of ESCC was
3.5 per 100,000 person-years in metformin users and 5.3 per 100,000
person-years in non-users of metformin. The risk reduction was more
pronounced in the new metformin users (HR 0.44, 95% CI 0.28-0.64)
and in participants aged 60-69 years (HR 0.45, 95% CI 0.31-0.66)
(18).
The preventive effects of metformin on ESCC
development have been demonstrated in vivo. Fan et al
(10) used N-nitroso-N-methyl
benzylamine (NMBzA), a specific carcinogen that induces and
promotes esophageal cancer, to create a model for the ESCC
progression in rats. They reported that metformin significantly
decreased the incidence of ESCC and the number of precancerous
lesions in rats treated with NMBzA (10).
These reports suggest that metformin reduces the
risk of ESCC. Thus, metformin has the potential to be an effective
prophylactic drug in patients with achalasia and other diseases
that are at high risk for the development of ESCC. Therefore,
further studies are warranted.
4. Antitumor and anti-inflammatory effects
on ESCC progression
Cell proliferation and programmed cell
death (apoptosis and pyroptosis)
Many studies have shown that metformin affects ESCC
cell proliferation and apoptosis both in vivo and in
vitro. In animal studies, metformin did not affect body weight
or vital signs (10). It also did
not cause significant changes in liver function, kidney function,
or blood glucose levels (19). In
addition, no noticeable toxic reactions were observed (11). This report provides evidence for
the safety of metformin.
Metformin has also been reported to inhibit
esophageal inflammation by decreasing the expression of inducible
nitric oxide synthase (iNOS), cyclooxygenase-2 (COX-2), and
interleukin-6 (IL-6) in vivo. Analysis of esophageal
epithelial cells showed that proliferating cell number antigen
(PCNA), a proliferation marker, was decreased, and cleaved
caspase-3, an apoptosis marker, was increased (10). In addition, metformin reduces tumor
size in an ESCC cell line xenograft model (11,15,20).
Analysis of tumors indicated that growth was inhibited while
apoptosis and autophagy were induced (14,15).
These mechanisms are proposed to involve increased expression of
AMPK, p53, p21CIP1, and p27KIP1, and repression of
cyclinD1(20), and the
mTOR-related genes, 4EBP1 and S6K1(11), in addition to reducing Stat3
activity and Bcl-2 expression (14).
On the level of the cell cycle, metformin induces
G0/G1 arrest, inhibits proliferation, increases apoptosis, and
inhibits colony and tumorsphere formation in human ESCC cells in
vitro (10,11,19).
The induction of G0/G1 phase arrest was mediated by increased
expression of p21CIP1 and p27KIP1(20). Concerning apoptosis, STAT3 and its
downstream target, Bcl-2, were inactivated, while the expression
levels of Bax and caspase-3 were increased (14,19).
Furthermore, it has been recently described that metformin induces
pyroptosis, a non-apoptotic inflammatory caspase-dependent
programmed cell death (PCD), via Gasdermin D (GSDMD) in
vitro and in vivo (21). These results suggest that metformin
could be an alternative treatment for ESSCs that are refractory to
chemotherapy and radiotherapy, as well as for other cancers with a
pyroptotic mechanism.
Surprisingly, the sensitivity of ESCC cells to
metformin has been reported to increase under conditions of glucose
deprivation (22). Yu et al
(22) noted that evaluating the
effect of metformin under glucose-depleted conditions was more
relevant considering the microenvironment of solid tumors
(typically lower than 0.5 mM) and that the glucose concentration of
the culture medium was influential in the in vitro
experimental setup. Further research is needed to elucidate the
underlying mechanism of the differential observed effects of
metformin based on the glucose concentration in the medium.
Interestingly, metformin selectively acts on cancer
cells, whereas Peng et al (23) reported that metformin acts
differently on ESCC cells (EC109) and normal cells (HEEC) because
metformin significantly inhibits growth and induces apoptosis in
cancer cells. Additionally, metformin suppressed STAT3
phosphorylation and Bcl-2 expression in ESCC cells but not in
normal cells (23). Feng et
al (14) also reported that
metformin selectively inhibited the growth of ESCC tumor cells but
not immortalized non-cancerous esophageal epithelial cells. These
results suggest that metformin acts specifically on cancer cells,
which may explain the low observed side effects of metformin
administration in vivo.
In a human clinical trial, Wang et al
(8) reported the effects of
low-dose metformin (250 mg/day for 7-14 days before surgery) on
ESCC and tumor immunity. Low-dose metformin did not affect ESCC
tumor growth and apoptosis, as assessed by immunostaining for Ki67
and cleaved caspase-3. As it is possible to use higher doses for DM
treatment, future analyses of the effects of metformin at higher
doses are also desirable.
Invasion, migration, metastasis
Metformin inhibits cancer cell invasion and
migration, which are essential hallmarks for invasion and
metastasis. In an in vitro study, metformin inhibited the
migration and invasion of cancer cells (10,15)
and increased the expression of the epithelial marker E-cadherin
(15). Furthermore, metformin
suppressed the expression of matrix metalloproteinase (MMP)-2,
MMP-9, and N-cadherin (16). This
expression signature has been suggested to be inhibited in a
phosphorylated AKT-dependent manner (16). Metformin also inhibits tumor
growth, suppresses lung metastasis, and decreases the expression of
MMP-2 and MMP-9 in vivo (19). Thus, metformin inhibits the
migration and invasion of esophageal cancer cells by regulating the
expression of migration- and invasion-related genes.
Nakayama et al (12) reported the effects of metformin on
EMT induction via ionizing radiation (IR). They first observed that
IR irradiation induced the expression of TGF-β, hypoxia-inducible
factor (HIF)-1α, mesenchymal markers (vimentin and N-cadherin),
transcription factors (Slug, Snail, and Twist), and MMPs. They
observed that metformin suppresses EMT-induced morphology and
motility, but the mechanism is not mediated through TGF-βbut
instead through phosphorylation of its downstream Smad2 and
Smad3(12). Metformin enhanced
IR-induced phosphorylation of AMPK. However, mTOR phosphorylation
was enhanced by radiation and inhibited by metformin (12).
Yang et al (24) reported an experiment to simulate
the ESCC microenvironment using a tumor-conditioned medium (TCM)
from ESCC cell culture's supernatant or human ESCC tissue
homogenate's supernatant. TCM promotes tumor angiogenesis by
transforming normal endothelial cells (NECs) into tumor endothelial
cells (TECs). However, metformin inhibited the transition of NECs
into TECs in the ESCC microenvironment by inhibiting the
JAK/STAT3/c-MYC signaling pathway. Yang et al (24) first validated metformin's
inhibitory effect on angiogenesis in vivo using a human ESCC
patient-derived xenograft (PDX) mouse model (24).
These results indicate that metformin inhibits
metastasis, invasion, and angiogenesis and exerts antitumor
effects. These properties directly relate to patient prognosis,
suggesting that metformin may improve prognosis.
Cancer immunity
Metformin improves the tumor immune microenvironment
(TIME) in esophageal cancer, as reported in a human clinical trial
conducted by Wang et al (8). Low-dose metformin (250 mg/day for
7-14 days before surgery) changes the TIME to an anticancer state
(8). Metformin reprogrammed TIME
to ‘infiltration-inflammation’ and increased the number of
infiltrating CD8+ cytotoxic T lymphocytes and CD20+ B-lymphocytes.
Furthermore, an increase in tumor-suppressive macrophages (CD11c+
M1 macrophages) and a decrease in tumor-promoting macrophages
(CD163+ M2 macrophages) have been observed (8).
In the ESCC mouse model (4-NQ O-induced orthotopic
ESCC mice 16 weeks old, metformin 50 mg/kg/day), short-term
metformin treatment reprogrammed TIME as previously observed in
humans, while long-term treatment further shifted TIME to an active
state (e.g., decreased CD4+ FoxP3+ regulatory T-cells) and
suppressed ESCC growth (8).
Regarding the mechanism by which metformin regulates CD4+ CD25+
regulatory T-cells in the microenvironment, it has been reported
that regulatory T-cells, which proliferate in the tumor mass, are
induced to undergo apoptosis, and their number is drastically
reduced. Detailed examination revealed that fatty acid-dependent
oxidative phosphorylation, the primary source of energy metabolism
of regulatory T-cells, was reduced. Instead, the glucose-dependent
glycolytic system was enhanced, activating the pathway that leads
to cell death (25). Others have
reported that metformin enhances the phagocytosis of ESCC cells by
macrophages in vitro (8)
and also alters the production of effector cytokines as tumor
necrosis factor-α(TNF-α) and IL-10 in immune cells by inducing AMPK
activation and STAT3 inactivation (6).
These results suggested that metformin modulated the
immune status of the host in a tumor-suppressive manner (Table I). These findings highlight the
promising potential of metformin in combination with treatment
focusing on cancer immunity, especially immunotherapy using immune
checkpoint inhibitors (ICIs).
 | Table IThe effects of metformin on immune
cells and cytokines. |
Table I
The effects of metformin on immune
cells and cytokines.
| A, Immune
cells |
|---|
| Target | Main function | Effect of
metformin | Mentioned
mechanism | (Refs.) |
|---|
| CD8+ T
cell | Cytotoxic
effects | ↑ | Increase p-AMPK
positive cells, TNF-α↑ | (8) |
| CD4+
Foxp3+ T cell | Immune suppression
(regulatory) | ↓ | | (8) |
| CD20+ B
cell | Antibody
production, T cell activation | ↑ | | (8) |
| CD11c+
macrophage (M1) | Immune
elimination | ↑ | Increase p-AMPK
positive cells, TNF-α↑, IL-10↓ | (8) |
| CD163+
macrophage (M2) | TAM; angiogenesis
promotion | ↓ | | (8) |
| B, Cytokines |
| Target | Main function | Effect of
metformin | Mentioned
mechanism | (Refs.) |
| IL-6 | Regulation of
immune response and inflammation | ↓ | Activate AMPK and
attenuate downstream signaling such as mTOR | (6,10) |
| IL-10 | Suppression of the
immune system | ↓ | Activate AMPK and
inactivate STAT3 | (6,8) |
| TNF-α | Elimination of
tumor cells | ↑ | Activate AMPK and
inactivate STAT3 | (6,8) |
| TGB-β | Regulates cell
proliferation and differentiation, and promotes cell death | ↑ | Activate AMPK and
suppress mTOR signal | (12) |
Antitumor effect via metabolism,
2-Deoxyglucose (2DG)
Since glycolysis is enhanced in tumors even in the
presence of oxygen due to the Warburg effect and ATP generation is
more dependent on aerobic glycolytic metabolism than on
mitochondrial metabolism (26),
glucose metabolism is one of the targets of cancer therapy.
2-Deoxyglucose (2DG), a glucose analog, inhibits hexokinase, the
first restriction enzyme in the glycolytic system, and acts as an
inhibitor of glucose metabolism (27). Both mitochondrial dysfunction and
aerobic glycolysis are signs of aggressive cancer growth, and the
glycolytic inhibitor, 2DG, appears to be a promising treatment tool
(28). As metformin is known to
decrease oxidative phosphorylation in ATP biosynthesis in the
mitochondrial inner membrane [27], the combination of metformin and
2-DG is expected to be effective.
The dual combination therapy of metformin and 2-DG
synergizes apoptosis induction by decreasing Bcl-2 expression and
increasing p53 expression in vitro (28). It has been reported that both
glycolysis and oxidative phosphorylation are simultaneously induced
in ESCC cancer stem cells (CSCs) depending on the Hsp27-AKT-HK2
pathway. In addition, the combination of 2-DG and metformin, which
inhibits the glycolytic system and oxidative phosphorylation, led
to the suppression of tumor growth, including tumor size and
weight, in a xenograft tumor model (29). The combinatorial therapy of
metformin and 2DG has already been used for positron emission
tomography (PET) scans, seizure disorders, and DM and is expected
to allow for rapid evaluation of clinical efficacy (28).
5. Combination with current therapies
Improving treatment outcomes
The use of metformin in ESCC treatment has been
reported to enhance the prognosis and prolong overall survival
(OS). Van De Voorde et al (30) reported that the use of metformin
improved OS and distant metastasis-free survival in 196 patients
with esophageal cancer treated with trimodality therapy (distant
metastasis-free survival, P=0.040; overall survival, P=0.012).
Although this study included a large number of patients with
esophageal cancer having adenocarcinoma (adenocarcinoma, n=137;
squamous cell carcinoma, n=36; other, n=4) and only a small number
of patients taking metformin (non-diabetic, n=172; diabetic without
metformin, n=5; diabetic taking metformin, n=19), the study was
conducted in a relatively large number of patients and was
considered meaningful.
Chemotherapy
Cisplatin and 5-FU, which are used in the treatment
of many patients, were also noted. As for cisplatin, there have
been a series of reports that its combination with metformin
enhances its antitumor effect. Basic experiments suggest that the
combination of metformin and chemotherapy is beneficial, as
metformin inhibits cell proliferation and promotes cell apoptosis,
thereby enhancing the effects of cisplatin (11).
Interestingly, metformin synergistically enhanced
the cytotoxicity of cisplatin under glucose-depleted conditions,
which is representative of the microenvironment of solid tumors.
Possible mechanisms include enhanced cytotoxicity by metformin,
markedly reduced intracellular ATP levels, AKT and AMPK signaling
pathway abnormalities, and impaired DNA repair (22).
Metformin exerts its antitumor effects by modulating
oxidation-reduction homeostasis. It acts as a pro-oxidant by
reducing intracellular glutathione, a major intracellular
antioxidant against reactive oxygen species (ROS). It has been
reported that the concomitant use of metformin suppresses the
cisplatin-induced increase in intracellular glutathione and
increases sensitivity to cisplatin in vitro and in
vivo (31). However, it was
reported that metformin protects cells from the cytotoxicity of
cisplatin by inducing a reductive intracellular environment that
decreases cisplatin-DNA adduct formation. They also noted that
caution should be taken when administering cisplatin to diabetic
patients on metformin (32).
Another chemotherapeutic agent used to treat ESCC is
5-fluorouracil (5-FU). Unlike cisplatin, metformin treatment
induced significant resistance to 5-FU in vitro (33). This resistance is thought to be due
to overall changes in nucleotide metabolism, such as increased
expression of thymidylate synthase and thymidine kinase 1, which
are established mechanisms of 5-FU resistance that increase the
intracellular dTTP pool and dilution of 5-FU assimilates (33).
Metformin in combination with cisplatin during
chemotherapy has been reported in both directions. However,
metformin in combination with 5-FU has the risk of inducing
resistance and should be treated with caution. As chemotherapy
plays a critical role in the treatment of ESCC, further studies on
this combination therapy are required.
Radiation therapy
Radiation therapy (RT) is essential for treating
ESCC as chemotherapy and surgery, and the effect of metformin on
its efficacy has also been studied. Clinical data suggest that
metformin improves progression-free and overall survival in various
cancer patients treated with RT, although the results are not
always consistent (34).
Metformin has been reported to inhibit
hypoxia-induced resistance to radiotherapy in ESCC. Hypoxia is a
critical cause of radioresistance because oxygen is a source of
free radicals necessary for ionizing radiation (IR) to kill tumor
cells. Hypoxia can induce a series of cellular biological
transformations to prevent the harmful effects of IR (35). In ESCC, miR-340-5p, highly
expressed in exosomes derived from hypoxic tumor cells, induces
radiotherapy resistance by targeting KLF10. However, metformin
increases the expression of KLF10 and enhances radiosensitivity
(35).
Although there are few reports related to ESCC,
reports on other carcinomas suggest that the combination of
metformin and RT is promising. RT shows an abscopal effect, which
is thought to be induced by tumor immune activation, and metformin
enhances tumor immunity, as mentioned earlier. Thus, a synergistic
effect is expected in ESCC, and further studies are required to
confirm this effect.
Effect on surgery
Since surgery is an essential treatment for ESCC, we
would like to discuss the effects of metformin on surgical
outcomes. Although there are no direct reports on the surgical
outcomes of ESCC, there are some reports on other cancers as
follows, and the impact is controversial. For colorectal cancer,
Fransgaard et al (36)
showed no association between diabetes or metformin treatment and
recurrence-free or disease-free survival after surgery for
colorectal cancer. In addition, Kaushik et al (37) reported that metformin did not
improve prostate cancer outcomes after radical prostatectomy.
In contrast, Chan et al (38) found that metformin improved the
prognosis of hepatocellular carcinoma (HCC) in patients with DM.
The hazard ratio of metformin use in HCC patients with DM was 0.65
(P<0.05, 95% CI=0.60-0.72) for HCC recurrence and 0.79
(P<0.05, 95% CI=0.72-0.88) for OS after liver resection.
Interestingly, the risk reduction in hepatocellular carcinoma
recurrence after liver resection was significantly associated with
a dose/duration dependent on accumulated metformin usage (38). Kaltenmeier et al (39) reported that metformin improved
outcomes for patients who underwent surgery for colorectal liver
metastasis (CRLM). They divided patients into metformin (n=62) or
no metformin (n=208), and patients on metformin had significantly
longer Recurrence-free survival (RFS) (HR: 0.44, 95% CI: 0.26-0.75,
P<0.002; Median RFS: 49 months vs. 33 months) and OS (HR 0.60,
95% CI 0.31-0.97, P<0.048, Median OS: 72 months vs. 60 months)
(39). Similarly, Luo et al
(40) showed that metformin usage
significantly improved OS in hepatitis B virus (HBV)-related HCC
patients with DM after radical resection (hazard ratio: 0.558, 95%
CI: 0.385-0.810).
These reports suggest that metformin could improve
the prognosis after surgery. However, the effects might depend on
the types of cancers and patients' conditions. Further studies
about the effects of metformin on ESCC after esophagectomy as a
radical surgery are required.
6. Future treatment strategy
This chapter discusses how to use metformin in the
treatment of ESCC. Of course, further study is needed, but it is
beneficial to consider this aspect.
The results of clinical trials should be given
particular attention. As mentioned, low doses of metformin
administered preoperatively improved TIME, although it did not
improve tumor growth or apoptosis (8), so perioperative metformin
administration is likely beneficial. Especially in diabetic
patients who are not on metformin, it is advisable to change the
therapeutic agent to metformin.
In addition, since the prognosis was improved in
treatment with trimodality therapy (30), it is desirable to administer
metformin after surgery. However, when 5-FU plus cisplatin is used
as chemotherapy, it might be necessary to switch to cisplatin alone
or different combination drugs, as metformin seems to produce 5-FU
resistance. However, the choice of concomitant drug is tough.
Docetaxel is under-reported, and 2DG needs further investigation.
Nivolumab is most likely useful because it improves the immune
status, and this also needs further investigation.
Therefore, even in the absence of diabetes
complications, it would make sense to take metformin under careful
follow-up, with attention to the above items, to improve treatment
outcomes.
7. Conclusion
In this review, we comprehensively searched and
summarized studies conducted on the effects of metformin on ESCC.
Many studies have reported that metformin exerts antitumor effects;
however, its actions are diverse. Hanahan and Weinberg identified
important cancer properties as hallmarks of cancer (41), and according to their categories,
metformin has been reported to affect cell proliferation, escape
from cell death, abnormal glucose metabolism, abnormal immune
function, increased inflammation, increased metastatic invasion,
and increased angiogenesis. Therefore, metformin is a promising
adjunct drug for treating these types of cancer, targeting their
specific hallmarks. Many studies have reported the enhanced
therapeutic effects of combination therapy, such as improved
efficacy of metabolic inhibitors and sensitivity to chemotherapy
and radiotherapy, thus nominating metformin as a promising
therapeutic approach for ESCC.
Although this paper is only a narrative review based
on the literature, metformin is expected to be helpful as an
adjunct to the treatment of ESCC, given its clinical effectiveness
in preventing ESCC and ultimately improving ESCC treatment
outcomes. Metformin is also an appealing therapeutic approach as it
is already in use clinically for the treatment of DM and is more
likely to be approved as a cancer treatment than a completely novel
drug. However, as mentioned earlier, the effects of metformin are
diverse and do not necessarily lead to antitumor effects or
improved treatment outcomes. Therefore, when treating ESCC, the use
of metformin depends on the choice of the treatment approach and
its underlying mechanism. In particular, chemotherapy using
cisplatin, immunotherapy, and metabolic therapy with 2DG should be
further investigated in combination with metformin.
Acknowledgements
Not applicable.
Funding
Funding: This study was supported by Surgery II Scholarship
Donation from the Department of Frontier Surgery, Graduate School
of Medicine, Chiba University (grant no. J09KF00261).
Availability of data and materials
Not applicable.
Authors' contributions
NS and MK conceived, designed and wrote the
manuscript. KM, TT, KH and GO critically reviewed and revised the
manuscript. HM conceived, designed and supervised the work. Data
authentication is not applicable. All authors read and approved the
final version of the manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Delman AM, Ammann AM, Turner KM, Vaysburg
DM and Van Haren RM: A narrative review of socioeconomic
disparities in the treatment of esophageal cancer. J Thorac Dis.
13:3801–3808. 2021.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Pennathur A, Gibson MK, Jobe BA and
Luketich JD: Oesophageal carcinoma. Lancet. 381:400–412.
2013.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Duan XF, Tang P, Shang XB, Jiang HJ and Yu
ZT: The prevalence of lymph node metastasis for pathological T1
esophageal cancer: A retrospective study of 143 cases. Surg Oncol.
27:1–6. 2018.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Tachimori Y, Ozawa S, Numasaki H,
Fujishiro M, Matsubara H, Oyama T, Shinoda M, Toh Y, Udagawa H and
Uno T: Registration Committee for Esophageal Cancer of the Japan
Esophageal Society. Comprehensive registry of esophageal cancer in
Japan, 2009. Esophagus. 13:110–137. 2016.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Kourelis TV and Siegel RD: Metformin and
cancer: New applications for an old drug. Med Oncol. 29:1314–1327.
2012.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Tahrani AA, Varughese GI, Scarpello JH and
Hanna FWF: Metformin, heart failure, and lactic acidosis: Is
metformin absolutely contraindicated? BMJ. 335:508–512.
2007.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Micic D, Cvijovic G, Trajkovic V, Duntas
LH and Polovina S: Metformin: Its emerging role in oncology.
Hormones (Athens). 10:5–15. 2011.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Wang S, Lin Y, Xiong X, Wang L, Guo Y,
Chen Y, Chen S, Wang G, Lin P, Chen H, et al: Low-dose metformin
reprograms the tumor immune microenvironment in human esophageal
cancer: Results of a phase II clinical trial. Clin Cancer Res.
26:4921–4932. 2020.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Hardie DG: Molecular pathways: Is AMPK a
friend or a foe in cancer? Clin Cancer Res. 21:3836–3840.
2015.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Fan H, Yu X, Zou Z, Zheng W, Deng X, Guo
L, Jiang W, Zhan Q and Lu SH: Metformin suppresses the esophageal
carcinogenesis in rats treated with NMBzA through inhibiting
AMPK/mTOR signaling pathway. Carcinogenesis. 40:669–679.
2019.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Wang F, Ding X, Wang T, Shan Z, Wang J, Wu
S, Chi Y, Zhang Y, Lv Z, Wang L and Fan Q: Metformin inhibited
esophageal squamous cell carcinoma proliferation in vitro and in
vivo and enhanced the anti-cancer effect of cisplatin. PLoS One.
12(e0174276)2017.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Nakayama A, Ninomiya I, Harada S, Tsukada
T, Okamoto K, Nakanuma S, Sakai S, Makino I, Kinoshita J, Hayashi
H, et al: Metformin inhibits the radiation-induced invasive
phenotype of esophageal squamous cell carcinoma. Int J Oncol.
49:1890–1898. 2016.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Wang L, Du L, Xiong X, Lin Y, Zhu J, Yao
Z, Wang S, Guo Y, Chen Y, Geary K, et al: Repurposing
dextromethorphan and metformin for treating nicotine-induced cancer
by directly targeting CHRNA7 to inhibit JAK2/STAT3/SOX2 signaling.
Oncogene. 40:1974–1987. 2021.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Feng Y, Ke C, Tang Q, Dong H, Zheng X, Lin
W, Ke J, Huang J, Yeung SCJ and Zhang H: Metformin promotes
autophagy and apoptosis in esophageal squamous cell carcinoma by
downregulating Stat3 signaling. Cell Death Dis.
5(e1088)2014.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Sekino N, Kano M, Matsumoto Y, Sakata H,
Akutsu Y, Hanari N, Murakami K, Toyozumi T, Takahashi M, Otsuka R,
et al: Antitumor effects of metformin are a result of inhibiting
nuclear factor kappa B nuclear translocation in esophageal squamous
cell carcinoma. Cancer Sci. 109:1066–1074. 2018.PubMed/NCBI View Article : Google Scholar
|
|
16
|
He Y, Tan X, Hu H, Wang Q, Hu X, Cai X,
Guan Y, Chen B and Jing X: Metformin inhibits the migration and
invasion of esophageal squamous cell carcinoma cells by
downregulating the protein kinase B signaling pathway. Oncol Lett.
15:2939–2945. 2018.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Noto H, Goto A, Tsujimoto T and Noda M:
Cancer risk in diabetic patients treated with metformin: A
systematic review and meta-analysis. PLoS One.
7(e33411)2012.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Wang QL, Santoni G, Ness-Jensen E,
Lagergren J and Xie SH: Association between metformin use and risk
of esophageal squamous cell carcinoma in a population-based cohort
study. Am J Gastroenterol. 115:73–78. 2020.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Liang F, Wang YG and Wang C: Metformin
inhibited growth, invasion and metastasis of esophageal squamous
cell carcinoma in vitro and in vivo. Cell Physiol Biochem.
51:1276–1286. 2018.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Cai X, Hu X, Tan X, Cheng W, Wang Q, Chen
X, Guan Y, Chen C and Jing X: Metformin induced AMPK activation,
G0/G1 phase cell cycle arrest and the inhibition of growth of
esophageal squamous cell carcinomas in vitro and in vivo. PLoS One.
10(e0133349)2015.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Wang L, Li K, Lin X, Yao Z, Wang S, Xiong
X, Ning Z, Wang J, Xu X, Jiang Y, et al: Metformin induces human
esophageal carcinoma cell pyroptosis by targeting the miR-497/PELP1
axis. Cancer Lett. 450:22–31. 2019.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Yu H, Bian X, Gu D and He X: Metformin
synergistically enhances cisplatin-induced cytotoxicity in
esophageal squamous cancer cells under glucose-deprivation
conditions. Biomed Res Int. 2016(8678634)2016.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Peng J, Jing X, Wu J, Hong D, Hu X, Wang
Q, Hu H and Cai X: Metformin's effects on apoptosis of esophageal
carcinoma cells and normal esophageal epithelial cells: An in vitro
comparative study. Biomed Res Int. 2020(1068671)2020.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Yang Y, Jin G, Liu H, Liu K, Zhao J, Chen
X, Wang D, Bai R, Li X, Jang Y, et al: Metformin inhibits
esophageal squamous cell carcinoma-induced angiogenesis by
suppressing JAK/STAT3 signaling pathway. Oncotarget. 8:74673–74687.
2017.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Kunisada Y, Eikawa S, Tomonobu N, Domae S,
Uehara T, Hori S, Furusawa Y, Hase K, Sasaki A and Udono H:
Attenuation of CD4+CD25+ regulatory T cells
in the tumor microenvironment by metformin, a type 2 diabetes drug.
EBioMedicine. 25:154–164. 2017.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Gatenby RA and Gillies RJ: Glycolysis in
cancer: A potential target for therapy. Int J Biochem Cell Biol.
39:1358–1366. 2007.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Zhu Z, Jiang W, McGinley JN and Thompson
HJ: 2-Deoxyglucose as an energy restriction mimetic agent: Effects
on mammary carcinogenesis and on mammary tumor cell growth in
vitro. Cancer Res. 65:7023–7030. 2005.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Shafaee A, Pirayesh Islamian J, Zarei D,
Mohammadi M, Nejati-Koshki K, Farajollahi A, Aghamiri SMR, Rahmati
Yamchi M, Baradaran B and Asghari Jafarabadi M: Induction of
apoptosis by a combination of 2-deoxyglucose and metformin in
esophageal squamous cell carcinoma by targeting cancer cell
metabolism. Iran J Med Sci. 44:99–107. 2019.PubMed/NCBI
|
|
29
|
Liu CC, Chou KT, Hsu JW, Lin JH, Hsu TW,
Yen DHT, Hung SC and Hsu HS: High metabolic rate and stem cell
characteristics of esophageal cancer stem-like cells depend on the
Hsp27-AKT-HK2 pathway. Int J Cancer. 145:2144–2156. 2019.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Van De Voorde L, Janssen L, Larue R,
Houben R, Buijsen J, Sosef M, Vanneste B, Schraepen MC, Berbée M
and Lambin P: Can metformin improve ‘the tomorrow’ of patients
treated for oesophageal cancer? Eur J Surg Oncol. 41:1333–1339.
2015.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Li PD, Liu Z, Cheng TT, Luo WG, Yao J,
Chen J, Zou ZW, Chen LL, Ma C and Dai XF: Redox-dependent
modulation of metformin contributes to enhanced sensitivity of
esophageal squamous cell carcinoma to cisplatin. Oncotarget.
8:62057–62068. 2017.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Damelin LH, Jivan R, Veale RB, Rousseau AL
and Mavri-Damelin D: Metformin induces an intracellular reductive
state that protects oesophageal squamous cell carcinoma cells
against cisplatin but not copper-bis(thiosemicarbazones). BMC
Cancer. 14(314)2014.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Mynhardt C, Damelin LH, Jivan R, Peres J,
Prince S, Veale RB and Mavri-Damelin D: Metformin-induced
alterations in nucleotide metabolism cause 5-fluorouracil
resistance but gemcitabine susceptibility in oesophageal squamous
cell carcinoma. J Cell Biochem. 119:1193–1203. 2018.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Chevalier B, Pasquier D, Lartigau EF,
Chargari C, Schernberg A, Jannin A, Mirabel X, Vantyghem MC and
Escande A: Metformin: (Future) best friend of the radiation
oncologist? Radiother Oncol. 151:95–105. 2020.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Chen F, Xu B, Li J, Yang X, Gu J, Yao X
and Sun X: Hypoxic tumour cell-derived exosomal miR-340-5p promotes
radioresistance of oesophageal squamous cell carcinoma via KLF10. J
Exp Clin Cancer Res. 40(38)2021.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Fransgaard T, Thygesen LC and Gögenur I:
Association between metformin use after surgery for colorectal
cancer and oncological outcomes: A nationwide register-based study.
Int J Cancer. 143:63–72. 2018.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Kaushik D, Karnes RJ, Eisenberg MS, Rangel
LJ, Carlson RE and Bergstralh EJ: Effect of metformin on prostate
cancer outcomes after radical prostatectomy. Urol Oncol.
32:43.e1–e7. 2014.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Chan KM, Kuo CF, Hsu JT, Chiou MJ, Wang
YC, Wu TH, Lee CF, Wu TJ, Chou HS and Lee WC: Metformin confers
risk reduction for developing hepatocellular carcinoma recurrence
after liver resection. Liver Int. 37:434–441. 2017.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Kaltenmeier C, Morocco B, Yazdani H, Reitz
K, Meyer K, Molinari M, Geller D and Tohme S: Impact of metformin
use on survival in patients undergoing liver resection for
colorectal cancer metastases. Am Surg. 87:1766–1774.
2021.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Luo CS, Lin Y, Zhou WP and Shi J: Survival
advantage associated with metformin usage in hepatocellular
carcinoma patients with diabetes mellitus receiving radical
resection: A propensity score matching analysis. Eur J
Gastroenterol Hepatol. 32:1030–1035. 2020.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Hanahan D and Weinberg RA: Hallmarks of
cancer: The next generation. Cell. 144:646–674. 2011.PubMed/NCBI View Article : Google Scholar
|