Introduction
Soft tissue sarcomas (STSs) are rare malignant
tumours, which account for approximately 1% of adult cancers and
include more than 50 histologic subtypes (1,2).
Although several treatments are available, the prognosis of
metastatic STSs is poor, with a median overall survival (OS) of 12
months (3). Undifferentiated
pleomorphic sarcoma (UPS) is characterized by no identifiable line
of differentiation and multiple cellular patternless forms. The
prognosis of patients with UPS with recurrence and metastasis is
poor (4,5).
Pazopanib is an oral tyrosine kinase inhibitor (TKI)
with activity against vascular endothelial growth factor receptor 1
(VEGFR1), VEGFR2, VEGFR3, platelet-derived growth factor receptor A
(PDGFRA), PDGFRB, and stem cell factor receptor (KIT). Pazopanib
has been approved for the treatment of advanced STSs (6). The phase III PALETTE trial included
patients with non-adipocytic STSs and progressive disease after
standard chemotherapy. Pazopanib improved progression-free survival
(PFS) by 3 months than placebo in this trial. However, OS did not
improve (2). The genetic features
of patients with STSs who responded to pazopanib are unclear.
Herein, we report the case of a patient with advanced UPS
characterized by coamplification of PDGFRA, VEGFR2,
and KIT genes who experienced a lasting and complete
response to pazopanib.
Case report
A 51-year-old man presented with abdominal pain and
was admitted to Mitsui Memorial Hospital (Tokyo, Japan) in March
2019, 10 months before visiting our hospital, Osaka International
Cancer Institute (Osaka, Japan). Computed tomography (CT) revealed
an unexplained intra-abdominal haemorrhage close to the pancreatic
head. Pain and haemorrhage spontaneously improved with rest. One
month before admission, the patient experienced swelling and pain
in the left thigh. Magnetic resonance imaging (MRI) revealed a
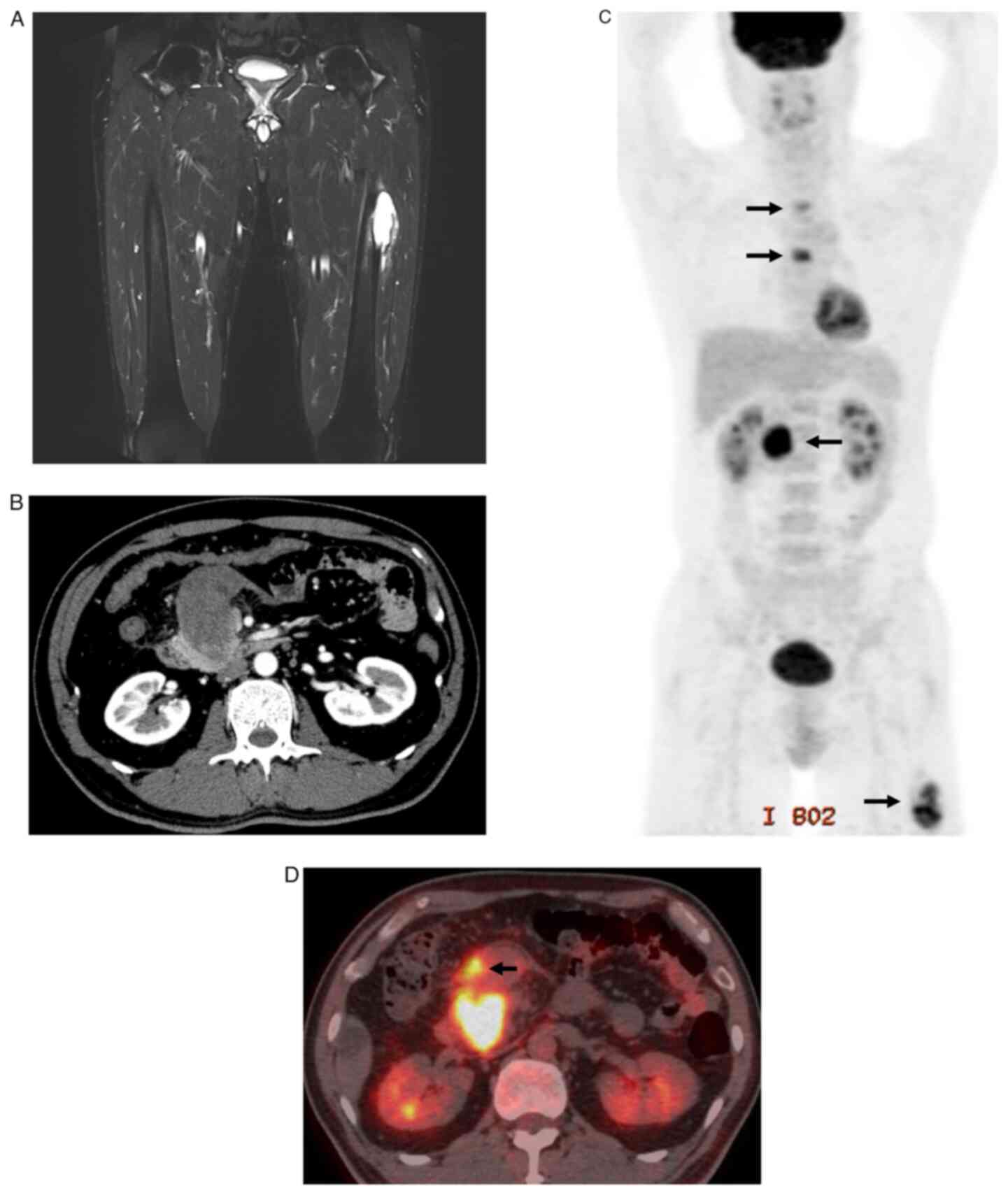
6.5-cm femoral bone tumour in the left thigh with haemorrhage and
cortex osteolysis (Fig. 1A).
Furthermore, 3 weeks before visiting our hospital, the abdominal
pain reoccurred. CT revealed a retroperitoneal tumour with bleeding
located in the groove adjacent to the pancreatic head (Fig. 1B). Hypermetabolism in the
retroperitoneal tumour was detected using positron emission
tomography-CT (PET/CT) at the time of the hospital visit. In
addition, pancreatic head lymph nodes, second/fifth thoracic
vertebrae, and left femoral bone tumours were detected (Fig. 1C and D). Blood test did not show any abnormal
condition, such as elevated inflammatory response, tumour marker
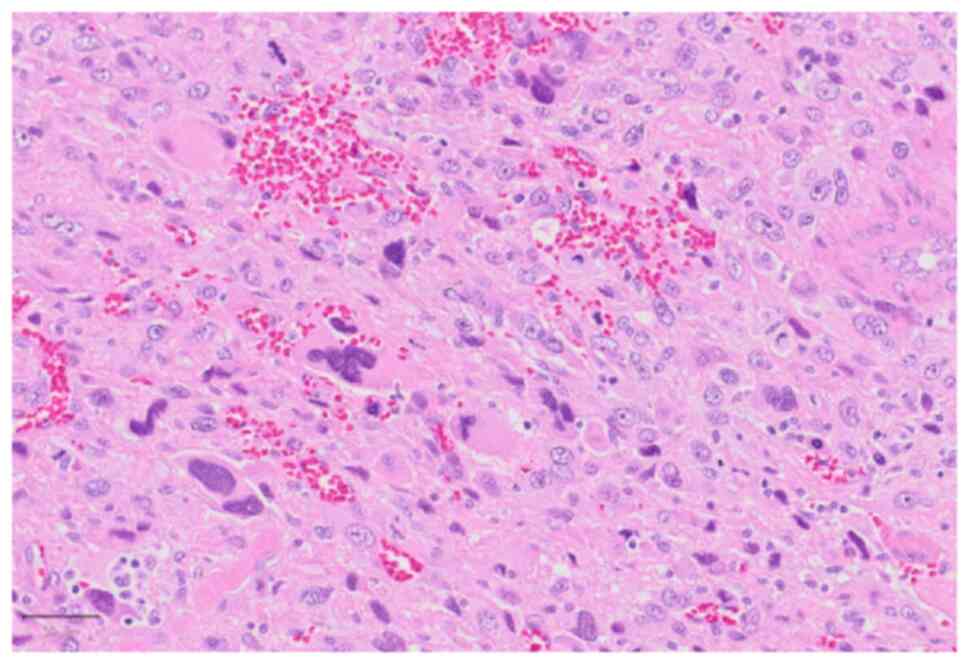
changes, or anaemia. Histological analysis of retroperitoneal and
left thigh tumour biopsies revealed pleomorphic cell proliferation
in a haphazard arrangement (Fig.
2). The tumour cells were focally positive for αSMA and CK
AE1/AE3 and negative for desmin, h-caldesmon, S-100, SOX10, and
MDM2. Based on the morphology, immune profile, and clinical
presentation, the patient was diagnosed with UPS arising from the
retroperitoneum with bone and lymph node metastases.
To prevent pathological fractures due to bone
metastases, the patient was treated with denosumab and radiation
therapy (35 Gy in 5 fractions) of the left femur and second/fifth
thoracic vertebrae. The patient was then treated with 75 mg/m²
doxorubicin administered on day 1 of a 21-day cycle for 8 cycles.
Due to fatigue, nausea, and vomiting, the dose was reduced to 80%
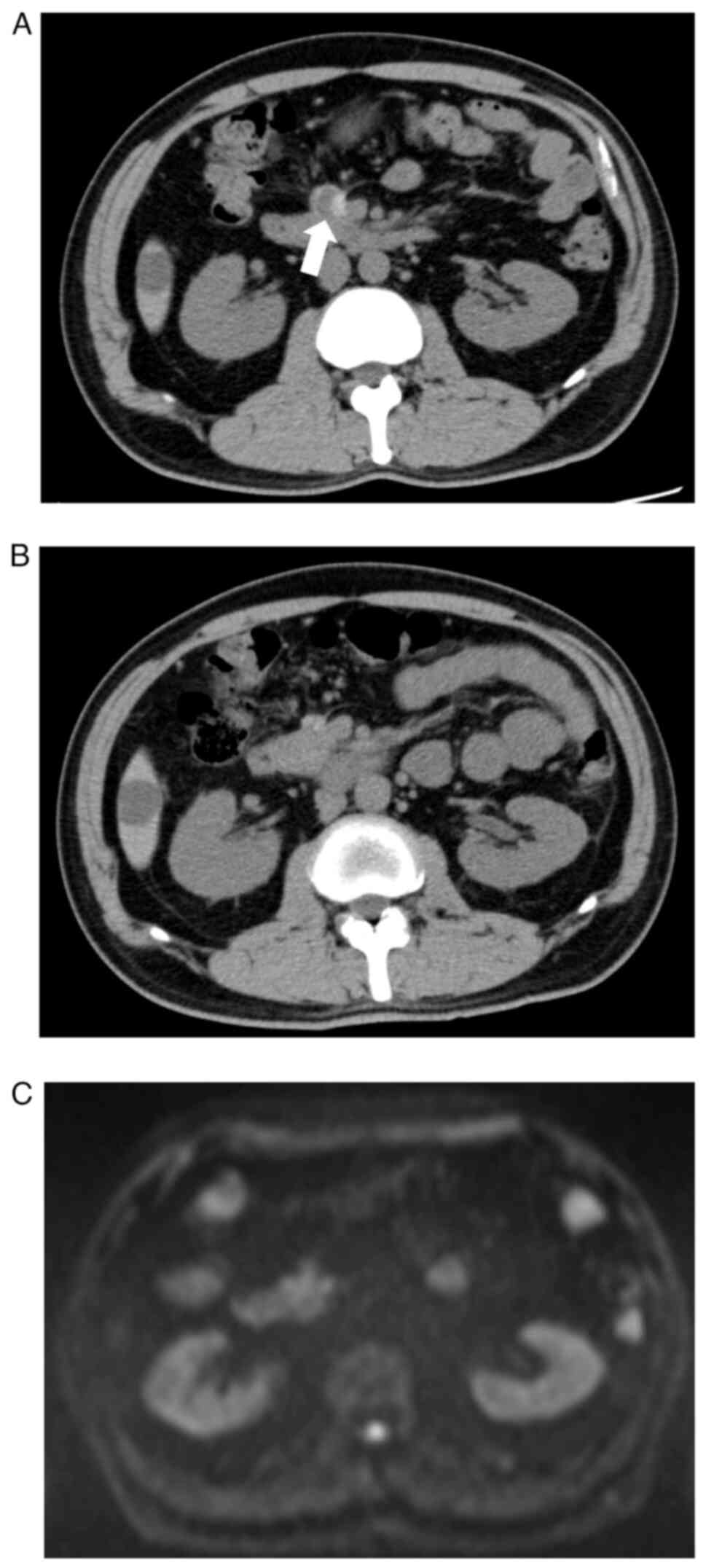
and then to 60%. After chemotherapy, the retroperitoneal primary
lesion and bone metastases shrank and no new lesions emerged,
suggesting tumour partial response (PR) (Fig. 3A). To determine the second-line
chemotherapy after doxorubicin reached the upper limit, a
comprehensive genomic profiling test (FoundationOne®
CDx, Foundation Medicine, Inc., Cambridge, MA, USA) was performed
when the patient was receiving doxorubicin. FoundationOne CDx gene
profiling can comprehensively identify 324 gene mutations using a
next-generation sequencer and evaluate biomarkers, such as
microsatellite status and tumour mutation burden (detailed
information available at https://www.foundationmedicine.com/genomic-testing/foundation-one-cdx).
Coamplification of the PDGFRA, VEGFR2, and KIT
genes was detected (Table I).
Pazopanib treatment was recommended by the expert, which is an
inhibitor of receptor tyrosine kinases (RTKs), based on
VEGFR2 amplification. Additionally, because pazopanib had
inhibitory effects on VEGFR2, PDGFRA and KIT, it could be suitable.
Therefore, 600 mg/day pazopanib was administered as second-line
chemotherapy. However, the dose was reduced to 400 mg/day because
of severe fatigue and diarrhoea. Surprisingly, the retroperitoneal
primary tumour shrank more, and no new lesions developed for 3
years after the start of pazopanib treatment, indicating complete
response (CR) (Fig. 3B and
C). We performed follow-up imaging
of the entire body by MRI and CT instead of PET-CT and did not
observe any recurrence or metastasis after CR. Although grade 1
hypothyroidism and grade 2 hypertension were observed during 400
mg/day pazopanib treatment, no serious adverse events such as grade
3 or higher cytopenia occurred. Spontaneous pneumothorax is an
evident and complicated side effect of pazopanib, and its
occurrence is associated with lung metastasis (7,8).
However, in this case, no lung metastasis or spontaneous
pneumothorax was observed. Denosumab was discontinued within 3
years of treatment initiation.
 | Table IGenomic findings. |
Table I
Genomic findings.
| Gene | Alteration |
|---|
| ATM | S2283 |
| DNMT3A | R882H |
| TP53 | R267P |
| CDKN2A/B | Loss |
| KIT | Amplification |
| PDGFRA | Amplification |
| VEGFR2 | Amplification |
| NRAS | Amplification |
| CDK6 | Amplification |
Discussion
Matching treatment strategies with tumour biology is
a central principle of precision medicine, and the ectopic
activation of RTKs is a universal theme in oncogenesis. Oncogenic
fusions involving RTKs such as anaplastic lymphoma kinase
(ALK) and proto-oncogene 1 (ROS1), and small
deletions in epidermal growth factor receptor (EGFR) predict
patient response to target-matched TKIs, especially in the context
of non-small-cell lung cancer (9,10).
Amplification of wild-type RTK genes, such as human epidermal
growth factor receptor 2 (HER2), in breast and gastric
cancers drives oncogenesis, and RTKs are therapeutic targets
(11,12). Thus, the coamplification of
PDGFRA, VEGFR2, and KIT, which is related to
chromosome 4q12 amplification, may be an oncogenic driver and
therapeutic target (13). Phase II
trial of axitinib, a TKI of PDGFRs, VEGFR2, and KIT, included
patients with recurrent adenoid cystic carcinoma. The longest
responder (nearly four times longer than the median PFS for the
study cohort) in this trial was a patient with amplified
PDGFRA, VEGFR2, and KIT (14). We detected the coamplification of
PDGFRA, VEGFR2, and KIT in UPS. Therefore, we
treated the patient with pazopanib with second-line therapy.
Interestingly, CR was achieved and maintained for 3 years with
pazopanib. To the best of our knowledge, this is the first report
to demonstrate that pazopanib was effective against STS with
coamplified PDGFRA, VEGFR2, and KIT.
PDGFRA, VEGFR2, and KIT genes
are located on chromosomal locus 4q12; hence, the concurrent
amplification of these genes may be due to the overall
amplification of chromosome 4q12. Coamplification of these RTKs is
present in 0.86% of TCGA cases across all cancers and is more
common in sarcomas and central nervous system neoplasms (13). In the Sarcoma Genome Project
dataset, putative high-level amplifications of PDGFRA,
VEGFR2, and KIT were reported in 3, 2 and 2.4%,
respectively, of cancer cases (15). Although rare in absolute numbers,
the coamplification frequency of these genes is consistent with
other uncommon but highly targetable oncogenic kinase alterations,
such as NTRK (16).
Pazopanib, which is the only approved noncytotoxic
STS therapy, is an orally available inhibitor of multiple RTKs,
including VEGFR1-3, PDGFRA/B, FGFR1/3/4, and KIT. The antitumor
activity of pazopanib is attributed to antiangiogenic effects and
inhibition of pro-proliferative signals mediated by RTKs on the
surface of STS cells (17-19).
Several clinical trials demonstrated survival benefits of pazopanib
in patients with STS. In the PALETTE trial with 246 patients, none
of the patients with STS had CR, 6% (14) had PR, 67% (164) had stable disease
(SD), and 23% (57) had progressive disease (PD) (2). In the placebo
arm, 0% (0 of 123) of patients had CR, 0% (0) had PR, 38% (47) had
SD, and 57% (70) had PD (2). In a study by the Japanese
Musculoskeletal Oncology Group, none of the 125 enrolled patients
achieved CR (8). Therefore, achieving CR with pazopanib is rare in
patients with STS. CR can be obtained in this case; however, it was
not maintained in advanced UPS as per our experience. Furthermore,
no standard treatment strategy has been established for treating
sarcoma. Therefore, making a decision regarding the discontinuation
of pazopanib was challenging. However, because CR persisted for a
long time, discontinuation of pazopanib will be an important issue
for future consideration.
The challenge of establishing reliable biomarkers
for responsiveness to pazopanib has been addressed by several
studies. Retrospective analyses of two EORTC studies and a
real-world cohort of pazopanib-treated patients revealed that
specific histologic types, including synovial sarcoma and
desmoplastic small round cell tumour, and clinical parameters,
including good performance status, low or intermediate tumour
grade, and a normal haemoglobin levels, correlated with better
outcomes (20,21). Several studies also explored the
importance of molecular characteristics in selecting patients who
may benefit from pazopanib. Heilig et al (22) analysed the molecular profiles and
clinical outcomes of sarcoma patients treated with pazopanib and
demonstrated that VEGFR2-high, NTRK3-high, and
IGF1R-low mRNA levels were independently associated with
PFS. In patients with advanced STS who exhibited short-term
high-grade PR or long-term SD, Suehara et al (23) demonstrated that amplified
GLI1 and elevated PDGFRB phosphorylation levels were linked
to high antitumor activity of pazopanib. Using gene panel
sequencing in 19 pazopanib-treated patients, Koehler et al
(24) demonstrated that
TP53 mutations correlated with better outcomes after
pazopanib therapy. The present case suggests that STSs with
amplified PDGFRA, VEGFR2, and KIT are rare;
however, patients with these features may benefit from pazopanib
therapy. A limitation of this study was the absence of
immunohistological analysis, including markers such as PDGFRA,
VEGFR2, KIT, VEGF, and PDGF. Therefore, further studies are
required to confirm the benefits of pazopanib in patients with
coamplification of PDGFRA, VEGFR2, and KIT,
and to establish predictive markers for pazopanib sensitivity.
Acknowledgements
Not applicable.
Funding
Funding: This research was supported by MEXT KAKENHI (grant no.
JP22K09421).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
HM, SN, KY and ST made substantial contributions to
the conception and design of the study, acquisition of data, and
analysis and interpretation of data. HM and SN confirm the
authenticity of all the raw data. RS, YI, HaT, MW, TW, HiT, HO, TY
and SK contributed to data acquisition, conception, and reviewed
and edited the manuscript. All authors read and approved the final
version of the manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Written informed consent was obtained from the
patient to publish this case report and all accompanying
images.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Casali PG, Abecassis N, Aro HT, Bauer S,
Biagini R, Bielack S, Bonvalot S, Boukovinas I, Bovee JVMG,
Brodowicz T, et al: Soft tissue and visceral sarcomas: ESMO-EURACAN
clinical practice guidelines for diagnosis, treatment and
follow-up. Ann Oncol. 29:iv51–iv67. 2018.PubMed/NCBI View Article : Google Scholar
|
|
2
|
van der Graaf WT, Blay JY, Chawla SP, Kim
DW, Bui-Nguyen B, Casali PG, Schöffski P, Aglietta M, Staddon AP,
Beppu Y, et al: Pazopanib for metastatic soft-tissue sarcoma
(PALETTE): A randomised, double-blind, placebo-controlled phase 3
trial. Lancet. 379:1879–1886. 2012.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Italiano A, Mathoulin-Pelissier S, Cesne
AL, Terrier P, Bonvalot S, Collin F, Michels JJ, Blay JY, Coindre
JM and Bui B: Trends in survival for patients with metastatic
soft-tissue sarcoma. Cancer. 117:1049–1054. 2011.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Vodanovich DA, Spelman T, May D, Slavin J
and Choong PFM: Predicting the prognosis of undifferentiated
pleomorphic soft tissue sarcoma: A 20-year experience of 266 cases.
ANZ J Surg. 89:1045–1050. 2019.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Winchester D, Lehman J, Tello T, Chimato
N, Hocker T, Kim S, Chang J, Markey J, Yom SS, Ryan W, et al:
Undifferentiated pleomorphic sarcoma: Factors predictive of adverse
outcomes. J Am Acad Dermatol. 79:853–859. 2018.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Schutz FA, Choueiri TK and Sternberg CN:
Pazopanib: Clinical development of a potent anti-angiogenic drug.
Crit Rev Oncol Hematol. 77:163–171. 2011.PubMed/NCBI View Article : Google Scholar
|
|
7
|
No authors listed. Pazopanib and
soft-tissue sarcomas. Too toxic. Prescrire Int. 22:145–147.
2013.PubMed/NCBI
|
|
8
|
Nakamura T, Matsumine A, Kawai A, Araki N,
Goto T, Yonemoto T, Sugiura H, Nishida Y, Hiraga H, Honoki K, et
al: The clinical outcome of pazopanib treatment in Japanese
patients with relapsed soft tissue sarcoma: A Japanese
musculoskeletal oncology group (JMOG) study. Cancer. 122:1408–1416.
2016.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Garraway LA, Verweij J and Ballman KV:
Precision oncology: An overview. J Clin Oncol. 31:1803–1805.
2013.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Shaw AT, Hsu PP, Awad MM and Engelman JA:
Tyrosine kinase gene rearrangements in epithelial malignancies. Nat
Rev Cancer. 13:772–787. 2013.PubMed/NCBI View
Article : Google Scholar
|
|
11
|
Santarius T, Shipley J, Brewer D, Stratton
MR and Cooper CS: A census of amplified and overexpressed human
cancer genes. Nat Rev Cancer. 10:59–64. 2010.PubMed/NCBI View
Article : Google Scholar
|
|
12
|
Bagci O and Kurtgöz S: Amplification of
cellular oncogenes in solid tumors. N Am J Med Sci. 7:341–346.
2015.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Disel U, Madison R, Abhishek K, Chung JH,
Trabucco SE, Matos AO, Frampton GM, Albacker LA, Reddy V,
Karadurmus N, et al: The pan-cancer landscape of coamplification of
the tyrosine kinases KIT, KDR, and PDGFRA. Oncologist. 25:e39–e47.
2020.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Ho AL, Dunn L, Sherman EJ, Fury MG, Baxi
SS, Chandramohan R, Dogan S, Morris LG, Cullen GD, Haque S, et al:
A phase II study of axitinib (AG-013736) in patients with incurable
adenoid cystic carcinoma. Ann Oncol. 27:1902–1908. 2016.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Barretina J, Taylor BS, Banerji S, Ramos
AH, Lagos-Quintana M, Decarolis PL, Shah K, Socci ND, Weir BA, Ho
A, et al: Subtype-specific genomic alterations define new targets
for soft-tissue sarcoma therapy. Nat Genet. 42:715–721.
2010.PubMed/NCBI View
Article : Google Scholar
|
|
16
|
Drilon A, Laetsch TW, Kummar S, DuBois SG,
Lassen UN, Demetri GD, Nathenson M, Doebele RC, Farago AF, Pappo
AS, et al: Efficacy of larotrectinib in TRK fusion-positive cancers
in adults and children. N Engl J Med. 378:731–739. 2018.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Kumar R, Knick VB, Rudolph SK, Johnson JH,
Crosby RM, Crouthamel MC, Hopper TM, Miller CG, Harrington LE,
Onori JA, et al: Pharmacokinetic-pharmacodynamic correlation from
mouse to human with pazopanib, a multikinase angiogenesis inhibitor
with potent antitumor and antiangiogenic activity. Mol Cancer Ther.
6:2012–2021. 2007.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Chellappan DK, Chellian J, Ng ZY, Sim YJ,
Theng CW, Ling J, Wong M, Foo JH, Yang GJ, Hang LY, et al: The role
of pazopanib on tumour angiogenesis and in the management of
cancers: A review. Biomed Pharmacother. 96:768–781. 2017.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Hamberg P, Verweij J and Sleijfer S:
(Pre-)clinical pharmacology and activity of pazopanib, a novel
multikinase angiogenesis inhibitor. Oncologist. 15:539–547.
2010.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Gelderblom H, Judson IR, Benson C,
Merimsky O, Grignani G, Katz D, Freivogel KW, Stein D, Jobanputra
M, Mungul A, et al: Treatment patterns and clinical outcomes with
pazopanib in patients with advanced soft tissue sarcomas in a
compassionate use setting: Results of the SPIRE study. Acta Oncol.
56:1769–1775. 2017.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Kasper B, Sleijfer S, Litière S, Marreaud
S, Verweij J, Hodge RA, Bauer S, Kerst JM and van der Graaf WTA:
Long-term responders and survivors on pazopanib for advanced soft
tissue sarcomas: Subanalysis of two European Organisation for
research and treatment of cancer (EORTC) clinical trials 62043 and
62072. Ann Oncol. 25:719–724. 2014.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Heilig CE, Laßmann A, Mughal SS, Mock A,
Pirmann S, Teleanu V, Renner M, Andresen C, Köhler BC, Aybey B, et
al: Gene expression-based prediction of pazopanib efficacy in
sarcoma. Eur J Cancer. 172:107–118. 2022.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Suehara Y, Kohsaka S, Yamaguchi S, Hayashi
T, Kurihara T, Sano K, Sasa K, Akaike K, Ueno T, Kojima S, et al:
Assessment of predictive biomarkers of the response to pazopanib
based on an integrative analysis of high-grade soft-tissue
sarcomas: Analysis of a tumor sample from a responder and patients
with other soft-tissue sarcomas. Clin Orthop Relat Res.
478:2461–2476. 2020.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Koehler K, Liebner D and Chen JL: TP53
mutational status is predictive of pazopanib response in advanced
sarcomas. Ann Oncol. 27:539–543. 2016.PubMed/NCBI View Article : Google Scholar
|