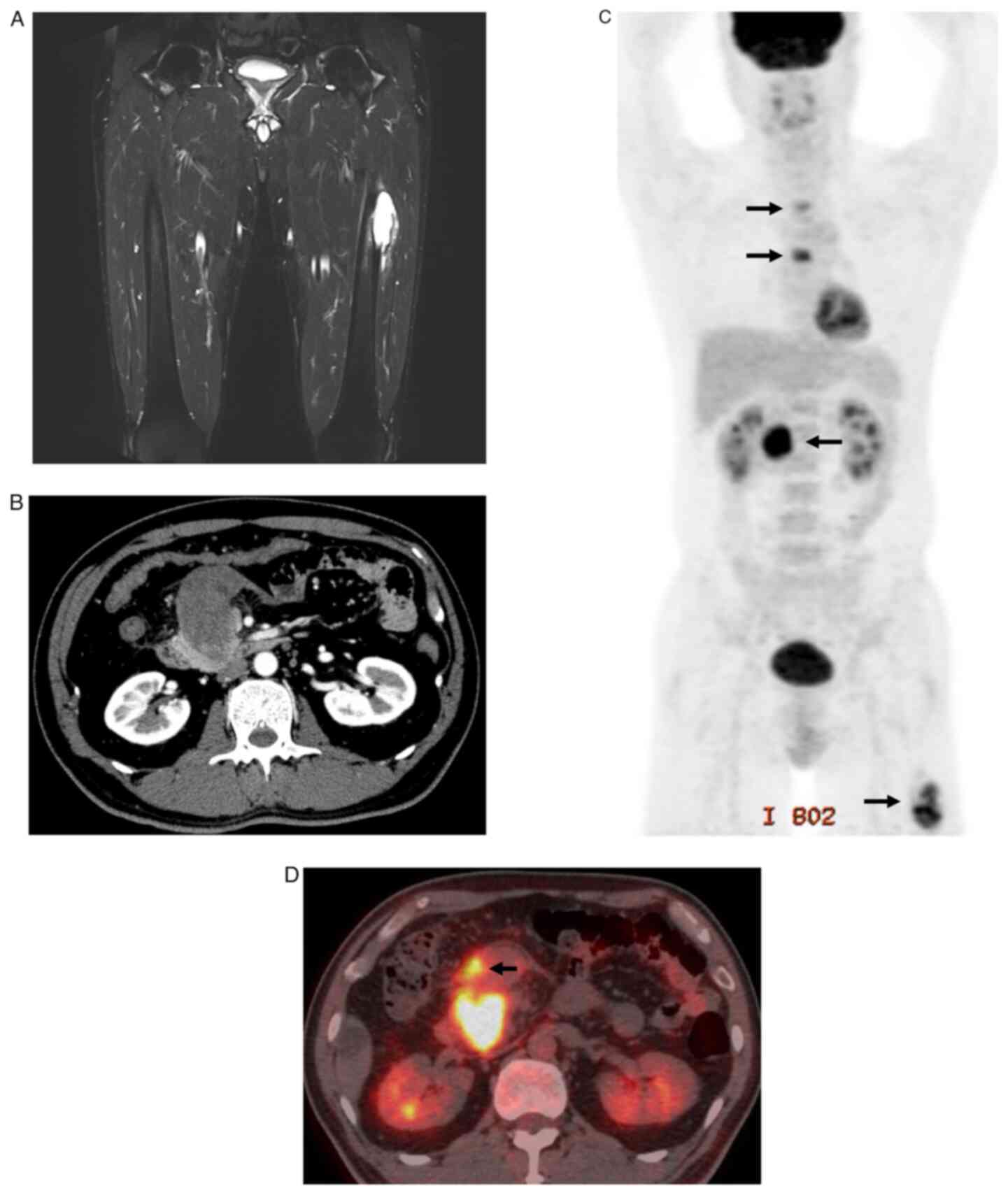
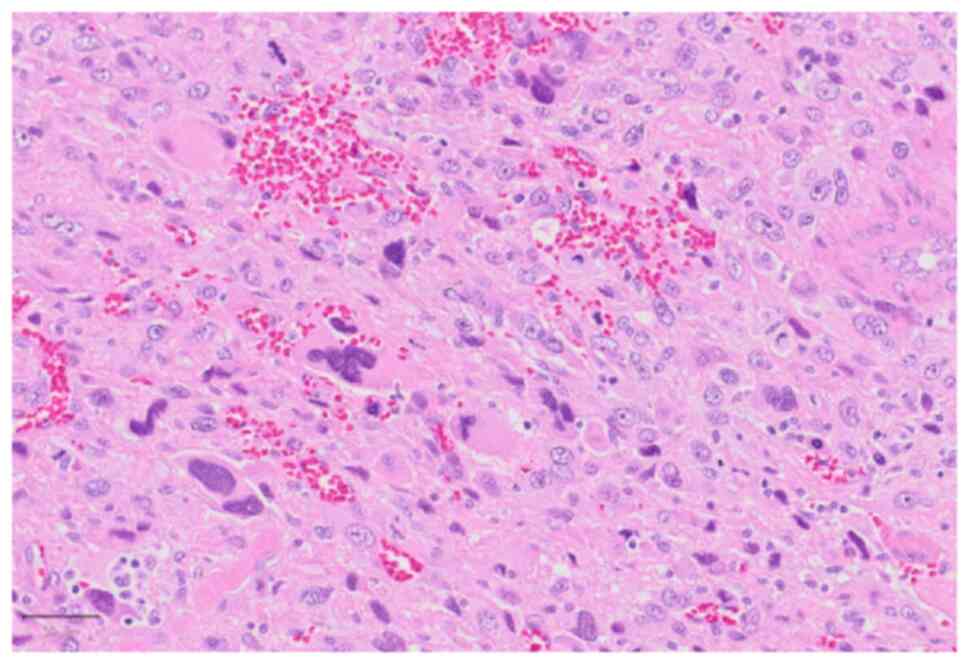
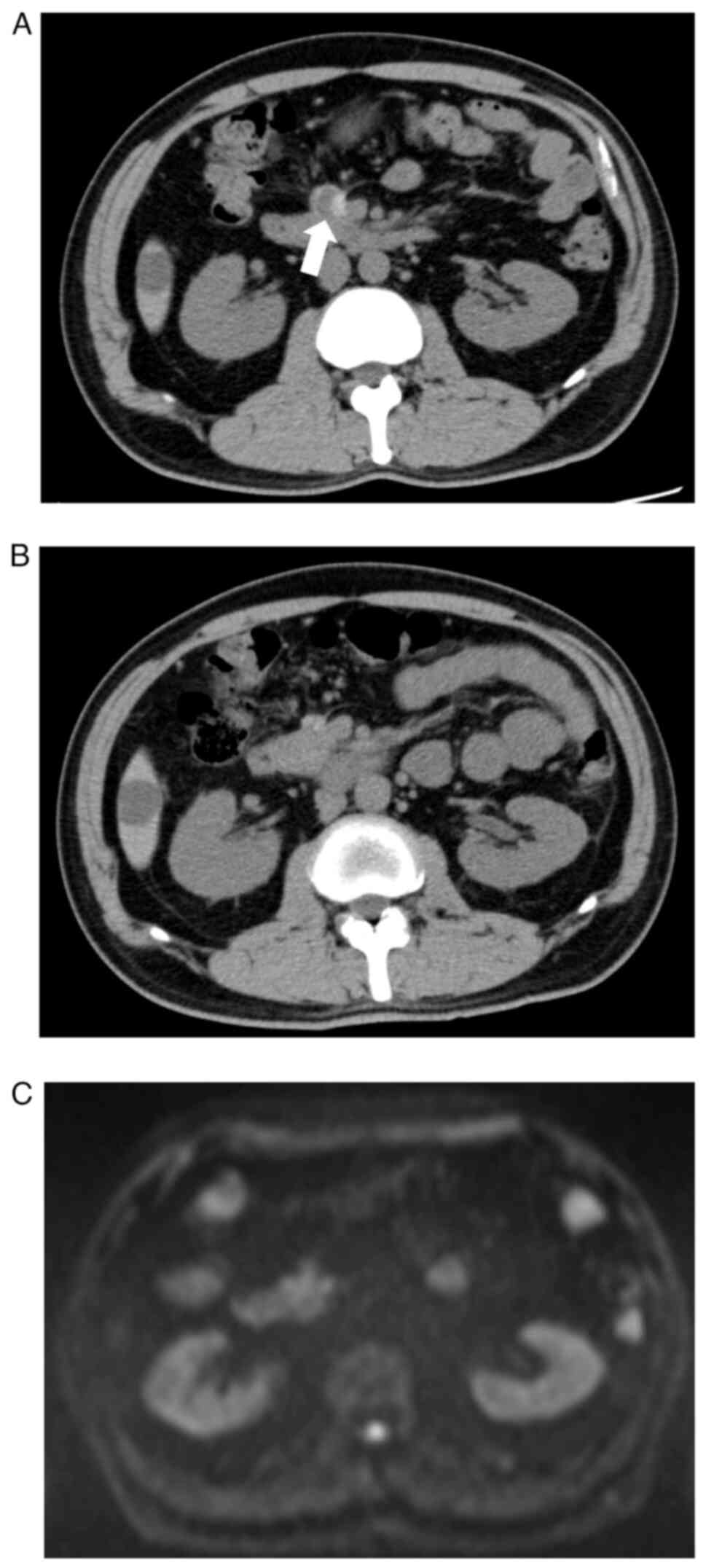
|
1
|
Casali PG, Abecassis N, Aro HT, Bauer S,
Biagini R, Bielack S, Bonvalot S, Boukovinas I, Bovee JVMG,
Brodowicz T, et al: Soft tissue and visceral sarcomas: ESMO-EURACAN
clinical practice guidelines for diagnosis, treatment and
follow-up. Ann Oncol. 29:iv51–iv67. 2018.PubMed/NCBI View Article : Google Scholar
|
|
2
|
van der Graaf WT, Blay JY, Chawla SP, Kim
DW, Bui-Nguyen B, Casali PG, Schöffski P, Aglietta M, Staddon AP,
Beppu Y, et al: Pazopanib for metastatic soft-tissue sarcoma
(PALETTE): A randomised, double-blind, placebo-controlled phase 3
trial. Lancet. 379:1879–1886. 2012.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Italiano A, Mathoulin-Pelissier S, Cesne
AL, Terrier P, Bonvalot S, Collin F, Michels JJ, Blay JY, Coindre
JM and Bui B: Trends in survival for patients with metastatic
soft-tissue sarcoma. Cancer. 117:1049–1054. 2011.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Vodanovich DA, Spelman T, May D, Slavin J
and Choong PFM: Predicting the prognosis of undifferentiated
pleomorphic soft tissue sarcoma: A 20-year experience of 266 cases.
ANZ J Surg. 89:1045–1050. 2019.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Winchester D, Lehman J, Tello T, Chimato
N, Hocker T, Kim S, Chang J, Markey J, Yom SS, Ryan W, et al:
Undifferentiated pleomorphic sarcoma: Factors predictive of adverse
outcomes. J Am Acad Dermatol. 79:853–859. 2018.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Schutz FA, Choueiri TK and Sternberg CN:
Pazopanib: Clinical development of a potent anti-angiogenic drug.
Crit Rev Oncol Hematol. 77:163–171. 2011.PubMed/NCBI View Article : Google Scholar
|
|
7
|
No authors listed. Pazopanib and
soft-tissue sarcomas. Too toxic. Prescrire Int. 22:145–147.
2013.PubMed/NCBI
|
|
8
|
Nakamura T, Matsumine A, Kawai A, Araki N,
Goto T, Yonemoto T, Sugiura H, Nishida Y, Hiraga H, Honoki K, et
al: The clinical outcome of pazopanib treatment in Japanese
patients with relapsed soft tissue sarcoma: A Japanese
musculoskeletal oncology group (JMOG) study. Cancer. 122:1408–1416.
2016.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Garraway LA, Verweij J and Ballman KV:
Precision oncology: An overview. J Clin Oncol. 31:1803–1805.
2013.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Shaw AT, Hsu PP, Awad MM and Engelman JA:
Tyrosine kinase gene rearrangements in epithelial malignancies. Nat
Rev Cancer. 13:772–787. 2013.PubMed/NCBI View
Article : Google Scholar
|
|
11
|
Santarius T, Shipley J, Brewer D, Stratton
MR and Cooper CS: A census of amplified and overexpressed human
cancer genes. Nat Rev Cancer. 10:59–64. 2010.PubMed/NCBI View
Article : Google Scholar
|
|
12
|
Bagci O and Kurtgöz S: Amplification of
cellular oncogenes in solid tumors. N Am J Med Sci. 7:341–346.
2015.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Disel U, Madison R, Abhishek K, Chung JH,
Trabucco SE, Matos AO, Frampton GM, Albacker LA, Reddy V,
Karadurmus N, et al: The pan-cancer landscape of coamplification of
the tyrosine kinases KIT, KDR, and PDGFRA. Oncologist. 25:e39–e47.
2020.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Ho AL, Dunn L, Sherman EJ, Fury MG, Baxi
SS, Chandramohan R, Dogan S, Morris LG, Cullen GD, Haque S, et al:
A phase II study of axitinib (AG-013736) in patients with incurable
adenoid cystic carcinoma. Ann Oncol. 27:1902–1908. 2016.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Barretina J, Taylor BS, Banerji S, Ramos
AH, Lagos-Quintana M, Decarolis PL, Shah K, Socci ND, Weir BA, Ho
A, et al: Subtype-specific genomic alterations define new targets
for soft-tissue sarcoma therapy. Nat Genet. 42:715–721.
2010.PubMed/NCBI View
Article : Google Scholar
|
|
16
|
Drilon A, Laetsch TW, Kummar S, DuBois SG,
Lassen UN, Demetri GD, Nathenson M, Doebele RC, Farago AF, Pappo
AS, et al: Efficacy of larotrectinib in TRK fusion-positive cancers
in adults and children. N Engl J Med. 378:731–739. 2018.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Kumar R, Knick VB, Rudolph SK, Johnson JH,
Crosby RM, Crouthamel MC, Hopper TM, Miller CG, Harrington LE,
Onori JA, et al: Pharmacokinetic-pharmacodynamic correlation from
mouse to human with pazopanib, a multikinase angiogenesis inhibitor
with potent antitumor and antiangiogenic activity. Mol Cancer Ther.
6:2012–2021. 2007.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Chellappan DK, Chellian J, Ng ZY, Sim YJ,
Theng CW, Ling J, Wong M, Foo JH, Yang GJ, Hang LY, et al: The role
of pazopanib on tumour angiogenesis and in the management of
cancers: A review. Biomed Pharmacother. 96:768–781. 2017.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Hamberg P, Verweij J and Sleijfer S:
(Pre-)clinical pharmacology and activity of pazopanib, a novel
multikinase angiogenesis inhibitor. Oncologist. 15:539–547.
2010.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Gelderblom H, Judson IR, Benson C,
Merimsky O, Grignani G, Katz D, Freivogel KW, Stein D, Jobanputra
M, Mungul A, et al: Treatment patterns and clinical outcomes with
pazopanib in patients with advanced soft tissue sarcomas in a
compassionate use setting: Results of the SPIRE study. Acta Oncol.
56:1769–1775. 2017.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Kasper B, Sleijfer S, Litière S, Marreaud
S, Verweij J, Hodge RA, Bauer S, Kerst JM and van der Graaf WTA:
Long-term responders and survivors on pazopanib for advanced soft
tissue sarcomas: Subanalysis of two European Organisation for
research and treatment of cancer (EORTC) clinical trials 62043 and
62072. Ann Oncol. 25:719–724. 2014.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Heilig CE, Laßmann A, Mughal SS, Mock A,
Pirmann S, Teleanu V, Renner M, Andresen C, Köhler BC, Aybey B, et
al: Gene expression-based prediction of pazopanib efficacy in
sarcoma. Eur J Cancer. 172:107–118. 2022.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Suehara Y, Kohsaka S, Yamaguchi S, Hayashi
T, Kurihara T, Sano K, Sasa K, Akaike K, Ueno T, Kojima S, et al:
Assessment of predictive biomarkers of the response to pazopanib
based on an integrative analysis of high-grade soft-tissue
sarcomas: Analysis of a tumor sample from a responder and patients
with other soft-tissue sarcomas. Clin Orthop Relat Res.
478:2461–2476. 2020.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Koehler K, Liebner D and Chen JL: TP53
mutational status is predictive of pazopanib response in advanced
sarcomas. Ann Oncol. 27:539–543. 2016.PubMed/NCBI View Article : Google Scholar
|