Introduction
The association between cancer and thrombosis was
first reported in 1865 by Trousseau in his book ‘Clinique Medicale
de l'Hotel-Dieu de Paris’ as ‘Phlegmatia Alba Dolens’ (1,2). In
1977, Sack et al reported that the Trousseau syndrome was a
chronic disseminated intravascular coagulation associated with
cancer (3). In recent years, the
risk of thromboembolism, including venous thromboembolism (VTE) and
arterial thromboembolism (ATE), has increased in patients with
cancer (4). Cancer-associated
thrombosis is a major cause of morbidity and mortality in cancer
patients.
Thromboembolism can occur at any stage of cancer and
often complicates the course of the disease and treatment. The
exact incidence of VTE, including deep venous thrombosis (DVT) and
pulmonary embolism (PE), in patients with cancer remains under
investigated. In total, 50% of autopsy cases in patients with
cancer have VTE (5); however, the
incidence rate of clinically significant VTE ranges from 0.4-43%
(6-8),
with 3.5-9% of VTE cases resulting in death (9,10).
However, there are few reports on the incidence of ATE, including
acute coronary syndrome, ischemic stroke, and peripheral arterial
disease (PAD). It has an incidence rate of 4.7% and a 2.2-fold
incidence risk compared with patients without cancer (11,12).
In addition, it is known that patients with cancer-associated
thrombosis also have a 2.2-fold increased risk of bleeding
(13). This increased risk in
cancer patients requires careful monitoring, and appropriate
management of thromboembolic events is necessary to improve their
overall prognosis and quality of life.
Pancreatic cancer has one of the poorest prognoses,
with a remarkably low 5-year survival rate of approximately 10% in
both Japan and the United States. It is the fourth leading cause of
cancer-related deaths (14,15).
Most recently, we reported the outcomes of 846 patients treated
with first-line chemotherapy for metastatic pancreatic cancer in a
real-world setting (16).
Pancreatic cancer also has a higher incidence of VTE than other
cancers (17); however, data on
ATE are scarce despite the presence of shared risk factors, such as
smoking, obesity, and diabetes mellitus. Understanding the
interplay between pancreatic cancer and thromboembolism,
particularly ATE, could lead to more effective prevention and
management strategies.
In this study, we aimed to examine the incidence of
cancer-associated thromboembolism (CAT), its prognosis background
factors affecting survival and the prognostic utility of the
Khorana score in predicting outcomes in patients with metastatic
pancreatic cancer treated with chemotherapy using the
aforementioned dataset. This study allows us to provide new
information on the clinical effects of cancer-associated ATE and
VTE.
Materials and methods
The Tokushukai REAl-world Data project is a
large-scale, retrospective cohort study that includes hospitals
from the Tokushukai Medical Group across Japan. Comprehensive
details regarding the study are outlined in a separate protocol
article (18). The project was
developed in compliance with Japanese ethical guidelines for
medical and biological research involving human subjects (19) and adheres to the principles of the
Declaration of Helsinki. The study was approved by the Ethics
Committee of the Tokushukai Group in April 2020 (approval no.
TGE01427-024). Patients were informed via an opt-out method.
Additionally, the study was registered with the UMIN Clinical Trial
Registry (http://www.umin.ac.jp/ctr/index.htm) under the
registration no. UMIN000050590 in March 2023.
Objective patients
We assessed patients with pathologically or
radiologically confirmed metastatic pancreatic cancer who received
first-line chemotherapy from April 1, 2010, to March 31, 2020, at
the Tokushukai Medical Group hospitals, comprising 46 hospitals
with 14,829 beds. They operate under a unified medical record
system (e-Karte and Newtons2, Software Service Inc.) and a
chemotherapy protocol system (srvApmDrop, Software Service
Inc.).
All patients were administered first-line
treatments, which included gemcitabine, S-1, a combination of
gemcitabine and S-1, a combination of gemcitabine and
nab-paclitaxel, or a regimen of fluorouracil, folinic acid,
oxaliplatin, and irinotecan (FOLFIRINOX). The study covered a range
of pathological diagnoses such as adenocarcinoma, adenosquamous
carcinoma, and other carcinomas/malignant neoplasms. However,
patients diagnosed with acinar and neuroendocrine carcinoma were
excluded from the study. Other significant exclusion criteria were
the presence of active concurrent cancers, an inadequate treatment
history, and missing essential patient data, including body weight
and height. Patients with an insufficient treatment history (e.g.,
those who received cancer treatment outside of the Tokushukai
Medical Group hospitals or lacked detailed treatment information)
were excluded from this study.
Data collection
We assessed eligible patients identified through
electronic medical records. Patient information including age, sex,
height, weight, body surface area, body mass index (BMI), most
recent survival data, survival outcomes, diagnosis from medical
receipts, and hospital classification (government-designated cancer
hospital, prefectural designated cooperative cancer hospital, or
non-designated general hospital) was extracted. Data related to
chemotherapy regimens, including start and end dates of treatment
and performance status (PS), were obtained from the chemotherapy
protocol system. Prescription information for oral medications
(antiplatelets, anticoagulants, antihypertensive medications,
diabetes medications, statins) was extracted from the electronic
medical record's commercial information for medications prescribed
within 30 days before and after the cancer diagnosis. Blood
laboratory data within 14 days of treatment were also extracted
from the electronic medical record. Data from the linked cancer
registry, encompassing diagnostic details (such as site, pathology,
and stage), treatment specifics (including surgery, endoscopic
procedures, radiotherapy, and chemotherapy), and prognosis data
(final survival confirmation date, date of death, and cause of
death), were obtained from the National Cancer Registry Data in
Japan (20). The date of the last
confirmed survival was extracted from both the cancer registry and
electronic medical record data, and the later date was used. The
incidence of VTE (DVT and PE) (21), ATE (acute coronary syndrome,
ischemic stroke, and PAD) (22-24),
bleeding complications (subarachnoid, intracranial, epidural,
cerebral, and upper and lower gastrointestinal bleeding) (25), and comorbidity (diabetes mellitus,
hypertension, hyperlipidemia, and chronic renal failure) (26), coded by the International
Classification of Diseases 10th Revision, were extracted from the
Diagnosis Procedure Combination (DPC), as listed in Table I. The DPC is diagnosis group
classification system in Japan, linked to the comprehensive payment
system for medical fees. To analyze secular trends and ensure
nearly equal distribution of patients, the study period was divided
into three intervals: Period A (2010-2013), Period B (2014-2016),
and Period C (2017-2020). In Japan, FOLFIRINOX were approved in
period A, gemcitabine plus nab-paclitaxel received approval in
period B, and nal-irinotecan received approval in period C.
 | Table IInternational Classification of
Diseases, 10th revision codes. |
Table I
International Classification of
Diseases, 10th revision codes.
| Category | Disease | Code |
|---|
| Venous
thromboembolism (24) | Deep vein
thrombosis | I80.1, I80.2,
I80.3, I80.8, I80.9, I82.8, I82.9, O22.3, O22.9, O87.1 |
| | Pulmonary
embolism | I26.0, I26.9 |
| Arterial
thromboembolism (25-27) | Acute coronary
syndrome | I20.0,
I21.x-I22.x |
| | Cerebrovascular
accident | G45.x,
I63.x-I64.x |
| | Peripheral arterial
disease | I70.0, I70.20-25,
I70.29, I70.9 |
| Major bleeding
(28) | Subarachnoid
hemorrhage | I60.x |
| | Intracranial
hemorrhage | I61.x |
| | Subdural
hemorrhage | I62.x |
| | Upper
gastrointestinal bleeding | I85.0, K22.1,
K22.6, K25.0, K25.2, K25.4, K25.6, K26.0, K26.2, K26.4, K26.6,
K27.0, K27.2, K27.4, K27.6, K28.0, K28.2, K28.4, K28.6, K29.0,
K31.80, K63.80, K92.0, K92.1, K92.2 |
| | Lower
gastrointestinal bleeding | K55.2, K51, K57,
K62.5, K92.0, K92.1, K92.2 |
| Preexisting
condition (29) | Diabetes
mellitus | E10.x-E14.x |
| | Hypertension | I10.x-I13.x,
I15.x |
| | Dyslipidemia | E78.x |
The Khorana score was calculated from the blood
collection data. The Khorana score, which consists of five clinical
and pre-chemotherapy laboratory parameters, primary tumor site (+1
or 2 points), platelet count of ≥350x109/l (+1 point),
hemoglobin concentration of ≤100 g/l or use of
erythropoiesis-stimulating agents (+1 point), leukocyte count of
≥11x109/l (+1 point), and a BMI of ≥35 kg/m2
(+1 point), predicts VTE development (27,28).
For pancreatic cancer, the minimum score is 2. The score increases
according to other parameters.
Statistical analyses
The primary endpoint of this study was overall
survival (OS), defined as the duration from the start date of the
initial palliative chemotherapy to the date of death or the last
confirmation of survival.
Basic statistics were calculated to summarize the
distribution of variables related to patient background factors,
complications, other prognostic factors, and primary endpoints.
These statistics included absolute and relative frequencies for
categorical variables, quartiles, maximum and minimum values, means
and standard deviations for continuous variables, and quartiles and
relative frequencies for discrete variables. The study period
spanned from April 1, 2010, to March 31, 2020. The time variable
was defined as the number of days from the start date of the
first-line chemotherapy treatment to the date of death. Censored
cases included patients who were alive at the end of the study or
those who dropped out for any reason. Fisher's exact tests were
used in these between-group comparisons.
For each prognostic score, Kaplan-Meier curves
(univariate analysis) for the occurrence of events associated with
the study endpoint (OS) were obtained and log-rank tests were
performed.
Additionally, several hierarchical predictive models
were developed by integrating explanatory variables anticipated to
impact the evaluated endpoints. Both single- and multi-tiered
proportional hazard models were established using each predictive
model. Stratified and conventional Cox multiple regression analyses
were performed. Conventional Cox regression was used when the
proportional hazards assumption was valid; otherwise, stratified
Cox regression was employed.
The Akaike information criterion (AIC), based on
partial likelihood, was utilized to identify the optimal model in
this study. When the number of eligible cases varied between
models, the average AIC per case was used. Hazard ratios (HRs) and
95% confidence intervals (CIs) were calculated for each category of
prognostic factors related to OS identified in the optimal model.
The effects of these prognostic factors were assessed using
likelihood tests, with associated p-values provided for each
factor.
All analyses were performed using R, version 4.2.2
(R Foundation for Statistical Computing, Vienna, Austria). All
statistical tests were two-sided, with significance set at
P<0.05.
Results
A total of 846 eligible patients were analyzed
(16). With a median follow-up of
5.4 months (95% confidence interval [CI], 4.8-6.0), 86 (10.2%) of
them had any form of CAT requiring hospitalization, comprising 21
patients with VTE (2.5%) and 70 patients with ATE (8.3%) (including
five patients with overlapping VTE and ATE). The patient
backgrounds for both groups are shown in Table II. There were no differences in
patient backgrounds, including Khorana scores, between the
CAT-positive and CAT-negative patients.
 | Table IIPatients' medical and demographic
characteristics. |
Table II
Patients' medical and demographic
characteristics.
|
Characteristics | CAT(-) (n=760), n
% | CAT(+) (n=86), n
% | P-value |
|---|
| Age | | | |
|
Median | 70 | 72 | 0.26 |
|
Quantile
(Min, Q1, Q2, Q3, Max) | (36, 64, 70, 76,
90) | (47, 68, 72, 77,
84) | |
|
≥75 | 232 (30.5) | 34 (39.5) | |
| Sex | | | |
|
Male | 451 (59.3) | 52 (60.5) | 0.91 |
|
Female | 309 (40.7) | 34 (39.5) | |
| Performance
status | | | |
|
0 | 210 (27.6) | 22 (25.6) | 0.84 |
|
1 | 260 (34.2) | 30 (34.9) | |
|
≥2 | 46 (6.1) | 7 (8.1) | |
|
Not
available | 244 (32.1) | 27 (31.4) | |
| BMI | | | |
|
Median | 19.7 | 20.3 | 0.55 |
|
Quantile
(Min, Q1, Q2, Q3, Max) | (11.2, 17.4, 19.7,
21.9, 35.4) | (13.6, 17.3, 20.3,
22.0, 34.3) | |
|
≥25 | 64 (8.4) | 9 (10.5) | |
| Smoking status | | | |
|
Current or
former (Brinkman index >0) | 197 (28.3) | 20 (23.3) | 0.44 |
|
Never smoked
(Brinkman index=0) | 500 (71.3) | 62 (72.1) | |
|
Not
available | 63 (8.4) | 4 (4.7) | |
| Pathological
confirmation | | | |
|
Yes | 666 (87.6) | 79 (91.9) | 0.20 |
|
Adenocarcinoma | 382 (50.3) | 36 (41.9) | |
|
Adenosquamous
carcinoma | 6 (0.8) | 1 (1.2) | |
|
Carcinoma/malignant
neoplasm | 278 (36.6) | 42 (48.3) | |
|
No
(radiological diagnosis only) | 94 (12.4) | 7 (8.1) | |
| Primary disease
site | | | |
|
Pancreas
head | 318 (41.8) | 41 (47.7) | 0.37 |
|
Pancreas
body | 215 (28.3) | 17 (19.8) | |
|
Pancreas
tail | 196 (25.8) | 24 (27.9) | |
|
Whole/not
evaluable | 31 (4.1) | 4 (4.7) | |
| Previous
procedures | | | |
|
Surgery | 115 (15.1) | 8 (9.3) | 0.77 |
|
Endoscopic
procedure | 40 (5.3) | 4 (4.7) | |
|
Radiotherapy | 43 (5.7) | 4 (4.7) | |
| Study period | | | |
|
Period A
(2010-2013) | 240 (31.6) | 28 (32.6) | 0.22 |
|
Period B
(2014-2016) | 232 (30.5) | 19 (22.1) | |
|
Period C
(2017-2020) | 288 (37.9) | 39 (45.3) | |
| Hospital scale (no.
of registered patients) | | | |
|
High volume
(n≥50) | 454 (59.7) | 55 (64.0) | 0.49 |
|
Low volume
(n<50) | 306 (40.3) | 31 (36.0) | |
| Hospital type | | | |
|
Government-designated
cancer hospital | 192 (25.3) | 26 (30.2) | 0.61 |
|
Prefectural
designated cooperative cancer hospital | 286 (37.6) | 30 (34.6) | |
|
General
hospital | 282 (37.1) | 30 (34.6) | |
| Platelet count | | | |
|
Median,
x109/l | 22 | 20.4 | 0.45 |
|
Quantile,
x109/l | (4.4, 17.1, 22.0,
28.5, 71.3) | (6.5, 16.0, 20.4,
27.9, 63.0) | |
|
≥350,
x109/l | 82 (10.8) | 6 (7.0) | |
| Hemoglobin | | | |
|
Median,
gl | 12.2 | 11.8 | 1.00 |
|
Quantile,
g/l | (7.2, 10.9, 12.2,
13.3, 18.4) | (8.6, 10.7, 11.8,
12.7, 16.1) | |
|
<100
g/l | 75 (9.9) | 8 (9.3) | |
| White blood cell
count | | | |
|
Median,
x109/l | 6.8 | 71 | 0.17 |
|
Quantile,
x109/l | (19, 53, 68, 88,
290) | (27, 56, 71, 92.7,
195) | |
|
>11x109/l | 87 (11.4) | 15 (17.4) | |
| Khorana score | | | |
|
2 | 502 (71.6) | 57 (65.1) | 0.50 |
|
3 | 155 (22.1) | 20 (23.3) | |
|
4 | 42 (6.0) | 3 (3.5) | |
|
5 | 2 (0.3) | 1 (1.2) | |
|
6 | 0 (0.0) | 0 (0.0) | |
|
Not
available | 59 (7.8) | 5 (5.8) | |
Patient comorbidities, concomitant medications
before and during first-line treatment, and first-line systemic
therapy regimens are shown in Table
III. Dyslipidemia was observed significantly more frequently in
CAT-positive patients than in CAT-negative patients. Patients in
the CAT-positive group used significantly more antiplatelet and
anticoagulant medications than those in the CAT-negative group.
Additionally, CAT-positive patients had a significantly higher rate
of gastrointestinal bleeding events than CAT-negative patients [13
of 86 (15.2%) vs. 46 of 760 (6.1%), P=0.01]. The detailed
thromboembolic and bleeding incidences are shown in Table IV.
 | Table IIIComorbidities, concomitant
medications and first-line systemic therapy. |
Table III
Comorbidities, concomitant
medications and first-line systemic therapy.
|
Characteristics | CAT(-) (n=760), n
(%) | CAT(+) (n=86), n
(%) | P-value |
|---|
| Pre-existing
condition | | | |
|
Diabetes
mellitus | 249 (32.8) | 39 (45.3) | 0.18 |
|
Hypertension | 290 (38.2) | 41 (47.7) | 0.3 |
|
Dyslipidemia | 145 (19.1) | 36 (41.9) | <0.001 |
|
Chronic
kidney disease | 122 (16.1) | 19 (22.1) | 0.24 |
| Concomitant
medication | | | |
|
Antidiabetics | 205 (27.0) | 31 (36.0) | 0.19 |
|
Antihypertensive | 279 (36.7) | 49 (57.0) | 0.03 |
|
Statins | 78 (10.3) | 25 (29.1) | <0.001 |
|
Antiplatelets | 51 (6.7) | 35 (40.7) | <0.001 |
|
Aspirin | 8 (5.0) | 25 (29.1) | <0.001 |
|
Thienopyridine | 13 (1.7) | 16 (18.6) | <0.001 |
|
Others | 11 (1.5) | 10 (11.6) | <0.001 |
|
Anticoagulants | 113 (14.9) | 38 (44.2) | <0.001 |
|
Warfarin | 14 (1.8) | 9 (10.5) | <0.001 |
|
Direct oral
anticoagulants | 7 (0.9) | 11 (12.8) | <0.001 |
|
Heparina | 101 (13.3) | 28 (32.6) | <0.001 |
|
Others | 1 (0.1) | 4 (4.7) | <0.001 |
| First-line systemic
therapy | | | |
|
Gemcitabine
alone | 269 (35.4) | 35 (40.7) | 0.9 |
|
S-1
alone | 178 (23.4) | 20 (23.3) | |
|
Gemcitabine+S-1 | 59 (7.8) | 8 (9.3) | |
|
Gemcitabine
+ nab-paclitaxel | 209 (27.5) | 23 (26.7) | |
|
FOLFIRINOX | 45 (5.9) | 7 (8.1) | |
 | Table IVIncidences of thromboembolism and
bleeding events. |
Table IV
Incidences of thromboembolism and
bleeding events.
|
Characteristics | CAT-
(n=760), n (%) | CAT+
(n=86), n (%)a | P-value |
|---|
| Thrombotic events
(%, CAT+/total) | | | |
| Venous
thromboembolism | | 21 (24.4/2.5) | |
|
Deep venous
thrombosis | | 16 (18.6/1.9) | |
|
Pulmonary
embolism | | 8 (9.3/0.9) | |
| Arterial
thromboembolism | | 70 (81.4/8.3) | |
|
Acute
coronary syndrome | | 24 (27.9/2.8) | |
|
Ischemic
stroke | | 39 (45.3/4.6) | |
|
Peripheral
arterial disease | | 12 (13.9/1.4) | |
| Bleeding
events | | | |
|
Cerebral
bleeding | 7 (0.9) | 1 (1.1) | 0.58 |
|
Gastrointestinal
bleeding | 46 (6.1) | 13 (15.2) | 0.01 |
We derived crude Kaplan-Meier curves and adjusted
results for ‘age,’ ‘sex,’ ‘BMI,’ ‘study period,’ and ‘PS,’ which
were correlated with prognosis in our previous analyses (16). The Kaplan-Meier curves of OS with
and without CAT are shown in Fig.
1. CAT-positive patients had a poor prognosis than those
without CAT in the crude data (HR, 1.28; 95% CI, 1.01-1.62;
P=0.044), and this trend persisted even after adjusting for
background factors (HR, 1.20; 95% CI, 0.94-1.52; P=0.131).
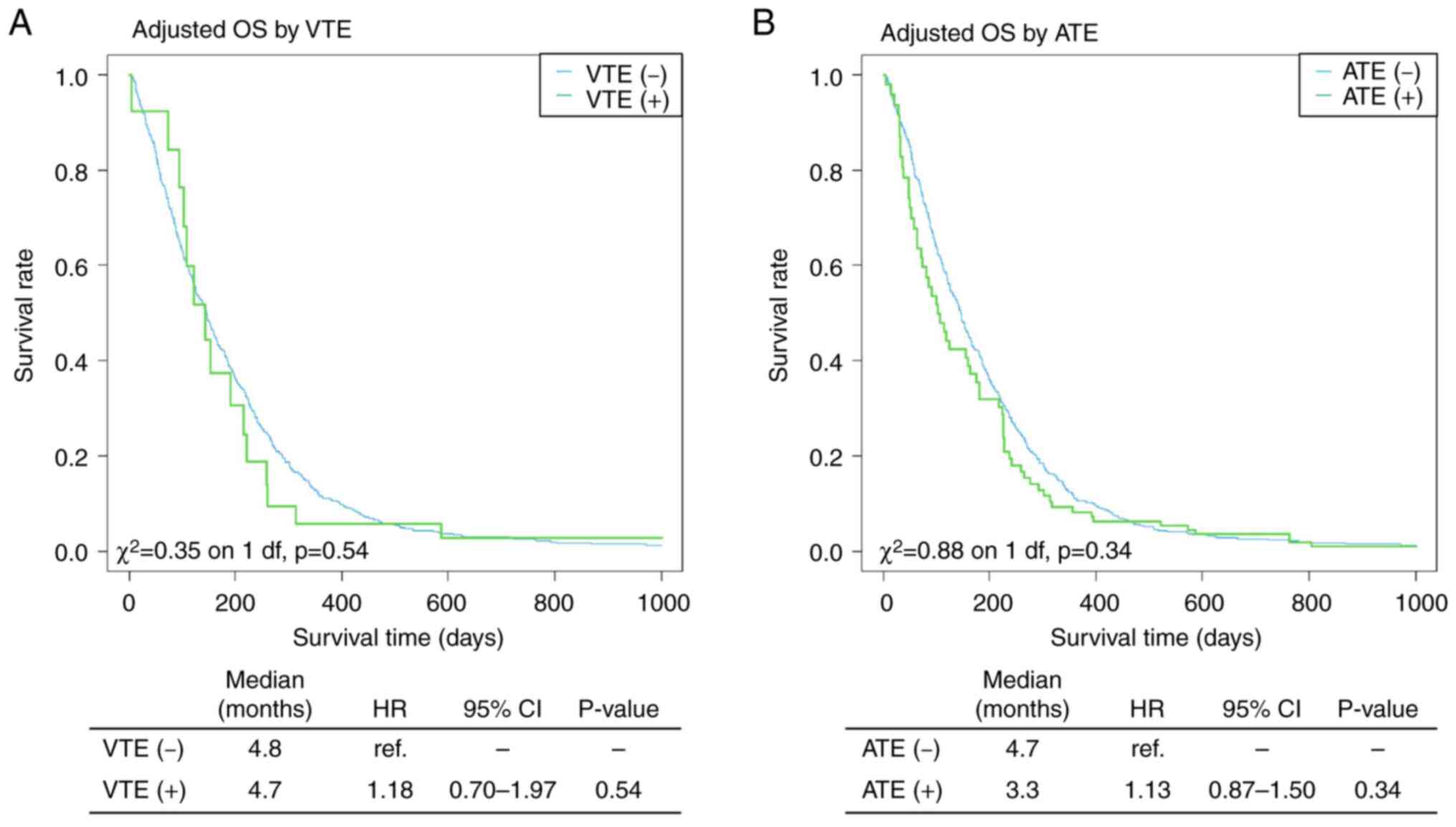
Prognostic analysis based on the presence of VTE and ATE was also
performed. However, no statistically significant difference was
found between the two groups, although there was a trend toward a
poorer prognosis in positive cases than in negative cases (Fig. 2). Kaplan-Meier curves of OS with
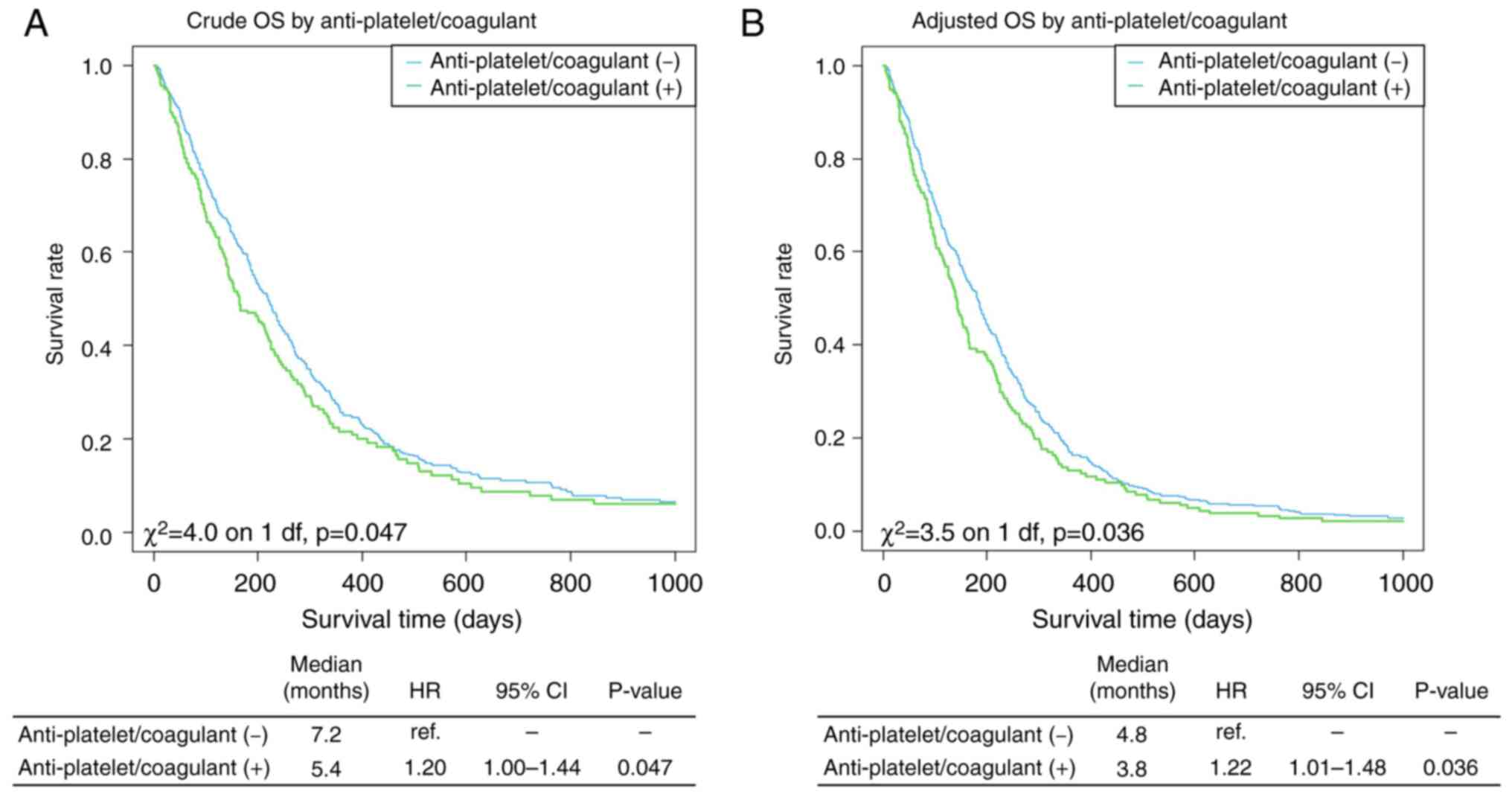
and without antiplatelet or anticoagulant use are shown in Fig. 3. Patients taking anticoagulants or
antiplatelets also had a poorer prognosis than those not taking
these medications (HR, 1.22; 95% CI, 1.01-1.48; P=0.036).
The Kaplan-Meier curves for OS based on the Khorana
score are shown in Figs. 4 and
5. The Khorana score was
significantly associated with poor prognosis (P<0.001), with
higher scores indicating a poorer prognosis. Although the Khorana
score was not associated with OS in the CAT-positive group, it was
a prognostic factor in the CAT-negative group (P<0.001).
Discussion
This real-world study revealed that the incidence
rate of CAT in patients with treatment-naive metastatic pancreatic
cancer was sufficiently high to be clinically alarming (10.2%). In
addition, a trend toward a poorer prognosis and higher risk of
gastrointestinal bleeding events in CAT-positive patients than in
CAT-negative patients was observed. A higher Khorana score did not
predict CAT events but had prognostic value in our cohort.
As noted in the Introduction section, the frequency
of clinically significant VTE ranges from 0.4-43% (6-8),
which is a significantly wide range. In a multicenter prospective
cohort study conducted in Japan, VTE was reported in 5.9% of the
patients (asymptomatic, 5.5%; symptomatic, 0.3%) (17). A retrospective study on VTE in
patients with pancreatic cancer in Japan reported a prevalence of
7.2% (29). Similarly, an Italian
and a German database study reported 26.0% (30) and 26.4%, respectively (31).
The incidence rate of VTE reported in this study
(2.5%) was significantly low compared with those of previous
studies. As the data in this study were based on DPC, only VTE
requiring hospitalization was extracted, and asymptomatic VTE
identified by ultrasonography or computed tomography imaging was
not included, as in other studies based on chart reviews. This may
explain the lower incidence of VTE in our study than that in
previous studies. In fact, the largest Japanese cohort study on
symptomatic VTE in metastatic pancreatic cancer reported a 1-year
cumulative incidence rate of only 1.4%, which is comparable to our
incidence rate (32).
The incidence rate of ATE (8.3%) in this study was
higher than that in previous studies. Although there are fewer
reports of ATE compared to VTE, the incidence rate of ATE in cancer
patients is 4.7-5.9% (33,34); in patients with pancreatic cancer,
the reported rate is 2.8-5.0% (35,36).
A retrospective study of thromboembolism in patients with gastric
and colorectal cancer in Japan reported that ATE was found in 12.9%
of patients (37). The incidence
rate of PAD is reported much less frequently. A case report of PAD
in a patient with pancreatic cancer described an incidence rate of
0.65% (38). However, a previous
study reported that 11.5% of hospitalized patients with PAD had
concurrent cancer (39).
Therefore, future studies should elucidate the incidence of PAD.
Additionally, the clinical significance of the incidence rates of
acute coronary syndrome (2.8%) and ischemic stroke (4.6%) warrants
further investigation.
Several reports have reported that CAT is associated
with a poor prognosis in patients with cancer, and this trend was
also observed in the present dataset. In the short term, VTE is an
independent prognostic factor for death in patients with cancer
receiving chemotherapy, with a remarkably high HR of 6.58 (4.50
after adjustment for background factors) (10). Inpatient mortality rates for
patients hospitalized with VTE were higher at 7, 14, and 30 days in
analyses of patient groups adjusted for background factors by
propensity score matching and were particularly high for
pancreatic, liver, and biliary tract cancers (40). Retrospective studies of patients
with pancreatic cancer have also demonstrated that patients with
VTE have shorter progression-free survival and OS, with HRs of 2.02
and 2.42, respectively (31).
Similarly, ATE is associated with poor prognosis (41) and is independently associated with
poor prognosis for OS (33).
In terms of patient background, our results
demonstrated a significant association between CAT onset and
dyslipidemia. Dyslipidemia is associated with hypercoagulability,
endothelial dysfunction, and increased platelet aggregation. A
meta-analysis showed that high triglyceride and low high-density
lipoprotein cholesterol levels were significantly associated with
VTE (42). Additionally, the
CAT-positive group was administered significantly more antiplatelet
and anticoagulant drugs than the CAT-negative group. In the overall
population, patients receiving antiplatelet and anticoagulant
medications have a poorer prognosis than those not receiving these
medications, which may be associated with a poorer prognosis in the
CAT-positive group than in the CAT-negative group. In this study,
patients with CAT had a higher risk of bleeding than those without.
This may reflect the fact that antiplatelet and anticoagulant
agents are used in patients with CAT (13).
Patients with cancer with VTE have a poor prognosis,
and the risk of death is 2.20 times higher than that in patients
without VTE (11). The Khorana
score was originally developed as a predictive model for
chemotherapy-related thrombosis (11,27),
and several studies have validated its effectiveness in predicting
VTE in cancer (28,43,44).
In addition, a cohort study demonstrated that the Khorana score had
a negative prognostic value in patients with resectable pancreatic
cancer (45). Similarly, the
Khorana score had a strong negative prognostic value in our
patients with metastatic pancreatic cancer. Notably, this
predictive significance remained consistent, even among patients
classified as CAT-negative. The Khorana score was originally
developed to estimate the risk of VTE in outpatients undergoing
chemotherapy and is not intended for patients with CAT requiring
hospitalization, which is the subject of this study. However, the
factors composing the Khorana score, which include elevated
platelets, leukocytosis, and anemia, may reflect an association
with the presence of cancer cachexia, a condition commonly observed
in pancreatic cancer (46,47). Therefore, it is not surprising that
the study found the Khorana score may be effective in predicting
the prognosis of metastatic pancreatic cancer. Calculation of the
Khorana score is straightforward, suggesting its potential
application as a prognostic marker in routine clinical
practice.
A clinical practice guideline was published in 2009
for CAT with detailed recommendations for diagnosis, treatment, and
prevention (48). Since the
publication of the European Society for Medical Oncology guideline
in 2011(49), similar guidelines
have subsequently been published and updated by several academic
societies (50-52).
The most current guidelines are the updated guidelines of the
American Society of Clinical Oncology and European Society of
Medical Oncology (53,54). Most guidelines recommend
direct-acting oral anticoagulants (DOACs), low-molecular-weight
heparin (LMWH) or unfractionated heparin for treatment induction,
DOACs or LMWH for maintenance of VTE, and antiplatelets with or
without anticoagulants for ATE. In addition, the guidelines
recommend the use of LMWH for VTE prevention. In the present study,
the CAT-positive group received both anticoagulants and
antiplatelet agents. The frequent use of these drugs in the CAT
group suggests that these guidelines are reflected in daily
clinical practice; however, LWMH has not been approved for VTE in
Japan. It is therefore believed that cases in which LMWH is
administered prophylactically are almost nonexistent, and that
virtually all of the heparin found in this study was unfractionated
heparin.
The present study had some limitations. First, the
incidence of CAT and bleeding events may have been underestimated
because our dataset was based on the DPC data. Virtually, only
symptomatic cases requiring hospitalization were recorded, while
asymptomatic cases and complications treated on an outpatient basis
were not counted. Additionally, fatal cases in the emergency
department that were not admitted to our hospitals were not
included. Furthermore, the cause of death cannot be accurately
determined due to lack of data, making it impossible to determine
if the cause was cancer, CAT, bleeding, etc. Second, VTE and ATE
were integrated into the analysis. Although VTE and ATE should have
been separately analyzed, the small number of cases and overlapping
cases made this difficult; therefore, they were integrated and
analyzed. Finally, the precise timing and intended use of
prescription oral medications remain uncertain because this study
only recorded whether each medication had been prescribed at any
point during the study period, without details on usage patterns.
Therefore, it is difficult to distinguish from these data whether
the dose administered was prophylactic or therapeutic against
thrombosis or other conditions, including whether the medication
was administered before or after the onset of CAT. Patients who
started antithrombotic therapy before the onset of CAT might have a
higher risk of CAT recurrence, while their survival rates may be
higher because they are already receiving treatment. This survivor
bias could lead to an overestimation of the efficacy of
antithrombotic therapy. Therefore, care should be taken when
interpreting the findings in connection with prescription.
Acknowledging these limitations, the strength of this study lies in
the notable incidence of CAT, particularly ATE, in patients with
metastatic pancreatic cancer treated with first-line
chemotherapy.
In conclusion, our real-world data demonstrated that
the incidence rate of CAT in patients with metastatic pancreatic
cancer was 10.2%. This clinically alarmingly high incidence rate
was not dependent on chemotherapy. At the same time,
gastrointestinal bleeding occurred more frequently in CAT-positive
patients than in CAT-negative patients. Additionally, patients with
CAT exhibited a trend towards a less favorable prognosis compared
to those without CAT. Moreover, the Khorana score may be useful in
predicting prognosis, even without CAT development. Further
analyses should be performed after accumulating a significant
number of cases, including metastatic pancreatic cancer and other
cancer types.
Acknowledgements
The authors express their gratitude for the
invaluable support provided by Dr Shinnichi Higashiue, Chair of the
Medical Corporation Tokushukai (Tokyo, Japan) and General
Incorporated Association Tokushukai (Tokyo, Japan) as well as Dr
Hisaaki Afuso, Chief Advisor of the Medical Corporation Tokushukai
(Tokyo, Japan) in facilitating clinical research within the
Tokushukai Group. Additionally, special thanks are extended to Mr.
Katsuhiko Ozaki, President of Tokushukai Information System, Inc.
(Osaka, Japan) for his assistance in accessing and utilizing the
medical database. The abstract of this study was presented at the
poster session (P10-1) of the 20th Annual Meeting of the Japanese
Society of Clinical Oncology Mar 16-18 2023, Fukuoka, Japan and it
was published as abstract no. P10-1 in Ann Oncol 34 (Suppl 3):
2023.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
RS, YI, and MO made significant contributions to the
study design and conception. RS, YF, MS, and MH were responsible
for data acquisition. YF and MS confirm the authenticity of all the
raw data. RS and YI interpreted the data and drafted the
manuscript. KU, TM, KO, NS, and HM provided valuable advice on the
research design and contributed to the critical interpretation of
the study findings. NS and HM thoroughly reviewed and approved the
final version of the manuscript. All authors have read and endorsed
the final version of the manuscript.
Ethics approval and consent to
participate
The project followed the ethical guidelines for
medical and biological research involving human subjects in Japan,
in accordance with the principles set forth in The Declaration of
Helsinki. Approval for the study was obtained from the Ethics
Committee of the Tokushukai Group in April 2020 (approval no.
TGE01427-024), and the study was registered in the UMIN Clinical
Trial Registry under the registration no. UMIN000050590. Patients
were informed about the study using opt-out methods, and all
patients chose to participate; none declined participation.
Patient consent for publication
Patient consent for publication was obtained through
opt-out methods.
Competing interests
The authors declare that they have no competing
interests.
Use of artificial intelligence tools
The authors used chatGPT 3.5 and DeepL as AI tools
during the preparation of this study to improve the English
translation, readability of the manuscript, and language. The
authors have revised and edited the content generated by the AI
tools as needed and have undertaken English language editing. The
authors take full responsibility for the final content of this
manuscript.
References
|
1
|
Trousseau A: Phlegmatia alba dolens. In:
Clinique Medicale de L'Hotel-Dieu de Paris; Vol 3. 2nd edition.
J.-B. Bailliere et fils, Paris, pp654-712, 1865.
|
|
2
|
Metharom P, Falasca M and Berndt MC: The
history of Armand Trousseau and cancer-associated thrombosis.
Cancers (Basel). 11(158)2019.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Sack GH Jr, Levin J and Bell WR:
Trousseau's syndrome and other manifestations of chronic
disseminated coagulopathy in patients with neoplasms: Clinical,
pathophysiologic, and therapeutic features. Medicine (Baltimore).
56:1–37. 1977.PubMed/NCBI
|
|
4
|
Ikushima S, Ono R, Fukuda K, Sakayori M,
Awano N and Kondo K: Trousseau's syndrome: Cancer-associated
thrombosis. Jpn J Clin Oncol. 46:204–208. 2016.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Thompson CM and Rodgers LR: Analysis of
the autopsy records of 157 cases of carcinoma of the pancreas with
particular reference to the incidence of thromboembolism. Am J Med
Sci. 223:469–478. 1952.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Walker AJ, Card TR, West J, Crooks C and
Grainge MJ: Incidence of venous thromboembolism in patients with
cancer-a cohort study using linked United Kingdom databases. Eur J
Cancer. 49:1404–1413. 2013.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Khorana AA, Francis CW, Culakova E,
Kuderer NM and Lyman GH: Frequency, risk factors, and trends for
venous thromboembolism among hospitalized cancer patients. Cancer.
110:2339–2346. 2007.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Khorana AA, Yannicelli D, McCrae KR,
Milentijevic D, Crivera C, Nelson WW and Schein JR: Evaluation of
US prescription patterns: Are treatment guidelines for
cancer-associated venous thromboembolism being followed? Thromb
Res. 145:51–53. 2016.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Khorana AA, Francis CW, Culakova E,
Kuderer NM and Lyman GH: Thromboembolism is a leading cause of
death in cancer patients receiving outpatient chemotherapy. J
Thromb Haemost. 5:632–634. 2007.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Khorana AA: Venous thromboembolism and
prognosis in cancer. Thromb Res. 125:490–493. 2010.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Sørensen HT, Mellemkjaer L, Olsen JH and
Baron JA: Prognosis of cancers associated with venous
thromboembolism. N Engl J Med. 343:1846–1850. 2000.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Khorana AA, Francis CW, Culakova E and
Lyman GH: Risk factors for chemotherapy-associated venous
thromboembolism in a prospective observational study. Cancer.
104:2822–2829. 2005.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Prandoni P, Lensing AWA, Piccioli A,
Bernardi E, Simioni P, Girolami B, Marchiori A, Sabbion P, Prins
MH, Noventa F and Girolami A: Recurrent venous thromboembolism and
bleeding complications during anticoagulant treatment in patients
with cancer and venous thrombosis. Blood. 100:3484–3488.
2002.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Siegel RL, Miller KD, Fuchs HE and Jemal
A: Cancer statistics, 2021. CA Cancer J Clin. 71:7–33.
2021.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Cancer Information Service, National
Cancer Center, Vital Statistics of Japan, Ministry of Health,
Labour and Welfare: Cancer statistics in Japan. https://ganjoho.jp/reg_stat/statistics/data/dl/en.html.
Accessed April 1, 2023.
|
|
16
|
Shimoyama R, Imamura Y, Uryu K, Mase T,
Fujimura Y, Hayashi M, Ohtaki M, Ohtani K, Shinozaki N and Minami
H: Real-world treatment outcomes among patients with metastatic
pancreatic cancer in Japan: The Tokushukai Real-world Data Project.
Mol Clin Oncol. 19(98)2023.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Ohashi Y, Ikeda M, Kunitoh H, Sasako M,
Okusaka T, Mukai H, Fujiwara K, Nakamura M, Oba MS, Kimura T, et
al: Venous thromboembolism in cancer patients: Report of baseline
data from the multicentre, prospective Cancer-VTE Registry. Jpn J
Clin Oncol. 50:1246–1253. 2020.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Shimoyama R, Imamura Y, Uryu K, Mase T,
Fujimura Y, Hayashi M, Ohtaki M, Ohtani K, Shinozaki N and Minami
H: Real-world outcomes of systemic therapy in Japanese patients
with cancer (Tokushkai REAl-world Data project: TREAD): Study
protocol for a nationwide cohort study. Healthcare (Basel).
10(2146)2022.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Eba J and Nakamura K: Overview of the
ethical guidelines for medical and biological research involving
human subjects in Japan. Jpn J Clin Oncol. 52:539–544.
2022.PubMed/NCBI View Article : Google Scholar
|
|
20
|
National Cancer Registry (Ministry of
Health, Labour and Welfare), tabulated by Cancer Information
Service, National Cancer Center, Japan. https://ganjoho.jp/med_pro/cancer_control/can_reg/hospital/index.html.
Accessed April 1, 2023.
|
|
21
|
Alotaibi GS, Wu C, Senthilselvan A and
McMurtry MS: The validity of ICD codes coupled with imaging
procedure codes for identifying acute venous thromboembolism using
administrative data. Vasc Med. 20:364–368. 2015.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Bosco E, Hsueh L, McConeghy KW,
Gravenstein S and Saade E: Major adverse cardiovascular event
definitions used in observational analysis of administrative
databases: A systematic review. BMC Med Res Methodol.
21(241)2021.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Winell K, Arffman M, Pietilä A and Salomaa
V: Regional differences in the incidence of diabetic cardiovascular
events reflect the quality of care. Scand Cardiovasc J. 52:232–237.
2018.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Reinecke H, Unrath M, Freisinger E,
Bunzemeier H, Meyborg M, Lüders F, Gebauer K, Roeder N, Berger K
and Malyar NM: Peripheral arterial disease and critical limb
ischaemia: Still poor outcomes and lack of guideline adherence. Eur
Heart J. 36:932–938. 2015.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Al-Ani F, Shariff S, Siqueira L, Seyam A
and Lazo-Langner A: Identifying venous thromboembolism and major
bleeding in emergency room discharges using administrative data.
Thromb Res. 136:1195–1198. 2015.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Quan H, Sundararajan V, Halfon P, Fong A,
Burnand B, Luthi JC, Saunders LD, Beck CA, Feasby TE and Ghali WA:
Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10
administrative data. Med Care. 43:1130–1139. 2005.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Khorana AA, Kuderer NM, Culakova E, Lyman
GH and Francis CW: Development and validation of a predictive model
for chemotherapy-associated thrombosis. Blood. 111:4902–4907.
2008.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Ay C, Dunkler D, Marosi C, Chiriac AL,
Vormittag R, Simanek R, Quehenberger P, Zielinski C and Pabinger I:
Prediction of venous thromboembolism in cancer patients. Blood.
116:5377–5382. 2010.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Suzuki T, Hori R, Takeuchi K, Yamamura R,
Katoh H, Noji Y, Yamaguchi M and Fujino S: Venous thromboembolism
in Japanese patients with pancreatic cancer. Clin Appl Thromb
Hemost. 27(10760296211051766)2021.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Mandalà M, Reni M, Cascinu S, Barni S,
Floriani I, Cereda S, Berardi R, Mosconi S, Torri V and Labianca R:
Venous thromboembolism predicts poor prognosis in irresectable
pancreatic cancer patients. Ann Oncol. 18:1660–1665.
2007.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Barrau M, Maoui K, Le Roy B, Roblin X,
Mismetti P, Phelip JM and Williet N: Early venous thromboembolism
is a strong prognostic factor in patients with advanced pancreatic
ductal adenocarcinoma. J Cancer Res Clin Oncol. 147:3447–3454.
2021.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Ohashi Y, Ikeda M, Kunitoh H, Sasako M,
Okusaka T, Mukai H, Fujiwara K, Nakamura M, Oba MS, Kimura T, et
al: One-year incidence of venous thromboembolism, bleeding, and
death in patients with solid tumors newly initiating cancer
treatment: Results from the Cancer-VTE Registry. Thromb Res.
213:203–213. 2022.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Navi BB, Reiner AS, Kamel H, Iadecola C,
Okin PM, Elkind MSV, Panageas KS and DeAngelis LM: Risk of arterial
thromboembolism in patients with cancer. J Am Coll Cardiol.
70:926–938. 2017.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Tuzovic M, Herrmann J, Iliescu C,
Marmagkiolis K, Ziaeian B and Yang EH: Arterial thrombosis in
patients with cancer. Curr Treat Options Cardiovasc Med.
20(40)2018.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Hung YS, Chen JS, Chen YY, Lu CH, Chang PH
and Chou WC: Incidence, risk factors, and outcomes of arterial
thromboembolism in patients with pancreatic cancer following
palliative chemotherapy. Cancers (Basel). 10(432)2018.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Riedl JM, Schwarzenbacher E, Moik F,
Horvath L, Gantschnigg A, Renneberg F, Posch F, Barth DA, Stotz M,
Pichler M, et al: Patterns of thromboembolism in patients with
advanced pancreatic cancer undergoing first-line chemotherapy with
FOLFIRINOX or gemcitabine/nab-paclitaxel. Thromb Haemost.
122:633–645. 2022.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Aonuma AO, Nakamura M, Sakamaki K, Murai
T, Matsuda C, Itaya K, Sone T, Yagisawa M, Koike Y, Endo A, et al:
Incidence of cancer-associated thromboembolism in Japanese gastric
and colorectal cancer patients receiving chemotherapy: A
single-institutional retrospective cohort analysis (Sapporo CAT
study). BMJ Open. 9(e028563)2019.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Schattner A, Klepfish A, Huszar M and
Shani A: Two patients with arterial thromboembolism among 311
patients with adenocarcinoma of the pancreas. Am J Med Sci.
324:335–338. 2002.PubMed/NCBI View Article : Google Scholar
|
|
39
|
El Sakka K, Gambhir RP, Halawa M, Chong P
and Rashid H: Association of malignant disease with critical leg
ischaemia. Br J Surg. 92:1498–1501. 2005.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Okushi Y, Kusunose K, Okayama Y, Zheng R,
Nakai M, Sumita Y, Ise T, Tobiume T, Yamaguchi K, Yagi S, et al:
Acute hospital mortality of venous thromboembolism in patients with
cancer from registry data. J Am Heart Assoc.
10(e019373)2021.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Ha J, Lee MJ, Kim SJ, Park BY, Park H, Cho
S, Chung JW, Seo WK, Kim GM, Bang OY and Chung CS: Prevalence and
impact of venous and arterial thromboembolism in patients with
embolic stroke of undetermined source with or without active
cancer. J Am Heart Assoc. 8(e013215)2019.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Ageno W, Becattini C, Brighton T, Selby R
and Kamphuisen PW: Cardiovascular risk factors and venous
thromboembolism: A meta-analysis. Circulation. 117:93–102.
2008.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Verso M, Agnelli G, Barni S, Gasparini G
and LaBianca R: A modified Khorana risk assessment score for venous
thromboembolism in cancer patients receiving chemotherapy: The
Protecht score. Intern Emerg Med. 7:291–292. 2012.PubMed/NCBI View Article : Google Scholar
|
|
44
|
van Es N, Di Nisio M, Cesarman G, Kleinjan
A, Otten HM, Mahé I, Wilts IT, Twint DC, Porreca E, Arrieta O, et
al: Comparison of risk prediction scores for venous thromboembolism
in cancer patients: A prospective cohort study. Haematologica.
102:1494–1501. 2017.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Sohal DP, Shrotriya S, Glass KT, Pelley
RJ, McNamara MJ, Estfan B, Shapiro M, Wey J, Chalikonda S,
Morris-Stiff G, et al: Predicting early mortality in resectable
pancreatic adenocarcinoma: A cohort study. Cancer. 121:1779–1784.
2015.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Aoyagi T, Terracina KP, Raza A, Matsubara
H and Takabe K: Cancer cachexia, mechanism and treatment. World J
Gastrointest Oncol. 7:17–29. 2015.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Poulia KA, Sarantis P, Antoniadou D,
Koustas E, Papadimitropoulou A, Papavassiliou AG and Karamouzis MV:
Pancreatic cancer and cachexia-metabolic mechanisms and novel
insights. Nutrients. 12(1543)2020.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Khorana AA: Cancer and thrombosis:
Implications of published guidelines for clinical practice. Ann
Oncol. 20:1619–1630. 2009.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Mandalà M, Falanga A and Roila F: ESMO
Guidelines Working Group. Management of venous thromboembolism
(VTE) in cancer patients: ESMO Clinical Practice Guidelines. Ann
Oncol 22 Suppl. 6:vi85–vi92. 2011.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Ramcharitar RK, Man L, Khaja MS, Barnett
ME and Sharma A: A review of the past, present and future of
cancer-associated thrombosis management. Heart Int. 16:117–123.
2022.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Farge D, Frere C, Connors JM, Khorana AA,
Kakkar A, Ay C, Muñoz A, Brenner B, Prata PH, Brilhante D, et al:
2022 international clinical practice guidelines for the treatment
and prophylaxis of venous thromboembolism in patients with cancer,
including patients with COVID-19. Lancet Oncol. 23:e334–e347.
2022.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Lyon AR, López-Fernández T, Couch LS,
Asteggiano R, Aznar MC, Bergler-Klein J, Boriani G, Cardinale D,
Cordoba R, Cosyns B, et al: 2022 ESC Guidelines on cardio-oncology
developed in collaboration with the European Hematology Association
(EHA), the European Society for Therapeutic Radiology and Oncology
(ESTRO) and the International Cardio-Oncology Society (IC-OS). Eur
Heart. 43:4229–4361. 2022.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Key NS, Khorana AA, Kuderer NM, Bohlke K,
Lee AYY, Arcelus JI, Wong SL, Balaban EP, Flowers CR, Gates LE, et
al: Venous thromboembolism prophylaxis and treatment in patients
with cancer: ASCO guideline update. J Clin Oncol. 41:3063–3071.
2023.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Falanga A, Ay C, Di Nisio M, Gerotziafas
G, Jara-Palomares L, Langer F, Lecumberri R, Mandala M, Maraveyas
A, Pabinger I, et al: Venous thromboembolism in cancer patients:
ESMO clinical practice guideline. Ann Oncol. 34:452–467.
2023.PubMed/NCBI View Article : Google Scholar
|