Introduction
According to recent studies, approximately 30% of
patients with prostate cancer (PCa) undergoing surgery and 40% of
patients undergoing radiation therapy experience biochemical
recurrence (BCR) within 10 years following local therapies
(1). Patients with BCR have
variable prognoses, with metastasis-free survival ranging from 1 to
>15 years (2).
Non-metastatic castration-resistant prostate cancer
(nmCRPC) is defined as an increase in prostate-specific antigen
(PSA) in the setting of castrate testosterone levels with no
detectable metastases on conventional imaging. Currently, the most
accepted definition of progression on androgen deprivation therapy
(ADT) is based on PSA increase and follows the Prostate Cancer
Clinical Trials Working Group 3 (PCWG3) consensus, which is
primarily intended to define endpoints for clinical trial design
(3).
Recently, three phase 3 trials (ARAMIS, PROSPER, and
SPARTAN) of nmCRPC demonstrated statistically significant
improvements in the primary endpoints of metastasis-free survival
and overall survival (OS) among patients who received androgen
receptor signaling inhibitors (ARSI; darolutamide, enzalutamide, or
apalutamide) (4-6).
Moreover, treatment with abiraterone acetate (1,000 mg) plus
prednisone (5 mg) resulted in a significant reduction in ≥50%
reduction of PSA, with encouraging results for time to PSA
progression, time to radiographic evidence of disease progression,
and safety in patients with high-risk nmCRPC (7). Ultimately, the treatment options for
patients with nmCRPC have significantly improved over the past 2
years (8).
Considering these findings, it is necessary to
understand the prognostic factors of ARSI for the first-line
treatment of Japanese patients with nmCRPC. Therefore, in the
current study, we retrospectively analyzed the prognostic outcomes
on Japanese patients with nmCRPC who received ARSI as a first-line
treatment. Moreover, we developed a novel system to stratify the
prognoses of these patients.
Materials and methods
Patients
In the current study, we retrospectively analyzed
the clinical data of 160 Japanese patients with nmCRPC who received
ARSI as a first-line treatment between January 2014 and December
2022 at four institutions belonging to the Tokai Urologic Oncology
Research Seminar group, including the Fujita Health University
School of Medicine, Nagoya City University Graduate School of
Medical Sciences, Hamamatsu University School of Medicine and Gifu
University Graduate School of Medicine. The study design was
approved by the ethics committees of the four institutions
<approval no: HM23-098 (Fujita Health University School of
Medicine), 60-23-0089 (Nagoya City University Graduate School of
Medical Sciences), 23-049 (Hamamatsu University School of
Medicine), and 023-120 (Gifu University Graduate School of
Medicine)>. The requirement for informed consent from all
patients included in this study was waived due to the retrospective
design.
Evaluation
Clinical characteristics and blood data, including
age, body mass index (BMI), PSA, PSA doubling time (PSADT), Gleason
score, treatment, Eastern Cooperative Oncology Group-Performance
status (ECOG-PS), Hb, ALP, LDH, neutrophil-to-lymphocyte ratio
(NLR), and Geriatric Nutritional Risk Index (GNRI) were assessed.
GNRI was proposed by Bouillanne et al (9) in 2005, and has been widely used for
nutritional assessment in older patients. Calculation of GNRI was
as follow: GNRI=14.89 X serum albumin (g/dL) + 41.7 X BMI/22.
Patients also underwent radiological examinations, including pelvic
magnetic resonance imaging, computed tomography, and radionuclide
bone scanning. Clinical staging of PCa was determined according to
the 8th edition of the American Joint Committee on Cancer manual
(10). Clinically, biochemically,
or radiographically progressive disease was defined according to
the criteria of the PCWG3.
The primary endpoint of the study was to evaluate
the efficacy and safety of ARSI in patients with nmCRPC. The
secondary endpoint was to develop a novel system to stratify the
prognoses of these patients.
Statistical analysis
All data were analyzed using IBM SPSS Statistics
version 23 (SPSS Japan). Each optimum cut-off value was determined
from the receiver operating characteristic (ROC) curve using
Youden's index. Statistical significance was set at P<0.05. OS
and progression-free survival (PFS) were estimated using the
Kaplan-Meier method, and differences were determined using the
log-rank test. Univariate and multivariate analyses were performed
using Cox proportional hazards regression.
Results
In the present study, we retrospectively analyzed
160 patients who received ARSI as a first-line treatment for nmCRPC
between January 2014 and December 2022 at four institutions
belonging to the Tokai Urologic Oncology Research Seminar group.
The clinical characteristics of the 160 patients are shown in
Table I.
 | Table IBaseline patient characteristics
(n=160). |
Table I
Baseline patient characteristics
(n=160).
| Baseline patient
characteristics | Value |
|---|
| Median age, years
(IQR) | 79 (73-83) |
| Median BMI,
kg/m2 (IQR) | 23.2 (20.5-25.5) |
| Median initial PSA,
ng/ml (IQR) | 20.5 (10.2-74.0) |
| Primary Gleason
score, n (%) | |
|
3+3 | 9 (5.6) |
|
3+4 | 17 (10.6) |
|
4+3 | 17 (10.6) |
|
4+4 | 44 (27.5) |
|
4+5 | 27 (16.9) |
|
5+4 | 27 (16.9) |
|
5+5 | 10 (6.3) |
|
Unknown | 9 (5.6) |
| Initial treatment, n
(%) | |
|
ADT | 12 (7.5) |
|
CAB | 89 (55.6) |
|
RP | 25 (15.6) |
|
ADT +
RP | 6 (3.8) |
|
Extrabeam | 6 (3.8) |
|
ADT or CAB +
extrabeam | 17 (10.6) |
|
BT | 1 (0.6) |
|
ADT or CAB +
BT + extrabeam | 4 (2.5) |
| Median time to CRPC,
months (IQR) | 48 (19-86) |
| Median PSA at nmCRPC,
ng/ml (IQR) | 3.7 (2.3-7.6) |
| PSA doubling time, n
(%) | |
|
<6
months | 83 (51.9) |
|
≥6
months | 6 (3.8) |
|
Unknown | 71 (44.4) |
| cN at nmCRPC, n
(%) | |
|
0 | 131 (81.9) |
|
1 | 26 (16.3) |
|
Unknown | 3 (1.9) |
| nmCRPC first-line
treatment, n (%) | |
|
Enzalutamide | 90 (56.3) |
|
Abiraterone | 28 (17.5) |
|
Apalutamide | 22 (13.8) |
|
Darolutamide | 20 (12.5) |
| ECOG PS, n (%) | |
|
0 | 103 (64.4) |
|
1 | 33 (20.6) |
|
2 | 4 (2.5) |
|
3 | 1 (0.6) |
|
Unknown | 19 (11.9) |
| Median Hb, g/dl
(IQR) | 12.7 (11.9-13.5) |
| Median ALP, IU/ml
(IQR) | 120.5
(86.0-245.5) |
| Median LDH, IU/l
(IQR) | 198.5
(175.3-229.0) |
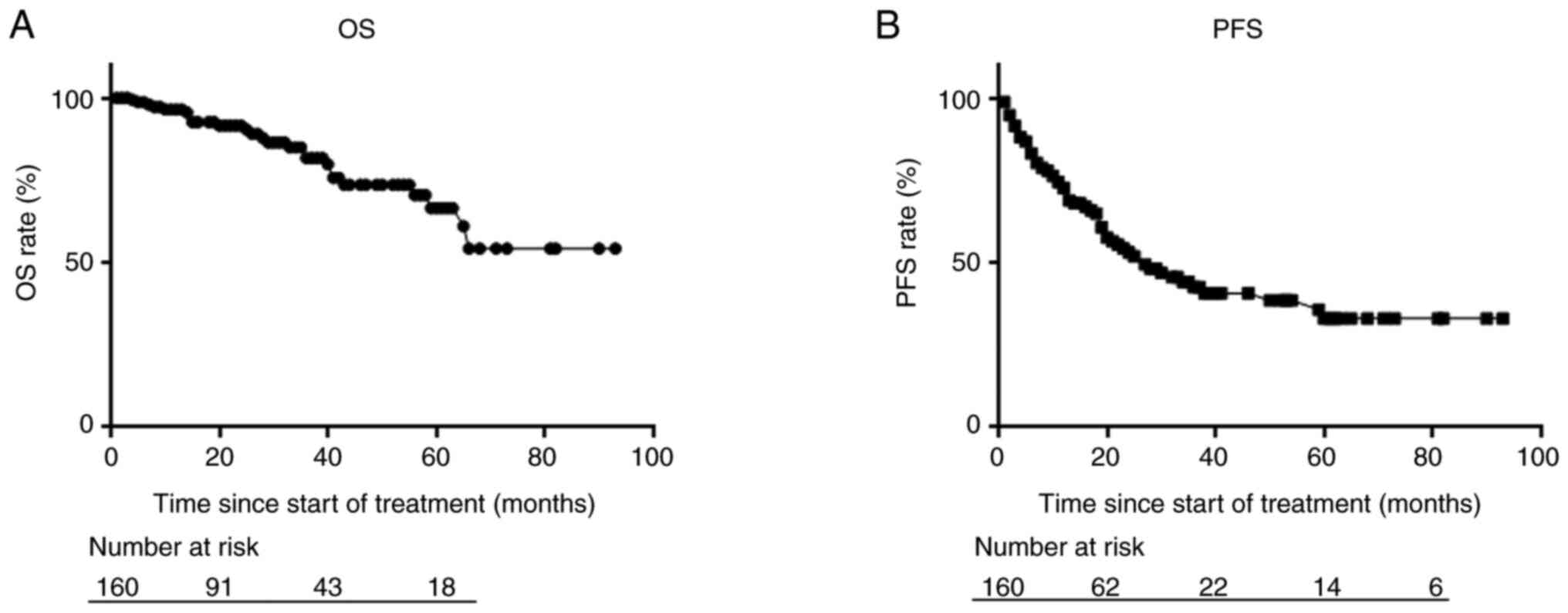
Within a median follow-up period of 23 months, the
median OS was not reached, whereas the median PFS was 26 months
(Fig. 1). The 2-year OS and PFS
rates were 91.6 and 53.1%, respectively.
Next, we performed Cox regression analyses for OS to
evaluate the prognostic significance of ARSI as a first-line
treatment. Univariate analysis demonstrated that the time to CRPC,
PSA level at the initiation of nmCRPC treatment, Hb level, and GNRI
affected OS (P=0.009, 0.021, 0.016, and 0.003, respectively).
Multivariate Cox regression analyses showed that the time to CRPC,
PSA level at the initiation of nmCRPC treatment, and GNRI were
independent predictors of OS (P=0.045, 0.031, and 0.018,
respectively) (Table II).
 | Table IIUnivariate and multivariate analyses
of the clinical parameters of overall survival. |
Table II
Univariate and multivariate analyses
of the clinical parameters of overall survival.
| | Univariate
analysis | Multivariate
analysis |
|---|
| Characteristics | HR (95% CI) | P-value | HR (95% CI) | P-value |
|---|
| Age (≥70 years vs.
<70 years) | 0.467
(0.110-1.984) | 0.302 | | |
| Initial PSA (≥41.7
ng/ml vs. <41.7 ng/ml) | 0.337
(0.150-0.754) | 0.008 | 0.786
(0.205-3.011) | 0.725 |
| Existence of Gleason
pattern 5 (yes vs. no) | 2.255
(0.671-7.582) | 0.189 | | |
| Initial
treatment | | | | |
|
ADT or
CAB | Ref. | | | |
|
RP | 3.040
(0.707-13.064) | 0.135 | | |
|
Radiation | 0.998
(0.166-5.981) | 0.998 | | |
| Time to CRPC (<38
months vs. ≥38 months) | 0.297
(0.119-0.743) | 0.009 | 0.244
(0.062-0.968) | 0.045 |
| PSA at nmCRPC
(≥2.89 ng/ml vs. <2.89 ng/ml) | 0.303
(0.110-0.834) | 0.021 | 0.116
(0.016-0.818) | 0.031 |
| cN (positive vs.
negative) | 0.436
(0.166-1.140) | 0.090 | | |
| nmCRPC first-line
treatment | | | | |
|
Enzalutamide | Ref. | | | |
|
Abiraterone | 0.821
(0.103-6.552) | 0.852 | | |
|
Apalutamide | 1.660
(0.199-13.817) | 0.639 | | |
|
Darolutamide | 0.000
(0.000-1.251x10233) | 0.965 | | |
| ECOG PS (≥1 vs.
0) | 0.625
(0.241-1.623) | 0.335 | | |
| Hb (<12.6 g/dl
vs. ≥12.6 g/dl) | 0.319
(0.125-0.809) | 0.016 | 0.514
(0.153-1.719) | 0.280 |
| ALP (≥174 IU/ml vs.
<174 IU/ml) | 0.720
(0.296-1.754) | 0.470 | | |
| LDH (≥212 IU/l vs.
<212 IU/l) | 0.612
(0.259-1.449) | 0.265 | | |
| NLR (≥2.45 IU/ml
vs. <2.45 IU/ml) | 2.638
(0.755-9.212) | 0.128 | | |
| GNRI (<101.6 vs.
≥101.6) | 0.214
(0.076-0.601) | 0.003 | 0.225
(0.066-0.774) | 0.018 |
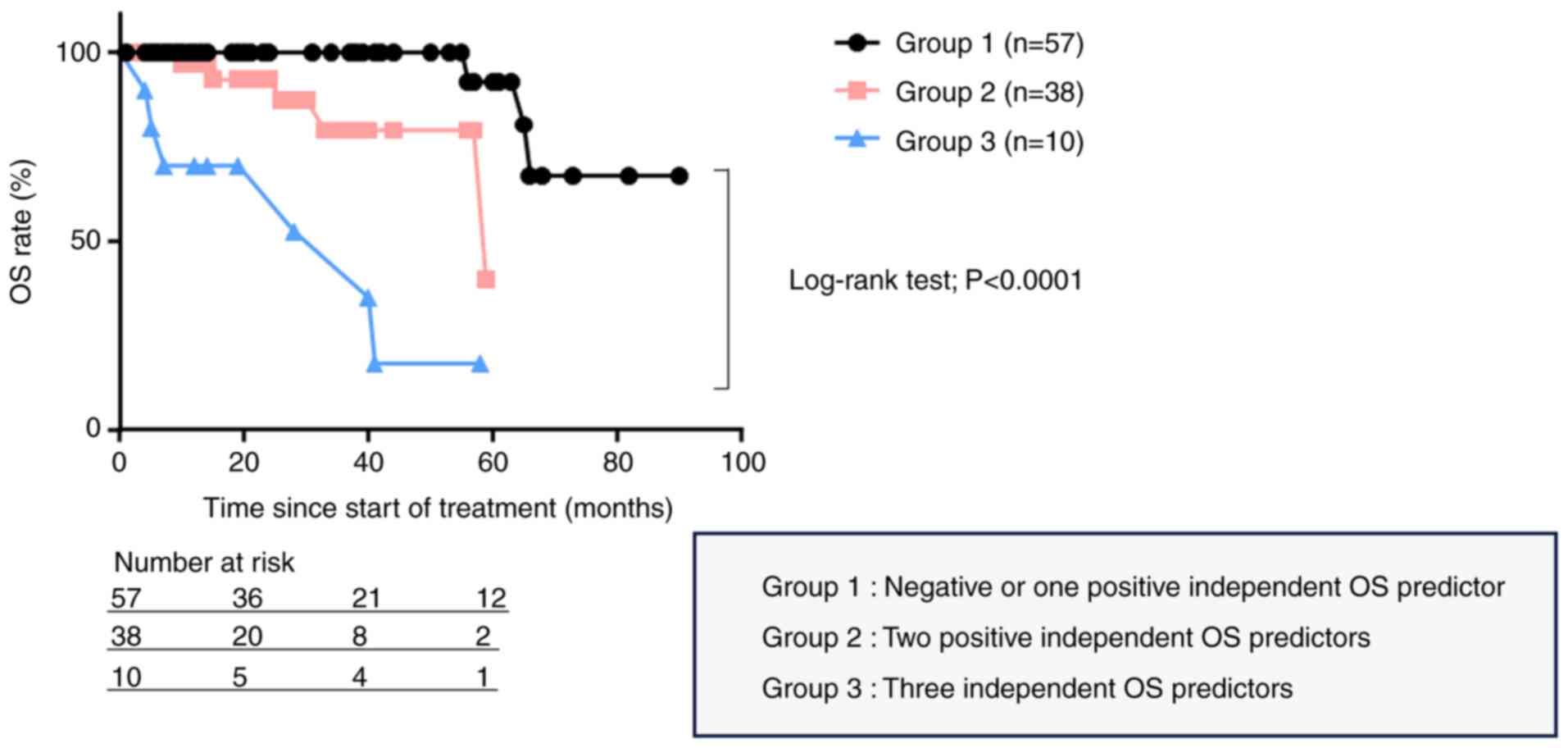
To properly predict the clinical outcomes of
Japanese patients with nmCRPC who received ARSI as a first-line
treatment, we attempted to develop a novel system for the
prognostic stratification of these patients using three independent
OS predictors. From the 160 patients, we selected the 105 for whom
information about all three independent OS predictors was
available. We stratified the 105 patients into three groups
according to these three independent predictors of OS as follows:
Group 1, 57 patients with negative or one positive independent OS
predictor; Group 2, 38 patients with two positive independent OS
predictors; and Group 3, 10 patients with three independent OS
predictors. The OS was significantly different among the three
groups (P<0.0001, Fig. 2).
Discussion
The treatment landscape for patients with nmCRPC has
significantly changed over the past year. First-generation
antiandrogen monotherapies (i.e., bicalutamide or flutamide) and
switching or withdrawal of antiandrogens provide short-term PSA
responses; however, no clinical trial has demonstrated a survival
benefit of such approaches (11-13).
On the other hand, regarding the treatment of nmCRPC, the recent
approval of potent ARSI is specifically linked to the nmCRPC
disease state, and these drugs have been shown to result in
improved outcomes in patients with nmCRPC (4-7).
However, according to a recent meta-analysis, similar results were
seen in sensitivity analyses conducted for OS between the PROSPER
and SPARTAN trials (14).
Collectively, further prognostication should be carried out to
provide more precise information regarding the Japanese patients
with nmCRPC who received ARSI as a first-line treatment.
In the current study, we retrospectively analyzed
the data of 160 Japanese patients with nmCRPC who received ARSI as
first-line treatment. Two recent phase 2 trials [PROSPER (6) for enzalutamide with a median
48-months follow-up and SPARTAN (5,15)
for apalutamide with a median 52-months follow-up] of nmCRPC
treatments reported a median OS of 67.0 and 73.9 months and a
median PFS of ‘not reached’ and 40.5 months, respectively. In the
present study, the median OS was ‘not reached’, whereas the median
PFS was 26 months. However, considering that our follow-up period
of 23 months was relatively short compared to those of the recent
trials described above, prospective studies with longer follow-up
periods are warranted to validate our findings regarding OS and
PFS.
In the present study, we did not obtain sufficient
patient information regarding PSADT. PSADT is a strong predictor of
metastasis, all-cause mortality, and PCa-specific mortality in
patients with nmCRPC. As with patients at earlier disease stages,
<3, 3-8.9, 9-14.9 and ≥15 months are reasonable PSADT thresholds
for risk stratification in men with nmCRPC (16). Considering this information about
PSADT as a strong predictor in patients with nmCRPC, because more
than half of the patients in the present cohort showed PSADT in
less than 6 months, we considered that PSADT is not needed to
assess OS.
Multivariate Cox regression analyses showed that the
time to CRPC, PSA level at the initiation of nmCRPC treatment, and
GNRI were independent predictors of OS, whereas local treatment,
including radiation therapy or prostatectomy, did not affect OS.
Considering these results of analyses, PSA level at the initiation
of nmCRPC treatment might be prognostic alternatives to PSADT for
the Japanese patients with nmCRPC who received ARSI as a first-line
treatment. Regarding the time to CRPC, several recent investigators
have advocated it as a significant prognosticator of OS (17-19).
The GNRI is a simple and objective screening tool
for clinicians to screen patients' nutritional status based on
serum albumin levels, weight, and height. Bouillanne et al
(9) first introduced the GNRI in
2005 to evaluate the 6-month midterm nutritional outcomes of
elderly patients admitted to a rehabilitation unit. They divided
the patients into four groups: a no-risk group (GNRI >98),
low-risk group (GNRI 92-98), moderate-risk group (GNRI 82 to
<92), and major risk group (GNRI <82), suggesting that the
risk of infectious complications or mortality was significantly
higher in the major-, moderate-, and low-risk groups than in the
no-risk group (9). Considering
this GNRI cutoff value, the cutoff value (101.6) obtained from the
ROC curve in the present study was reasonable. Regarding PCa,
Okamoto et al (20)
reported that a GNRI <92.0 was an independent prognostic factor
for cancer-specific survival and OS in patients with metastatic
hormone-naïve PCa. Moreover, in the context of metastatic CRPC
(mCRPC), Chang et al (21)
demonstrated that poor nutritional status with a GNRI <92 was
associated with shorter PFS and OS in patients with mCRPC treated
with docetaxel.
In the current study, to properly predict the
clinical outcomes of Japanese patients with nmCRPC who received
ARSI as a first-line treatment, we attempted to develop a novel
system for the prognostic stratification of these patients using
three independent OS predictors (time to CRPC, PSA at the
initiation of nmCRPC treatment, and GNRI). We divided the patients
into three groups based on the presence of none, one, two, or three
independent OS predictors. We then compared the OS among these
three groups and found that the OS was significantly different
among them. As described above, the treatment options for patients
with nmCRPC have significantly improved over the past 2 years
(8); however, there was no
significant OS difference among patients with nmCRPC who received
ARSI as first-line treatment (14). Considering these findings, we
believe that our novel stratification system based on the positive
number of independent OS predictors could be a useful tool for the
management of Japanese patients with nmCRPC who received ARSI as
first-line treatment.
This study had several limitations. First, it was
retrospectively conducted with a small sample size; thus, a
selection bias may have affected the results. Regarding the
selection of ARSI as a first-line treatment for patients with
nmCRPC, there was no criteria. Second, the cutoff points used in
the current analyses should be assessed in a large-scale study.
Third, we could not obtain sufficient patient information regarding
PSADT. Prospective studies with larger sample sizes and longer
follow-up periods are warranted to confirm our findings.
In conclusion, we identified that ARSI might provide
favorable outcomes for Japanese patients with nmCRPC as a
first-line treatment. Time to CRPC, PSA level at the initiation of
nmCRPC treatment, and GNRI are potential predictors of OS in
Japanese patients with nmCRPC who received ARSI as a first-line
treatment. Furthermore, our novel stratification system based on
the positive numbers of these three independent OS predictors could
help guide decision-making for patients who received ARSI as a
first-line treatment.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
KT, TN, HM, TK, TY and RS conceived and designed the
study. KT, TN, KN and HW acquired the data. KT and TN analyzed and
interpreted the data and drafted the manuscript. KT, TN, KN and HW
confirm the authenticity of all the raw data. All authors have read
and approved the final manuscript.
Ethics approval and consent to
participate
The study design was approved by the ethics
committees of the four institutions [approval nos. HM23-098 (Ethics
Review Committee, Fujita Health University, Toyoake, Japan),
60-23-0089 (Clinical Research Management Center, Nagoya City
University Hospital, Nagoya, Japan), 23-049 (Ethics Committee of
Hamamatsu University School of Medicine, Hamamatsu, Japan) and
023-120 (Medical Review Board of Gifu University Graduate School of
Medicine, Gifu, Japan)], and it was conducted in line with the
guidelines of The Declaration of Helsinki. The requirement for
informed consent from all patients included in the present study
was waived due to the retrospective design.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Paller CJ and Antonarakis ES: Management
of biochemically recurrent prostate cancer after local therapy:
Evolving standards of care and new directions. Clin Adv Hematol
Oncol. 11:14–23. 2013.PubMed/NCBI
|
|
2
|
Antonarakis ES, Feng Z, Trock BJ,
Humphreys EB, Carducci MA, Partin AW, Walsh PC and Eisenberger MA:
The natural history of metastatic progression in men with
prostate-specific antigen recurrence after radical prostatectomy:
Long-term follow-up. BJU Int. 109:32–39. 2012.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Scher HI, Morris MJ, Stadler WM, Higano C,
Basch E, Fizazi K, Antonarakis ES, Beer TM, Carducci MA, Chi KN, et
al: Trial design and objectives for castration-resistant prostate
cancer: Updated recommendations from the prostate cancer clinical
trials working group 3. J Clin Oncol. 34:1402–1418. 2016.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Fizazi K, Shore N, Tammela TL, Ulys A,
Vjaters E, Polyakov S, Jievaltas M, Luz M, Alekseev B, Kuss I, et
al: Nonmetastatic, castration-resistant prostate cancer and
survival with darolutamide. N Engl J Med. 383:1040–1049.
2020.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Smith MR, Saad F, Chowdhury S, Oudard S,
Hadaschik BA, Graff JN, Olmos D, Mainwaring PN, Lee JY, Uemura H,
et al: Apalutamide treatment and metastasis-free survival in
prostate cancer. N Engl J Med. 378:1408–1418. 2018.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Sternberg CN, Fizazi K, Saad F, Shore ND,
De Giorgi U, Penson DF, Ferreira U, Efstathiou E, Madziarska K,
Kolinsky MP, et al: Enzalutamide and survival in nonmetastatic,
castration-resistant prostate cancer. N Engl J Med. 382:2197–206.
2020.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Ryan CJ, Crawford ED, Shore ND, Underwood
W III, Taplin ME, Londhe A, Francis PSJ, Phillips J, McGowan T and
Kantoff PW: The IMAAGEN study: effect of abiraterone acetate and
prednisone on prostate specific antigen and radiographic disease
progression in patients with nonmetastatic castration resistant
prostate cancer. J Urol. 200:344–352. 2018.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Esther J, Maughan BL, Anderson N, Agarwal
N and Hahn AW: Management of nonmetastatic castration-resistant
prostate cancer: Recent advances and future direction. Curr Treat
Options Oncol. 20(14)2019.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Bouillanne O, Morineau G, Dupont C,
Coulombel I, Vincent JP, Nicolis I, Benazeth S, Cynober L and
Aussel C: Geriatric nutritional risk index: A new index for
evaluating at-risk elderly medical patients. Am J Clin Nutr.
82:777–783. 2005.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Buyyounouski MK, Choyke PL, McKenney JK,
Sartor O, Sandler HM, Amin MB, Kattan MW and Lin DW: Prostate
cancer-major changes in the American joint committee on cancer
eighth edition cancer staging manual. CA Cancer J Clin. 67:245–253.
2017.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Lodde M, Lacombe L and Fradet Y: Salvage
therapy with bicalutamide 150 mg in nonmetastatic
castration-resistant prostate cancer. Urology. 76:1189–1193.
2010.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Sartor AO, Tangen CM, Hussain MH,
Eisenberger MA, Parab M, Fontana JA, Chapman RA, Mills GM, Raghavan
D and Crawford ED: Southwest Oncology Group. Antiandrogen
withdrawal in castrate-refractory prostate cancer: A southwest
oncology group trial (SWOG 9426). Cancer. 112:2393–2400.
2008.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Suzuki H, Okihara K, Miyake H, Fujisawa M,
Miyoshi S, Matsumoto T, Fujii M, Takihana Y, Usui T, Matsuda T, et
al: Alternative nonsteroidal antiandrogen therapy for advanced
prostate cancer that relapsed after initial maximum androgen
blockade. J Urol. 180:921–927. 2008.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Tombal B, Sternberg CN, Hussain M, Ganguli
A, Li Y, Sandin R, Bhadauria H, Oh M and Saad F: Matching-adjusted
indirect treatment comparison of the efficacy of enzalutamide
versus apalutamide for the treatment of nonmetastatic
castration-resistant prostate cancer. ESMO Open.
7(100510)2022.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Smith MR, Saad F, Chowdhury S, Oudard S,
Hadaschik BA, Graff JN, Olmos D, Mainwaring PN, Lee JY, Uemura H,
et al: Apalutamide and overall survival in prostate cancer. Eur
Urol. 79:150–158. 2021.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Howard LE, Moreira DM, De Hoedt A, Aronson
WJ, Kane CJ, Amling CL, Cooperberg MR, Terris MK and Freedland SJ:
Thresholds for PSA doubling time in men with non-metastatic
castration-resistant prostate cancer. BJU Int. 120:E80–E86.
2017.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Frees S, Akamatsu S, Bidnur S, Khalaf D,
Chavez-Munoz C, Struss W, Eigl BJ, Gleave M, Chi KN and So A: The
impact of time to metastasis on overall survival in patients with
prostate cancer. World J Urol. 36:1039–1046. 2018.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Miyake H, Matsushita Y, Watanabe H, Tamura
K, Motoyama D, Ito T, Sugiyama T and Otsuka A: Prognostic
significance of time to castration resistance in patients with
metastatic castration-sensitive prostate cancer. Anticancer Res.
39:1391–1396. 2019.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Wenzel M, Preisser F, Hoeh B, Schroeder M,
Würnschimmel C, Steuber T, Heinzer H, Banek S, Ahrens M, Becker A,
et al: Impact of time to castration resistance on survival in
metastatic hormone sensitive prostate cancer patients in the era of
combination therapies. Front Oncol. 11(659135)2021.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Okamoto T, Hatakeyama S, Narita S,
Takahashi M, Sakurai T, Kawamura S, Hoshi S, Ishida M, Kawaguchi T,
Ishidoya S, et al: Impact of nutritional status on the prognosis of
patients with metastatic hormone-naive prostate cancer: A
multicenter retrospective cohort study in Japan. World J Urol.
37:1827–1835. 2019.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Chang LW, Hung SC, Li JR, Chiu KY, Yang
CK, Chen CS, Lu K, Chen CC, Wang SC, Lin CY, et al: Geriatric
nutritional risk index as a prognostic marker for patients with
metastatic castration-resistant prostate cancer receiving
docetaxel. Front Pharmacol. 11(601513)2020.PubMed/NCBI View Article : Google Scholar
|