Introduction
Gastric cancer (GC) constitutes one of the most
wide-ranging cancers, with >1 million affected patients each
year (1), and usually recurs as
metastasis to the liver and peritoneum (2). However, brain metastasis (BM) is very
uncommon (<1%), and the prognosis is markedly unfavorable
compared with CG metastasis to other organs, with a median survival
at this stage of the cancer approximately 2 to 4 months (3).
Due to the relative rarity of the disease, a
significant number of patients with GC quickly succumb to the
disease after receiving a diagnosis of BM, or BM is identified
after death in numerous autopsies (4). In addition, there are relatively few
studies with GC and developed BM, and management options such as
stereotactic radiosurgery (SRS) or chemotherapy, whole-brain
radiotherapy (WBRT), and surgical resection are still under
examination (5).
In this respect, the present meta-analysis assessed
the relationship between no-surgical treatment (SRS, WBRT or
chemotherapy) vs. the additional microsurgical BM resection in
terms of the patient's quality of life and potential survival
advantage.
Materials and methods
Literature search strategy
The meta-analysis investigated studies that compared
no-surgical treatments (SRS, WBRT or chemotherapy) with studies
that involved surgery for BM resection. The studies were found in
electronic databases such as PubMed (https://www.ncbi.nlm.nih.gov/pmc/?db=PMC) (1980-April
2024), Medline (https://www.nlm.nih.gov/medline/medline_home.html)
(1980-April 2024), Cochrane Library (https://library.udel.edu/databases/cochrane/), and
EMBASE (https://libguides.lib.cuhk.edu.hk/medicine/database/embase)
(1980-April 2024). A protocol and documentation plan was created by
applying the Preferred Reporting Items for Systematic Reviews and
Meta-Analyses (PRISMA) guidelines (6). The following key words were used for
the search: ‘Gastric cancer’, ‘brain metastasis’, and ‘gastric
cancer and brain metastasis’.
Inclusion and exclusion criteria
The current meta-analysis assembled the PICOS
parameters from the included studies (7). Inclusion of studies was based on the
following: i) The population was limited to patients with GC and
BM; ii) An additional surgical intervention for BM was implemented;
iii) survival outcomes were compared and analyzed; and iv) the
overall survival of GC patients with BM who received additional
surgical management was quantified. In order to mitigate
publication bias, the ultimate goal was to gather a uniform set of
studies that solely assessed two modalities: A comparison between
no-surgical treatments such as SRS, WBRT or chemotherapy, and an
additional surgical BM resection for patients with GC.
All the articles that were case reports, reviews,
editorials, and not in English were excluded. Articles with
pediatric populations, novel procedures in the investigational
phase, those that included only one of the two management options,
and those that disclosed doubtful results were also excluded. Two
investigators (GF, a neurosurgeon and GC, a gastric cancer surgeon)
individually extracted information from the enclosed articles using
the epidemiology principles of meta-analysis. In cases of
disagreement, the decision of an additional author was considered.
The post-interventional outcomes stated in the last collection of
articles were evaluated at least 6 months following surgical
treatment (surgical resection of BM in patients with GC). In
addition, to reduce the risk of bias in the included articles, a
quality assessment tool (the Newcastle-Ottawa Scale) was used
(Table I) (8). All patients with GC were divided into
two groups: Those with no-surgical treatment (SRS, WBRT or
chemotherapy) and those with an additional surgical BM
resection.
 | Table INewcastle-Ottawa scale quality
assessment of the final article pool. |
Table I
Newcastle-Ottawa scale quality
assessment of the final article pool.
| | Newcastle-Ottawa
scale | |
|---|
| First author,
year | Study design | Selection | Comparability | Exposure | Total scores | (Refs.) |
|---|
| York et al,
1999 | One single center,
retrospective | 3 | 3 | 3 | 9 | (5) |
| Kasakura et
al, 2000 | One single center,
retrospective | 3 | 2 | 2 | 7 | (9) |
| Qiu et al,
2018 | Multicenter,
retrospective | 3 | 3 | 3 | 9 | (10) |
| Li et al,
2020 | Multicenter,
retrospective | 3 | 2 | 2 | 7 | (11) |
| Ishizuka et
al, 2023 | One single center,
retrospective | 3 | 2 | 2 | 7 | (12) |
| Baccili Cury Megid
et al, 2024 | One single center,
retrospective | 3 | 3 | 3 | 9 | (13) |
Statistical analysis
All data were evaluated via Review Manager Software
(RevMan), version 5.4 (https://www.risetku.com/blog/revman). I2
statistics assessed heterogeneity among studies. Α meta-analysis
was evaluated using a random-effect model. P<0.05 was considered
to indicate a statistically significant difference.
Results
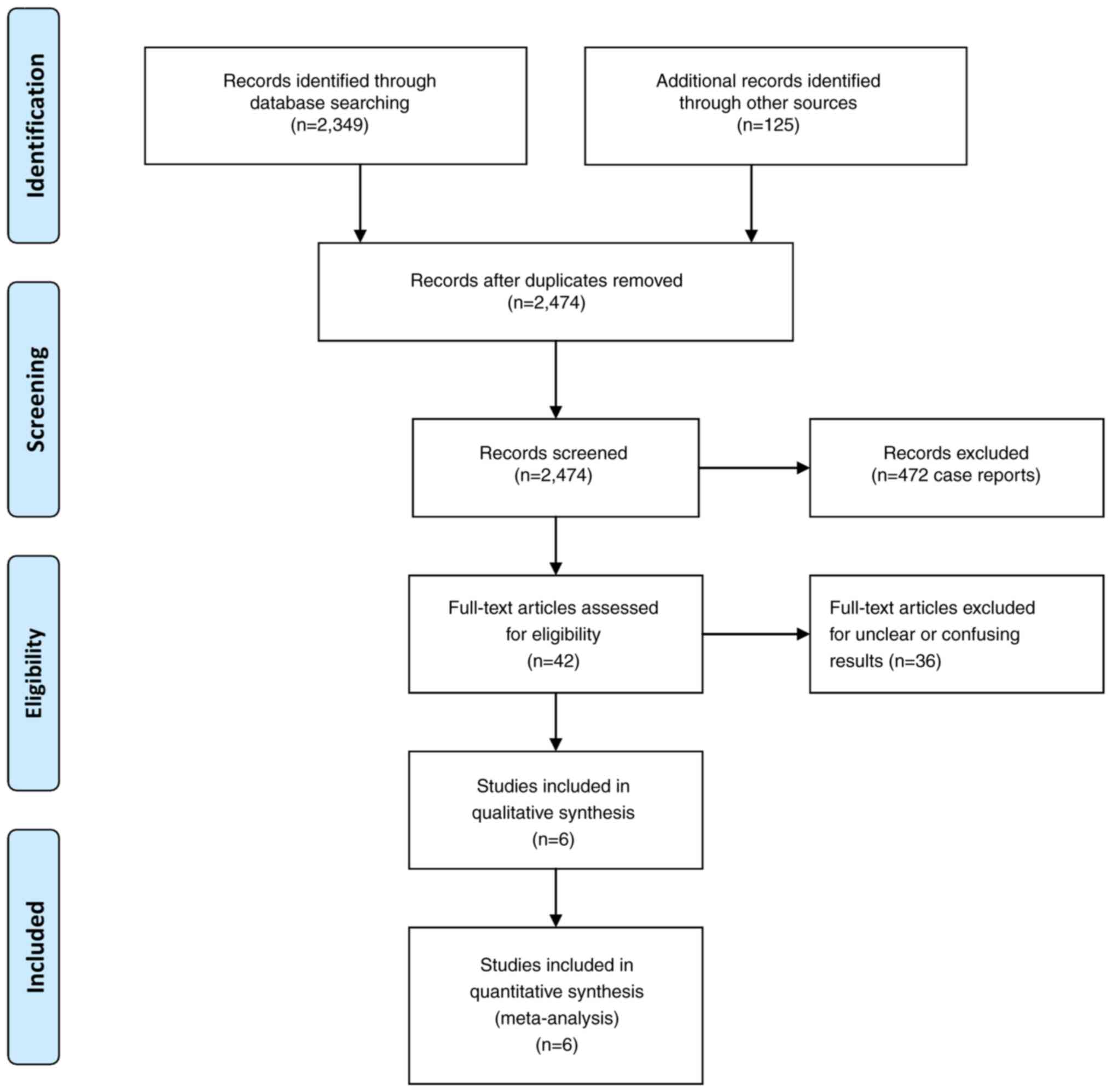
Baseline characteristics. Following the prime
literature search, 42 studies were suitable for additional
investigation. When all the criteria were applied, six articles
were included in the final study pool (Fig. 1) (5,9-13).
The entire data of these studies are presented in Table II. The total sample of patients
collected from these six articles with GC was 32.372, and from
these patients 361 (1.1%) were identified with BM. The number of
patients with BM and no-surgical treatment was 289 (80.1%) compared
with those that underwent an additional surgical resection which
was 72 (19.9%). The mean age of the patients was 59.2 years, and
the males were 195 (73.9%) of the 264 available from five studies
(5,9-12)
(Table II).
 | Table IIDesign and baseline characteristics of
the included study trials. |
Table II
Design and baseline characteristics of
the included study trials.
| | Sample size | | >6-month
survival | |
|---|
| First author,
year | Total no. of patients
with GC | Total no. of patients
with GC and BM | BM no-surgical
treatment | BM plus surgical
treatment | Mean age of patients
with BM (years) | No. of male patients
with BM | Follow-up
(years) | Stage of advanced
cancer | Location of BM | Time from GC to BM
(months) | BM no-surgical
Treatment | BM plus Surgical
Treatment | (Refs.) |
|---|
| York et al,
1999 | 3,320 | 24 | 14 | 10 | 53 | 18 | 40 | III or more | 14 supratentorial, 3
infratentorial, 7 multiple | 9 (1-23) | 5 | 6 | (5) |
| Kasakura et
al, 2000 | 2,322 | 11 | 8 | 3 | 54.6 | 9 | 18 | III or more | 7 multiple, 4
solitary supratentorial | 9.6 (0.1-43.7) | 1 | 2 | (9) |
| Qiu et al,
2018 | 19,022 | 151 | 139 | 12 | 61.3 | 113 | 5 | NR | 99 multiple, 52
solitary supratentorial | NR | 4 | 2 | (10) |
| Li et al,
2020 | 4,221 | 59 | 41 | 18 | 51.1 | 44 | 2 | NR | 12 multiple, 47
solitary supratentorial | NR | 12 | 12 | (11) |
| Ishizuka et
al, 2023 | 1,257 | 16 | 12 | 4 | 71 | 11 | 10 | II or III | 12 multiple, 7
solitary supratentorial | 12.9 | 2 | 2 | (12) |
| Baccili Cury Megid
et al, 2024 | 2,230 | 100 | 75 | 25 | 64.4 | NR | 14 | III or more | NR | 6.7 (3,4-13,8) | 15 | 13 | (13) |
| Summary | 32.372 | 361 | 289 (80.1%) | 72 (19.9%) | 59.2 | 195 of 264
(73.9%) | - | - | - | - | 39 of 289
(13.5%) | 37 of 72
(51.4%) | - |
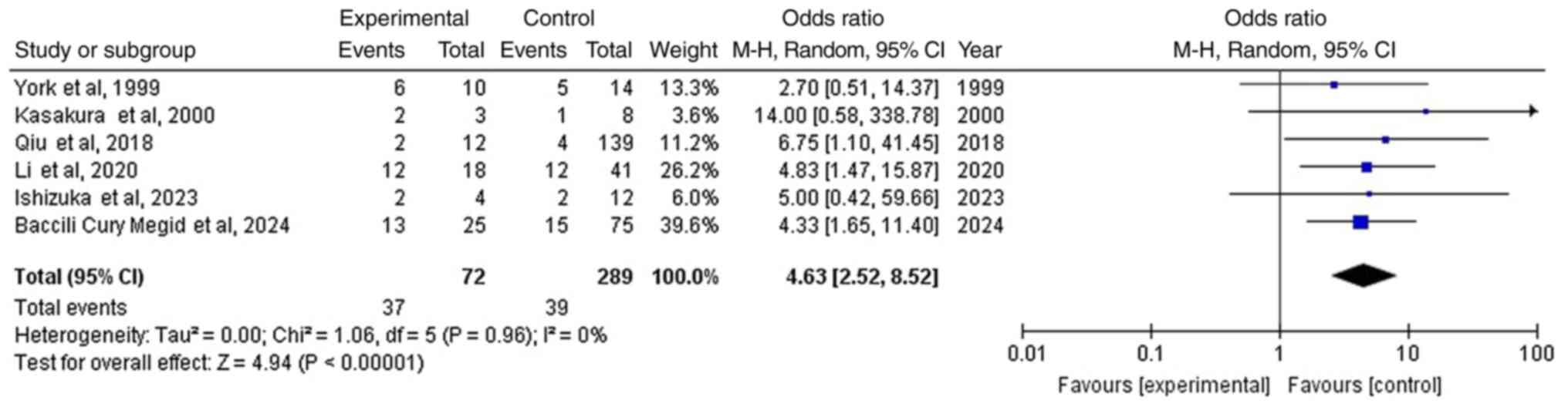
Survival >6 months
Data was gathered from six articles (5,9-13).
In the entire group of patients with GC and BM, there were 76 out
of the 361 (21.1%) patients [39 of 289 (13.5%) in the no-surgical
treatment group, and 37 out of the 72 (51.4%) with an additional
surgical BM resection], showing a statistically significant
difference between the groups (OR, 4.63; 95% CI, 2.52 to 8.52;
P<0.05) with no heterogeneity (P=0.96 and
I2=0%) (Fig. 2
and Table III), and thus the
superiority of the additional surgical BM resection group compared
with no-surgical treatment group; Fig.
2) (Table III). When
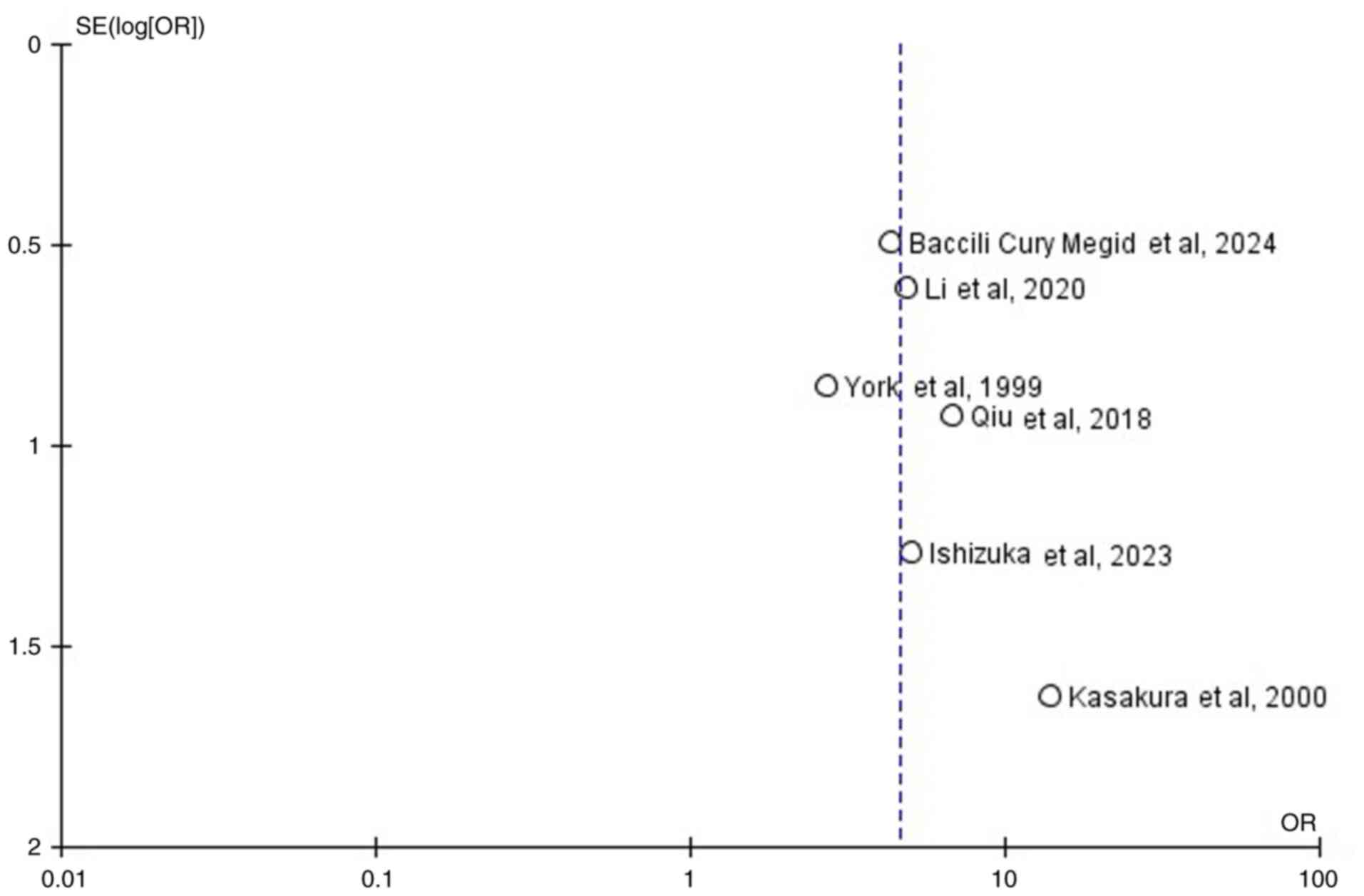
studying the funnel plot of the same parameter, it was observed
that the study results showed no publication bias (Fig. 3).
 | Table IIIThe outcome results of the
meta-analysis. |
Table III
The outcome results of the
meta-analysis.
| | Groups | Overall effect | Heterogeneity |
|---|
| Parameters | No. of studies | Total no. of
patients with BM and no-surgical treatment | Total no. of
patients with BM plus surgical treatment | >6-month
survival of patients with BM and no-surgical treatment | >6-month
survival of patients with BM plus surgical treatment | Effect
estimate | 95% CI | P-value | I2
(%) | P-value |
|---|
| >6-month
survival | 6 | 289 | 72 | 39 | 37 | 4.63 | (2.52-8.52) | <0.05 | 0 | 0.96 |
Discussion
Prognosis of patients with BM from
GC
BM constitutes ~13% of all brain tumors, with the
primary malignancy mostly found in the lung and secondarily in the
breast (14). Considering that BM
from GC is extremely rare and usually occurs hematogenously with a
markedly unfavorable outcome, the present meta-analysis revealed
that additional surgical treatment of BM was associated with an
improved prognosis (survival, >6 months) than no-surgical
management (SRS, WBRT or chemotherapy). It was determined that in
the entire group of patients with GC and BM, there were 51.4% of
patients with an additional surgical BM resection compared with
13.5% in those with no-surgical treatment, which had improved
outcomes (survival, >6 months).
Frequency of BM and GC
BM accounts for ~13% of central nervous system (CNS)
tumors and mainly originates from melanoma, chorioepitheliomas and
lung cancer (14). On the other
hand, GC is the 5th most frequent tumor metastasizing to various
organs, with markedly unfavorable outcomes (15). BM in patients with GC is relatively
rare (0.5-0.7%), and in most cases, the diagnosis occurs at a late
stage, which may signify that the survival of those patients is
markedly short (16). In the
present meta-analysis, BM was identified in 1.1% of the total
number of patients with GC.
Conversion therapy of GC with BM and
survival
The main approach for managing GC according to
literature is palliative chemotherapy (17). On the other hand, conversion
therapy, an expansion of exchange chemotherapy, aids in achieving
surgical resection of a primary tumor that was initially considered
to be technically difficult to approach or inoperable, encompassing
the utililization of radiotherapy, chemotherapy, or target therapy
for a locally advanced tumor. In terms of palliative management,
conversion therapy can result in extended survival times and
improved outcomes for patients with metastatic GC (18).
Surgical resection as the sole
treatment for the primary tumor of GC with BM and survival
As only 10% of patients with metastatic GC underwent
surgical removal, surgical procedures on the primary tumor mostly
improved the outcome of these patients (5). Conversely, compared with patients
with BM, patients with GC with lung and liver metastases exhibited
an improved prognosis (5). In
addition, the location and the number of BMs also influenced the
outcome of patients with GC. Thus, the prognosis of metastatic GC
is not easy to detect, and the resection alone of the primary tumor
may be better when it includes a BM site. The meta-analysis showed
that an additional surgical removal of BM is related to favorable
outcomes.
Prognosis in patients with GC and
BM
A median age of >65 years old, signet ring cell
carcinoma histological type, and the IV stage of GC constitute some
of the main parameters related to unfavorable outcomes and low
patient survival with GC and BM (19). According to the literature, the
prognosis of patients with metastatic GC depends on the metastatic
location, with the most unfavorable outcome in those patients with
BM compared with metastasis in the lung and liver (20,21).
In addition, the number and site of the metastatic lesions in the
brain could also influence the survival of patients (5). New therapeutic protocols and the
development of imaging equipment have led to early detection of
patients with GC and BM, ultimately improving the quality of life
of these patients (22). In
addition, surgical management of both the primary tumor and BM in
patients with GC, in combination with chemotherapy, SRS or WBRT,
has extended the survival time of this fatal disease (18). The present meta-analysis revealed
that the additional surgical treatment of BM compared with
no-surgical management (SRS, WBRT or chemotherapy) achieved a
>6-month survival in 21.1% of patients with GC and BM.
Limitation
A limitation of the present study is that the
meta-analysis pool consisted of relatively small sample sizes;
consequently, the results require further validation with a
large-scale sample size.
Conclusion of the findings
The findings of the present meta-analysis revealed
that the curative effect of BM tumor resection on patients with GC
compared with additional no-surgical treatment using SRS, WBRT or
chemotherapy was favorable for their survival. However, further
studies on carefully selected patients are necessary to confirm
these findings.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
GF and NF conceptualized the present study. VEG,
DAS, GC, PS, KP, NT, GF and NF evaluated the data and wrote and
prepared the draft of the manuscript. NF and GF applied critical
revisions. All authors contributed to manuscript revision and have
read and approved the final version of the manuscript. Data
authentication is not applicable.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
DAS is the Editor-in-Chief for the journal, but had
no personal involvement in the reviewing process, or any influence
in terms of adjudicating on the final decision, for this article.
The other authors declare that they have no competing
interests.
References
|
1
|
Rawla P and Barsouk A: Epidemiology of
gastric cancer: Global trends, risk factors and prevention. Prz
Gastroenterol. 14:26–38. 2019.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Deng J, Liang H, Wang D, Sun D, Pan Y and
Liu Y: Investigation of the recurrence patterns of gastric cancer
following a curative resection. Surg Today. 41:210–215.
2011.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Gaspar L, Scott C, Rotman M, Asbell S,
Phillips T, Wasserman T, McKenna WG and Byhardt R: Recursive
partitioning analysis (RPA) of prognostic factors in three
radiation therapy oncology group (RTOG) brain metastases trials.
Int J Radiat Oncol Biol Phys. 37:745–751. 1997.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Abrams Hl, Spiro R and Goldstein N:
Metastases in carcinoma; analysis of 1000 autopsied cases. Cancer.
3:74–85. 1950.PubMed/NCBI View Article : Google Scholar
|
|
5
|
York JE, Stringer J, Ajani JA, Wildrick DM
and Gokaslan ZL: Gastric cancer and metastasis to the brain. Ann
Surg Oncol. 6:771–776. 1999.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Foster RL: Reporting guidelines: CONSORT,
PRISMA, and SQUIRE. J Spec Pediatr Nurs. 17:1–2. 2012.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Amir-Behghadami M and Janati A:
Population, intervention, comparison, outcomes and study (PICOS)
design as a framework to formulate eligibility criteria in
systematic reviews. Emerg Med J. 37(387)2020.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Bae JM: A suggestion for quality
assessment in systematic reviews of observational studies in
nutritional epidemiology. Epidemiol Health.
38(e2016014)2016.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Kasakura Y, Fujii M, Mochizuki F, Suzuki T
and Takahashi T: Clinicopathological study of brain metastasis in
gastric cancer patients. Surg Today. 30:485–490. 2000.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Qiu MZ, Shi SM, Chen ZH, Yu HE, Sheng H,
Jin Y, Wang DS, Wang FH, Li YH, Xie D, et al: Frequency and
clinicopathological features of metastasis to liver, lung, bone,
and brain from gastric cancer: A SEER-based study. Cancer Med.
7:3662–3672. 2018.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Li Y, Xie D, Chen X, Hu T, Lu S and Han Y:
Prognostic value of the site of distant metastasis and surgical
interventions in metastatic gastric cancer: A population-based
study. Technol Cancer Res Treat.
19(1533033820964131)2020.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Ishizuka Y, Omori T, Shinno N, Yamamoto M,
Hara H, Otsuka T, Nishio M, Nishida N, Fujisawa F, Sugimoto N, et
al: Early detection of brain metastases and appropriate local
therapy followed by systemic chemotherapy may improve the prognosis
of gastric cancer. Sci Rep. 13(20805)2023.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Baccili Cury Megid T, Baskurt Z, Ma LX,
Barron CC, Farooq A, Saltiel MP, Wang X, Bach Y, Ayoama H, Jang RW,
et al: Leptomeningeal carcinomatosis and brain metastases in
gastroesophageal carcinoma: A real-world analysis of clinical and
pathologic characteristics and outcomes. J Neurooncol. 167:111–122.
2024.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Global Burden of Disease Cancer
Collaboration. Fitzmaurice C, Abate D, Abbasi N, Abbastabar H,
Abd-Allah F, Abdel-Rahman O, Abdelalim A, Abdoli A, Abdollahpour I,
et al: Global, regional, and national cancer incidence, mortality,
years of life lost, years lived with disability, and
disability-adjusted life-years for 29 cancer groups, 1990 to 2017:
A systematic analysis for the global burden of disease study. JAMA
Oncol. 5:1749–1768. 2019.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Riihimäki M, Hemminki A, Sundquist K,
Sundquist J and Hemminki K: Metastatic spread in patients with
gastric cancer. Oncotarget. 7:52307–52316. 2016.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Davis FG, Dolecek TA, McCarthy BJ and
Villano JL: Toward determining the lifetime occurrence of
metastatic brain tumors estimated from 2007 United States cancer
incidence data. Neuro Oncol. 14:1171–1177. 2012.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Japanese Gastric Cancer Association.
Japanese gastric cancer treatment guidelines 2010 (ver. 3). Gastric
Cancer. 14:113–123. 2011.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Guo Q, Li Q, Wang J, Liu M, Wang Y, Chen
Z, Ye Y, Guan Q and Zhou Y: A comprehensive evaluation of clinical
efficacy and safety of celecoxib in combination with chemotherapy
in metastatic or postoperative recurrent gastric cancer patients: A
preliminary, three-center, clinical trial study. Medicine
(Baltimore). 98(e16234)2019.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Liu G, Xu M, Gao T, Xu L, Zeng P, Bo H, Li
F, Zhang W and Wang Z: Surgical compliance and outcomes in gastric
cancer: A population-based cohort study. J Cancer. 10:779–788.
2019.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Inoue K, Nakane Y, Kogire M, Fujitani K,
Kimura Y, Imamura H, Tamura S, Okano S, Kwon AH, Kurokawa Y, et al:
Phase II trial of preoperative S-1 plus cisplatin followed by
surgery for initially unresectable locally advanced gastric cancer.
Eur J Surg Oncol. 38:143–149. 2012.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Yamaguchi K, Yoshida K, Tanahashi T,
Takahashi T, Matsuhashi N, Tanaka Y, Tanabe K and Ohdan H: The
long-term survival of stage IV gastric cancer patients with
conversion therapy. Gastric Cancer. 21:315–323. 2018.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Luo D, Liu Q, Yu W, Ma Y, Zhu J, Lian P,
Cai S, Li Q and Li X: Prognostic value of distant metastasis sites
and surgery in stage IV colorectal cancer: A population-based
study. Int J Colorectal Dis. 33:1241–1249. 2018.PubMed/NCBI View Article : Google Scholar
|