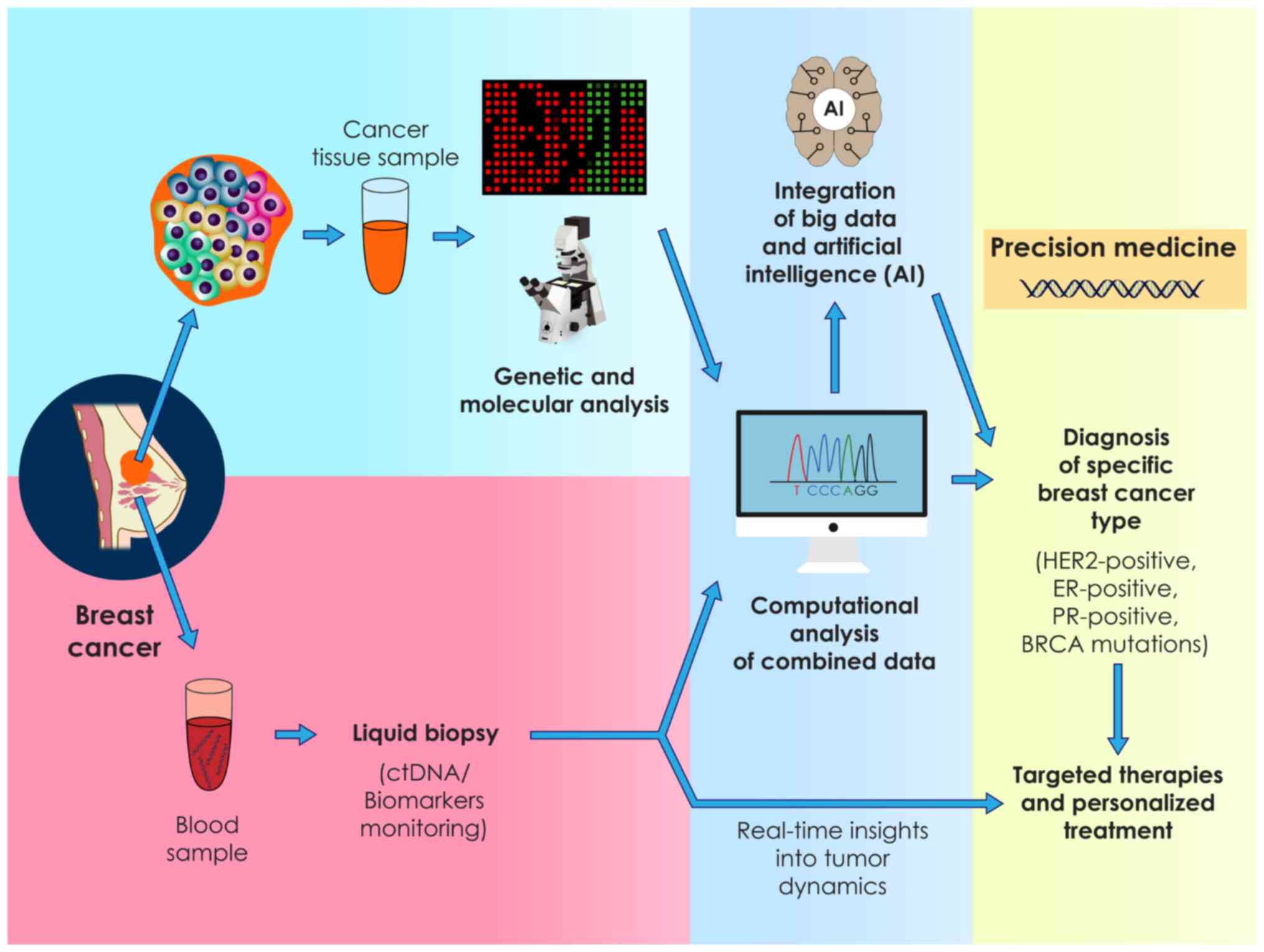
1. Introduction
Precision medicine in breast cancer stands at the
forefront of transformative healthcare, reshaping the landscape of
diagnosis, treatment, and management strategies. This innovative
approach, tailored to the individual characteristics of both the
patient and their tumor, marks a paradigm shift from traditional
one-size-fits-all cancer therapies to a more personalized, targeted
and effective treatment regimen (1,2).
Breast cancer remains a major global health concern,
being the most common cancer type in women worldwide. According to
the World Health Organization, in 2020, there were ~2.3 million new
cases and 685,000 deaths globally. In the United States alone, it
is estimated that ~1 in 8 women (13%) will develop invasive breast
cancer over the course of their lifetime (3). Despite advancements in early
detection and treatment, the heterogeneous nature of breast cancer
poses significant challenges in achieving optimal patient outcomes.
This heterogeneity encompasses various subtypes driven by distinct
genetic mutations, molecular profiles and cellular characteristics,
which significantly influence the behavior of tumors and their
response to treatment (4).
Genetic testing, particularly for BRCA DNA repair
associated 1 (BRCA1) and BRCA2 mutations, plays a critical role in
assessing hereditary risk factors and guiding treatment decisions.
Individuals carrying these mutations have a higher risk of
developing breast cancer, necessitating personalized surveillance
and preventive strategies (5). The
classification of breast cancer into subtypes such as hormone
receptor-positive, human epidermal growth factor receptor 2
(HER2)-positive and triple-negative breast cancer (TNBC) allows for
more precise treatment strategies. Each subtype responds
differently to therapies, emphasizing the need for tailored
treatment plans (6-8).
Advances in targeted therapies, including HER2
inhibitors and hormonal treatments, have significantly improved
outcomes for patients with specific subtypes of breast cancer.
These therapies offer a more effective approach by directly
targeting the molecular abnormalities driving tumor growth.
Emerging treatments like immune checkpoint inhibitors show promise,
particularly in TNBC, and ongoing research aims to expand the
applicability of immunotherapy across various breast cancer
subtypes (7,9).
Additionally, the use of big data analytics and
artificial intelligence (AI) enhances our understanding of breast
cancer biology, facilitating the development of predictive models
and personalized treatment strategies (10). Non-invasive liquid biopsies provide
real-time insights into tumor dynamics, enabling continuous
monitoring and timely adjustments in therapy (11).
While significant progress has been made, challenges
remain, including the accessibility of advanced medical
technologies, managing tumor heterogeneity and addressing acquired
resistance to therapies. Continued research and innovation are
essential to overcoming these barriers and achieving the full
potential of precision medicine in breast cancer. By leveraging the
advancements in genomics, targeted therapies, immunotherapy, data
analytics and innovative diagnostics, precision medicine aims to
optimize treatment efficacy, minimize adverse effects and
ultimately enhance the quality of life for individuals affected by
breast cancer (12,13).
2. Understanding breast cancer
diversity
The diversity within breast cancer is a pivotal
aspect that shapes its behavior and determines treatment responses
and patient outcomes. It's not a singular disease but a complex
spectrum comprising various subtypes, each exhibiting unique
genetic, molecular and cellular characteristics. This inherent
heterogeneity profoundly influences how tumors behave and how they
respond to different treatments, and ultimately impacts patient
prognosis (14,15).
The different subtypes of breast cancer arise from
distinct genetic mutations or alterations within the DNA of breast
cells. These alterations drive the growth and behavior of the
tumor. For instance, the presence or absence of hormone receptors
[estrogen receptors (ER), progesterone receptors (PR)] and the
overexpression of the HER2/neu gene play crucial roles in defining
specific breast cancer subtypes (16,17).
The recognition of these variations in breast cancer
subtypes has given rise to the paradigm of precision medicine.
Instead of a universal method, precision medicine recognizes and
embraces the variations within breast cancer. It emphasizes the
need for tailored and personalized treatment strategies that
account for the unique characteristics of each patient's tumor
(18,19).
This shift in approach is significant, as it
acknowledges that what works for one subtype of breast cancer may
not be as effective for another. For instance, hormone
receptor-positive breast cancers respond well to hormonal therapies
that target these receptors, while HER2-positive tumors benefit
from therapies specifically designed to inhibit the HER2 protein
(20).
Furthermore, this diversity influences the behavior
of tumors, impacting their aggressiveness, growth rates, likelihood
of spreading (metastasis) and response to various treatments.
Certain subtypes may be more aggressive and fast-growing, while
others may respond better to certain therapies but poorly to
others.
Appreciating and understanding this diversity is
fundamental to developing targeted therapies. By identifying
specific genetic or molecular alterations within each subtype,
oncologists can tailor treatments to attack these specific
vulnerabilities in the tumor. This approach leads to more effective
treatments that precisely target the unique characteristics of a
patient's cancer while minimizing side effects (21,22).
3. Genetic insights and risk assessment
Genetic testing has become an integral part of
precision medicine in the realm of breast cancer. This innovative
approach involves the examination of specific genes, notably the
breast cancer genes BRCA1 and BRCA2, to uncover potential mutations
or alterations that significantly contribute to a heightened risk
of developing breast cancer (23).
However, in addition to the geneticist's
perspective, it is crucial to incorporate insights from medical
professionals and patients to provide a comprehensive view of
precision medicine. Medical professionals can offer practical
insights into the clinical implementation and ethical
considerations of precision medicine, while patients' experiences
and preferences are essential for tailoring treatments that improve
their quality of life (24,25).
Furthermore, the concept of precision medicine, which involves
calculating personal risks based on genetic data in the context of
a referential population, can be prone to errors. These errors may
arise from the complexity of genetic information, variability in
genetic databases and potential misinterpretations, particularly
when dealing with rare or poorly understood genetic variants
(21).
The discovery of mutations within the BRCA1 and
BRCA2 genes has profound implications, particularly in assessing
hereditary risk factors associated with breast cancer. Individuals
carrying mutations in these genes have a notably higher risk of
developing breast and ovarian cancers compared to the general
population. This knowledge has revolutionized risk assessment,
enabling healthcare professionals to identify individuals who may
be predisposed to these hereditary forms of cancer (26,27).
Genetic testing offers a personalized understanding
of an individual's risk profile. For those identified as carrying
BRCA mutations, personalized risk assessment becomes crucial. It
not only aids in understanding their likelihood of developing
breast cancer but also extends to assessing the risk of other
associated cancers, such as ovarian cancer. Armed with this
knowledge, patients and healthcare providers can make
well-considered choices regarding proactive steps to handle and
reduce these risks.
Increased surveillance, such as more frequent
screenings or specific imaging modalities, is often recommended for
individuals identified as having a higher genetic predisposition to
breast cancer. In addition, risk-reducing surgeries, such as
prophylactic mastectomy or oophorectomy, may be considered
preventive measures in high-risk individuals, significantly
reducing the chances of developing breast or ovarian cancer
(24,25).
Furthermore, genetic insights are not limited to
risk assessment alone; they also play a pivotal role in guiding
treatment decisions. In cases where a patient's tumor exhibits
specific genetic mutations or alterations, this information can
influence the selection of targeted therapies. For instance,
individuals with BRCA mutations may benefit from specific drugs or
treatments that target these genetic vulnerabilities within the
tumor, leading to more effective and tailored treatment plans
(28,29).
Understanding an individual's genetic predisposition
to breast cancer is a cornerstone of precision medicine. It not
only informs personalized risk assessment but also guides treatment
strategies, empowering patients and healthcare providers to make
informed decisions about proactive measures and tailored
treatments. This personalized approach significantly enhances the
efficacy of interventions and improves patient outcomes in the
realm of breast cancer management (30,31).
4. Genomic profiling and molecular
subtyping
Advancements in genomic profiling techniques have
ushered in a new era of understanding the intricacies of breast
cancer biology. These sophisticated molecular analysis methods
delve deep into the genetic makeup of tumors, unraveling specific
genetic mutations, alterations or expression patterns that underpin
the disease's development and progression (32).
One of the key contributions of genomic profiling is
the ability to molecularly subtype breast cancers. These subtypes
are determined by analyzing various molecular characteristics
present in the tumor. Hormone receptor status, primarily the
presence of ER and PR, serves as a fundamental classification
criterion. Tumors that express these receptors (ER-positive or
PR-positive) respond differently to treatments compared to those
lacking these receptors (ER-negative or PR-negative) (33,34).
In addition, the expression of HER2 represents
another crucial molecular marker. HER2-positive tumors overexpress
the HER2 protein, leading to aggressive tumor behavior.
Identification of the HER2 status is pivotal, as it influences
treatment decisions, such as targeted therapies like trastuzumab,
specifically designed to inhibit HER2 (35-37).
Furthermore, gene expression profiling techniques,
such as the Prosigna Breast Cancer Prognostic Gene Signature
(PAM50) assay, have emerged as valuable tools in molecular
subtyping. The PAM50 assay evaluates the expression of a panel of
genes within the tumor, enabling the categorization of breast
cancers into distinct molecular subgroups. These subtypes, such as
Luminal A, Luminal B, HER2-enriched and TNBC, provide deeper
insights into tumor behavior and response to treatments (38,39).
Molecular subtyping offers a refined understanding
of breast cancer heterogeneity. It allows oncologists to categorize
tumors into specific subgroups based on their unique molecular
characteristics, guiding tailored therapeutic interventions. For
instance, hormone receptor-positive tumors typically respond well
to hormonal therapies, while HER2-positive tumors benefit from
HER2-targeted treatments. Conversely, TNBCs, lacking these
receptors, often require different approaches, such as chemotherapy
(40).
This molecular categorization aids in personalized
treatment planning, facilitating the selection of therapies that
precisely target the biological characteristics of each subtype. By
aligning treatments with the molecular profile of the tumor,
clinicians can optimize treatment efficacy while minimizing
unnecessary side effects (41).
5. Targeted therapies and personalized
treatment
The emergence of targeted therapies marks a
groundbreaking advancement in the field of breast cancer
management. These therapies represent a sophisticated approach that
aims to target precisely the underlying molecular abnormalities
driving the growth and spread of tumors.
One of the remarkable success stories in targeted
therapies is the development of treatments specifically designed
for HER2-positive breast cancer. HER2, a protein overexpressed in
certain breast cancers, plays a crucial role in promoting
aggressive tumor behavior. Targeted agents like trastuzumab and
pertuzumab are monoclonal antibodies that act by binding to the
HER2 receptor, inhibiting its signaling pathways and effectively
slowing down tumor growth. These therapies have transformed the
outlook for patients with HER2-positive breast cancer,
significantly improving survival rates and reducing the risk of
recurrence (42,43).
Another pivotal area of targeted therapy lies in
addressing hormone receptor-positive breast cancers. Hormone
receptor-positive tumors, particularly those expressing ER, rely on
hormone signaling pathways for their growth. Targeted hormonal
therapies aim to disrupt these pathways, thereby impeding tumor
proliferation. Aromatase inhibitors and selective ER modulators
(SERMs) are among the tailored treatments used for this subtype.
Aromatase inhibitors, for example, inhibit the production of
estrogen in postmenopausal women, while SERMs like tamoxifen block
estrogen from binding to receptors in breast cells, effectively
hindering tumor growth (44,45).
These targeted therapies exemplify the shift towards
personalized treatment approaches in breast cancer. By focusing on
the specific molecular characteristics of each subtype, these
therapies offer more effective and tailored interventions. This
tailored method not only boosts the effectiveness of treatments but
also reduces negative impacts on healthy tissues, ultimately
enhancing the life quality of patients receiving these therapies
(Fig. 1) (46).
6. Immunotherapy and its promise
Immunotherapy has emerged as a promising frontier in
the realm of breast cancer treatment, heralding a novel approach
that harnesses the body's immune system to combat cancer cells. At
the forefront of this innovative approach are immune checkpoint
inhibitors, such as pembrolizumab and nivolumab, which have shown
significant potential in transforming cancer therapy. These
inhibitors function by blocking proteins like programmed cell death
1/programmed cell death ligand 1 (PD-L1) and cytotoxic T-lymphocyte
associated protein 4, which tumors use to evade immune detection.
By inhibiting these checkpoints, these therapies enhance the immune
system's ability to recognize and attack cancer cells. The
effectiveness of these inhibitors can be significantly improved
through precision medicine approaches that involve genetic and
molecular profiling to identify biomarkers indicative of a likely
response to immunotherapy (47-49).
While the efficacy of immunotherapy, particularly
checkpoint inhibitors, has been more conspicuous in certain subsets
of breast cancer, such as TNBC, ongoing research endeavors aim to
expand its scope across various breast cancer subtypes. TNBC,
characterized by the absence of hormone receptors and HER2
expression, has shown particular promise in responding to
immunotherapy due to its heightened immune response and increased
presence of immune cells within the tumor microenvironment
(50,51).
However, the efficacy of immunotherapy in other
breast cancer subtypes, such as hormone receptor-positive or
HER2-positive cancers, has been more modest. Researchers are
actively exploring combination therapies that combine immunotherapy
with other treatment modalities, including chemotherapy, targeted
therapies or other immune-modulating agents. These combinations aim
to augment the immune system's response and enhance the
effectiveness of immunotherapy across a broader spectrum of
patients with breast cancer (52,53).
Furthermore, ongoing efforts to identify predictive
biomarkers play a crucial role in refining patient selection for
immunotherapy. Biomarkers, such as PD-L1 expression on tumor cells
or tumor-infiltrating lymphocytes, serve as indicators of potential
responsiveness to checkpoint inhibitors. Incorporating these
biomarkers into clinical decision-making helps identify patients
who are more likely to benefit from immunotherapy, ensuring a more
tailored and effective treatment approach.
The evolving landscape of immunotherapy in breast
cancer treatment holds promise for advancing therapeutic options.
While the current success has been more pronounced in specific
subtypes, ongoing research endeavors aim to broaden its
applicability across a wider spectrum of patients with breast
cancer. Through combination therapies, biomarker identification and
ongoing clinical trials, the goal is to optimize the efficacy of
immunotherapy and integrate it into the paradigm of personalized
breast cancer treatment (54,55).
7. Integration of big data and AI
The integration of big data analytics and AI has
heralded a transformative era in precision medicine, particularly
in the context of breast cancer research and treatment.
Incorporating multi-omics approaches, which include genomics,
proteomics, metabolomics and transcriptomics, can provide a more
comprehensive understanding of cancer biology. This multi-faceted
approach allows for a deeper insight into tumor heterogeneity and
can lead to more effective and personalized treatment strategies
(56,57).
These cutting-edge technologies empower researchers
and clinicians to navigate and analyze vast and intricate datasets
encompassing genetic information, treatment responses, patient
outcomes and a multitude of clinical variables (58).
At the core of this integration lies the capacity to
process and derive insights from immense volumes of diverse data.
Big data analytics, leveraging advanced computational techniques,
sifts through this wealth of information to identify subtle
patterns, correlations and associations that may elude traditional
analytical methods. Within the realm of breast cancer, these
datasets include genetic profiles obtained through genomic
sequencing, molecular data characterizing tumor subtypes, treatment
history, patient demographics and clinical outcomes (59).
AI, particularly machine learning algorithms, has a
significant role in decoding this complex data landscape. These
algorithms possess the capacity to learn and adapt autonomously,
recognizing intricate relationships and deciphering patterns within
the datasets. By processing this information, AI algorithms can
generate predictive models that aid clinicians in crafting
well-informed choices customized for each patient. For instance,
these models can predict treatment responses, anticipate potential
side effects, forecast disease progression or identify personalized
therapeutic strategies based on a patient's unique genetic makeup
and clinical profile (60).
In the context of breast cancer, AI algorithms,
particularly machine learning, can process and analyze vast
datasets comprising genetic information, clinical records and
treatment outcomes, uncovering patterns and insights that are
beyond human capability. These insights can inform personalized
treatment strategies, predicting which therapies will be most
effective for individual patients based on their unique genetic and
molecular profiles. Future advancements in AI and big data
analytics will continue to refine these approaches, enabling more
precise and effective cancer treatments. The ability to
continuously learn and adapt from new data will allow AI to stay at
the forefront of precision medicine, ultimately leading to better
patient outcomes (61).
Furthermore, the ongoing evolution of these
technologies continues to refine their capabilities. With ongoing
progression in machine learning algorithms and data analytics, the
precision and accuracy of predictive models are improving.
Integration with real-time patient data from diverse sources
further enhances the depth and breadth of insights generated by
these AI-driven approaches. However, it is important to note that,
while the potential of big data and AI in breast cancer research
and treatment is promising, challenges remain. Issues related to
data privacy, data standardization and the need for validation and
clinical translation of AI-generated insights pose significant
hurdles that need to be addressed (62).
8. Liquid biopsies and real-time
monitoring
Liquid biopsies represent a groundbreaking,
non-invasive technique in cancer diagnosis and surveillance,
providing exceptional real-time understanding of tumor genetics and
behavior. This innovative approach involves analyzing circulating
tumor DNA (ctDNA) or specific biomarkers present in blood samples,
presenting a promising avenue for monitoring treatment response,
detecting potential resistance mechanisms and guiding timely
adjustments in therapy (63,64).
One of the primary advantages of liquid biopsies
lies in their non-invasive nature. Unlike traditional tissue
biopsies, which involve invasive procedures to extract tissue
samples from the tumor site, liquid biopsies utilize blood samples.
These blood samples contain circulating tumor components, such as
ctDNA, exosomes, circulating tumor cells and other tumor-specific
biomarkers shed by the tumor into the bloodstream.
The analysis of these components provides a dynamic
and comprehensive view of the tumor's genetic makeup and behavior.
By examining ctDNA, which comprises fragments of tumor DNA released
into the bloodstream, liquid biopsies can reveal specific genetic
alterations, mutations or genomic rearrangements present in the
tumor. This information is invaluable in understanding the tumor's
heterogeneity, predicting treatment responses and identifying
potential resistance mechanisms that may emerge during the course
of treatment (65,66).
Furthermore, the real-time nature of liquid biopsies
allows for continuous monitoring of the tumor's status throughout
the treatment journey. These tests offer the ability to track
changes in the tumor profile over time, enabling clinicians to
promptly detect any alterations in the tumor's genetic landscape.
This capability is crucial in identifying early signs of treatment
response or disease progression and facilitating timely
interventions and adjustments in therapy regimens (67).
Liquid biopsies hold immense promise in various
facets of cancer management, including breast cancer. In the
context of breast cancer treatment, these tests enable clinicians
to monitor the effectiveness of targeted therapies or chemotherapy,
assess the emergence of treatment resistance and make informed
decisions regarding treatment modifications or switches to
alternative therapies.
In addition, liquid biopsies have the potential to
revolutionize post-treatment surveillance. They can detect minimal
residual disease or early signs of recurrence more sensitively than
conventional imaging techniques, allowing for earlier intervention
and potentially improving patient outcomes.
However, while liquid biopsies offer tremendous
potential, several challenges remain. Standardization of
methodologies, optimization of sensitivity and specificity, and
validation of their clinical utility in large-scale studies are
essential to ensuring their widespread adoption in routine clinical
practice. Overcoming these challenges will further enhance the role
of liquid biopsies as a crucial tool in the armamentarium of cancer
diagnostics and monitoring, shaping a more personalized and dynamic
approach to cancer care (68-70).
9. Challenges and future directions
Despite the remarkable strides, challenges persist
in implementing precision medicine universally. One of the primary
obstacles is ensuring equitable access to these advanced medical
technologies. Socioeconomic disparities, geographic limitations and
variations in healthcare infrastructure can lead to unequal access
to genomic testing and personalized treatments. Addressing these
barriers is crucial to ensure that the benefits of precision
medicine are available to all populations, regardless of their
socioeconomic status or geographic location (69,70).
Access to advanced genomic testing, targeted therapies and
specialized treatments may be limited in certain healthcare
settings or regions. In addition, tumor heterogeneity, acquired
resistance to targeted therapies and the evolving complexity of
cancer biology pose ongoing challenges that necessitate continued
research and innovation (69).
The future of precision medicine for breast cancer
holds immense promise. Progressions in technology, such as
single-cell sequencing, spatial genomics and multi-omics
approaches, offer a deeper understanding of tumor heterogeneity and
evolution. Integrating diverse treatment modalities, including
targeted therapies, immunotherapy and emerging novel agents, in a
personalized approach based on comprehensive molecular profiling
holds potential to further improve patient outcomes (70).
Furthermore, precision prevention emerges as a
significant aspect, focusing on identifying high-risk individuals
through genetic screening and implementing personalized
interventions to prevent breast cancer development or detect it at
an early, more curable stage (56). Another future promising field of
precision medicine recommends radiogenomics that combines genomic
profiles and particular imaging features of every individual that
have been recognized and show promising results in the diagnosis
and prognosis of breast cancer (71).
In conclusion, precision medicine in breast cancer
epitomizes a tailored, patient-centric approach that leverages the
advancements in genomics, targeted therapies, immunotherapy, data
analytics and innovative diagnostics. By harnessing the power of
personalized medicine, the goal is to optimize treatment efficacy,
minimize adverse effects, and ultimately enhance the quality of
life for individuals affected by breast cancer. However, achieving
widespread implementation requires concerted efforts in research,
accessibility, healthcare infrastructure and interdisciplinary
collaborations to fully harness the potential of precision medicine
in combating breast cancer.
Acknowledgements
The authors would like to thank Dr Meletios Verras
(Department of General Biology, Medical School, University of
Patras, Patras, Greece) for the professional assistance with the
figure design.
Funding
Funding: No funding was received.
Availability of data and materials
Not applicable.
Authors' contributions
PP and VEG conceptualized the study. PP, VEG, PVD,
ET, AN, ACL, GCZ, DAS, NK and GET made substantial contributions to
data interpretation and analysis and wrote and prepared the draft
of the manuscript. DAS and VEG analyzed the data and provided
critical revisions. All authors have read and approved the final
manuscript. Data authentication is not applicable.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
DAS is the Editor-in-Chief for the journal, but had
no personal involvement in the reviewing process, or any influence
in terms of adjudicating on the final decision, for this article.
The other authors declare that they have no competing
interests.
Use of AI tools
During the preparation of this work, the AI tool
Chat GPT was used to improve the readability and language of the
manuscript, and subsequently, the authors revised and edited the
content produced by the AI tool as necessary, taking full
responsibility for the ultimate content of the present
manuscript.
References
|
1
|
Harris EER: Precision medicine for breast
cancer: The paths to truly individualized diagnosis and treatment.
Int J Breast Cancer. 2018(4809183)2018.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Sarhangi N, Hajjari S, Heydari SF,
Ganjizadeh M, Rouhollah F and Hasanzad M: Breast cancer in the era
of precision medicine. Mol Biol Rep. 49:10023–10037.
2022.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Arnold M, Morgan E, Rumgay H, Mafra A,
Singh D, Laversanne M, Vignat J, Gralow JR, Cardoso F, Siesling S
and Soerjomataram I: Current and future burden of breast cancer:
Global statistics for 2020 and 2040. Breast. 66:15–23.
2022.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Mavrommati I, Johnson F, Echeverria GV and
Natrajan R: Subclonal heterogeneity and evolution in breast cancer.
NPJ Breast Cancer. 7(155)2021.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Singh DN, Daripelli S, Elamin Bushara MO,
Polevoy GG and Prasanna M: Genetic testing for successful cancer
treatment. Cureus. 15(e49889)2023.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Orrantia-Borunda E, Anchondo-Nuñez P,
Acuña-Aguilar LE, Gómez-Valles FO and Ramírez-Valdespino CA:
Subtypes of breast cancer. In: Breast Cancer [Internet]. Mayrovitz
HN (ed). Exon Publications, Brisbane, AU, 2022.
|
|
7
|
Damaskos C, Garmpis N, Garmpi A,
Nikolettos K, Sarantis P, Georgakopoulou VE, Nonni A, Schizas D,
Antoniou EA, Karamouzis MV, et al: Investigational drug treatments
for triple-negative breast cancer. J Pers Med.
11(652)2021.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Papalexis P, Georgakopoulou VE, Keramydas
D, Vogiatzis R, Taskou C, Anagnostopoulou FA, Nonni A, Lazaris AC,
Zografos GC, Kavantzas N and Thomopoulou GE: Clinical,
histopathological, and immunohistochemical characteristics of
predictive biomarkers of breast cancer: A retrospective study.
Cancer Diagn Progn. 4:340–351. 2024.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Mercogliano MF, Bruni S, Mauro FL and
Schillaci R: Emerging targeted therapies for HER2-positive breast
cancer. Cancers (Basel). 15(1987)2023.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Sebastian AM and Peter D: Artificial
intelligence in cancer research: Trends, challenges and future
directions. Life (Basel). 12(1991)2022.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Adhit KK, Wanjari A, Menon S and K S:
Liquid biopsy: An evolving paradigm for non-invasive disease
diagnosis and monitoring in medicine. Cureus.
15(e50176)2023.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Feng Y, Spezia M, Huang S, Yuan C, Zeng Z,
Zhang L, Ji X, Liu W, Huang B, Luo W, et al: Breast cancer
development and progression: Risk factors, cancer stem cells,
signaling pathways, genomics, and molecular pathogenesis. Genes
Dis. 5:77–106. 2018.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Yersal O and Barutca S: Biological
subtypes of breast cancer: Prognostic and therapeutic implications.
World J Clin Oncol. 5:412–424. 2014.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Testa U, Castelli G and Pelosi E: Breast
cancer: A molecularly heterogenous disease needing subtype-specific
treatments. Med Sci (Basel). 8(18)2020.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Zhao S, Zuo WJ, Shao ZM and Jiang YZ:
Molecular subtypes and precision treatment of triple-negative
breast cancer. Ann Transl Med. 8(499)2020.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Subhan MA, Parveen F, Shah H, Yalamarty
SSK, Ataide JA and Torchilin VP: Recent advances with precision
medicine treatment for breast cancer including triple-negative
sub-type. Cancers (Basel). 15(2204)2023.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Bettaieb A, Paul C, Plenchette S, Shan J,
Chouchane L and Ghiringhelli F: Precision medicine in breast
cancer: Reality or utopia? J Transl Med. 15(139)2017.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Patel A, Unni N and Peng Y: The changing
paradigm for the treatment of HER2-positive breast cancer. Cancers
(Basel). 12(2081)2020.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Ottaiano A, Ianniello M, Santorsola M,
Ruggiero R, Sirica R, Sabbatino F, Perri F, Cascella M, Di Marzo M,
Berretta M, et al: From chaos to opportunity: decoding cancer
heterogeneity for enhanced treatment strategies. Biology (Basel).
12(1183)2023.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Wang Q, Shao X, Zhang Y, Zhu M, Wang FXC,
Mu J, Li J, Yao H and Chen K: Role of tumor microenvironment in
cancer progression and therapeutic strategy. Cancer Med.
12:11149–11165. 2023.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Olopade OI, Grushko TA, Nanda R and Huo D:
Advances in breast cancer: Pathways to personalized medicine. Clin
Cancer Res. 14:7988–7999. 2008.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Nelson HD, Huffman LH, Fu R, Harris EL,
Walker M and Bougatsos C: Genetic risk assessment and brca mutation
testing for breast and ovarian cancer susceptibility [Internet].
Rockville (MD): Agency for Healthcare Research and Quality (US);
2005.
|
|
23
|
Kinney AY, Simonsen SE, Baty BJ, Mandal D,
Neuhausen SL, Seggar K, Holubkov R and Smith K: Acceptance of
genetic testing for hereditary breast ovarian cancer among study
enrollees from an African American kindred. Am J Med Genet A.
140:813–826. 2006.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Mehrgou A and Akouchekian M: The
importance of BRCA1 and BRCA2 genes mutations in breast cancer
development. Med J Islam Repub Iran. 30(369)2016.PubMed/NCBI
|
|
25
|
Casaubon JT, Kashyap S and Regan JP: BRCA1
and BRCA2 mutations. [Updated 2023 Jul 23]. In: StatPearls
[Internet]. Treasure Island (FL): StatPearls Publishing; 2024.
|
|
26
|
Maioru OV, Radoi VE, Coman MC, Hotinceanu
IA, Dan A, Eftenoiu AE, Burtavel LM, Bohiltea LC and Severin EM:
Developments in genetics: Better management of ovarian cancer
patients. Int J Mol Sci. 24(15987)2023.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Weitzel JN, Blazer KR, MacDonald DJ,
Culver JO and Offit K: Genetics, genomics, and cancer risk
assessment: State of the art and future directions in the era of
personalized medicine. CA Cancer J Clin. 61:327–359.
2011.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Mehmood S, Faheem M, Ismail H, Farhat SM,
Ali M, Younis S and Asghar MN: ‘Breast cancer resistance likelihood
and personalized treatment through integrated multiomics’. Front
Mol Biosci. 9(783494)2022.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Wang RC and Wang Z: Precision medicine:
Disease subtyping and tailored treatment. Cancers (Basel).
15(3837)2023.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Eralp Y: The role of genomic profiling in
advanced breast cancer: The two faces of Janus. Transl
Oncogenomics. 8 (Suppl 1):S1–S7. 2016.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Masood S: Breast cancer subtypes:
Morphologic and biologic characterization. Womens Health (Lond).
12:103–119. 2016.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Tariq M, Richard V and Kerin MJ: MicroRNAs
as molecular biomarkers for the characterization of basal-like
breast tumor subtype. Biomedicines. 11(3007)2023.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Iqbal N and Iqbal N: Human epidermal
growth factor receptor 2 (HER2) in cancers: Overexpression and
therapeutic implications. Mol Biol Int. 2014(852748)2014.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Krishnamurti U and Silverman JF: HER2 in
breast cancer: A review and update. Adv Anat Pathol. 21:100–107.
2014.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Dank M, Mühl D, Pölhös A, Csanda R, Herold
M, Kovacs AK, Madaras L, Kulka J, Palhazy T, Tokes AM, et al: The
prediction analysis of microarray 50 (PAM50) gene expression
classifier utilized in indeterminate-risk breast cancer patients in
hungary: A consecutive 5-year experience. Genes (Basel).
14(1708)2023.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Archer SG, Eliopoulos A, Spandidos D,
Barnes D, Ellis IO, Blamey RW, Nicholson RI and Robertson JF:
Expression of ras p21, p53 and c-erbB-2 in advanced breast cancer
and response to first line hormonal therapy. Br J Cancer.
72:1259–1266. 1995.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Caan BJ, Sweeney C, Habel LA, Kwan ML,
Kroenke CH, Weltzien EK, Quesenberry CP Jr, Castillo A, Factor RE,
Kushi LH and Bernard PS: Intrinsic subtypes from the PAM50 gene
expression assay in a population-based breast cancer survivor
cohort: prognostication of short- and long-term outcomes. Cancer
Epidemiol Biomarkers Prev. 23:725–734. 2014.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Cho N: Molecular subtypes and imaging
phenotypes of breast cancer. Ultrasonography. 35:281–288.
2016.PubMed/NCBI View Article : Google Scholar
|
|
39
|
El-Deiry WS, Goldberg RM, Lenz HJ, Shields
AF, Gibney GT, Tan AR, Brown J, Eisenberg B, Heath EI, Phuphanich
S, et al: The current state of molecular testing in the treatment
of patients with solid tumors, 2019. CA Cancer J Clin. 69:305–343.
2019.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Masoud V and Pagès G: Targeted therapies
in breast cancer: New challenges to fight against resistance. World
J Clin Oncol. 8:120–134. 2017.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Jallah JK, Dweh TJ, Anjankar A and Palma
O: A Review of the advancements in targeted therapies for breast
cancer. Cureus. 15(e47847)2023.PubMed/NCBI View Article : Google Scholar
|
|
42
|
El Sayed R, El Jamal L, El Iskandarani S,
Kort J, Abdel Salam M and Assi H: Endocrine and targeted therapy
for hormone-receptor-positive, HER2-negative advanced breast
cancer: insights to sequencing treatment and overcoming resistance
based on clinical trials. Front Oncol. 9(510)2019.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Andrahennadi S, Sami A, Manna M, Pauls M
and Ahmed S: Current landscape of targeted therapy in hormone
receptor-positive and HER2-negative breast cancer. Curr Oncol.
28:1803–1822. 2021.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Nandy A, Gangopadhyay S and Mukhopadhyay
A: Individualizing breast cancer treatment-The dawn of personalized
medicine. Exp Cell Res. 320:1–11. 2014.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Hu ZI and McArthur HL: Immunotherapy in
breast cancer: The new frontier. Curr Breast Cancer Rep. 10:35–40.
2018.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Vasileiou M, Papageorgiou S and Nguyen NP:
Current advancements and future perspectives of immunotherapy in
breast cancer treatment. Immuno. 3:195–216. 2023.
|
|
47
|
Lao Y, Shen D, Zhang W, He R and Jiang M:
Immune checkpoint inhibitors in cancer therapy-how to overcome drug
resistance? Cancers (Basel). 14(3575)2022.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Abdou Y, Goudarzi A, Yu JX, Upadhaya S,
Vincent B and Carey LA: Immunotherapy in triple negative breast
cancer: Beyond checkpoint inhibitors. NPJ Breast Cancer.
8(121)2022.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Liu Y, Hu Y, Xue J, Li J, Yi J, Bu J,
Zhang Z, Qiu P and Gu X: Advances in immunotherapy for
triple-negative breast cancer. Mol Cancer. 22(145)2023.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Ayoub NM, Al-Shami KM and Yaghan RJ:
Immunotherapy for HER2-positive breast cancer: Recent advances and
combination therapeutic approaches. Breast Cancer (Dove Med Press).
11:53–69. 2019.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Debien V, De Caluwé A, Wang X,
Piccart-Gebhart M, Tuohy VK, Romano E and Buisseret L:
Immunotherapy in breast cancer: An overview of current strategies
and perspectives. NPJ Breast Cancer. 9(7)2023.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Chen N, Higashiyama N and Hoyos V:
Predictive biomarkers of immune checkpoint inhibitor response in
breast cancer: Looking beyond tumoral PD-L1. Biomedicines.
9(1863)2021.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Kossai M, Radosevic-Robin N and
Penault-Llorca F: Refining patient selection for breast cancer
immunotherapy: Beyond PD-L1. ESMO Open. 6(100257)2021.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Pasic MD, Samaan S and Yousef GM: Genomic
medicine: New frontiers and new challenges. Clin Chem. 59:158–167.
2013.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Di Sario G, Rossella V, Famulari ES,
Maurizio A, Lazarevic D, Giannese F and Felici C: Enhancing
clinical potential of liquid biopsy through a multi-omic approach:
A systematic review. Front Genet. 14(1152470)2023.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Panahiazar M, Chen N, Lituiev D and Hadley
D: Empowering study of breast cancer data with application of
artificial intelligence technology: Promises, challenges, and use
cases. Clin Exp Metastasis. 39:249–254. 2022.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Xu Y, Liu X, Cao X, Huang C, Liu E, Qian
S, Liu X, Wu Y, Dong F, Qiu CW, et al: Artificial intelligence: A
powerful paradigm for scientific research. Innovation (Camb).
2(100179)2021.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Dembrower K, Crippa A, Colón E, Eklund M
and Strand F: ScreenTrustCAD Trial Consortium. Artificial
intelligence for breast cancer detection in screening mammography
in Sweden: A prospective, population-based, paired-reader,
non-inferiority study. Lancet Digit Health. 5:e703–e711.
2023.PubMed/NCBI View Article : Google Scholar
|
|
59
|
Roelands J, Mall R, Almeer H, Thomas R,
Mohamed MG, Bedri S, Al-Bader SB, Junejo K, Ziv E, Sayaman RW, et
al: Ancestry-associated transcriptomic profiles of breast cancer in
patients of African, Arab, and European ancestry. NPJ Breast
Cancer. 7(10)2021.PubMed/NCBI View Article : Google Scholar
|
|
60
|
Johnson KB, Wei WQ, Weeraratne D, Frisse
ME, Misulis K, Rhee K, Zhao J and Snowdon JL: Precision medicine,
ai, and the future of personalized health care. Clin Transl Sci.
14:86–93. 2021.PubMed/NCBI View Article : Google Scholar
|
|
61
|
Karachaliou N, Mayo-de-Las-Casas C,
Molina-Vila MA and Rosell R: Real-time liquid biopsies become a
reality in cancer treatment. Ann Transl Med. 3(36)2015.PubMed/NCBI View Article : Google Scholar
|
|
62
|
Finotti A, Allegretti M, Gasparello J,
Giacomini P, Spandidos DA, Spoto G and Gambari R: Liquid biopsy and
PCR-free ultrasensitive detection systems in oncology (review). Int
J Oncol. 53:1395–1434. 2018.PubMed/NCBI View Article : Google Scholar
|
|
63
|
Noor J, Chaudhry A, Noor R and Batool S:
Advancements and applications of liquid biopsies in oncology: A
narrative review. Cureus. 15(e42731)2023.PubMed/NCBI View Article : Google Scholar
|
|
64
|
Shegekar T, Vodithala S and Juganavar A:
The emerging role of liquid biopsies in revolutionising cancer
diagnosis and therapy. Cureus. 15(e43650)2023.PubMed/NCBI View Article : Google Scholar
|
|
65
|
Lone SN, Nisar S, Masoodi T, Singh M,
Rizwan A, Hashem S, El-Rifai W, Bedognetti D, Batra SK, Haris M, et
al: Liquid biopsy: A step closer to transform diagnosis, prognosis
and future of cancer treatments. Mol Cancer. 21(79)2022.PubMed/NCBI View Article : Google Scholar
|
|
66
|
Tay TKY and Tan PH: Liquid biopsy in
breast cancer: A focused review. Arch Pathol Lab Med. 145:678–686.
2021.PubMed/NCBI View Article : Google Scholar
|
|
67
|
Mazzitelli C, Santini D, Corradini AG,
Zamagni C, Trerè D, Montanaro L and Taffurelli M: Liquid biopsy in
the management of breast cancer patients: Where are we now and
where are we going. Diagnostics (Basel). 13(1241)2023.PubMed/NCBI View Article : Google Scholar
|
|
68
|
Mithraprabhu S, Chen M, Savvidou I, Reale
A and Spencer A: Liquid biopsy: An evolving paradigm for the
biological characterisation of plasma cell disorders. Leukemia.
35:2771–2783. 2021.PubMed/NCBI View Article : Google Scholar
|
|
69
|
Christofyllakis K, Bittenbring JT, Thurner
L, Ahlgrimm M, Stilgenbauer S, Bewarder M and Kaddu-Mulindwa D:
Cost-effectiveness of precision cancer medicine-current challenges
in the use of next generation sequencing for comprehensive tumour
genomic profiling and the role of clinical utility frameworks
(review). Mol Clin Oncol. 16(21)2022.PubMed/NCBI View Article : Google Scholar
|
|
70
|
Pashayan N, Antoniou AC, Ivanus U,
Esserman LJ, Easton DF, French D, Sroczynski G, Hall P, Cuzick J,
Evans DG, et al: Personalized early detection and prevention of
breast cancer: ENVISION consensus statement. Nat Rev Clin Oncol.
17:687–705. 2020.PubMed/NCBI View Article : Google Scholar
|
|
71
|
Demetriou D, Lockhat Z, Brzozowski L,
Saini KS, Dlamini Z and Hull R: The convergence of radiology and
genomics: Advancing breast cancer diagnosis with radiogenomics.
Cancers (Basel). 16(1076)2024.PubMed/NCBI View Article : Google Scholar
|