1. Introduction
Given its constant state of evolution and
adaptation, modern medicine is keeping pace with current trends by
integrating herbal extracts that have long been traditionally
utilized as complementary components to medical products. The
difference between herbal extracts and pharmaceutical preparations
is that the former contains more than one active chemical
ingredient, while the latter usually contains only one such
ingredient that demonstrates clear pharmacological activities
(1). However, the composition of
chemical extracts depends on the geographical distribution of the
herbs (2). In addition, active
pharmacophores may be identified in herbal extracts, such as
curcumin, which is found in the curcuma root (3); epigallocatechin, which is derived
from green tea (3,4); and resveratrol, which is obtained
from red wine (3).
Natural compounds have been gaining considerable
attention in the pharmaceutical industry with regard to the
prevention and treatment of cancer. Several natural compounds have
been reported to have anticancer effects, such as curcumin,
quercetin, matrine and andrographolide (5). Derived from the plant Andrographis
paniculata, the andrographolide compound offers numerous
advantages, particularly as a herbal medicine, which is favored in
Asian countries such as India and China. As a herbal medicine,
andrographolide has several advantages, including being used as a
therapy for common illnesses and infections such as colds,
tonsillitis, diarrhea, wounds and ulcers. Meanwhile, the plant
broth can be utilized to treat jaundice, dermatitis, and liver
diseases (6). Varying quantities
of this compound can be acquired from distinct plant components and
different geographical locations. Plants growing in locations with
moderate temperatures (25-28˚C) have a higher andrographolide
concentration compared with plants in locations with temperatures
<25˚C or >30˚C (6).
Andrographolide can be obtained both naturally and synthetically.
Naturally, the compound can be extracted from the plant,
Andrographis paniculata, through a process of extraction,
isolation and purification to obtain the pure andrographolide
compound (7). The concentration of
andrographolide extracted from a plant ranges between 0.054-4.686%,
with the leaves containing the highest concentration compared with
the stem of the plant, flowering tops and roots (8). The leaves and flowers of the
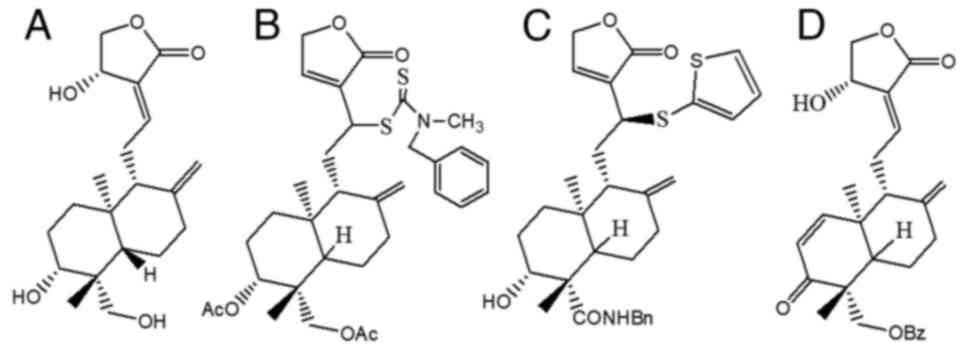
Andrographis paniculata plant are shown in Fig. 1. Andrographolide belongs to the
class of terpenes, namely diterpenes (9). The molecular formula for
andrographolide is C20H30O5, and
its IUPAC name reported on PubChem is
(3E,4S)-3-[2-[(1R,4aS,5R,6R,8aS)-6-hydroxy-5-(hydroxymethyl)-5,8a-dimethyl-2-methylidene-3,4,4a,6,7,8-hexahydro-1H-naphthalen-1-yl]ethylidene]-4-hydroxyoxolan-2-one
(Fig. 2A) (10-12).
Andrographolide has attracted interest due to its
extensive range of pharmacological effects, which include
anticancer, antimalarial, anti-inflammatory, antidiabetic,
antiviral and antibacterial benefits (13). Andrographolide and its derivatives
may be able to prevent and treat cancer, as well as exhibit
anticancer effects through the inhibition of the growth,
multiplication and metastasis of numerous types of cancer cells,
such as breast, lung, colorectal, bladder and colon cancer cells as
well as prostate carcinoma and leukemic cells (14). A potent compound,
19-(2-furoyl)-1,2-didehydro-3-ox-andrographolide (Fig. 2D), can inhibit the proliferation of
breast cancer cell lines, especially HCT116 and MCF-7, with an
IC50 <8 for both cell lines (15). Furthermore, the potent
andrographolide derivative,
12-dithiocarbamate-14-deoxyandrographolide analogue (Fig. 2B), has an anticancer effect on nine
different types of cancer cells, especially MCF-7 and KKU-055,
which are human breast cancer cells and human cholangiocarcinoma
cells, respectively (16).
Additionally, 3,19-analogue of 12-thioether andrographolide
(Fig. 2C) has a cytotoxic effect
on MCF-7 human breast cancer cell lines (17). The anticancer effects of
andrographolide are also demonstrated in relation to human
epidermoid carcinoma, breast cancer and human ovarian cancer cells
via the nuclear factor-κB (NF-κB) signaling pathway. The results of
the study demonstrate that andrographolide effectively inhibits the
proliferation and growth of these cancer cell lines via the
inhibition of this pathway (18).
The chemical structures of andrographolide (19) and its derivatives are shown in
Fig. 2.
Andrographolide has been tested with severe acute
respiratory syndrome (SARS)-coronavirus (CoV)-2 to determine its
ability to inhibit SARS-CoV-2 activity in human lung epithelial
cells, Calu-3. Andrographolide inhibits the production of
SARS-CoV-2 virions, with an IC50 of 0.034 µM. The
results reveal that the anti-viral mechanism of action of
andrographolide on SARS-CoV-2 is to target the non-structural
proteins of the virions (20).
Andrographolide also exhibits a bacteriostatic antibacterial action
against bacteria (21).
Furthermore, an in vivo analysis using
Plasmodium berghei and three different andrographolide
fractions in tablet form reveals that the inhibition rate of the
growth of this parasite ranges between 70-80%, demonstrating the
anti-parasitic effect of andrographolide (22). Another potent pharmacological
effect of andrographolide is its anti-inflammatory property.
Andrographolide can suppress the inflammatory mediators in
ovalbumin-stimulated mice by inhibiting the activation of the Janus
kinase 1 (JAK1)/signal transducer and activator of transcription 3
(STAT3) signaling pathway by attenuating the production of
TH17-regulated cytokines. This further demonstrates that
andrographolide can treat inflammation-mediated diseases (23). In addition, andrographolide also
has an antidiabetic effect, which is indicated in an in
vitro study conducted on 3T3-LI mouse adipocytes.
Andrographolide increases the glucose uptake by increasing the main
components of glucose homeostasis, such as peroxisome
proliferator-activated receptor γ and glucose transporter type
4(24).
Of these activities, the anticancer properties of
andrographolide have received the most attention, as reflected by
the number of studies investigating its potential in combating
cancer (16-18).
Previous reviews have addressed the anticancer effects of
andrographolide but have not investigated the associated
biochemical signaling pathways. Thus, the present review focused on
the anticancer effects of andrographolide, specifically on breast,
colorectal and lung cancer through the NF-κB, hypoxia-inducible
factor 1 (HIF-1) and the JAK/STAT signaling pathways. Therefore,
the Google Scholar, PubMed and ScienceDirect databases were used to
search for references to these prevalent cancers and the anticancer
mechanisms of andrographolide. The following key words were used:
Andrographolide, anticancer, NF-κB, HIF-1, JAK/STAT,
phosphatidylinositol-3-kinase (PI3K)/AKT/mammalian target of
rapamycin (mTOR), Wnt/β-catenin and mitogen-activated protein
kinase (MAPK) pathways, and the literature was limited to studies
published between 2010 to 2023. The present review article detailed
the different involvements of the signaling pathways in the
anticancer mechanisms of andrographolide.
2. Types of cancer and current
treatments
The anticancer effects of andrographolide and its
derivatives have been gaining considerable attention among
researchers due to its low toxicity towards the human body
(13). The present section
introduces three different types of cancer: Breast, lung and
colorectal cancer.
Breast cancer
According to data collected by the World Health
Organization in 2020, breast cancer is a prevalent form of cancer
(surpassing lung cancer), with 2.26 million newly diagnosed cases
recorded worldwide in 2020 alone (25). Breast cancer is recognized as one
of the most prevalent types of cancer to affect women worldwide.
However, the most severe type of breast cancer is triple-negative
breast cancer (TNBC) which has notable long-term adverse effects as
it is more aggressive compared with the other types (25). Only a small number of effective
targeted treatments are available for this type of breast cancer,
hence the importance to investigate the causes and effects of
breast cancer in order to address the current need for its
effective treatment. Currently, the available treatment to stop
metastasis of TNBC cells and early-stage TNBC is cytotoxic
chemotherapy (26,27), which causes an array of side
effects such as hair loss, peripheral neuropathy, anemia and
fatigue (28). However, several
costly drugs have been approved by the United States Food and Drug
Administration, such as capecitabine, bevacizumab, albumin
bound-paclitaxel and docetaxel (29). Traditional Chinese medicine (TCM)
is another option that patients may resort to when modern medicine
fails. TCM for breast cancer prevention often involves acupuncture
and herbal medicine, which are used 19.4 and 100% of the time in
TCM, respectively (30,31). The numerous herbal agents often
used to prevent breast cancer include Astagaslia
macrocephalia, Salvia miltorrhizae, Atractlodes
and Poria cocos (31).
Studies on patients with chronic fibrocystic breast disorder
treated with TCM suggest that this approach is effective and does
not have adverse side effects (31,32).
Natural components can also be useful in cancer therapy and
prevention. Studies demonstrate that andrographolide can suppress
levels of different factors, such as tumor necrosis factor-α
(TNF-α), monocyte chemoattractant protein-1, high sensitivity
c-reactive protein and interleukin (IL)-1β. Therefore,
andrographolide is recognized as an anti-inflammatory agent in
macrophages for different types of diseases such as breast cancer,
hepatitis, coronary heart and pulmonary fibrosis (33-35).
Women have a higher incidence of developing breast
cancer compared with men, as the lifetime risk of developing breast
cancer for a man is 1:100 compared with 1:8 for a woman. In men,
the rate of developing all types of breast cancer, primarily
estrogen-receptor-positive, is only ~1% (36). Therefore, it is important to
understand how tumor progression and metastasis work. The tumor
microenvironment, which refers to the ecosystem surrounding a tumor
in the body, can influence tumor progression and metastasis. In a
tumor microenvironment, elements that serve a major role in tumor
development are angiogenesis, fibrosis, immune cells and
macrophages (25). However, that
tumor-associated macrophages (TAM) have the greatest influence on
breast cancer development as they comprise 5-40% of the mass of
solid tumors (37,38). The two main subtypes of TAM are
classically activated macrophages, M1, and alternatively activated
macrophages, M2. They have contrasting roles in TAM, where M1
macrophages are activated by the microbial product interferon-γ
(IFN-γ), while M2 macrophages are induced by Type 2 helper T-cell
cytokines. M1 are known as proinflammatory and cancer-cell-killing
macrophages, while M2 are anti-inflammatory macrophages that aid in
tissue repair processes and the expression of scavenger receptors,
such as CD163, CD23 and CD206, to initiate various signaling
pathways, such as the NF-κB and PI3K/AKT/mTOR pathways, in the
tumor microenvironment, including those that result in tumor
progression (38,39). Consequently, agents that
re-polarize macrophages from a pro-inflammatory to an
anti-inflammatory subset or regulate secreted cytokines of the M1
subtype are useful tools for cancer therapy (38,39).
Colorectal cancer
With a malignancy rate that is ranked as second
worldwide, colorectal cancer has a high mortality rate of 35%, and
147,950 individuals were diagnosed with this type of cancer in 2020
alone (40). Patients were mainly
>50 years old, with a mortality rate of 20% (41). This complex multifactorial disease
involves genetic mutations and epigenetic mutations, resulting in
gene changes associated with processes such as cellular
differentiation and colonocyte regulation (42). In the initial phase, the cancer
cells develop from the colon mucosa crypt, which is a tube-shaped
gland in the colon wall, and start to proliferate. In the first
stage, the cancer cells originating from the mucosa wall start to
emerge onto the muscularis propria and submucosa. At this point,
patients have >90% chance of surviving 5 years. In the second
stage, which has a 50% survival rate, cancer cells continue to
develop into the attached organs, visceral peritoneum and
peri-colorectal tissues. In the third stage, the cells further
metastasize to nearby tissues and lymph nodes (43,44).
The cancer cells then metastasize to other organs such as the
lungs, liver or ovaries, indicating the start of stage four of
colorectal cancer, for which the survival rate is only 10%
(43,44). The high invasiveness and metastatic
behavior of colon cancer cells mean they surpass lung cancer in
terms of the mortality rate. The nature of this disease makes it
challenging to detect until it reaches an advanced stage (45).
Several treatments are now available, such as
chemotherapy, surgery and targeted therapy. However, these
treatments are not promising because chemotherapeutic drugs can
cause adverse toxicity and drug resistance could potentially
develop over time. In addition, targeted therapy demonstrates a
lack of cost-effectiveness and specificity, and it has the
potential to be associated with adverse events such as leukopenia
or hypertension (45). Common
systemic therapy consists of chemotherapy with the drug
fluoropyrimidine, taken in isolation. However, treatment might also
involve other types of drugs, such as oxaliplatin or irinotecan, as
well as biological therapy that targets vascular endothelial growth
factor (VEGF), multiple receptor tyrosine kinases or epidermal
growth factor receptors (46,47).
TCM can also be applied in treating colorectal cancer as it helps
to induce apoptosis and cycle arrest, suppress migration and
invasion, and target the tumor microenvironment. TCM compounds
associated with anti-colorectal cancer agents are berberine,
evodiamine, shikonin, quercetin, resveratrol and curcumin (46). Berberine is a traditional herbal
medicine obtained from the plant Coptis chinensis (48). It downregulated 33 genes
differently involved in the cell cycle, epithelial-mesenchymal
transition (EMT) and differentiation when tested on HCA-7 cell
lines with doses of between 10 and 100 µM (49). Phytochemicals are natural products
that can inhibit the activities of cancer cells, such as cell
proliferation, angiogenesis and inflammation, but have minimal side
effects (50). Andrographolide, a
phytochemical with anticancer properties, has been used worldwide
as a cancer treatment. This compound acts on regulatory pathways
such as the cell adhesion process, the cell cycle and apoptosis
(51). A previous study using
human colorectal carcinoma LoVo cells treated with andrographolide
reveals an anti-proliferative effect on the cells in a time- and
dose-dependent manner. Even when treatment involves a low
concentration of andrographolide, such as 0-30 µM, andrographolide
still causes cell arrest at the G1-S phase, an interphase stage in
the cell cycle (52).
Additionally, andrographolide induces protein expression in p53,
p21 and p16, resulting in suppression of cyclin D1/Cdk4, cyclin
A/Cdk2 and the Rb phosphorylation process. Thus, andrographolide
can inhibit the proliferation of LoVo cells (52).
Lung cancer
According to the Global Cancer Observatory, the
global mortality rate of lung cancer caused by neoplasms of the
lungs was 1.8 million, with 2.2 million cases of lung cancer
reported in 2020 alone, making it the most common cancer-related
cause of mortality worldwide (25). The two types of lung cancer are
small-cell lung cancer (SCLC) and non-small lung cancer (NSCLC). Of
these, SCLC is known to be the more malignant, and it comes from
cells with neuroendocrine features. Most cases of lung cancer
originate from NSCLC, accounting for 85% of cases, compared with
SCLC that accounts for 15% of cases. Furthermore, NSCLC can be
divided into three pathologic subtypes: Large cell carcinoma,
squamous cell carcinoma and adenocarcinoma (53). The high number of NSCLC cases is
due to poor diagnosis and tumors worsening over time (53).
There are several uncontrollable risk factors for
lung cancer, such as age, sex, ethnicity and family history. In the
United States, the average age for lung cancer diagnosis is 70
years old, with >50% of cases being in the age range of 55-74
years (54,55). This is because mutagen exposure
occurs over a long period of time often spanning several years or
even decades before causing carcinogenesis, which delays the
detection of the illness. Furthermore, as individuals age, the
telomeres shorten, leading to a decrease in the metabolite NAD+ and
cells no longer being able to repair and withstand DNA damage or
detect abnormal cells (56). In
terms of sex differences, men are more likely to be diagnosed with
and die from lung cancer, with the lifetime risk of diagnosis being
3.80% among men and 1.77% among women. This increased risk among
men is attributed to a higher frequency of tobacco smoking in men,
which is 36.7% globally compared with 7.8% for women (55,57).
Additionally, one theory suggests that hormones might influence the
relatively low occurrence of lung cancer in women. This hypothesis
stems from the overexpression of estrogen receptors in
adenocarcinomas and the antitumor effects of antiestrogen compounds
observed in in vitro studies (55,58).
Ethnicity also serves a major role in the number of lung cancer
cases. Despite having lower rates of cigarette consumption (~2%),
Chinese women are equally as likely to be diagnosed with lung
cancer as Western European women, who have a smoking prevalence of
20-25%. This is due to air pollution and charcoal burning, which
exposes Chinese women to smoke more frequently compared with
Western European women (59).
However, patients with lung cancer may have no history of smoking
tobacco, suggesting that the disease could be due to heritable
components (55). According to
studies by the Genome-wide Association, certain variants in several
chromosomal regions, such as the 5p15 locus (60), 6p21 locus (61) and 15q25-26 loci, might increase the
probability of being diagnosed with lung cancer (55,62).
The current available treatments for lung cancer are
targeted therapy, chemotherapy, radiotherapy and surgical
resection. Patients with stages I and II of NSCLC are suggested to
undergo surgical tumor resection as this is the most effective
therapy, but it has limited effects among patients in the advanced
stages (53). The two types of
chemotherapy are platinum-based chemotherapy for NSCLC (63) and standard chemotherapy for SCLC
(64). Targeted therapy and
platinum-based chemotherapy have demonstrated a number of
therapeutic benefits (65).
However, these treatments are also associated with a number of
systemic toxicities and adverse effects such as diarrhea, vomiting,
fatigue and neutropenia (66,67).
The novel drugs resulting from pharmaceutical developments are
known to have less severe side effects, unlike other common
cytotoxic drugs. The available drugs include sorafenib, sunitinib
and ASA404(68). TCM is also
applied in lung cancer treatment paired with chemotherapy. The most
popular therapy is a combination of TCM and platinum-based
chemotherapy as this offers synergistic therapeutic effects and is
reported to decrease severe toxicities by 36%. The TCM injection is
called an Aidi injection, which is a common adjuvant chemotherapy
drug used in China (69). Natural
compounds such as andrographolide have good inhibitory effects on
pro-survival autophagic processes. These processes involve the
suppression of the PI3K/AKT signaling pathway, leading to a
decrease in the primary factor responsible for tumor growth in
NSCLC cells, which is HIF-1α (70,71).
A recent study on NSCLC cell lines, specifically H460 and H1650,
treated with andrographolide demonstrates that the compound is able
to induce ferroptosis, an iron-dependent cell death, which inhibits
cell line growth and metastasis in H460 and H1650. The induction of
ferroptosis was confirmed by the high levels of glutathione (GSH),
malondialdehyde, reactive oxygen species (ROS), intracellular iron
content and lipid ROS-reduced GSH. This further demonstrates that
andrographolide can suppress NSCLC cell proliferation and
metastasis (72).
Other types of cancer
One of the leading causes of cancer-related
mortality is liver cancer. This starts in hepatocytes, cells in the
liver tissue that have a number of key functions, such as
detoxification, clotting and lipid metabolism. Hepatocellular
carcinoma is the most common type of primary liver cancer,
comprising 75-85% of all liver cancer cases, and starts and
progresses in hepatocytes (73).
In the early stage of hepatocellular carcinoma, patients are
asymptomatic but start to show symptoms such as fatigue and liver
dysfunction as the cancer advances (73). The current treatment for this
disease can involve surgical resection, liver transplant, ablation
or embolization but these have numerous potential side effects such
as a risk of infection, fever, nausea, damage to healthy liver
tissue, bleeding and low survival rates, depending on the cancer
stage (74). Drugs given to
patients with hepatocellular carcinoma vary depending on the
severity and progress of the cancer. Currently, sorafenib is given
to patients as the first-line treatment, followed by negorafenib as
the second-line treatment. These drugs help prevent cancer cells
from multiplying and inhibit the tumor growth-promoting pathway
(75). TCM may also be used to
treat hepatocellular carcinoma, with herbal remedies, acupuncture,
dietary therapy, exercise and massages being utilized in hospitals
in China. Various Chinese herbal medicines are recognized by the
International Organization for Standardization and may be
prescribed to treat patients with hepatocellular carcinoma, such as
Poria (Fuling), Rhizoma Atractylodis Macrocephalae
and Radix Astragali Mongolici (76). These herbs inhibit proliferation
and tumor growth while reducing the side effects of common liver
cancer treatments (76). Recently,
andrographolide was investigated for its ability to treat liver
cancer. Andrographolide coupled to phytosomes, which enhances the
solubility and delivery of the compound to HepG2 liver cancer
cells, demonstrates an ability to inhibit HepG2 cell proliferation.
These findings indicate that andrographolide coupled to phytosomes
notably suppresses HepG2 cell proliferation, with an
IC50 value of 4.02±0.14 µM (73).
In 2024, it is predicted that there will be 2
million new cancer cases in the United States, with an estimated
299,010 cases of prostate cancer. Additionally, a high number of
mortalities, totaling 35,250 cases, are expected in the United
States due to this cancer (77).
In the United States, prostate cancer is the leading cancer-related
cause of mortality among the male population (78). The currently available first-line
treatments of prostate cancer are surgery, radiation therapy and
proton beam therapy. Additionally, chemotherapy, hormonal therapy,
cryosurgery and high intensity focused ultrasound are also
conducted to treat patients with prostate cancer, depending on the
severity and stage of the cancer. However, these treatments expose
patients to a number of side effects that may hinder aspects of
their quality of life such as urinary issues and erectile
dysfunction (79). Several drugs
categorized as second-generation non-steroidal anti-androgens are
currently used as a pharmacotherapy for patients with prostate
cancer, namely enzalutamide, apalutamide and darolutamide. These
drugs compete against testosterone and dihydrotestosterone to bind
to the androgenic receptor, inhibiting cell proliferation and the
tumor growth of prostate cancer cells. However, a number of these
drugs may cause side effects such as hypertension, bone injury and
diarrhea (80). TCM has also been
used among patients with prostate cancer as an alternative
treatment. A study on the Chinese medicine extract Ganoderma
lucidum demonstrates its ability to induce apoptosis, inhibit
cell proliferation and suppress PC3 human prostate cancer cells
from metastasizing to other organs, with a concentration of
0.125-0.5 mg/ml (81). A previous
study investigated the ability of andrographolide to inhibit
prostate cancer cell activity. It reveals that andrographolide can
suppress tumor growth in mice induced with a castration-resistant
DU145 human prostate tumor by inhibiting the protein expression of
IL-6. This was indicated by a 50% reduction in the IL-6 protein in
DU145 cells after treatment with 3 µM andrographolide (82). Similar results are observed in the
human prostate cancer cell line PC3(82).
Leukemia is a type of cancer that originates in the
bone marrow and causes the production of large numbers of abnormal
white blood cells. The four types of leukemia are: Acute
lymphoblastic leukemia (ALL), acute myelogenous leukemia, chronic
lymphocytic leukemia and chronic myelogenous leukemia (83). Of these, ALL is the most aggressive
and is known for its ability to metastasize to other organs. The
current treatments available for leukemia are induction
chemotherapy and risk-directed therapy. The current survival rate
of young patients aged 1-9 years with ALL is 80-90% due to
implementing newly developed chemotherapy schedules and optimized
risk-directed therapy (84,85).
However, studies have reported that cell resistance and relapse are
still the greatest challenge in treating ALL (84,86,87).
Drugs have also been used with chemotherapy; a method known as
combination therapy. Common drugs used in this way are
glucocorticoids (GCs) and dexamethasone. GCs aid in regulating
genes that cause apoptosis in leukemia cells by activating the
glucocorticoids receptor; however, long-term exposure to GCs may
cause adverse reactions, resulting in resistance to this drug
(84). TCM herbs are used to treat
young patients with leukemia, including treatments based on
Radix Astragali membranaceus, Bulbus Fritillariae
thunbergii and Herba Hedyotisdiffusa. Furthermore, three
common types of TCM used to treat adult patients (aged 19-80 years
old) with leukemia are Radix Astragali membranaceus,
Radix et Rhizoma Salviae miltiorrhizae and Fructus
Ligustri Lucidi. These herbs are known for their ability to
increase the white blood cell count of a patient (88). The anti-tumor activity of
andrographolide toward human T-ALL Jurkat cells was investigated
using both in vitro and in vivo (nude mice models)
analysis. The results demonstrate the ability of andrographolide to
induce apoptosis in Jurkat cells, with a concentration of 10 µg/ml.
Additionally, the in vivo study on T-ALL Jurkat cells with
three different concentrations of andrographolide (50, 100 and 200
mg/kg), demonstrates that the compound notably inhibits the growth
of T-ALL Jurkat cells in mice (89). This further demonstrates the
anti-tumor activity of andrographolide in leukemia.
3. Mechanisms of andrographolide activation
and its derivatives
Andrographolide, known for its function as a natural
antibiotic, has various other abilities and can act to reduce
fever, remove toxins, relieve pain and reduce inflammation
(90). A number of pathways allow
andrographolide to exert its anticancer effects; however, the main
signaling pathways are the NF-κB, HIF-1 and JAK/STAT signaling
pathways. Other pathways have also been discussed in the present
review, such as the PI3K/AKT, mTOR, Wnt/β-catenin and MAPK
signaling pathways (91). Table I summarizes the anticancer effects
of andrographolide through these pathways.
 | Table IAnticancer effects of andrographolide
due to the inhibition of NF-κB, HIF-1, JAK/STAT, PI3K/AKT/mTOR,
Wnt/β-catenin or MAPK signaling pathways. |
Table I
Anticancer effects of andrographolide
due to the inhibition of NF-κB, HIF-1, JAK/STAT, PI3K/AKT/mTOR,
Wnt/β-catenin or MAPK signaling pathways.
| Signaling
pathway | Type of cancer | Process | Cancer cell
lines | Mechanism of
action | (Refs.) |
|---|
| NF-κB | Breast cancer | Gene
expression | MMTV-PyMT and
MCF-7 | Downregulates the
protein expression levels of p65 and pp65 (Ser536) | (119) |
| | Colorectal
cancer | Proliferation | SW620 | Downregulates the
MMP-9 signaling pathway | (112) |
| | Prostate
cancer | Proliferation | PC3 and 22RV1 | Decreases the
expression level of MMP-11 | (78) |
| HIF-1 | Lung cancer | Metastasis and
invasion | A549 | Decreases the
HIF-1α cellular protein level | (71) |
| | Breast cancer | Angiogenesis and
cancer cell growth | MDA-MB-231 and
T47D | Reduces the protein
and mRNA levels of HIF-1α | (128) |
| | Liver cancer | Cancer cell
growth | Hep3B | Decreases the
protein expression levels of HIF-1α | (130) |
| JAK/STAT | Prostate
cancer | Apoptosis | DU145 | Suppresses the
expression of IL-6 as well as the signaling pathway | (82) |
| | Breast cancer | Apoptosis | MDA-MB-231 | Reduces the STAT3
luciferase activity | (137) |
| | Liver cancer | Cancer cell
growth | AD-293 | Inhibits the
phosphorylation and dimerization of STAT3 proteins | (138) |
| | Lung cancer | Proliferation | H1975 and
H1299 | Downregulates the
phosphorylation of STAT3 proteins | (136) |
| | Pancreatic
cancer | Proliferation | AsPC-1 and
Panc-1 | Suppresses the
activation of IL-6-induced STAT3 | (135) |
| PI3K/AKT/mTOR | Breast cancer | Apoptosis | MCF-7 and
MDA-MB-231 | Inhibits the
expression of Bcl-2 and increases the expression of Bax | (142) |
| Wnt/β-catenin | Colorectal
cancer | Proliferation | HCT116 and
SW480 | Dysregulates the
WNT16, TCF7L2 and AXIN2 protein expression levels | (153) |
| MAPK | Glioblastoma
multiforme | Metastasis | GBM8401 and
U251 | Inhibits the
expression of MMP-2 at the transcriptional level | (160) |
Inhibition of the NF-κB signaling
pathway
NF-κB is a signaling pathway that regulates various
cellular processes such as the production of anti-tumor cytokines,
gene transcription and the inhibition of apoptosis (92,93).
Aside from the ability of anti-tumor cytokines to combat cancer,
they can also manipulate signaling pathways (such as the NF-κB,
PI3K/Akt and JAK/STAT pathways) to avoid immune detection and
destruction (94-96).
In response to chemotherapy, cancer cells can inhibit apoptosis and
produce anti-tumor cytokines in order to evade the immune system
through different processes such as chronic inflammation, over
expression of anti-apoptotic proteins and a modulation of the
immune response (97). This
immunoevasive strategy allows cancer cells to survive and
proliferate despite the immune system (92). The NF-κB pathway is one of the
transcription factors that serves a major role in a number of
physiological and pathological processes. Physiological processes
include immunological responses, inflammation and apoptosis,
whereas pathological processes include cancer, autoimmune diseases
and metabolic disorders (98,99).
The pathway is divided into two different pathways, canonical and
non-canonical, with each having different activating mechanisms
(98,100). Activation of the canonical NF-κB
pathway causes a number of different responses, which are involved
in survival, differentiation, cell proliferation, immune response
and inflammation (98,101). The non-canonical NF-κB pathway is
mainly associated with the development of immune cells in various
stages, and it is a critical regulator in the development of
secondary lymphoid organs (98,100), the development of tertiary
lymphoid organs and chronic inflammation (102). For immune cells that are
associated with the development of T cells in the thymus, this
pathway is needed for thymus epithelium cell maturation and
function (98,103). The NF-κB canonical pathway begins
at the cell membrane, where different stimuli (such as carcinogens,
tumor promoters, stress, endotoxin, apoptosis-inducers, infection,
reactive oxygen intermediate inducers and cytokines (104) bind to their corresponding
receptors, such as the TNF, toll-like receptors and T/B cell
receptors (105). This causes the
activation of the IκB kinase (IKK) complex by TGF-β activated
kinase-1, which takes place in the cytoplasm. Subsequently, IκBα is
phosphorylated in an IKK-mediated manner, and the phosphorylated
IκBα is degraded by the proteasome. Thus, the degraded
phosphorylated IκBα forms a heterodimer, RelA/p50, which leads to
the nuclear translocation of this heterodimer and results in gene
transcription. In contrast, the non-canonical pathway begins with
stimuli binding to a restricted set of cell surface receptors,
namely TNF and B cell activating factor receptors, leading to the
activation of IKKα by NF-κB inducing kinase. Subsequently, IKKα is
phosphorylated and the RelB/p100 complex undergoes partial
proteolysis by proteasome-activating p52. RelB dimerizes with p52
and forms a heterodimer, which translocates to the nucleus and
causes gene transcription (106-109).
Fig. 3 indicates the regulation of
the NF-κB signaling pathway by andrographolide.
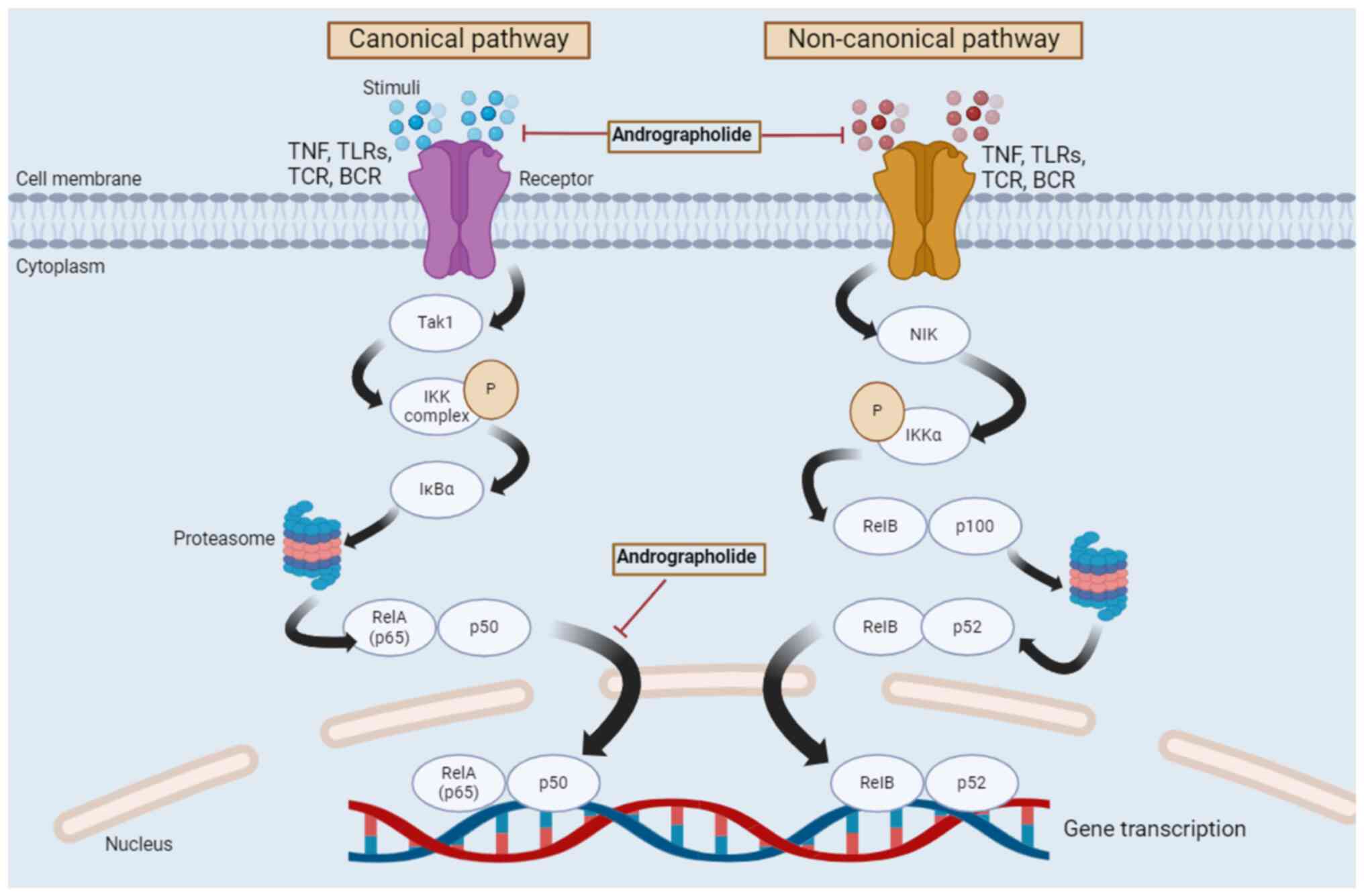 | Figure 3Regulation of canonical and
non-canonical NF-κB signaling pathways by andrographolide. Two
different pathways, namely the canonical and non-canonical
pathways, form the NF-κB signaling pathway. The canonical pathway
begins at the cell membrane, where different stimuli bind to their
corresponding receptors. This causes the activation of the IKK
complex by Tak1. Subsequently, IκBα is phosphorylated in an
IKK-mediated manner, and the phosphorylated IκBα is degraded by the
proteasome. The degraded and phosphorylated IκBα then forms a
heterodimer, RelA/p50, which leads to the nuclear translocation of
this heterodimer and results in gene transcription. The
non-canonical pathway begins with stimuli binding to a restricted
set of cell surface receptors, namely TNF and B cell activating
factor receptors, leading to the activation of IKKα by NIK.
Subsequently, IKKα is phosphorylated and the RelB/p100 complex
undergoes a partial proteolysis by the proteasome, which activates
p52. RelB dimerizes with p52 and forms a heterodimer, which then
translocates to the nucleus and causes gene transcription. Tak1,
TGF-β activated kinase-1; IKK, IκB kinase; NF-κB, nuclear
factor-κB; NIK, NF-κB inducing kinase; RelA, REL-associated
protein; RelB, RELB-associated protein; p65, REL-associated
protein; TNF, tumor necrosis factor; TLRs, toll-like receptors;
TCR, T-cell receptor; BCR, B-cell receptor; IκBα, inhibitory κ B α;
P, phosphorylation. |
NF-κB is a gene transcription factor that serves an
important role in immune responses and cellular inflammation. Due
to its pro-inflammatory function, the NF-κB signaling pathway may
cause several autoimmune diseases including cancer, as well as
aiding the survival, proliferation, metastasis, angiogenesis and
further development of cancer cells (110). Therefore, the NF-κB signaling
pathway has gained considerable attention, especially in terms of
developing drugs that can inhibit this signaling pathway to
overcome the different types of cancer to which it is associated
with (111). Different stimuli
that bind to different receptors may activate the transcription and
expression of various genes, such as, invasion-related genes and
anti-apoptotic genes. This process also regulates cell
proliferation and angiogenesis. The gene transcription process
induces matrix metalloproteinases (MMPs), which are
invasion-related genes that promote cancer cell invasion, adhesion
and metastasis (109) MMP-9 is an
MMP protein that specifically causes breast cancer cells to
metastasize to other organs, while MMP-11 is known to cause the
proliferation of prostate cancer cells (112,113). Therefore, the downregulation of
these MMP proteins using andrographolide could prevent these cancer
cells from metastasizing and proliferating by inhibiting the
phosphorylation process, which in turn, halts the gene
transcription process (109,114). TNF-α and IL-8 are cytokines that
bind to the TNF and CXC chemokine receptors, respectively to
initiate the NF-κB signaling pathway, which causes the angiogenesis
of colorectal cancer (115-117).
Andrographolide downregulates inflammatory factors such as TNF-α
and IL-8, and thus, inhibits cytokine binding, resulting in an
inhibition of angiogenesis-associated gene transcription (114). In a lipopolysaccharide
(LPS)-induced RAW 264.7 cell line (originating from an Abelson
leukemia virus-transformed cell line derived from BALB/C mice),
andrographolide suppresses the activation of the NF-κB pathway, and
thus hinders the inflammation induced by LPS. This also occurs due
to the inhibition of the release of cytokines that activate the
NF-κB pathway, such as TNF-α, IL-6 and IL-1β, when treated with
doses of andrographolide ranging from 6.25 to 25.00 g/ml. The
expression of these cytokines decreases as the andrographolide dose
increases from 6.25-25.00 g/ml. Furthermore, the expression of the
transcription factor p65 protein notably reduced, leading to the
cessation of the gene transcription process in the nucleus
(118). Another study using the
human colon cancer SW620 cell line treated with 20 µM of
andrographolide, reveals a notable reduction in the p65 protein
expression levels, which are involved in the NF-κB signaling
pathway, leading to the inactivation of the pathway (112). Additionally, a previous study
using two different breast cancer cell lines of spontaneous
luminal-like breast cancer, MMTV-PyMT and MCF-7, demonstrates a
reduction in tumor growth after andrographolide treatment is
applied. The high expression of p65 and pp65 (Ser536) proteins in
breast cancer cell lines is reduced by andrographolide, which helps
to prevent gene expression processes in the nucleus during the
NF-κB signaling pathway (119).
Furthermore, another study on pelvic inflammatory disease also
indicates the ability of andrographolide to block the
pathogen-induced activation of the NF-κB pathway by reducing the
excessive production of chemokines and cytokines such as IL-1β,
IL-6, C-X-C motif chemokine ligand 1, monocyte chemoattractant
protein-1 and regulated on activation, normal T cell expressed and
secreted (120). A notable
decrease in pp65 levels is observed in the epidermoid carcinoma
cell line, A431, and the breast cancer cell line, MDA-MB-231, as
the andrographolide concentration increases from 10-50 µM. This
prevents the growth of cancer cell and the proliferation process by
inhibiting the NF-κB signaling pathway (18). Uncontrolled DNA damage in response
to genotoxic stress, such as radiation or chemotherapy, will
provide a challenge to cell homeostasis because it can disrupt
genomic stability and survival, leading to cancer cell formation.
DNA damage is able to initiate both the canonical and non-canonical
NF-κB signaling pathways (121).
A previous study using osteosarcoma cell lines demonstrates the
ability of DNA damage to initiate the NF-κB signaling pathway
following the phosphorylation of p100 and processing of the p52
protein through a non-canonical pathway (121). However, it remains unclear how
DNA damage can activate the NF-κB signaling pathway through a
non-canonical pathway and the role of IKKα in this pathway. Further
research into the non-canonical NF-κB signaling pathway triggered
by DNA damage may aid the prevention of cancer cell development
(121).
Inhibition of the HIF-1 signaling
pathway
HIF is a transcription factor, which activates genes
that regulate cellular oxygen homeostasis, participate in
physiological processes and react to environmental stressors.
Physiological processes include angiogenesis and cell
proliferation, whereas environmental stressors include hypoxia and
inflammation (122). The
deregulation of HIF expression is involved in cancer progression
and metastasis. The HIF-1 signaling pathway is activated in cancer
cells through growth factors such as TGF-β3 and epidermal growth
factors (123). Two conditions
are associated with the HIF-1 signaling pathway: Normoxia and
hypoxia. Under normoxic conditions, proline residues on HIF-1α are
hydroxylated by the prolyl hydroxylase domain protein, forming a
binding site for the von Hippel-Lindau protein. Subsequently,
ubiquitylation (Ub) of HIF-1α is followed by degradation of
polyubiquitylated HIF-1α in the 26S proteasome (124,125). Under hypoxic conditions, HIF-1α
is not hydroxylated, but HIF-1α and HIF-1β form a heterodimer
(124,125) that binds to the
hypoxia-responsive element on DNA, activating hypoxia-responsive
gene transcription. This results in the expression of genes such as
VEGF, erythropoietin and adrenomedullin, which cause angiogenesis,
erythropoiesis and cell survival (123). The regulation of the HIF
signaling pathway by andrographolide is presented in Fig. 4.
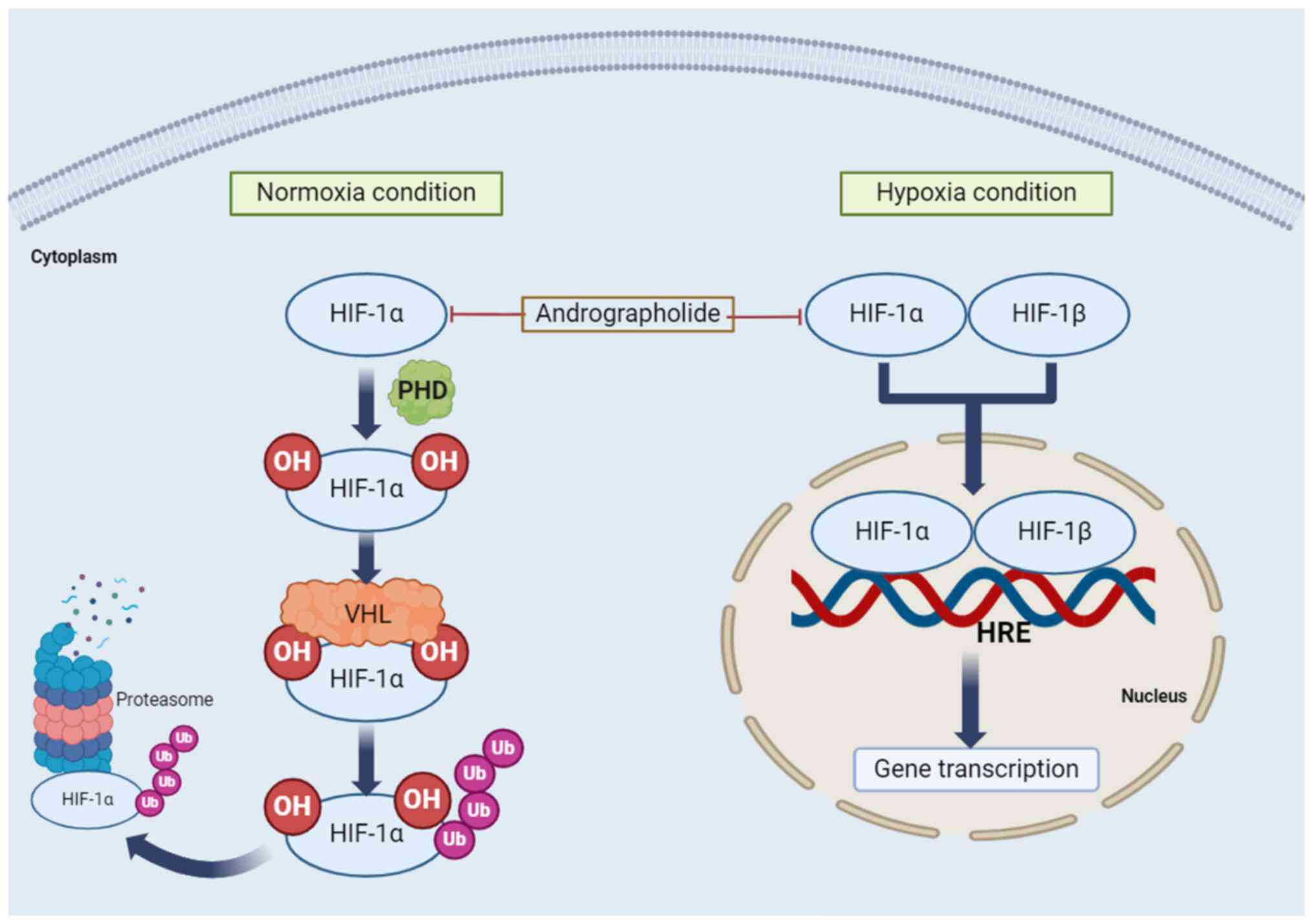 | Figure 4Regulation of HIF signaling pathways
by andrographolide. Two conditions are associated with the HIF-1
signaling pathway: Normoxia and hypoxia. Under normoxic conditions,
proline residues on HIF-1α are hydroxylated by the PHD protein,
forming a binding site for the VHL protein. Subsequently, Ub of
HIF-1α is followed by degradation of polyubiquitylated HIF-1α in
the 26S proteasome. Under hypoxic conditions, HIF-1α and HIF-1β
form a heterodimer that binds to the HRE on DNA, activating
hypoxia-responsive gene transcription. HIF, hypoxia-inducible
factor; PHD, prolyl hydroxylase domain; VHL, von Hippel-Lindau;
HRE, hypoxia responsive element; OH, hydroxylation; Ub,
ubiquitylation. |
Metastasis and tumorigenesis can be promoted by
altering the tumor microenvironment through inflammation and
hypoxia (126). Tumorigenesis
causes vessels to be leaky and abnormal, resulting in enlarged
hypoxic regions. This promotes metastasis and makes the tumor
resilient to current treatments such as radiotherapy and
chemotherapy (126,127). HIF activity in immune cells, HIF
pathway stabilization and HIF expression can be triggered by
hypoxia and pathological stress such as cancer or inflammation
(126). Inhibition of the HIF-1
signaling pathway by andrographolide occurs in MDA-MB-231 and T47D
breast cancer cells under hypoxic conditions through its targeting
of the expression of HIF-1α mRNA and HIF-1α protein levels via
processes such as protein translation or degradation, which
terminates the proliferation process of these breast cancer cells
(123,126,128). Additionally, in Hep3B liver
cancer cells, andrographolide can reduce HIF-1α protein expression,
reducing its nuclear translocation. Andrographolide also
downregulates a pro-angiogenic growth factor, VEGFA, which
activates the HIF-1 signaling pathway in Hep3B cancer cells
(129,130). Furthermore, HIF-1 is responsible
for lung cancer growth in A549 cells and NSCLC. A previous study
reveals that andrographolide inhibits the HIF-1 signaling pathway
in A549 cells by reducing VEGF, leading to the inactivation of the
HIF-1 signaling pathway (71).
Thus, andrographolide serves a role as an anti-angiogenesis and is
potentially a chemotherapeutic drug for treating NSCLC (14). Histone deacetylase (HDAC) is a
redox-sensing deacetylase that can oppose the target gene
expression of HIF-1α, but during hypoxia, it may cause activation
of the HIF-1α pathway. It remains unclear how HDAC inhibitors can
inhibit the HIF-1α signaling pathway (123). Therefore, in-depth research on
the inhibition of HDAC by andrographolide will enhance the
understanding of how it can inhibit this signaling pathway by
disrupting HIF-1α stabilization.
Inhibition of the JAK/STAT signaling
pathway
The JAK/STAT pathway is crucial for gene expression
and controlling cell functions (131). However, abnormal activation,
mainly due to the inappropriate stimulation or constitutive binding
of a ligand to its receptor, can lead to tumorigenesis (132). The JAK/STAT signaling pathway
mainly involves three proteins: Cell-surface, JAK and STAT
receptors. The activation process begins with reactions outside of
the cell, where cytokines, IFN and IL, attach to their specific
receptors, resulting in JAK proteins phosphorylating each other.
The receptors involved are growth factor, IL-specific and
G-protein-associated receptors (82,131,133). JAK phosphorylates the STAT
protein receptor allowing the STAT protein to bind before JAK then
phosphorylates the STAT protein on its retained tyrosine residues,
leading to the formation of a STAT protein complex in the
cytoplasm. This molecule travels to the nucleus and is attached to
the DNA, initiating the expression of genes that aid in tumor cell
differentiation, proliferation and activation (133). Regulation of the JAK/STAT
signaling pathway by andrographolide occurs as shown in Fig. 5.
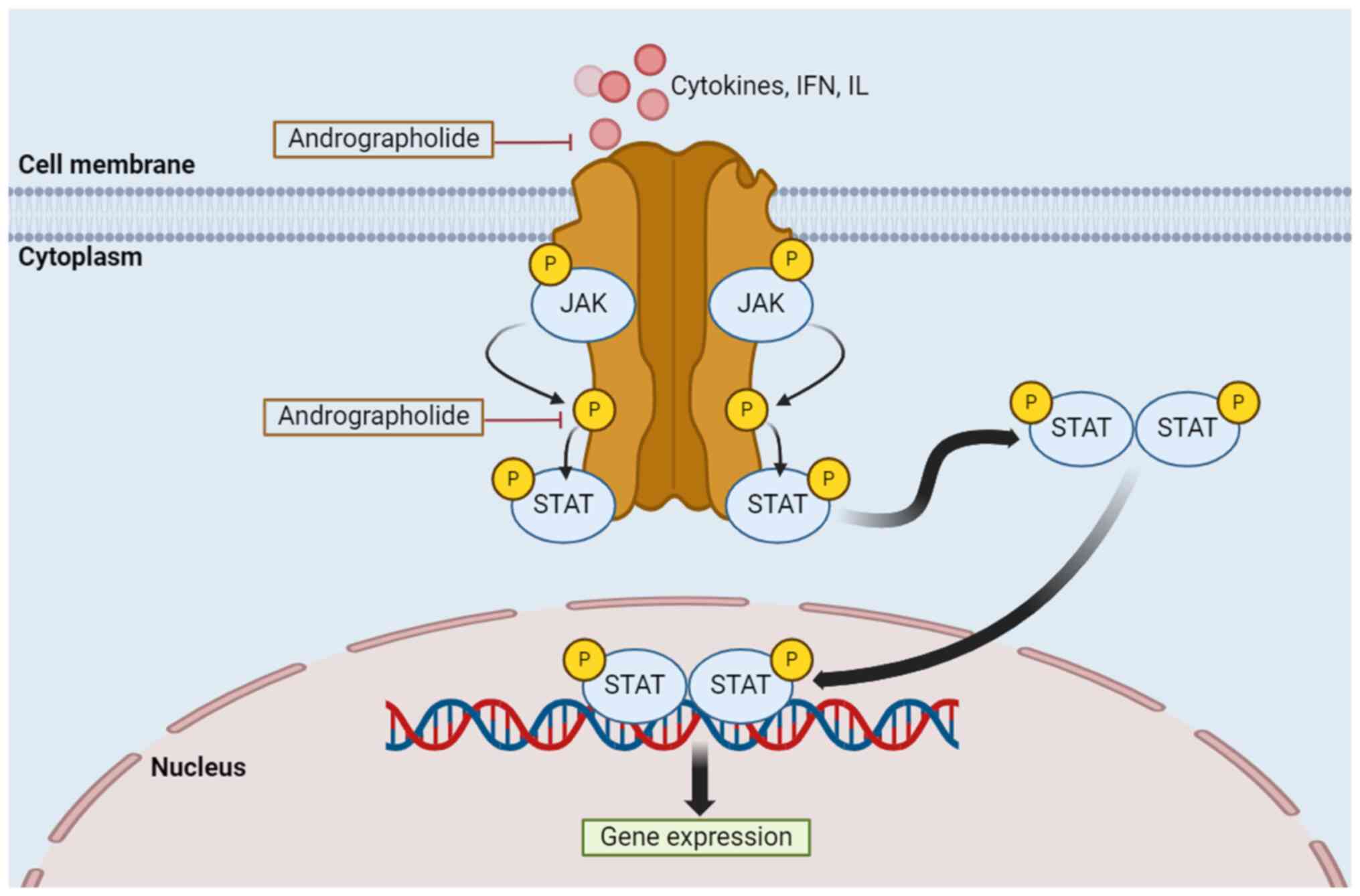 | Figure 5Regulation of the JAK/STAT signaling
pathway by andrographolide. Activation of this process is the
result of reactions outside of the cell, in which cytokines, IFN
and IL, attach to their specific receptors, resulting in JAK
proteins phosphorylating each other. JAK phosphorylates both the
binding site of the STAT protein receptor and the STAT protein on
its retained tyrosine residues, forming a complex STAT molecule in
the cytoplasm. This molecule travels to the nucleus and attaches to
the DNA, initiating the expression of genes. Types of receptors
include growth factor receptors, IL receptors and
G-protein-associated receptors. JAK, Janus kinase; STAT, signal
transducer and activator of transcription; IFN, interferon; IL,
interleukin; P, phosphorylation. |
The inhibition of the JAK/STAT pathway by
andrographolide is further demonstrated in previous studies using
different types of human cancer cells. IL is a type of cytokine
that binds to a receptor on the cell membrane to initiate the
JAK/STAT pathway. IL-6 is one of the cytokines produced by various
types of lymphocytes as well as non-lymphocytes such as endothelial
cells, monocytes, fibroblasts, and T and B lymphocytes (134). It facilitates the EMT process by
activating the JAK/STAT pathway, which results in inflammation
(134). Previous studies using
DU145, AsPC-1 and Panc-1 cancer cells reveal that andrographolide
can reduce the IL-6 expression levels and signaling, which is
directly associated with the inhibition of the JAK/STAT pathway
(82,135). Additionally, in the MDA-MB-231,
AD-293, HI975 and HI299 cancer cell lines, andrographolide inhibits
the phosphorylation of STAT3, hindering cell apoptosis, growth and
proliferation (136-138).
Hindering one of the steps in the JAK/STAT pathway may lead to
cancer cell death (82). Other
receptors that will be attached to the cell membrane include
G-protein-coupled receptors and homodimeric hormone receptors with
ligands such as bradykinin receptor B2 and thrombopoietin binding
to them, respectively. The binding of these ligands to their
receptors may cause ovarian and myeloproliferative cancer (139). Therefore, studies investigating
how andrographolide suppresses these biomarkers will further the
understanding of how it inhibits the JAK/STAT pathway and may
elucidate its anticancer effects.
Other signaling pathways
Andrographolide inhibits tumor growth in a number of
other pathways. One of these is the PI3K/AKT/mTOR signaling
pathway, which serves a major role in cancer cell growth and tumor
proliferation by responding to different types of factors such as
nutrients, hormones and growth factors. In this pathway, receptor
tyrosine kinases bound to the cell membrane activate the PI3K
molecule. This pathway begins with growth factors binding to ligand
binding sites, causing the dimerization of the receptors, with one
receptor phosphorylating a tyrosine residue on the other receptor.
This allows the docking of proteins, such as insulin receptor
substrate-1 and the GRB-2-associated binder, which are then
activated by tyrosine kinases PI3K (140). The PI3K molecule phosphorylates a
lipid bound to two phosphates, namely PiP2, to cause PiP3
activation, which further activates the AKT molecule. AKT will
further initiate the downstream effects and activate mTOR.
Subsequently, mTOR will upregulate cell translation to initiate the
synthesis of proteins from mRNA as well as the biosynthesis of
lipids. mTOR has roles in cell survival, cell cycle progression and
cell division. The disturption or upregulation of mTOR pathways
will lead to uncontrollable cell division, causing cancer (141). The Bcl-2 protein promotes cell
survival when phosphorylated by AKT, unlike the Bax protein, which
is a pro-apoptotic protein. The treatment of breast cancer cell
lines, MCF-7 and MDA-MB-231, with andrographolide, demonstrates an
inhibition of Bcl-2 and an increase in the expression and protein
levels of Bax, further demonstrating the apoptotic effect of
andrographolide on these breast cancer cell lines (142).
The Wnt/β-catenin pathway involves Wnt, a series of
growth simulating factors, which is a protein with palmitoleic acid
attached to it for binding purposes (143). β-catenin is a molecule mainly
regulated through degradation. In an activated state, β-catenin is
bound to a destruction complex, which is a large protein complex
including axis inhibition protein, glycogen synthase kinase 3β,
casein kinase I, adenomatous polyposis coli, dishevelled and
β-transducin repeat-containing protein (144-146).
The destruction complex is so named because it causes
phosphorylation, Ub and proteasome degradation of β-catenin
(147,148). This pathway begins with the
activation of the Wnt receptors, such as Frizzled Class Receptor 1
and Frizzled Class Receptor 2, by Wnt, which leads to low-density
lipoprotein receptor-related protein (LRP) phosphorylation. This
induces the translocation of the destruction complex to the region
of the membrane near the frizzled and LRP receptors (148,149). Subsequently, dishevelled binds to
LRP and becomes activated, leading to destruction complex
inhibition (150). This increases
the levels of β-catenin in the cytosol because it does not undergo
phosphorylation, Ub or degradation in the proteasome (150). The transcription factor (TCF)
mediates gene expression as the β-catenin translocates into the
mitochondria, dislodging Groucho from the TCF (150). Subsequently, β-catenin binds to
the TCF, leading to the transcription of the Wnt target genes and
the growth and proliferation of the cell (150-152).
A study on the anticancer effect of andrographolide (using
colorectal cancer lines, HCT116 and SW480, and both in vitro
and in vivo analysis) demonstrates a dysregulation of the
WNT16, transcription factor 7-like 2 and axis inhibition protein 2
protein expression levels in the cancer cells following treatment
with andrographolide. The co-administration of andrographolide
along with 5-fluorouracil-based chemotherapeutic regimens notably
reduces the tumor volume in mice. These results further indicate
the anticancer effect of andrographolide through the dysregulation
of the Wnt/β-catenin pathway (153).
The MAPK pathway regulates processes such as cell
death, cell differentiation and cell proliferation (154). An epidermal growth factor
signaling molecule, located outside of the cell, binds to the
epidermal growth factor receptor to activate the MAPK pathway. This
process causes the dimerization and autophosphorylation of a
tyrosine residue on the receptor, which leads to the recruitment of
the growth factor receptor-bound protein 2 (GRB-2) protein, which
contains the Src-homology 2 (sh2) domain (155,156). This protein is activated as it
binds to the phosphorylated tyrosine through the sh2 domain, which
then recruits another molecule, son of sevenless (SOS) protein,
which is attached to the sh2 domain of GRB-2(157). Subsequently, the SOS protein
activates the RAS molecule containing bound GDP by replacing it
with GTP. Activated RAS phosphorylates the RAF molecule, which then
activates the MEK molecule (156-158).
The activated MEK molecule then targets and phosphorylates the ERK
1/2 molecule, which is the outcome of the kinase cascade. The
ERK1/2 molecule acts on different transcription factors such as
C-Fos and C-Jun, causing them to dimerize into activator protein-1
(AP-1) (156,157). AP-1 then enters the nucleus and
binds to the DNA molecule, initiating gene transcription (156,157). Glioblastoma multiforme (GBM) is
the most common type of primary malignant brain tumor in adults
globally, accounting for 50.1% of all primary malignant brain
tumors in the United States (with 14,490 new cases reported in the
United States in 2023 alone), and it can be fatal due to its
metastatic activity (159). An
in vitro analysis using the brain tumor cell lines GBM8401
and U251, reports the prevention of GBM cell metastasis by
andrographolide. The results reveal that andrographolide inhibits
MMP-2 expression at the transcriptional level in the MAPK pathway
(160). Since AP-1 is a key
transcription factor that promotes MMP-2 transcription, decreasing
the expression of MMP-2 facilitates the prevention of cancer
progression and metastasis. Thus, it is crucial to target MMP-2 and
the MAPK pathway to prevent tumor progression and enhance treatment
efficiency.
4. Conclusion
In conclusion, the present review provides a
comprehensive description of how andrographolide regulates the
signaling pathways involved in the development of cancer cells,
mainly NF-κB, HIF-1, JAK/STAT, PI3K/AKT/mTOR, Wnt/β-catenin and
MAPK. Andrographolide has the potential to be an effective
anticancer agent as it can target the important biomarkers involved
in each of the signaling pathways. In the NF-κB signaling pathway,
it mainly downregulates the MMP biomarkers. In addition,
andrographolide specifically reduces the HIF-1α protein expression
levels in the HIF-1 pathway, leading to unsuccessful gene
transcription. In the JAK/STAT pathway, andrographolide targets
both the IL-6 cytokine and the STAT3 protein, inhibiting the gene
expression process, which can cause apoptosis as well as reduce
cell growth and proliferation. Hence, andrographolide has been
highlighted as a promising anticancer agent in various types of
signaling pathways.
5. Future perspectives
The present review discusses the most prevalent
types of cancer, such as lung, colorectal and breast cancer, as
well as signaling pathways such as NF-κB, HIF-1, JAK/STAT,
PI3K/AKT/mTOR, Wnt/β-catenin and MAPK. The present review also
discusses the ability of andrographolide to inhibit these signaling
pathways to prevent cancer cells from growing efficiently. However,
numerous biomarkers are involved in each signaling pathway, and
their association with andrographolide are yet to be revealed. In
the HIF-1 pathway, HDAC may activate this pathway under hypoxic
conditions. Therefore, inhibition of this HDAC biomarker will also
inhibit this pathway. Further in-depth in vivo and in
vitro analyses of how andrographolide could aid this inhibition
process would help reveal its ability to inhibit the HIF-1 pathway
(161). Furthermore, at present,
only a small number of studies have investigated the ability of
andrographolide to inhibit the JAK/STAT pathway by downregulating
two specific biomarkers, bradykinin receptor B2 and thrombopoietin.
These biomarkers are known to be associated with cancer cell growth
in myeloproliferative and ovarian cancers. In addition, previous
studies using osteosarcoma cell lines demonstrate the ability of
DNA damage to initiate the NF-κB signaling pathway following
phosphorylation of p100 and processing of p52 protein through a
non-canonical pathway. However, it remains unclear how DNA damage
can activate the NF-κB signaling pathway through a non-canonical
pathway and the role of IKKα in this pathway. Further research into
the non-canonical NF-κB signaling pathway triggered by DNA damage
could aid the development of cancer cell treatments.
Acknowledgements
Not applicable.
Funding
Funding: Funding was provided by the Ministry of Higher
Education under the Fundamental Research Grant Scheme (grant no.
FRGS/1/2022/STG01/UITM/02/06) and Universiti Teknologi MARA
Research Entity Collaboration Grant [grant no. KEPU 5/3
(008/2023)].
Availability of data and materials
Not applicable.
Authors' contributions
NS was involved in the literature search and
writing the manuscript draft. GS, SS and TK contributed to
developing the idea and revising the draft. All authors read and
approved the final version of the manuscript. Data authentication
is not applicable.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Fotsing Yannick Stéphane F, Kezetas Jean
Jules B, El-Saber Batiha G, Ali I and Ndjakou Bruno L: Extraction
of bioactive compounds from medicinal plants and herbs. In:
El-Shemy HA (ed). Natural Medicinal Plants. IntechOpen; London, UK,
2022.
|
|
2
|
Balekundri A and Mannur V: Quality control
of the traditional herbs and herbal products: A review. Futur J
Pharm Sci. 6(67)2020.
|
|
3
|
Cione E, La Torre C, Cannataro R, Caroleo
MC, Plastina P and Gallelli L: Quercetin, epigallocatechin gallate,
curcumin, and resveratrol: from dietary sources to human MicroRNA
modulation. Molecules. 25(63)2019.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Lambert JD, Lee MJ, Lu H, Meng X, Hong JJ,
Seril DN, Sturgill MG and Yang CS: Epigallocatechin-3-gallate is
absorbed but extensively glucuronidated following oral
administration to mice. J Nutr. 133:4172–4177. 2003.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Chunarkar-Patil P, Kaleem M, Mishra R, Ray
S, Ahmad A, Verma D, Bhayye S, Dubey R, Singh HN and Kumar S:
Anticancer Drug discovery based on natural products: From
computational approaches to clinical studies. Biomedicines.
12(201)2024.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Pandey AK, Gulati S, Gupta A and Tripathi
YC: Variation in andrographolide content among different accessions
of Andrographis paniculata. Pharma Innov J. 8:140–144.
2019.
|
|
7
|
Liang D, Zhang WM, Liang X, Tian HY, Zhang
XM, Li X and Gao WY: A review on the extraction and separation of
andrographolide from Andrographis paniculata: Extraction
selectivity, current challenges and strategies. Tradit Med Res.
8(38)2023.
|
|
8
|
Sharma S, Sharma YP and Bhardwaj C: HPLC
quantification of andrographolide in different parts of
Andrographis paniculata (Burm.f.) Wall. ex Nees. J
Pharmacogn Phytochem. 7:168–171. 2018.
|
|
9
|
Kandanur SGS, Tamang N, Golakoti NR and
Nanduri S: Andrographolide: A natural product template for the
generation of structurally and biologically diverse diterpenes. Eur
J Med Chem. 176:513–533. 2019.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Tran QTN, Tan WSD, Wong WSF and Chai CLL:
Polypharmacology of andrographolide: Beyond one molecule one
target. Nat Prod Rep. 38:682–692. 2021.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Phunikom N, Boonmuen N, Kheolamai P,
Suksen K, Manochantr S, Tantrawatpan C and Tantikanlayaporn D:
Andrographolide promotes proliferative and osteogenic potentials of
human placenta-derived mesenchymal stem cells through the
activation of Wnt/β-catenin signaling. Stem Cell Res Ther.
12(241)2021.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Nair DS and Manjula S: Induction of root
endosymbiosis as a highly sustainable and efficient strategy for
overproduction of the medicinally important diterpenoid
lactone-andrographolide in Andrographis paniculata (Burm.
F.) Wall. ex Nees. Ind Crops Prod. 156(112835)2020.
|
|
13
|
Dai Y, Chen SR, Chai L, Zhao J and Wang Y
and Wang Y: Overview of pharmacological activities of
Andrographis paniculata and its major compound
andrographolide. Crit Rev Food Sci Nutr. 59 (Suppl 1):S17–S29.
2019.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Tundis R, Patra JK, Bonesi M, Das S, Nath
R, Das Talukdar A, Das G and Loizzo MR: Anti-cancer agent: The
labdane diterpenoid-andrographolide. Plants (Basel).
12(1969)2023.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Cai W, Li J, Chen C, Wu J, Li J and Xue X:
Design, synthesis, and anticancer evaluation of novel
andrographolide derivatives bearing an α,β-unsaturated ketone
moiety. Bioorg Chem. 112(104941)2021.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Arsakhant P, Sirion U, Chairoungdua A,
Suksen K, Piyachaturawat P, Suksamrarn A and Saeeng R: Design and
synthesis of C-12 dithiocarbamate andrographolide analogues as an
anticancer agent. Bioorg Med Chem Lett. 30(127263)2020.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Cheng CR, Zheng Z, Liang RM, Li XF, Jiang
QQ, Yue L, Wang Q, Ding J and Liu Y: Preparation and cytotoxic
Activity of 3,19-analogues of 12-thioether andrographolide. Chem
Nat Compd. 56:264–269. 2020.
|
|
18
|
Beesetti SL, Jayadev M, Subhashini GV,
Mansour L, Alwasel S and Harrath AH: Andrographolide as a
therapeutic agent against breast and ovarian cancers. Open Life
Sci. 14:462–469. 2019.PubMed/NCBI View Article : Google Scholar
|
|
19
|
He X, Li J, Gao H, Qiu F, Hu K, Cui X and
Yao X: Identification of a rare sulfonic acid metabolite of
andrographolide in rats. Drug Metab Dispos. 31:983–985.
2003.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Sa-ngiamsuntorn K, Suksatu A, Pewkliang Y,
Thongsri P, Kanjanasirirat P, Manopwisedjaroen S,
Charoensutthivarakul S, Wongtrakoongate P, Pitiporn S, Chaopreecha
J, et al: Anti-SARS-CoV-2 activity of Andrographis
paniculata extract and its major component andrographolide in
human lung epithelial cells and cytotoxicity evaluation in major
organ cell representatives. J Nat Prod. 84:1261–1270.
2021.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Banerjee M, Parai D, Chattopadhyay S and
Mukherjee SK: Andrographolide: Antibacterial activity against
common bacteria of human health concern and possible mechanism of
action. Folia Microbiol (Praha). 62:237–244. 2017.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Widyawaruyanti A, Asrory M, Ekasari W,
Setiawan D, Radjaram A, Tumewu L and Hafid AF: In vivo antimalarial
activity of Andrographis paniculata tablets. Procedia Chem.
13:101–104. 2014.
|
|
23
|
Yu Q, Shi Y, Shu C, Ding X, Zhu S, Shen Z
and Lou Y: Andrographolide inhibition of Th17-regulated cytokines
and JAK1/STAT3 signaling in OVA-stimulated asthma in mice. Evid
Based Complement Alternat Med. 2021(6862073)2021.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Astuti NT, Novitasari PR, Tjandrawinata R,
Nugroho AE and Pramono S: Anti-diabetic effect of andrographolide
from Sambiloto herbs (Andrographis paniculata (Burm.f.)
Nees) through the expression of PPARγ and GLUT-4 in adipocytes.
Indones J Biotechnol. 27(203)2022.
|
|
25
|
Sung H, Ferlay J, Siegel RL, Laversanne M,
Soerjomataram I, Jemal A and Bray F: Global cancer statistics 2020:
GLOBOCAN estimates of incidence and mortality worldwide for 36
cancers in 185 countries. CA Cancer J Clin. 71:209–249.
2021.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Gadi VK and Davidson NE: Practical
approach to triple-negative breast cancer. J Oncol Pract.
13:293–300. 2017.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Li L, Yang LL, Yang SL, Wang RQ, Gao H,
Lin ZY, Zhao YY, Tang WW, Han R, Wang WJ, et al: Andrographolide
suppresses breast cancer progression by modulating tumor-associated
macrophage polarization through the Wnt/β-catenin pathway. Phyther
Res. 36:4587–4603. 2022.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Anand U, Dey A, Chandel AKS, Sanyal R,
Mishra A, Pandey DK, De Falco V, Upadhyay A, Kandimalla R,
Chaudhary A, et al: Cancer chemotherapy and beyond: Current status,
drug candidates, associated risks and progress in targeted
therapeutics. Genes Dis. 10:1367–1401. 2022.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Aggarwal S, Verma SS, Aggarwal S and Gupta
SC: Drug repurposing for breast cancer therapy: Old weapon for new
battle. Semin Cancer Biol. 68:8–20. 2021.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Hung Y, Leung S, Chiu SP, Li PY, Kan AC,
Lo CC, Wong SZ, Luk SL, Lai CC, El Helali A and Chan WW:
Perceptions about traditional Chinese medicine use among Chinese
breast cancer survivors: A qualitative study. Cancer Med.
12:1997–2007. 2023.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Cohen I, Tagliaferri M and Tripathy D:
Traditional Chinese medicine in the treatment of breast cancer.
Semin Oncol. 29:563–574. 2002.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Wang R, Wang Y, Fang L, Xie Y, Yang S, Liu
S, Fang Y and Zhang Y: Efficacy and safety of traditional Chinese
medicine in the treatment of menopause-like syndrome for breast
cancer survivors: A systematic review and meta-analysis. BMC
Cancer. 24(42)2024.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Shu J, Huang R, Tian Y, Liu Y, Zhu R and
Shi G: Andrographolide protects against endothelial dysfunction and
inflammatory response in rats with coronary heart disease by
regulating PPAR and NF-κB signaling pathways. Ann Palliat Med.
9:1965–1975. 2020.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Xia YF, Ye BQ, Li YD, Wang JG, He XJ, Lin
X, Yao X, Ma D, Slungaard A, Hebbel RP, et al: Andrographolide
attenuates inflammation by inhibition of NF-kappa B activation
through covalent modification of reduced cysteine 62 of p50. J
Immunol. 173:4207–4217. 2004.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Li Z and Wu JC, Sheikh AY, Kraft D, Cao F,
Xie X, Patel M, Gambhir SS, Robbins RC, Cooke JP and Wu JC:
Differentiation, survival, and function of embryonic stem cell
derived endothelial cells for ischemic heart disease. Circulation.
116 (11 Suppl):I46–I54. 2007.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Giordano SH: Breast cancer in men. N Engl
J Med. 378:2311–2320. 2018.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Sousa S, Brion R, Lintunen M, Kronqvist P,
Sandholm J, Mönkkönen J, Kellokumpu-Lehtinen PL, Lauttia S,
Tynninen O, Joensuu H, et al: Human breast cancer cells educate
macrophages toward the M2 activation status. Breast Cancer Res.
17(101)2015.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Yang Q, Guo N, Zhou Y, Chen J, Wei Q and
Han M: The role of tumor-associated macrophages (TAMs) in tumor
progression and relevant advance in targeted therapy. Acta Pharm
Sin B. 10:2156–2170. 2020.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Hagemann T, Lawrence T, McNeish I, Charles
KA, Kulbe H, Thompson RG, Robinson SC and Balkwill FR:
‘Re-educating’ tumor-associated macrophages by targeting NF-κB. J
Exp Med. 205:1261–1268. 2008.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Siegel RL, Miller KD, Goding Sauer A,
Fedewa SA, Butterly LF, Anderson JC, Cercek A, Smith RA and Jemal
A: Colorectal cancer statistics, 2020. CA Cancer J Clin.
70:145–164. 2020.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Ohri A, Robinson A, Liu B, Bhuket T and
Wong R: Updated assessment of colorectal cancer incidence in the
U.S. by age, sex, and race/ethnicity. Dig Dis Sci. 65:1838–1849.
2020.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Eslami M, Yousefi B, Kokhaei P, Hemati M,
Nejad ZR, Arabkari V and Namdar A: Importance of probiotics in the
prevention and treatment of colorectal cancer. J Cell Physiol.
234:17127–17143. 2019.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Lai Y, Wang C, Civan JM, Palazzo JP, Ye Z,
Hyslop T, Lin J, Myers RE, Li B, Jiang B, et al: Effects of cancer
stage and treatment differences on racial disparities in survival
from colon cancer: A United States population-based study.
Gastroenterology. 150:1135–1146. 2016.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Xynos ID, Kavantzas N, Tsaousi S,
Zacharakis M, Agrogiannis G, Kosmas C, Lazaris A, Sarantonis J,
Sougioultzis S, Tzivras D, et al: Factors influencing survival in
stage IV colorectal cancer: The influence of DNA ploidy. ISRN
Gastroenterol. 2013(490578)2013.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Xie YH, Chen YX and Fang JY: Comprehensive
review of targeted therapy for colorectal cancer. Signal Transduct
Target Ther. 5(22)2020.PubMed/NCBI View Article : Google Scholar
|
|
46
|
García-Alfonso P, Muñoz Martín AJ, Ortega
Morán L, Soto Alsar J, Torres Pérez-Solero G, Blanco Codesido M,
Calvo Ferrandiz PA and Grasso Cicala S: Oral drugs in the treatment
of metastatic colorectal cancer. Ther Adv Med Oncol.
13(17588359211009001)2021.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Van Cutsem E, Cervantes A, Adam R, Sobrero
A, Van Krieken JH, Aderka D, Aranda Aguilar E, Bardelli A, Benson
A, Bodoky G, et al: ESMO consensus guidelines for the management of
patients with metastatic colorectal cancer. Ann Oncol.
27:1386–1422. 2016.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Feng X, Sureda A, Jafari S, Memariani Z,
Tewari D, Annunziata G, Barrea L, Hassan STS, Šmejkal K, Malaník M,
et al: Berberine in cardiovascular and metabolic diseases: From
mechanisms to therapeutics. Theranostics. 9:1923–1951.
2019.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Palmieri A, Scapoli L, Iapichino A,
Mercolini L, Mandrone M, Poli F, Giannì AB, Baserga C and
Martinelli M: Berberine and Tinospora cordifolia exert a potential
anticancer effect on colon cancer cells by acting on specific
pathways. Int J Immunopathol Pharmacol.
33(2058738419855567)2019.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Ranjan A, Ramachandran S, Gupta N, Kaushik
I, Wright S, Srivastava S, Das H, Srivastava S, Prasad S and
Srivastava SK: Role of phytochemicals in cancer prevention. Int J
Mol Sci. 20(4981)2019.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Islam MT, Ali ES, Uddin SJ, Islam MA, Shaw
S, Khan IN, Saravi SSS, Ahmad S, Rehman S, Gupta VK, et al:
Andrographolide, a diterpene lactone from Andrographis
paniculata and its therapeutic promises in cancer. Cancer Lett.
420:129–145. 2018.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Shi MD, Lin HH, Lee YC, Chao JK, Lin RA
and Chen JH: Inhibition of cell-cycle progression in human
colorectal carcinoma Lovo cells by andrographolide. Chem Biol
Interact. 174:201–210. 2008.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Norouzi M and Hardy P: Clinical
applications of nanomedicines in lung cancer treatment. Acta
Biomater. 121:134–142. 2021.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Torre LA, Siegel RL and Jemal A: Lung
cancer statistics. Adv Exp Med Biol. 893:1–19. 2016.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Thandra KC and Barsouk A, Saginala K,
Aluru JS and Barsouk A: Epidemiology of lung cancer. Contemp Oncol
(Pozn). 25:45–52. 2021.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2020. CA Cancer J Clin. 70:7–30. 2020.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Commar A, Prasad V and D'Espaignet ET: WHO
global report on trends in prevalence of tobacco use 2000-2025,
2021. https://www.who.int/publications/i/item/who-global-report-on-trends-in-prevalence-of-tobacco-use-2000-2025-third-edition.
|
|
58
|
Dougherty SM, Mazhawidza W, Bohn AR,
Robinson KA, Mattingly KA, Blankenship KA, Huff MO, McGregor WG and
Klinge CM: Gender difference in the activity but not expression of
estrogen receptors alpha and beta in human lung adenocarcinoma
cells. Endocr Relat Cancer. 13:113–134. 2006.PubMed/NCBI View Article : Google Scholar
|
|
59
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018.PubMed/NCBI View Article : Google Scholar
|
|
60
|
Landi MT, Chatterjee N, Yu K, Goldin LR,
Goldstein AM, Rotunno M, Mirabello L, Jacobs K, Wheeler W, Yeager
M, et al: A genome-wide association study of lung cancer identifies
a region of chromosome 5p15 associated with risk for
adenocarcinoma. Am J Hum Genet. 85:679–691. 2009.PubMed/NCBI View Article : Google Scholar
|
|
61
|
Yokota J, Shiraishi K and Kohno T: Genetic
basis for susceptibility to lung cancer: Recent progress and future
directions. Adv Cancer Res. 109:51–72. 2010.PubMed/NCBI View Article : Google Scholar
|
|
62
|
Thorgeirsson TE, Geller F, Sulem P, Rafnar
T, Wiste A, Magnusson KP, Manolescu A, Thorleifsson G, Stefansson
H, Ingason A, et al: A variant associated with nicotine dependence,
lung cancer and peripheral arterial disease. Nature. 452:638–642.
2008.PubMed/NCBI View Article : Google Scholar
|
|
63
|
Hussain S: Nanomedicine for treatment of
lung cancer. In: Ahmad A, Gadgeel S (eds). Lung Cancer and
Personalized Medicine: Novel Therapies and Clinical Management.
Advances in Experimental Medicine and Biology. Vol. 890. Springer,
Cham, pp137-147, 2016.
|
|
64
|
Sun A, Durocher-Allen LD, Ellis PM, Ung
YC, Goffin JR, Ramchandar K and Darling G: Initial management of
small-cell lung cancer (limited- and extensive-stage) and the role
of thoracic radiotherapy and first-line chemotherapy: A systematic
review. Curr Oncol. 26:e372–e384. 2019.PubMed/NCBI View Article : Google Scholar
|
|
65
|
Bahman F, Elkaissi S, Greish K and Taurin
S: Polymeric micelles in management of lung cancer. In:
Nanotechnology-Based Targeted Drug Delivery Systems for Lung
Cancer. Elsevier, pp193-216, 2019.
|
|
66
|
Norouzi M, Amerian M, Amerian M and Atyabi
F: Clinical applications of nanomedicine in cancer therapy. Drug
Discov Today. 25:107–125. 2020.PubMed/NCBI View Article : Google Scholar
|
|
67
|
Norouzi M, Yathindranath V, Thliveris JA
and Miller DW: Salinomycin-loaded iron oxide nanoparticles for
glioblastoma therapy. Nanomaterials (Basel). 10(477)2020.PubMed/NCBI View Article : Google Scholar
|
|
68
|
Ricciardi S, Tomao S and de Marinis F:
Toxicity of targeted therapy in non-small-cell lung cancer
management. Clin Lung Cancer. 10:28–35. 2009.PubMed/NCBI View Article : Google Scholar
|
|
69
|
Su XL, Wang JW, Che H, Wang CF, Jiang H,
Lei X, Zhao W, Kuang HX and Wang QH: Clinical application and
mechanism of traditional Chinese medicine in treatment of lung
cancer. Chin Med J (Engl). 133:2987–2997. 2020.PubMed/NCBI View Article : Google Scholar
|
|
70
|
Paul S, Roy D, Pati S and Sa G: The
adroitness of andrographolide as a natural weapon against
colorectal cancer. Front Pharmacol. 12(731492)2021.PubMed/NCBI View Article : Google Scholar
|
|
71
|
Lin HH, Tsai CW, Chou FP, Wang CJ, Hsuan
SW, Wang CK and Chen JH: Andrographolide down-regulates
hypoxia-inducible factor-1α in human non-small cell lung cancer
A549 cells. Toxicol Appl Pharmacol. 250:336–345. 2011.PubMed/NCBI View Article : Google Scholar
|
|
72
|
Jiaqi L, Siqing H, qin W, di Z, bei Z and
jialin Y: Andrographolide promoted ferroptosis to repress the
development of non-small cell lung cancer through activation of the
mitochondrial dysfunction. Phytomedicine.
109(154601)2023.PubMed/NCBI View Article : Google Scholar
|
|
73
|
Neamatallah T, Malebari AM, Alamoudi AJ,
Nazreen S, Alam MM, Bin-Melaih HH, Abuzinadah OA, Badr-Eldin SM,
Alhassani G, Makki L and Nasrullah MZ: Andrographolide
nanophytosomes exhibit enhanced cellular delivery and pro-apoptotic
activities in HepG2 liver cancer cells. Drug Deliv.
30(2174209)2023.PubMed/NCBI View Article : Google Scholar
|
|
74
|
Shannon AH, Ruff SM and Pawlik TM: Expert
insights on current treatments for hepatocellular carcinoma:
Clinical and molecular approaches and bottlenecks to progress. J
Hepatocell Carcinoma. 9:1247–1261. 2022.PubMed/NCBI View Article : Google Scholar
|
|
75
|
Rimassa L and Gish GR: HCC in focus:
Current developments in the management of hepatocellular carcinoma.
Gastroenterol Hepatol (N Y). 14:542–544. 2018.
|
|
76
|
Xi SY and Minuk GY: Role of traditional
Chinese medicine in the management of patients with hepatocellular
carcinoma. World J Hepatol. 10:799–806. 2018.PubMed/NCBI View Article : Google Scholar
|
|
77
|
Siegel RL, Giaquinto AN and Jemal A:
Cancer statistics, 2024. CA Cancer J Clin. 74:12–49.
2024.PubMed/NCBI View Article : Google Scholar
|
|
78
|
Forestier-Román IS, López-Rivas A,
Sánchez-Vázquez MM, Rohena-Rivera K, Nieves-Burgos G, Ortiz-Zuazaga
H, Torres-Ramos CA and Martínez-Ferrer M: Andrographolide induces
DNA damage in prostate cancer cells. Oncotarget. 10:1085–1101.
2019.PubMed/NCBI View Article : Google Scholar
|
|
79
|
Chen FZ and Zhao XK: Prostate cancer:
Current treatment and prevention strategies. Iran Red Crescent Med
J. 15:279–284. 2013.PubMed/NCBI View Article : Google Scholar
|
|
80
|
Malinowski B, Wiciński M, Musiała N,
Osowska I and Szostak M: Previous, current, and future
pharmacotherapy and diagnosis of prostate cancer-a comprehensive
review. Diagnostics (Basel). 9(161)2019.PubMed/NCBI View Article : Google Scholar
|
|
81
|
Jiang J, Slivova V, Valachovicova T,
Harvey K and Sliva D: Ganoderma lucidum inhibits
proliferation and induces apoptosis in human prostate cancer cells
PC-3. Int J Oncol. 24:1093–1099. 2004.PubMed/NCBI
|
|
82
|
Chun JY, Tummala R, Nadiminty N, Lou W,
Liu C, Yang J, Evans CP, Zhou Q and Gao AC: Andrographolide, an
herbal medicine, inhibits interleukin-6 expression and suppresses
prostate cancer cell growth. Genes Cancer. 1:868–876.
2010.PubMed/NCBI View Article : Google Scholar
|
|
83
|
Munir AH and Khan MI: Pattern of basic
hematological parameters in acute and chronic leukemias. J Med Sci.
27:125–129. 2019.
|
|
84
|
Li X, Wu T, Chen W, Zhang J, Jiang Y, Deng
J, Long W, Qin X and Zhou Y: Andrographolide acts with
dexamethasone to inhibit the growth of acute lymphoblastic leukemia
CEM-C1 cells via the regulation of the autophagy-dependent
PI3K/AKT/mTOR signaling pathway. Biomed Reports.
20(43)2024.PubMed/NCBI View Article : Google Scholar
|
|
85
|
Hunger SP and Mullighan CG: Acute
lymphoblastic leukemia in children. N Engl J Med. 373:1541–1552.
2015.PubMed/NCBI View Article : Google Scholar
|
|
86
|
Samii B, Jafarian A, Rabbani M, Zolfaghari
B, Rahgozar S and Pouraboutaleb E: The effects of Astragalus
polysaccharides, tragacanthin, and bassorin on
methotrexate-resistant acute lymphoblastic leukemia. Res Pharm Sci.
18:381–391. 2023.PubMed/NCBI View Article : Google Scholar
|
|
87
|
Follini E, Marchesini M and Roti G:
Strategies to overcome resistance mechanisms in T-cell acute
lymphoblastic leukemia. Int J Mol Sci. 20(3021)2019.PubMed/NCBI View Article : Google Scholar
|
|
88
|
Wang YJ, Liao CC, Chen HJ, Hsieh CL and Li
TC: The effectiveness of traditional Chinese medicine in treating
patients with leukemia. Evid Based Complement Alternat Med.
2016(8394850)2016.PubMed/NCBI View Article : Google Scholar
|
|
89
|
Yang T, Yao S, Zhang X and Guo Y:
Andrographolide inhibits growth of human T-cell acute lymphoblastic
leukemia Jurkat cells by downregulation of PI3K/AKT and
upregulation of p38 MAPK pathways. Drug Des Devel Ther.
10:1389–1397. 2016.PubMed/NCBI View Article : Google Scholar
|
|
90
|
Cai Q, Zhang W, Sun Y, Xu L, Wang M, Wang
X, Wang S and Ni Z: Study on the mechanism of andrographolide
activation. Front Neurosci. 16(977376)2022.PubMed/NCBI View Article : Google Scholar
|
|
91
|
Li X, Yuan W, Wu J, Zhen J, Sun Q and Yu
M: Andrographolide, a natural anti-inflammatory agent: An update.
Front Pharmacol. 13(920435)2022.PubMed/NCBI View Article : Google Scholar
|
|
92
|
Harrington BS and Annunziata CM: NF-κB
signaling in ovarian cancer. Cancers (Basel).
11(1182)2019.PubMed/NCBI View Article : Google Scholar
|
|
93
|
Khan H, Ullah H, Castilho PCMF, Gomila AS,
D'Onofrio G, Filosa R, Wang F, Nabavi SM, Daglia M, Silva AS, et
al: Targeting NF-κB signaling pathway in cancer by dietary
polyphenols. Crit Rev Food Sci Nutr. 60:2790–2800. 2020.PubMed/NCBI View Article : Google Scholar
|
|
94
|
Yu H and Jove R: The STATs of cancer-new
molecular targets come of age. Nat Rev Cancer. 4:97–105.
2004.PubMed/NCBI View Article : Google Scholar
|
|
95
|
Vivanco I and Sawyers CL: The
phosphatidylinositol 3-Kinase-AKT pathway in human cancer. Nat Rev
Cancer. 2:489–501. 2002.PubMed/NCBI View
Article : Google Scholar
|
|
96
|
Baldwin AS Jr: Series introduction: The
transcription factor NF-kappaB and human disease. J Clin Invest.
107:3–6. 2001.PubMed/NCBI View Article : Google Scholar
|
|
97
|
Showalter A, Limaye A, Oyer JL, Igarashi
R, Kittipatarin C, Copik AJ and Khaled AR: Cytokines in immunogenic
cell death: Applications for cancer immunotherapy. Cytokine.
97:123–132. 2017.PubMed/NCBI View Article : Google Scholar
|
|
98
|
Mirzaei S, Zarrabi A, Hashemi F, Zabolian
A, Saleki H, Ranjbar A, Seyed Saleh SH, Bagherian M, Sharifzadeh
SO, Hushmandi K, et al: Regulation of nuclear factor-kappaB (NF-κB)
signaling pathway by non-coding RNAs in cancer: Inhibiting or
promoting carcinogenesis? Cancer Lett. 509:63–80. 2021.PubMed/NCBI View Article : Google Scholar
|
|
99
|
Singh V, Gupta D and Arora R: NF-kB as a
key player in regulation of cellular radiation responses and
identification of radiation countermeasures. Discoveries (Craiova).
3(e35)2015.PubMed/NCBI View Article : Google Scholar
|
|
100
|
Sun SC: The non-canonical NF-κB pathway in
immunity and inflammation. Nat Rev Immunol. 17:545–558.
2017.PubMed/NCBI View Article : Google Scholar
|
|
101
|
Kawai T and Akira S: The role of
pattern-recognition receptors in innate immunity: Update on
Toll-like receptors. Nat Immunol. 11:373–384. 2010.PubMed/NCBI View Article : Google Scholar
|
|
102
|
Lin L, Hu X, Zhang H and Hu H: Tertiary
lymphoid organs in cancer immunology: Mechanisms and the new
strategy for immunotherapy. Front Immunol. 10(1398)2019.PubMed/NCBI View Article : Google Scholar
|
|
103
|
Abramson J and Anderson G: Thymic
epithelial cells. Annu Rev Immunol. 35:85–118. 2017.PubMed/NCBI View Article : Google Scholar
|
|
104
|
Serasanambati M and Chilakapati SR:
Function of nuclear factor kappa B (NF-kB) in human diseases-a
review. South Indian J Biol Sci. 2(368)2016.
|
|
105
|
Ben Hamouda S and Essafi-Benkhadir K:
Interplay between signaling pathways and tumor microenvironment
components: A paradoxical role in colorectal cancer. Int J Mol Sci.
24(5600)2023.PubMed/NCBI View Article : Google Scholar
|
|
106
|
Sun SC: Non-canonical NF-κB signaling
pathway. Cell Res. 21:71–85. 2011.PubMed/NCBI View Article : Google Scholar
|
|
107
|
Jost PJ and Ruland J: Aberrant NF-kappaB
signaling in lymphoma: Mechanisms, consequences, and therapeutic
implications. Blood. 109:2700–2707. 2007.PubMed/NCBI View Article : Google Scholar
|
|
108
|
Zhang T, Ma C, Zhang Z, Zhang H and Hu H:
NF-κB signaling in inflammation and cancer. MedComm (2020).
2:618–653. 2021.PubMed/NCBI View Article : Google Scholar
|
|
109
|
Liu T, Zhang L, Joo D and Sun SC: NF-κB
signaling in inflammation. Signal Transduct Target Ther.
2(17023)2017.PubMed/NCBI View Article : Google Scholar
|
|
110
|
Oeckinghaus A and Ghosh S: The NF-kappaB
family of transcription factors and its regulation. Cold Spring
Harb Perspect Biol. 1(a000034)2009.PubMed/NCBI View Article : Google Scholar
|
|
111
|
Guan C, Zhou X, Li H, Ma X and Zhuang J:
NF-κB inhibitors gifted by nature: The anticancer promise of
polyphenol compounds. Biomed Pharmacother.
156(113951)2022.PubMed/NCBI View Article : Google Scholar
|
|
112
|
Zhang R, Zhao J, Xu J, Jiao DX, Wang J,
Gong ZQ and Jia JH: Andrographolide suppresses proliferation of
human colon cancer SW620 cells through the TLR4/NF-κB/MMP-9
signaling pathway. Oncol Lett. 14:4305–4310. 2017.PubMed/NCBI View Article : Google Scholar
|
|
113
|
Im NK, Jang WJ, Jeong CH and Jeong GS:
Delphinidin suppresses PMA-induced MMP-9 expression by blocking the
NF-κB activation through MAPK signaling pathways in MCF-7 human
breast carcinoma cells. J Med Food. 17:855–861. 2014.PubMed/NCBI View Article : Google Scholar
|
|
114
|
Liu MY, Li HJ, Yang C, Zang WD, Liu ZD,
Zhang L, Li PH, Zhu YJ, Zhao YY, Liu RZ and Gao YZ: Insight into
the pharmacological effects of andrographolide in musculoskeletal
disorders. Biomed Pharmacother. 146(112583)2022.PubMed/NCBI View Article : Google Scholar
|
|
115
|
Oh A, Pardo M, Rodriguez A, Yu C, Nguyen
L, Liang O, Chorzalska A and Dubielecka PM: NF-κB signaling in
neoplastic transition from epithelial to mesenchymal phenotype.
Cell Commun Signal. 21(291)2023.PubMed/NCBI View Article : Google Scholar
|
|
116
|
Naserian S, Abdelgawad ME, Afshar Bakshloo
M, Ha G, Arouche N, Cohen JL, Salomon BL and Uzan G: The TNF/TNFR2
signaling pathway is a key regulatory factor in endothelial
progenitor cell immunosuppressive effect. Cell Commun Signal.
18(94)2020.PubMed/NCBI View Article : Google Scholar
|
|
117
|
Hoesel B and Schmid JA: The complexity of
NF-κB signaling in inflammation and cancer. Mol Cancer.
12(86)2013.PubMed/NCBI View Article : Google Scholar
|
|
118
|
Li Y, He S, Tang J, Ding N, Chu X, Cheng
L, Ding X, Liang T, Feng S, Rahman SU, et al: Andrographolide
inhibits inflammatory cytokines secretion in LPS-stimulated
RAW264.7 cells through suppression of NF-κB/MAPK signaling pathway.
Evid Based Complement Alternat Med. 2017(8248142)2017.PubMed/NCBI View Article : Google Scholar
|
|
119
|
Li J, Huang L, He Z, Chen M, Ding Y, Yao
Y, Duan Y, Zixuan L, Qi C, Zheng L, et al: Andrographolide
suppresses the growth and metastasis of luminal-like breast cancer
by inhibiting the NF-κB/miR-21-5p/PDCD4 signaling pathway. Front
Cell Dev Biol. 9(643525)2021.PubMed/NCBI View Article : Google Scholar
|
|
120
|
Zou W, Xiao Z, Wen X, Luo J, Chen S, Cheng
Z, Xiang D, Hu J and He J: The anti-inflammatory effect of
Andrographis paniculata (Burm. f.) Nees on pelvic
inflammatory disease in rats through down-regulation of the NF-κB
pathway. BMC Complement Altern Med. 16(483)2016.PubMed/NCBI View Article : Google Scholar
|
|
121
|
Wang W, Mani AM and Wu ZH: DNA
damage-induced nuclear factor-kappa B activation and its roles in
cancer progression. J Cancer Metastasis Treat. 3:45–59.
2017.PubMed/NCBI View Article : Google Scholar
|
|
122
|
Han S, Huang T, Li W, Liu S, Yang W, Shi
Q, Li H, Ren J and Hou F: Association between hypoxia-inducible
factor-2α (HIF-2α) expression and colorectal cancer and its
prognostic role: A systematic analysis. Cell Physiol Biochem.
48:516–527. 2018.PubMed/NCBI View Article : Google Scholar
|
|
123
|
Masoud GN and Li W: HIF-1α pathway: Role,
regulation and intervention for cancer therapy. Acta Pharm Sin B.
5:378–389. 2015.PubMed/NCBI View Article : Google Scholar
|
|
124
|
Walsh JC, Lebedev A, Aten E, Madsen K,
Marciano L and Kolb HC: The clinical importance of assessing tumor
hypoxia: Relationship of tumor hypoxia to prognosis and therapeutic
opportunities. Antioxidants Redox Signal. 21:1516–1554.
2014.PubMed/NCBI View Article : Google Scholar
|
|
125
|
Cerychova R and Pavlinkova G: HIF-1,
metabolism, and diabetes in the embryonic and adult heart. Front
Endocrinol (Lausanne). 9(460)2018.PubMed/NCBI View Article : Google Scholar
|
|
126
|
Glaus Garzon JF, Pastrello C, Jurisica I,
Hottiger MO, Wenger RH and Borsig L: Tumor cell endogenous HIF-1α
activity induces aberrant angiogenesis and interacts with TRAF6
pathway required for colorectal cancer development. Neoplasia.
22:745–758. 2020.PubMed/NCBI View Article : Google Scholar
|
|
127
|
Carmeliet P and Jain RK: Principles and
mechanisms of vessel normalization for cancer and other angiogenic
diseases. Nat Rev Drug Discov. 10:417–427. 2011.PubMed/NCBI View Article : Google Scholar
|
|
128
|
Li J, Zhang C, Jiang H and Cheng J:
Andrographolide inhibits hypoxia-inducible factor-1 through
phosphatidylinositol 3-kinase/AKT pathway and suppresses breast
cancer growth. Onco Targets Ther. 8:427–435. 2015.PubMed/NCBI View Article : Google Scholar
|
|
129
|
Kubaichuk K and Kietzmann T: USP10
contributes to colon carcinogenesis via mTOR/S6K mediated HIF-1α
but Not HIF-2α protein synthesis. Cells. 12(1585)2023.PubMed/NCBI View Article : Google Scholar
|
|
130
|
Shi L, Zhang G, Zheng Z, Lu B and Ji L:
Andrographolide reduced VEGFA expression in hepatoma cancer cells
by inactivating HIF-1α: The involvement of JNK and MTA1/HDCA. Chem
Biol Interact. 273:228–236. 2017.PubMed/NCBI View Article : Google Scholar
|
|
131
|
Bose S, Banerjee S, Mondal A, Chakraborty
U, Pumarol J, Croley CR and Bishayee A: Targeting the JAK/STAT
signaling pathway using phytocompounds for cancer prevention and
therapy. Cells. 9(1451)2020.PubMed/NCBI View Article : Google Scholar
|
|
132
|
Firdous P, Nissar K, Sabba A, Hassan T and
Maqbool MT: Application of plasma membrane proteomics to identify
cancer biomarkers. In: Ali S, Majid S and Rehman MU (eds).
Proteomics: A Promising Approach for Cancer Research. Elsevier,
pp287-317, 2023.
|
|
133
|
Hu Q, Bian Q, Rong D, Wang L, Song J,
Huang HS, Zeng J, Mei J and Wang PY: JAK/STAT pathway:
Extracellular signals, diseases, immunity, and therapeutic
regimens. Front Bioeng Biotechnol. 11(1110765)2023.PubMed/NCBI View Article : Google Scholar
|
|
134
|
Huang B, Lang X and Li X: The role of
IL-6/JAK2/STAT3 signaling pathway in cancers. Front Oncol.
12(1023177)2022.PubMed/NCBI View Article : Google Scholar
|
|
135
|
Bao GQ, Shen BY, Pan CP, Zhang YJ, Shi MM
and Peng CH: Andrographolide causes apoptosis via inactivation of
STAT3 and Akt and potentiates antitumor activity of gemcitabine in
pancreatic cancer. Toxicol Lett. 222:23–35. 2013.PubMed/NCBI View Article : Google Scholar
|
|
136
|
Wang XR, Jiang ZB, Xu C, Meng WY, Liu P,
Zhang YZ, Xie C, Xu JY, Xie YJ, Liang TL, et al: Andrographolide
suppresses non-small-cell lung cancer progression through induction
of autophagy and antitumor immune response. Pharmacol Res.
179(106198)2022.PubMed/NCBI View Article : Google Scholar
|
|
137
|
Zhou J, Ong CN, Hur GM and Shen HM:
Inhibition of the JAK-STAT3 pathway by andrographolide enhances
chemosensitivity of cancer cells to doxorubicin. Biochem Pharmacol.
79:1242–1250. 2010.PubMed/NCBI View Article : Google Scholar
|
|
138
|
Chen SR, Li F, Ding MY, Wang D, Zhao Q and
Wang Y, Zhou GC and Wang Y: Andrographolide derivative as STAT3
inhibitor that protects acute liver damage in mice. Bioorg Med
Chem. 26:5053–5061. 2018.PubMed/NCBI View Article : Google Scholar
|
|
139
|
Bousoik E and Montazeri Aliabadi H: ‘Do we
know Jack’ About JAK? A closer look at JAK/STAT signaling pathway.
Front Oncol. 8(287)2018.PubMed/NCBI View Article : Google Scholar
|
|
140
|
Lemmon MA and Schlessinger J: Cell
signaling by receptor tyrosine kinases. Cell. 141:1117–1134.
2010.PubMed/NCBI View Article : Google Scholar
|
|
141
|
Su YC, Lee WC, Wang CC, Yeh SA, Chen WH
and Chen PJ: Targeting PI3K/AKT/mTOR signaling pathway as a
radiosensitization in head and neck squamous cell carcinomas. Int J
Mol Sci. 23(15749)2022.PubMed/NCBI View Article : Google Scholar
|
|
142
|
Tohkayomatee R, Reabroi S, Tungmunnithum
D, Parichatikanond W and Pinthong D: Andrographolide exhibits
anticancer activity against breast cancer cells (MCF-7 and
MDA-MB-231 cells) through suppressing cell proliferation and
inducing cell apoptosis via inactivation of ER-α receptor and
PI3K/AKT/mTOR signaling. Molecules. 27(3544)2022.PubMed/NCBI View Article : Google Scholar
|
|
143
|
Weerackoon N, Gunawardhana KL and Mani A:
Wnt signaling cascades and their role in coronary artery health and
disease. J Cell Signal. 2:52–62. 2021.PubMed/NCBI View Article : Google Scholar
|
|
144
|
Reyes M, Flores T, Betancur D,
Peña-Oyarzún D and Torres VA: Wnt/β-catenin signaling in oral
carcinogenesis. Int J Mol Sci. 21(4682)2020.PubMed/NCBI View Article : Google Scholar
|
|
145
|
MacDonald BT, Tamai K and He X:
Wnt/beta-catenin signaling: components, mechanisms, and diseases.
Dev Cell. 17:9–26. 2009.PubMed/NCBI View Article : Google Scholar
|
|
146
|
MacDonald BT and He X: Frizzled and LRP5/6
receptors for Wnt/β-catenin signaling. Cold Spring Harb Perspect
Biol. 4(a007880)2012.PubMed/NCBI View Article : Google Scholar
|
|
147
|
Steinhart Z and Angers S: Wnt signaling in
development and tissue homeostasis. Development.
145(dev146589)2018.PubMed/NCBI View Article : Google Scholar
|
|
148
|
Chatterjee A, Paul S, Bisht B,
Bhattacharya S, Sivasubramaniam S and Paul MK: Advances in
targeting the WNT/β-catenin signaling pathway in cancer. Drug
Discov Today. 27:82–101. 2022.PubMed/NCBI View Article : Google Scholar
|
|
149
|
Jackstadt R, Hodder MC and Sansom OJ: WNT
and β-catenin in cancer: Genes and therapy. Annu Rev Cancer Biol.
4:177–196. 2020.
|
|
150
|
Liu J, Xiao Q, Xiao J, Niu C, Li Y, Zhang
X, Zhou Z, Shu G and Yin G: Wnt/β-catenin signalling: Function,
biological mechanisms, and therapeutic opportunities. Signal
Transduct Target Ther. 7(3)2022.PubMed/NCBI View Article : Google Scholar
|
|
151
|
Cruciat CM and Niehrs C: Secreted and
transmembrane Wnt inhibitors and activators. Cold Spring Harb
Perspect Biol. 5(a015081)2013.PubMed/NCBI View Article : Google Scholar
|
|
152
|
Skronska-Wasek W, Mutze K, Baarsma HA,
Bracke KR, Alsafadi HN, Lehmann M, Costa R, Stornaiuolo M,
Novellino E, Brusselle GG, et al: Reduced frizzled receptor 4
expression prevents WNT/β-catenin-driven alveolar lung repair in
chronic obstructive pulmonary disease. Am J Respir Crit Care Med.
196:172–185. 2017.PubMed/NCBI View Article : Google Scholar
|
|
153
|
Sharma P, Shimura T, Banwait JK and Goel
A: Andrographis-mediated chemosensitization through activation of
ferroptosis and suppression of β-catenin/Wnt-signaling pathways in
colorectal cancer. Carcinogenesis. 41:1385–1394. 2020.PubMed/NCBI View Article : Google Scholar
|
|
154
|
Rubinfeld H and Seger R: The ERK cascade:
A prototype of MAPK signaling. Mol Biotechnol. 31:151–174.
2005.PubMed/NCBI View Article : Google Scholar
|
|
155
|
Karnoub AE and Weinberg RA: Ras oncogenes:
Split personalities. Nat Rev Mol Cell Biol. 9:517–531.
2008.PubMed/NCBI View Article : Google Scholar
|
|
156
|
Zlobin A, Bloodworth JC and Osipo C:
Mitogen-activated protein kinase (MAPK) signaling. In: Badve S,
Kumar G (eds). Predictive Biomarkers in Oncology. Springer, Cham,
pp213-221, 2019.
|
|
157
|
Guo YJ, Pan WW, Liu SB, Shen ZF, Xu Y and
Hu LL: ERK/MAPK signalling pathway and tumorigenesis. Exp Ther Med.
19:1997–2007. 2020.PubMed/NCBI View Article : Google Scholar
|
|
158
|
Dhillon AS, Hagan S, Rath O and Kolch W:
MAP kinase signalling pathways in cancer. Oncogene. 26:3279–3290.
2007.PubMed/NCBI View Article : Google Scholar
|
|
159
|
Ballestín A, Armocida D, Ribecco V and
Seano G: Peritumoral brain zone in glioblastoma: Biological,
clinical and mechanical features. Front Immunol.
15(1347877)2024.PubMed/NCBI View Article : Google Scholar
|
|
160
|
Yang SL, Kuo FH, Chen PN, Hsieh YH, Yu NY,
Yang WE, Hsieh MJ and Yang SF: Andrographolide suppresses the
migratory ability of human glioblastoma multiforme cells by
targeting ERK1/2-mediated matrix metalloproteinase-2 expression.
Oncotarget. 8:105860–105872. 2017.PubMed/NCBI View Article : Google Scholar
|
|
161
|
Zhang C, Yang C, Feldman MJ, Wang H, Pang
Y, Maggio DM, Zhu D, Nesvick CL, Dmitriev P, Bullova P, et al:
Vorinostat suppresses hypoxia signaling by modulating nuclear
translocation of hypoxia inducible factor 1 alpha. Oncotarget.
8:56110–56125. 2017.PubMed/NCBI View Article : Google Scholar
|