Hepatocellular carcinoma (HCC) is the third most
common cause of cancer-related mortality measured in a total of 185
countries in 2020, following only colorectal cancer among digestive
system tumors, and its fatality rate is increasing (1,2). A
global review suggested that there are hundreds of thousands of new
HCC cases annually, which demonstrates a progressive rise in the
prevalence of this disease (3,4).
Numerous factors can contribute to the development of HCC. Viral
hepatitis B and C infection, alcohol abuse, non-alcoholic fatty
liver disease and other metabolic liver disorders may induce
mutations in hepatocytes, thereby precipitating the onset of liver
cancer (5). Due to the
asymptomatic or insidious onset of HCC, detection is often
challenging, with the majority of patients being diagnosed at
advanced stages of the disease (6). Currently, the treatment options for
HCC are relatively limited, mainly including surgical resection,
liver transplantation, radiofrequency ablation and arterial
embolization (7-9).
However, these treatment modalities each come with their own set of
limitations, and due to the varying physical conditions of
patients, there are also constraints on the selection of
appropriate therapies (10).
Therefore, targeted biological therapies for HCC have increasingly
garnered attention from researchers (11). As a multi-kinase inhibitor,
sorafenib is the first oral medication approved for the treatment
of unresectable HCC (12,13). Previous studies have reported that
sorafenib exerts inhibitory effects on the proliferation and
migration of tumor cells (14,15).
Additionally, it regulates vascular endothelial growth factors,
suppresses tumor angiogenesis and promotes apoptosis in cancer
cells by inhibiting related signaling pathways (16-18).
However, sorafenib is associated with serious side effects and
comes with a high cost, which limits its scope of application
(19,20). Lenvatinib exerts its therapeutic
effects on HCC by targeting multiple kinase receptors, such as
VEGFR1-3, fibroblast growth factor receptor 1-4 and c-KIT, albeit
with the limitation of potential resistance development due to
various mechanisms, which include tumor microenvironment
alterations and drug transport activation (21,22).
Combination therapy with atezolizumab and bevacizumab is associated
with limited improvements in survival and may lead to adverse
reactions, including hypertension, cardiac dysfunction and thyroid
function changes (23,24). Therefore, it is necessary to
develop a more efficacious and targeted drug for the treatment of
HCC.
The protein encoded by the human
apurinic/apyrimidinic endonuclease 1 (APE1) gene is also referred
to as APEX1, APE, APE1, APEX, APX, HAP1 and REF1. The APE1 protein
possesses two distinct domains [DNA repair and reduction-oxidation
(redox)], serving a crucial role in the DNA base excision repair
(BER) pathway, which is closely associated with tumor cell
proliferation (25). Additionally,
APE1 is critically involved in the repair of DNA damage induced by
a wide array of carcinogens, encompassing both those generated
internally through cellular metabolism and externally through
environmental exposure, thereby safeguarding the integrity of the
genetic material and mitigating the risk of carcinogenesis
(25,26). In the tumor microenvironment, APE1
may serve a role in inflammation and stromal cells, affecting tumor
development and therapy efficacy due to its key role in regulating
oxidative stress responses and inflammatory processes (27,28).
Furthermore, APE1 is involved in the regulation of multiple
transcription factors associated with cancer-related signaling
pathways, including p53, NF-κB, activator protein 1 (AP-1), paired
box (Pax)-5, Pax-8, hypoxia-inducible factor 1 (HIF-1), cAMP
response element binding protein, activating transcription factor
and HIF-1α. Previous studies have reported that, following the
malignant transformation of tissues, the expression levels of APE1
are elevated in lung cancer (29),
prostate cancer (30), breast
cancer (31), HCC (32) and pancreatic cancer (33), which suggests that APE1 is a
tumor-associated factor.
In normal hepatocytes, APE1 is expressed at lower
levels and is primarily localized in the nuclei (34). However, in liver cancer cells, the
expression level of APE1 is increased, is ectopically localized and
can also be detected in the cytoplasm (35,36).
Therefore, APE1 has been considered to be an important diagnostic
indicator for hepatocellular carcinogenesis.
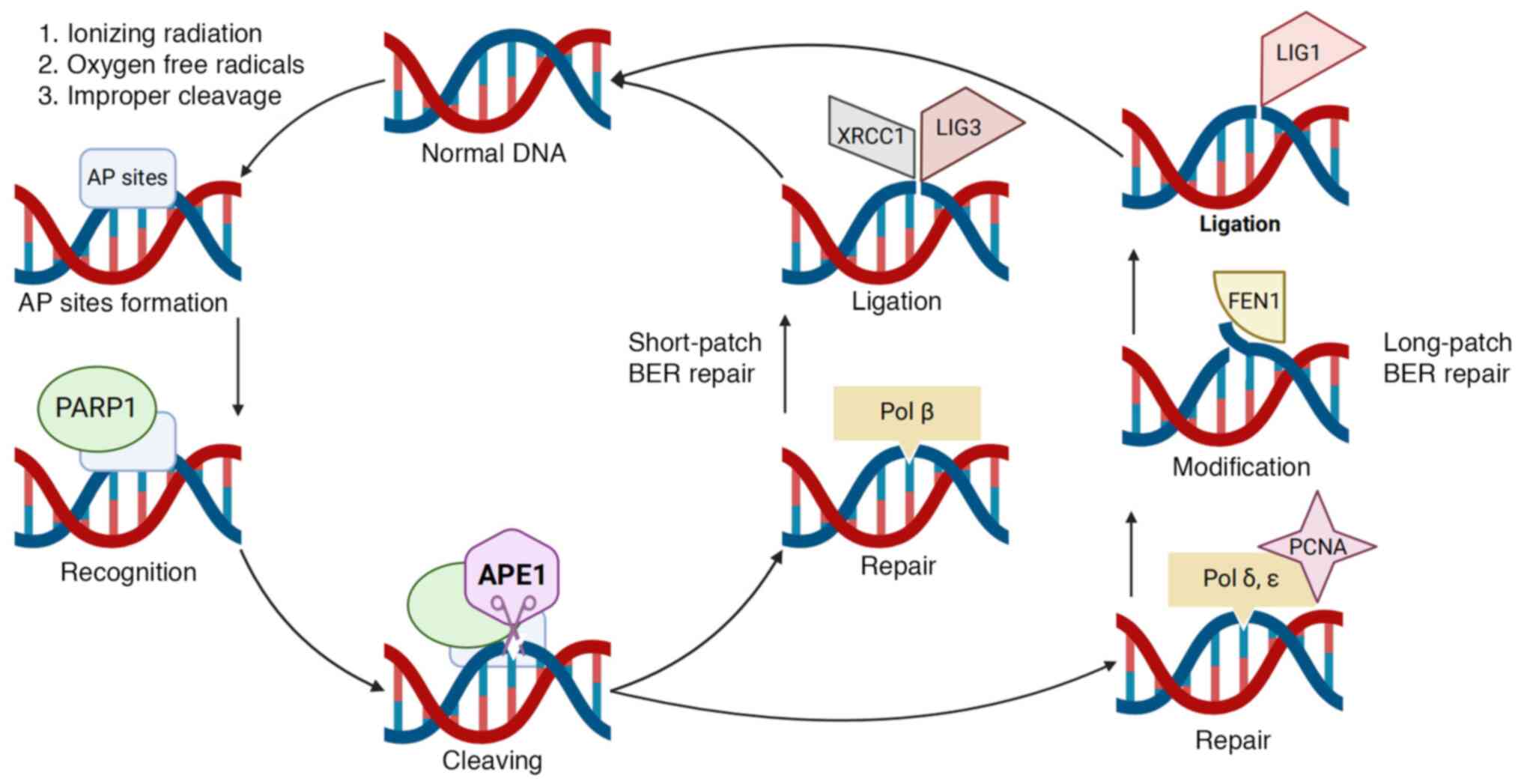
When cells are exposed to ionizing radiation,
assault from oxygen free radicals or improper cleavage by DNA
endonucleases, apurinic/apyrimidinic sites (AP sites) can be
readily formed (37). AP sites, a
common form of DNA damage, can result from exposure to X-ray and
ultraviolet radiation. The absence of a nucleotide base at an AP
site can disrupt the action of DNA/RNA polymerases during
transcription and synthesis, which leads to interruptions in
nucleotide substitution and insertion. Furthermore, the chemical
reactivity of AP sites can cause the breakage of cross-links
between DNA molecules, as well as DNA-protein and DNA-DNA
cross-links (38,39). These factors contribute to the high
mutagenicity and cytotoxicity within radiation exposed cells.
Consequently, the repair of AP sites is a crucial mechanism for
maintaining genomic stability. The BER pathway is the primary
pathway for repairing DNA damage, including AP sites, and APE1 is a
key rate-limiting enzyme in this pathway (40,41).
During the DNA repair process, APE1 interacts with proteins
involved in the BER pathway, such as poly(ADP-ribose) polymerase
1(42), X-ray repair cross
complementing 1(43), DNA
polymerase β (44), DNA ligase
III, proliferating cell nuclear antigen and flap structure-specific
endonuclease 1, and exerts certain stimulatory effects on these
proteins, allowing it to participate in and regulate BER (37) (Fig.
1).
APE1 is also capable of cleaving AP sites present in
single-stranded RNA molecules (45). Previous studies have reported that
numerous non-coding RNAs and a number of specific microRNAs
(miRNAs) directly bind to APE1 in cancer cells in specific ways
(46,47). Early in vitro evidence and
indirect observations have demonstrated the ability of APE1 to bind
to and cleave RNA, as well as the relationship between the
downregulation of APE1 and miRNA expression (25,47).
Malfatti et al (48)
demonstrated that APE1 can bind to the drosha ribonuclease III
(DROSHA)-processing complex, which is associated with the
regulation of primary miRNAs (pri-miRs) in cervical cancer in
response to oxidative stress. The deletion of APE1 leads to
increased oxidation of pri-miR-221/222 and enhances its interaction
with DROSHA. The endoribonuclease activity of APE1 towards
pri-miR-221/222 affects PTEN expression and is directly related to
cancer progression (48).
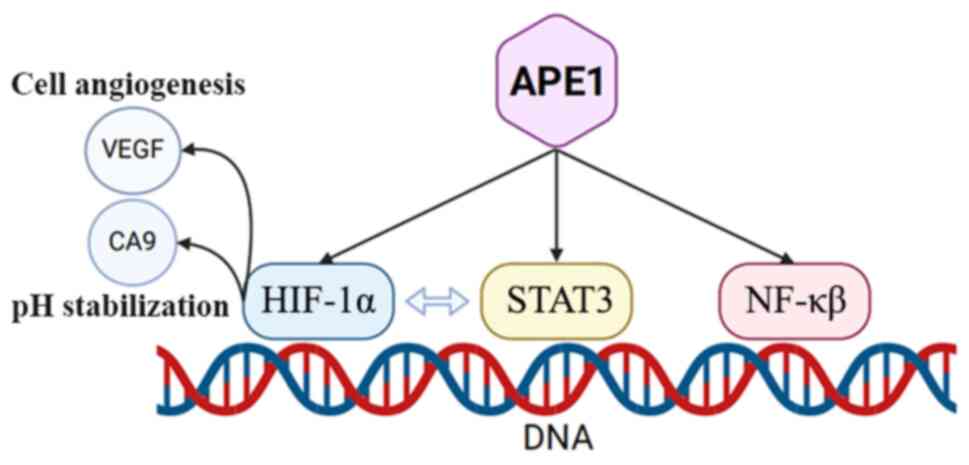
APE1 can also act as a redox factor, stimulating the
binding of multiple redox factors, including AP-1, NF-κB, HIF-1α
and p53, to DNA, thereby participating in processes such as cell
proliferation, migration and inflammatory reactions (49,50).
For instance, APE1 reduces the binding of the transcription factor
Oct1 to the lectin-like oxidized low-density lipoprotein receptor-1
(LOX1) promoter, which leads to the downregulation of LOX1.
Consequently, this suppression inhibits the activation of
macrophages and the formation of foam cells induced by oxidized
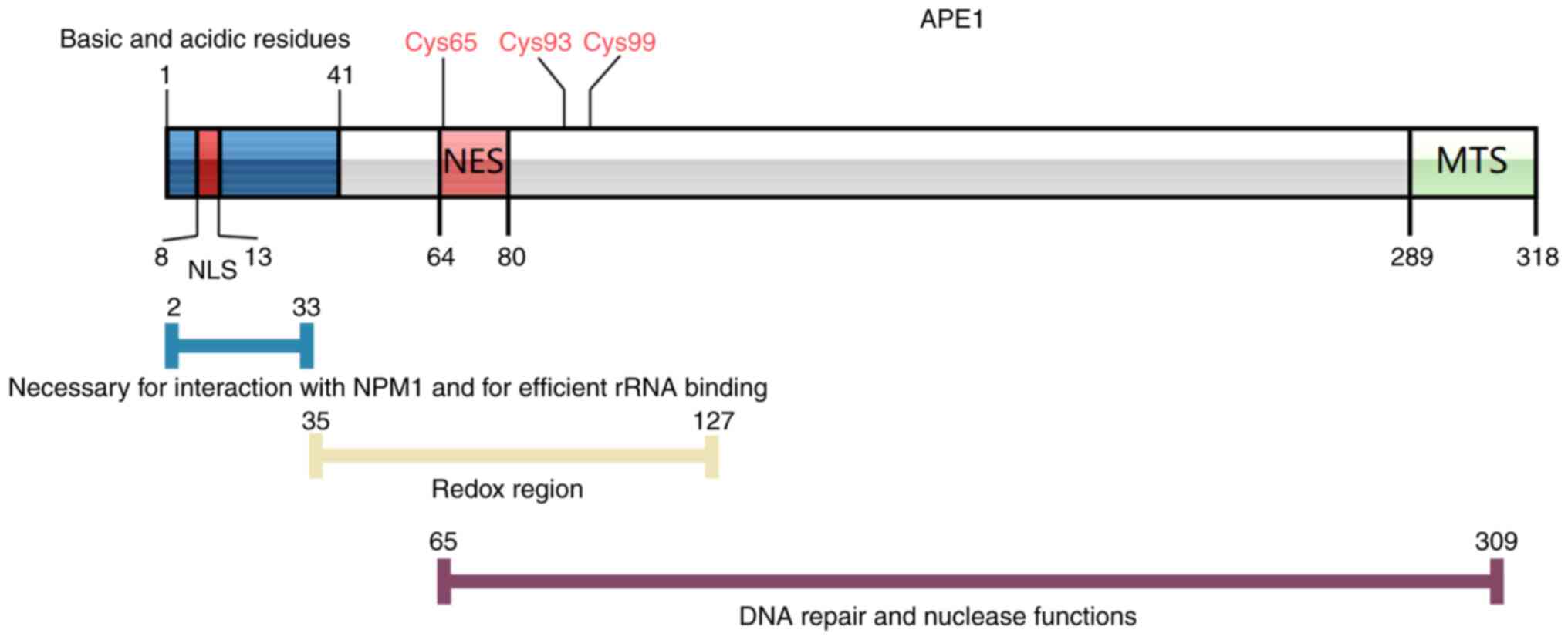
low-density lipoprotein in THP-1 cells (51). Of the seven cysteine residues
present in the APE1 protein (Cys65, Cys93, Cys99, Cys138, Cys208,
Cys296 and Cys310), Cys65, Cys93 and Cys99 are associated with the
redox activity of APE1 (52,53)
(Fig. 2). Furthermore, since only
Cys65 is present in mammalian cells, mutations in the structure of
Cys65 may affect the redox activity of APE1, whereas its DNA repair
function remains unaffected.
Research into the functions of APE1 initially
focused on its repair activity as an endonuclease for DNA damage
(38,48,54).
However, as research progresses, an increasing number of studies
have indicated that APE1 not only functions as a DNA repair enzyme
but also acts as a redox protein to regulate the activation of
various transcription factors, and it is increasingly recognized as
a key factor in multiple signaling pathways (25,55)
(Fig. 3). The APE1 pathway is a
signaling pathway that is highly conserved from prokaryotes to
humans, whereas its redox regulation of other signaling pathways is
unique to mammals (56). APE1
inhibits adipocyte differentiation by suppressing the expression of
transcription factors such as CCAAT-enhancer binding protein α,
peroxisome proliferator-activated receptor γ and adaptor protein
complex 2 in 3T3-L1 cells (57).
APE1 has been reported to be associated with a large number of
signaling pathways, including the STAT3, HIF-1α, NF-κB, AP-1 and
P53 signaling pathways, which are typically closely related to the
occurrence and development of tumor cells (26,33,58,59).
APE1 has increased activity levels in pediatric acute lymphoblastic
leukemia when compared with normal cells, and testing on patient
samples has shown that signaling pathways associated with APE1,
such as the NF-κB signaling pathway, are activated under conditions
of oxidative and DNA damage (60,61).
E3330 is an inhibitor that specifically targets the redox activity
of APE1 and has been demonstrated in cell experiments to suppress
the survival of lymphoma cells by interfering with the redox
activity of APE1, downregulating the expression levels of the
downstream target genes of APE1 and promoting lymphoma cell
apoptosis (60,61). However, in vivo experiments
using xenogeneic cell transplantation in nude mice have shown that
the growth rate of tumor cells in E3330-treated mice is decreased,
the expression of APE1-associated downstream target genes is
downregulated (62) and E3330 has
an inhibitory effect on tumors (63,64).
STAT3 serves a vital role in both normal and
cancerous cells. During the processes of cell proliferation and
survival, STAT3 can be phosphorylated by Janus kinase to form
dimers, which are then translocated into the nucleus to regulate
gene transcription (65-67).
In tumor cells, the STAT3 signaling pathway is often activated, and
APE1 is also active, regulating gene transcription by affecting the
sensitivity of STAT3 to DNA binding sites (68). Studies have shown that inhibition
of the redox activity of APE1 by inhibitors against REF1/APE1 can
reduce the expression levels of downstream target genes of STAT3
and suppress cancer cell proliferation (68,69).
Therefore, the combined use of the STAT3 signaling pathway and APE1
inhibitors to inhibit tumor cell proliferation may represent a
novel direction for clinical cancer treatment.
HIFs are a class of cellular factors produced by
cells in a specific hypoxic environment, serving an essential role
in various tissues and organs, and are abnormally expressed during
the process of cellular carcinogenesis. Research has shown that
HIF-1 is closely associated with the APE1 signaling pathway
(33,70). The redox function of APE1 can
promote the activation of HIF-1. Under hypoxic conditions,
inhibiting the activity of APE1 can reduce the expression levels of
downstream genes of HIF-1 and decrease the survival of tumor cells
(33). HIF-1 and STAT3 also
exhibit a synergistic effect against cell carcinogenesis (70-72).
Furthermore, inhibitors of APE1 can be used to simultaneously
suppress the transcription of genes downstream of both signaling
pathways (73).
NF-κB is an intracellular transcription factor
generated in response to both intracellular and extracellular
signals, serving a vital role in various physiological processes of
cells, including proliferation, migration, regeneration and the
immune response (74). Research
has demonstrated that the NF-κB signaling pathway also serves an
important role in tumor formation (74). Once activated, it can lead to
carcinogenesis through a series of changes, including inhibiting
apoptosis or altering the tumor cell microenvironment. During the
cell repair process following DNA damage, the NF-κB signaling
pathway is also activated, promoting the generation of reactive
oxygen species (ROS). Subsequently, ROS cause DNA damage in cells
(75), which further activates the
NF-κB signaling pathway and increases the expression levels of
related anti-apoptotic and pro-proliferation genes, promoting the
malignant transformation of cells (76).
APE1 is essential for the activation of the NF-κB
signaling pathway, and the DNA binding of NF-κB depends on the
redox activity of APE1. Research has demonstrated that inhibition
of APE1 leads to decreased transcription of downstream genes
regulated by NF-κB (62,77), and that APE1 can reduce the
proliferative capacity of tumor cells and promote tumor cell
apoptosis (34,69).
During cell carcinogenesis, APE1 expression in the
nuclei and cytoplasm is elevated, and there are changes to the DNA
repair and redox functions involving APE1(78). Furthermore, high intracellular APE1
expression levels have been associated with poor outcomes of
anticancer treatment, poor response to chemotherapy, low survival
rates and shorter relapse-free intervals (79,80).
Plasma APE1 levels are elevated in patients with colorectal,
kidney, liver and pancreatic cancers and cutaneous squamous cell
carcinoma (cSCC). APE1 is upregulated in human pancreatic cancer
cells, and modulating its redox activity using APE1 inhibitors
blocks the proliferation and migration of cancer cells (62), indicating that the redox activity
of APE1 is closely related to cell proliferation and migration. A
similar phenomenon has been observed in ovarian tumors, where
ovarian tumor cells exhibited increased APE1 expression, and its
knockdown inhibited tumor cell proliferation (81). Additionally, APE1 promotes the
proliferation of cSCC cells. The deletion of APE1 can inhibit the
viability of cSCC cells, while the upregulation of APE1 promotes
cell proliferation (82). This has
also been reported in HCC (62).
Conventionally, the notion persists that APE1 is exclusively
located within the nucleus, but emerging research has demonstrated
that APE1 is expressed in the cytoplasm of both lung tumor and HCC
cells (36,74). In HCC, all signaling pathways
involving APE1 can stimulate cells to enhance their proliferation,
metastasis and anti-apoptotic capabilities (83). Therefore, the downregulation of
APE1 expression in tumor cells is likely to become a treatment for
tumors in the future, and it may be ideal for patients who cannot
tolerate chemotherapy or radiotherapy.
The development and progression of tumors involves
the abnormal expression of multiple related genes (84). APE1 is a transcription factor that
serves a vital role in DNA repair and redox functions, regulating
cancer-related pathways (78,85),
and is closely associated with carcinogenesis and proliferation of
tumor cells. Research has shown that APE1 expression is associated
with the staging and classification, degree of invasion and
recurrence of tumors. Therefore, plasma APE1 expression levels can
be used as biomarkers for the detection of bladder cancer (34). However, to date, there have been
few reports on the relationship between APE1 and HCC. Current
research suggests that, compared with that in non-cancerous
tissues, APE1 expression in HCC tissues is increased, and it serves
a crucial role in the carcinogenesis and progression of HCC
(35). The downregulation of APE1
expression can effectively reduce the proliferation of Hep3B cells
and promote tumor cell apoptosis (34). This suggests that APE1 may be able
to promote tumor growth by inhibiting cell apoptosis. Tumor cells
exhibit an enhanced proliferative capacity and a low apoptosis rate
compared with non-tumor cells. The cytoplasmic localization of APE1
has been implicated in the carcinogenesis of various cancer types,
including ovarian, lung and colorectal cancer, suggesting its
potential as a prognostic marker and therapeutic target (86,87).
Enhanced cytoplasmic APE1 expression, often associated with p53
aberrations, may predict survival and relapse in patients with
cancer, highlighting the importance of the subcellular distribution
of APE1 in tumor progression and aggression (74,88,89).
Therefore, for cancer treatment, inhibiting cell proliferation and
differentiation by promoting tumor cell apoptosis can be an
effective method for tumor suppression.
APE1 serves an important role in the development and
progression of cancer, and its expression is closely associated
with the prognosis of patients (90). In HCC, APE1 expression is higher in
cancerous tissue cells compared with that in para-neoplastic
tissues (32). Elevated APE1
levels dysregulate homologous recombination and the cell cycle,
contributing to chemoresistance (91). Research has indicated that APE1
inhibitors can enhance the efficacy of cisplatin chemotherapy,
photodynamic therapy and radiotherapy (69,92-94).
High APE1 expression is inversely associated with CD4+
naive T cell infiltration, which is a predictor of recurrence-free
survival in patients with non-small cell lung cancer, with improved
survival in patients with high APE1 expression levels (95). High APE1 expression in breast
cancer nuclei is associated with poor disease-free survival, and is
associated with the luminal A subtype and estrogen receptor
positivity, while low APE1 expression in patients with low Ki-67
cases predicts worse overall survival rates (96). Combining APE1/REF1 redox inhibitors
with the standard-of-care chemotherapy drug cisplatin in
vitro more effectively inhibits bladder cancer cell
proliferation when compared with cisplatin alone (69). Therefore a number of studies have
proposed methods to treat HCC by inhibiting the action of APE1. By
administering APE1 inhibitors, the functions of APE1 are inhibited,
which further suppresses tumor cell proliferation and promotes
tumor cell apoptosis (97-100)
(Table I).
Resveratrol is also known as
3,4',5-trihydroxy-trans-stilbene and is a type of natural
polyphenol compound. Experiments have shown that resveratrol
pretreatment enhances human melanoma cell sensitivity to the
chemotherapeutic drug dacarbazine (97,101). However, it has been shown in
vitro that resveratrol effectively reduces the activities of
AP-1 and NF-κB by inhibiting the redox function of APE1, and such
an effect has been observed in a wide range of cancer types
(102). Furthermore, resveratrol
can be used as a selective inhibitor of APE1, laying the foundation
for its clinical application.
Isoflavones are a class of natural compounds that
have an important protective effect against cancer (98,103). It has been reported in
vitro that isoflavones can effectively inhibit tumor cell
proliferation and potentiate cell death by radiation (104). In non-small cell lung cancer,
isoflavones sensitize tumor cells to radiation by inhibiting the
DNA repair function of APE1(105). In addition, in prostate cancer,
soy isoflavones can downregulate NF-κB and HIF-1 simultaneously by
inhibiting APE1, potentiating tumor cell apoptosis, inhibiting
tumor tissue angiogenesis and sensitizing tumor cells to radiation
(102). From this perspective,
isoflavones, as inhibitors of APE1, have an inhibitory effect on
tumor progression. This also suggests that inhibiting APE1 activity
could be a potential effective treatment strategy for cancer.
Tanshinone is a Traditional Chinese Medicine that
can inhibit the redox activity of APE1(99). It can effectively inhibit the
proliferation of human cervical and colon cancer cells.
Furthermore, tanshinone pre-treatment can enhance the sensitivity
of certain tumor cell lines, such as HeLa and HCT116 cells, to
ionizing radiation and chemotherapy drugs. As an inhibitor of APE1,
tanshinone may have a promising future in cancer treatment.
E3330 is also known as
(2E)-3-[5-(2,3-dimethoxy-6-methyl-1,4-benzoquinoyl)]-2-nonyl-2-propenoic
acid and is a compound that can selectively inhibit the redox
activity of APE1 without having any impact on its DNA repair
function (100). Therefore, E3330
has no inhibitory effect on the BER pathway. By increasing
disulfide bond formation involving Cys65 and/or Cys93, E3330
effectively decreases the redox-active population of APE1 molecules
(100,106). A previous study reported that
E3330 can effectively inhibit the proliferation of tumor cells in
ovarian, colon, lung, breast, brain, pancreatic and prostate
cancers and multiple myeloma but does not significantly inhibit the
proliferation of normal cells (107). By inhibiting the activity of
APE1, the activities of some transcriptional regulators, including
NF-κB, activator protein and HIF-1, are blocked, which have marked
effects on the proliferation, invasion and metabolism of tumor
cells, thereby inhibiting tumor progression (62). In addition, E3330 can effectively
inhibit tumor cell proliferation and migration in pancreatic cancer
(64).
A number of studies have reported that the redox
domain of APE1 is indispensable for tumor-associated epithelial
cell differentiation, function and angiogenesis following tumor
cell migration (77,108). In liver cancer, APE1 can
facilitate the development of HCC both in vitro and in
vivo (35). APE1
overexpression and the increase in enzyme activity are related to
the survival and drug resistance of cancer cells (109). Furthermore, the inhibition of
APE1 leads to the accumulation of lipid peroxidation and enhanced
ferroptosis in HCC (110).
Western blotting analysis has demonstrated that diethylnitrosamine
(DEN) treatment enhanced APE1 protein expression (111). The antioxidant effect of
Licochalcone B and fullerene C60 may be the mechanism by which
these compounds reduce the expression of APE1, which is
predominantly activated by oxidative stress (111,112). This has a protective effect
against DEN-induced HCC.
Inhibition of APE1 has the potential to be an
effective treatment approach for tumors. It can be concluded from
the aforementioned findings that APE1 serves a vital role in the
development and progression of tumors. The inhibition of APE1
activity can effectively control tumor cell proliferation and
spread, suggesting that targeting of APE1 may be a novel direction
for the treatment of HCC, especially for patients with poor
response to surgical therapy and chemotherapy.
Not applicable.
Funding: The present study was supported by the National Natural
Science Foundation of China (grant nos. 81930119, 82090050 and
82090053) and the CAMS Innovation Fund for Medical Sciences (grant
no. 2019-I2M-5-056).
Not applicable.
LY and ZS equally contributed to the present
manuscript, the conception and design of the study, literature
review and analysis, and drafting, critical revision and editing of
the manuscript. Data authentication is not applicable. All authors
have read and approved the final version of the manuscript.
Not applicable.
Not applicable.
The authors declare that they have no competing
interests.
|
1
|
Sung H, Ferlay J, Siegel RL, Laversanne M,
Soerjomataram I, Jemal A and Bray F: Global Cancer Statistics 2020:
GLOBOCAN estimates of incidence and mortality worldwide for 36
cancers in 185 countries. CA Cancer J Clin. 71:209–249.
2021.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Singal AG, Kanwal F and Llovet JM: Global
trends in hepatocellular carcinoma epidemiology: Implications for
screening, prevention and therapy. Nat Rev Clin Oncol. 20:864–884.
2023.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Yang JD, Hainaut P, Gores GJ, Amadou A,
Plymoth A and Roberts LR: A global view of hepatocellular
carcinoma: Trends, risk, prevention and management. Nat Rev
Gastroenterol Hepatol. 16:589–604. 2019.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Clark T, Maximin S, Meier J, Pokharel S
and Bhargava P: Hepatocellular carcinoma: Review of epidemiology,
screening, imaging diagnosis, response assessment, and treatment.
Curr Probl Diagn Radiol. 44:479–486. 2015.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Sia D, Villanueva A, Friedman SL and
Llovet JM: Liver cancer cell of origin, molecular class, and
effects on patient prognosis. Gastroenterology. 152:745–761.
2017.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Zhang H, Su X, Burley SK and Zheng XFS:
mTOR regulates aerobic glycolysis through NEAT1 and nuclear
paraspeckle-mediated mechanism in hepatocellular carcinoma.
Theranostics. 12:3518–3533. 2022.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Vogel A, Meyer T, Sapisochin G, Salem R
and Saborowski A: Hepatocellular carcinoma. Lancet. 400:1345–1362.
2022.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Chang Y, Jeong SW, Young Jang J and Jae
Kim Y: Recent updates of transarterial chemoembolilzation in
hepatocellular carcinoma. Int J Mol Sci. 21(8165)2020.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Galle PP, Dufour JF, Peck-Radosavljevic M,
Trojan J and Vogel A: Systemic therapy of advanced hepatocellular
carcinoma. Future Oncol. 17:1237–1251. 2021.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Llovet JM, De Baere T, Kulik L, Haber PK,
Greten TF, Meyer T and Lencioni R: Locoregional therapies in the
era of molecular and immune treatments for hepatocellular
carcinoma. Nat Rev Gastroenterol Hepatol. 18:293–313.
2021.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Llovet JM, Montal R, Sia D and Finn RS:
Molecular therapies and precision medicine for hepatocellular
carcinoma. Nat Rev Clin Oncol. 15:599–616. 2018.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Cheng AL, Kang YK, Chen Z, Tsao CJ, Qin S,
Kim JS, Luo R, Feng J, Ye S, Yang TS, et al: Efficacy and safety of
sorafenib in patients in the Asia-Pacific region with advanced
hepatocellular carcinoma: A phase III randomised, double-blind,
placebo-controlled trial. Lancet Oncol. 10:25–34. 2009.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Llovet JM, Castet F, Heikenwalder M, Maini
MK, Mazzaferro V, Pinato DJ, Pikarsky E, Zhu AX and Finn RS:
Immunotherapies for hepatocellular carcinoma. Nat Rev Clin Oncol.
19:151–172. 2022.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Kong FH, Ye QF, Miao XY, Liu X, Huang SQ,
Xiong L, Wen Y and Zhang ZJ: Current status of sorafenib
nanoparticle delivery systems in the treatment of hepatocellular
carcinoma. Theranostics. 11:5464–5490. 2021.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Ladd AD, Duarte S, Sahin I and Zarrinpar
A: Mechanisms of drug resistance in HCC. Hepatology. 79:926–940.
2024.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Dattachoudhury S, Sharma R, Kumar A and
Jaganathan BG: Sorafenib inhibits proliferation, migration and
invasion of breast cancer cells. Oncology. 98:478–486.
2020.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Tang W, Chen Z, Zhang W, Cheng Y, Zhang B,
Wu F, Wang Q, Wang S, Rong D, Reiter FP, et al: The mechanisms of
sorafenib resistance in hepatocellular carcinoma: Theoretical basis
and therapeutic aspects. Signal Transduct Target Ther.
5(87)2020.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Tian C, Liu Y, Xue L, Zhang D, Zhang X, Su
J, Chen J, Li X, Wang L and Jiao S: Sorafenib inhibits ovarian
cancer cell proliferation and mobility and induces radiosensitivity
by targeting the tumor cell epithelial-mesenchymal transition. Open
Life Sci. 17:616–625. 2022.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Llovet JM, Ricci S, Mazzaferro V, Hilgard
P, Gane E, Blanc JF, de Oliveira AC, Santoro A, Raoul JL, Forner A,
et al: Sorafenib in advanced hepatocellular carcinoma. N Engl J
Med. 359:378–390. 2008.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Gupta N, Verma RK, Prinja S and Dhiman RK:
Cost-effectiveness of sorafenib for treatment of advanced
hepatocellular carcinoma in India. J Clin Exp Hepatol. 9:468–475.
2019.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Zschäbitz S and Grüllich C: Lenvantinib: A
tyrosine kinase inhibitor of VEGFR 1-3, FGFR 1-4, PDGFRα, KIT and
RET. Recent Results Cancer Res. 211:187–198. 2018.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Bo W and Chen Y: Lenvatinib resistance
mechanism and potential ways to conquer. Front Pharmacol.
14(1153991)2023.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Liu X, Lu Y and Qin S: Atezolizumab and
bevacizumab for hepatocellular carcinoma: Mechanism,
pharmacokinetics and future treatment strategies. Future Oncol.
17:2243–2256. 2021.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Gao X, Zhao R, Ma H and Zuo S: Efficacy
and safety of atezolizumab plus bevacizumab treatment for advanced
hepatocellular carcinoma in the real world: A single-arm
meta-analysis. BMC Cancer. 23(635)2023.PubMed/NCBI View Article : Google Scholar
|
|
25
|
López DJ, Rodríguez JA and Bañuelos S:
Molecular mechanisms regulating the DNA repair protein APE1: A
focus on its flexible N-terminal tail domain. Int J Mol Sci.
22(6308)2021.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Caston RA, Gampala S, Armstrong L,
Messmann RA, Fishel ML and Kelley MR: The multifunctional APE1 DNA
repair-redox signaling protein as a drug target in human disease.
Drug Discov Today. 26:218–228. 2021.PubMed/NCBI View Article : Google Scholar
|
|
27
|
He H, Liu X, Wu Y, Qi L, Huang J, Zhou Y,
Zeng J, Wang K and He X: DNA nanotechnology-empowered fluorescence
imaging of APE1 Activity. Chemistry. 5:1815–1831. 2023.
|
|
28
|
An SY, Jin SA, Seo HJ, Lee YR, Kim S, Jeon
BH and Jeong JO: Protective effect of secretory APE1/Ref-1 on
doxorubicin-induced cardiotoxicity via suppression of ROS and p53
pathway. ESC Heart Fail. 11:1182–1193. 2024.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Zhang S, He L, Dai N, Guan W, Shan J, Yang
X, Zhong Z, Qing Y, Jin F, Chen C, et al: Serum APE1 as a
predictive marker for platinum-based chemotherapy of non-small cell
lung cancer patients. Oncotarget. 7:77482–77494. 2016.PubMed/NCBI View Article : Google Scholar
|
|
30
|
McIlwain DW, Fishel ML, Boos A, Kelley MR
and Jerde TJ: APE1/Ref-1 redox-specific inhibition decreases
survivin protein levels and induces cell cycle arrest in prostate
cancer cells. Oncotarget. 9:10962–10977. 2018.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Lee YR, Park MS, Joo HK, Kim KM, Kim J,
Jeon BH and Choi S: Therapeutic positioning of secretory acetylated
APE1/Ref-1 requirement for suppression of tumor growth in
triple-negative breast cancer in vivo. Sci Rep.
8(8701)2018.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Di Maso V, Mediavilla MG, Vascotto C, Lupo
F, Baccarani U, Avellini C, Tell G, Tiribelli C and Crocè LS:
Transcriptional Up-Regulation of APE1/Ref-1 in hepatic tumor: Role
in hepatocytes resistance to oxidative stress and apoptosis. PLoS
One. 10(e0143289)2015.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Logsdon DP, Grimard M, Luo M, Shahda S,
Jiang Y, Tong Y, Yu Z, Zyromski N, Schipani E, Carta F, et al:
Regulation of HIF1α under Hypoxia by APE1/Ref-1 Impacts CA9
expression: Dual targeting in patient-derived 3D pancreatic cancer
models. Mol Cancer Ther. 15:2722–2732. 2016.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Sun Z, Zhu Y, Aminbuhe Fan Q, Peng J and
Zhang N: Differential expression of APE1 in hepatocellular
carcinoma and the effects on proliferation and apoptosis of cancer
cells. Biosci Trends. 12:456–462. 2018.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Lu X, Zhao H, Yuan H, Chu Y and Zhu X:
High nuclear expression of APE1 correlates with unfavorable
prognosis and promotes tumor growth in hepatocellular carcinoma. J
Mol Histol. 52:219–231. 2021.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Di Maso V, Avellini C, Crocè LS, Rosso N,
Quadrifoglio F, Cesaratto L, Codarin E, Bedogni G, Beltrami CA,
Tell G and Tiribelli C: Subcellular localization of APE1/Ref-1 in
human hepatocellular carcinoma: Possible prognostic significance.
Mol Med. 13:89–96. 2007.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Hegde ML, Hazra TK and Mitra S: Early
steps in the DNA base excision/single-strand interruption repair
pathway in mammalian cells. Cell Res. 18:27–47. 2008.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Demple B, Herman T and Chen DS: Cloning
and expression of APE, the cDNA encoding the major human apurinic
endonuclease: Definition of a family of DNA repair enzymes. Proc
Natl Acad Sci U S A. 88:11450–11454. 1991.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Kciuk M, Marciniak B, Mojzych M and Kontek
R: Focus on UV-Induced DNA damage and repair-disease relevance and
protective strategies. Int J Mol Sci. 21(7264)2020.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Krokan HE and Bjørås M: Base excision
repair. Cold Spring Harb Perspect Biol. 5(a012583)2013.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Hindi NN, Elsakrmy N and Ramotar D: The
base excision repair process: Comparison between higher and lower
eukaryotes. Cell Mol Life Sci. 78:7943–7965. 2021.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Khodyreva SN, Prasad R, Ilina ES,
Sukhanova MV, Kutuzov MM, Liu Y, Hou EW, Wilson SH and Lavrik OI:
Apurinic/apyrimidinic (AP) site recognition by the 5'-dRP/AP lyase
in poly(ADP-ribose) polymerase-1 (PARP-1). Proc Natl Acad Sci USA.
107:22090–22095. 2010.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Vidal AE, Boiteux S, Hickson ID and
Radicella JP: XRCC1 coordinates the initial and late stages of DNA
abasic site repair through protein–protein interactions. EMBO J.
20(6530-6539-6539)2001.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Bennett RA, Wilson DM III, Wong D and
Demple B: Interaction of human apurinic endonuclease and DNA
polymerase beta in the base excision repair pathway. Proc Natl Acad
Sci USA. 94:7166–7169. 1997.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Antoniali G, Serra F, Lirussi L, Tanaka M,
D'Ambrosio C, Zhang S, Radovic S, Dalla E, Ciani Y, Scaloni A, et
al: Mammalian APE1 controls miRNA processing and its interactome is
linked to cancer RNA metabolism. Nat Commun. 8(797)2017.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Berquist BR, McNeill DR and Wilson DM III:
Characterization of abasic endonuclease activity of human Ape1 on
alternative substrates, as well as effects of ATP and sequence
context on AP site incision. J Mol Biol. 379:17–27. 2008.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Antoniali G, Dalla E, Mangiapane G, Zhao
X, Jing X, Cheng Y, De Sanctis V, Ayyildiz D, Piazza S, Li M and
Tell G: APE1 controls DICER1 expression in NSCLC through miR-33a
and miR-130b. Cell Mol Life Sci. 79(446)2022.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Malfatti MC, Antoniali G, Codrich M and
Tell G: Coping with RNA damage with a focus on APE1, a BER enzyme
at the crossroad between DNA damage repair and RNA
processing/decay. DNA Repair (Amst). 104(103133)2021.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Kladova OA, Bazlekowa-Karaban M, Baconnais
S, Piétrement O, Ishchenko AA, Matkarimov BT, Iakovlev DA, Vasenko
A, Fedorova OS, Le Cam E, et al: The role of the N-terminal domain
of human apurinic/apyrimidinic endonuclease 1, APE1, in DNA
glycosylase stimulation. DNA Repair (Amst). 64:10–25.
2018.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Oliveira TT, Coutinho LG, de Oliveira LOA,
Timoteo ARS, Farias GC and Agnez-Lima LF: APE1/Ref-1 role in
inflammation and immune response. Front Immunol.
13(793096)2022.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Hu Z, Hui B, Hou X, Liu R, Sukhanov S and
Liu X: APE1 inhibits foam cell formation from macrophages via LOX1
suppression. Am J Transl Res. 12:6559–6568. 2020.PubMed/NCBI
|
|
52
|
Luo M, Zhang J, He H, Su D, Chen Q, Gross
ML, Kelley MR and Georgiadis MM: Characterization of the Redox
activity and disulfide bond formation in apurinic/apyrimidinic
endonuclease. Biochemistry. 51:695–705. 2012.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Pekhale K, Haval G, Perween N, Antoniali
G, Tell G and Ghaskadbi S and Ghaskadbi S: DNA repair enzyme APE1
from evolutionarily ancient Hydra reveals redox activity
exclusively found in mammalian APE1. DNA Repair (Amst). 59:44–56.
2017.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Kelley MR, Logsdon D and Fishel ML:
Targeting DNA repair pathways for cancer treatment: What's new?
Future Oncol. 10:1215–1237. 2014.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Kelley MR, Georgiadis MM and Fishel ML:
APE1/Ref-1 role in redox signaling: Translational applications of
targeting the redox function of the DNA repair/redox protein
APE1/Ref-1. Curr Mol Pharmacol. 5:36–53. 2012.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Georgiadis MM, Luo M, Gaur RK, Delaplane
S, Li X and Kelley MR: Evolution of the redox function in mammalian
Apurinic/apyrimidinic endonuclease. Mutat Res. 643:54–63.
2008.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Lee EO, Joo HK, Lee YR, Kim S, Lee KH, Lee
SD and Jeon BH: APE1/Ref-1 inhibits adipogenic transcription
factors during adipocyte differentiation in 3T3-L1 cells. Int J Mol
Sci. 24(3251)2023.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Shah F, Logsdon D, Messmann RA,
Fehrenbacher JC, Fishel ML and Kelley MR: Exploiting the Ref-1-APE1
node in cancer signaling and other diseases: From bench to clinic.
NPJ Precis Oncol. 1(19)2017.PubMed/NCBI View Article : Google Scholar
|
|
59
|
Garcia-Bailo B, El-Sohemy A, Haddad PS,
Arora P, Benzaied F, Karmali M and Badawi A: Vitamins D, C, and E
in the prevention of type 2 diabetes mellitus: Modulation of
inflammation and oxidative stress. Biologics. 5:7–19.
2011.PubMed/NCBI View Article : Google Scholar
|
|
60
|
Biswas A, Khanna S, Roy S, Pan X, Sen CK
and Gordillo GM: Endothelial cell tumor growth is Ape/ref-1
dependent. Am J Physiol Cell Physiol. 309:C296–C307.
2015.PubMed/NCBI View Article : Google Scholar
|
|
61
|
Ding J, Fishel ML, Reed AM, McAdams E,
Czader MB, Cardoso AA and Kelley MR: Ref-1/APE1 as a
transcriptional regulator and novel therapeutic target in pediatric
T-cell Leukemia. Mol Cancer Ther. 16:1401–1411. 2017.PubMed/NCBI View Article : Google Scholar
|
|
62
|
Fishel ML, Jiang Y, Rajeshkumar NV,
Scandura G, Sinn AL, He Y, Shen C, Jones DR, Pollok KE, Ivan M, et
al: Impact of APE1/Ref-1 redox inhibition on pancreatic tumor
growth. Mol Cancer Ther. 10:1698–1708. 2011.PubMed/NCBI View Article : Google Scholar
|
|
63
|
Vasko MR, Guo C, Thompson EL and Kelley
MR: The repair function of the multifunctional DNA repair/redox
protein APE1 is neuroprotective after ionizing radiation. DNA
Repair (Amst). 10:942–952. 2011.PubMed/NCBI View Article : Google Scholar
|
|
64
|
Zou GM and Maitra A: Small-molecule
inhibitor of the AP endonuclease 1/REF-1 E3330 inhibits pancreatic
cancer cell growth and migration. Mol Cancer Ther. 7:2012–2021.
2008.PubMed/NCBI View Article : Google Scholar
|
|
65
|
Huynh J, Chand A, Gough D and Ernst M:
Therapeutically exploiting STAT3 activity in cancer-using tissue
repair as a road map. Nat Rev Cancer. 19:82–96. 2019.PubMed/NCBI View Article : Google Scholar
|
|
66
|
Hu X, li J, Fu M, Zhao X and Wang W: The
JAK/STAT signaling pathway: From bench to clinic. Signal Transduct
Target Ther. 6(402)2021.PubMed/NCBI View Article : Google Scholar
|
|
67
|
Seif F, Khoshmirsafa M, Aazami H,
Mohsenzadegan M, Sedighi G and Bahar M: The role of JAK-STAT
signaling pathway and its regulators in the fate of T helper cells.
Cell Commun Signal. 15(23)2017.PubMed/NCBI View Article : Google Scholar
|
|
68
|
Cardoso AA, Jiang Y, Luo M, Reed AM,
Shahda S, He Y, Maitra A, Kelley MR and Fishel ML: APE1/Ref-1
regulates STAT3 transcriptional activity and APE1/Ref-1-STAT3
dual-targeting effectively inhibits pancreatic cancer cell
survival. PLoS One. 7(e47462)2012.PubMed/NCBI View Article : Google Scholar
|
|
69
|
Fishel ML, Xia H, McGeown J, McIlwain DW,
Elbanna M, Craft AA, Kaimakliotis HZ, Sandusky GE, Zhang C, Pili R,
et al: Antitumor activity and mechanistic characterization of
APE1/Ref-1 inhibitors in bladder cancer. Mol Cancer Ther.
18:1947–1960. 2019.PubMed/NCBI View Article : Google Scholar
|
|
70
|
Pawlus MR, Wang L and Hu CJ: STAT3 and
HIF1α cooperatively activate HIF1 target genes in MDA-MB-231 and
RCC4 cells. Oncogene. 33:1670–1679. 2014.PubMed/NCBI View Article : Google Scholar
|
|
71
|
Dinarello A, Betto RM, Diamante L,
Tesoriere A, Ghirardo R, Cioccarelli C, Meneghetti G, Peron M,
Laquatra C, Tiso N, et al: STAT3 and HIF1α cooperatively mediate
the transcriptional and physiological responses to hypoxia. Cell
Death Discov. 9(226)2023.PubMed/NCBI View Article : Google Scholar
|
|
72
|
Rad E, Dodd K, Thomas L, Upadhyaya M and
Tee A: STAT3 and HIF1α signaling drives oncogenic cellular
phenotypes in malignant peripheral nerve sheath tumors. Mol Cancer
Res. 13:1149–1160. 2015.PubMed/NCBI View Article : Google Scholar
|
|
73
|
Bhakat KK, Mantha AK and Mitra S:
Transcriptional regulatory functions of mammalian AP-endonuclease
(APE1/Ref-1), an essential multifunctional protein. Antioxid Redox
Signal. 11:621–638. 2009.PubMed/NCBI View Article : Google Scholar
|
|
74
|
Wu HH, Cheng YW, Chang JT, Wu TC, Liu WS,
Chen CY and Lee H: Subcellular localization of apurinic
endonuclease 1 promotes lung tumor aggressiveness via NF-kappaB
activation. Oncogene. 29:4330–4340. 2010.PubMed/NCBI View Article : Google Scholar
|
|
75
|
Huang TT, Wuerzberger-Davis SM, Wu ZH and
Miyamoto S: Sequential modification of NEMO/IKKgamma by SUMO-1 and
ubiquitin mediates NF-kappaB activation by genotoxic stress. Cell.
115:565–576. 2003.PubMed/NCBI View Article : Google Scholar
|
|
76
|
Xia L, Tan S, Zhou Y, Lin J, Wang H, Oyang
L, Tian Y, Liu L, Su M, Wang H, et al: Role of the NFκB-signaling
pathway in cancer. Onco Targets Ther. 11:2063–2073. 2018.PubMed/NCBI View Article : Google Scholar
|
|
77
|
Siqueira PB, de Sousa Rodrigues MM, de
Amorim ÍSS, da Silva TG, da Silva Oliveira M, Rodrigues JA, de
Souza da Fonseca A and Mencalha AL: The APE1/REF-1 and the
hallmarks of cancer. Mol Biol Rep. 51(47)2024.PubMed/NCBI View Article : Google Scholar
|
|
78
|
Shin JH, Choi S, Lee YR, Park MS, Na YG,
Irani K, Lee SD, Park JB, Kim JM, Lim JS and Jeon BH: APE1/Ref-1 as
a serological biomarker for the detection of bladder cancer. Cancer
Res Treat. 47:823–833. 2015.PubMed/NCBI View Article : Google Scholar
|
|
79
|
Luo M and Kelley MR: Inhibition of the
human apurinic/apyrimidinic endonuclease (APE1) repair activity and
sensitization of breast cancer cells to DNA alkylating agents with
lucanthone. Anticancer Res. 24:2127–2134. 2004.PubMed/NCBI
|
|
80
|
Long K, Gu L, Li L, Zhang Z, Li E, Zhang
Y, He L, Pan F, Guo Z and Hu Z: Small-molecule inhibition of APE1
induces apoptosis, pyroptosis, and necroptosis in non-small cell
lung cancer. Cell Death Dis. 12(503)2021.PubMed/NCBI View Article : Google Scholar
|
|
81
|
Fishel ML, He Y, Reed AM, Chin-Sinex H,
Hutchins GD, Mendonca MS and Kelley MR: Knockdown of the DNA repair
and redox signaling protein Ape1/Ref-1 blocks ovarian cancer cell
and tumor growth. DNA Repair (Amst). 7:177–186. 2008.PubMed/NCBI View Article : Google Scholar
|
|
82
|
Deng X, Zhen P, Niu X, Dai Y, Wang Y and
Zhou M: APE1 promotes proliferation and migration of cutaneous
squamous cell carcinoma. J Dermatol Sci. 100:67–74. 2020.PubMed/NCBI View Article : Google Scholar
|
|
83
|
Yang Z, Yang S, Misner BJ, Liu-Smith F and
Meyskens FL: The role of APE/Ref-1 signaling pathway in
hepatocellular carcinoma progression. Int J Oncol. 45:1820–1828.
2014.PubMed/NCBI View Article : Google Scholar
|
|
84
|
Singh AK, Kumar R and Pandey AK:
Hepatocellular carcinoma: Causes, mechanism of progression and
biomarkers. Curr Chem Genom Transl Med. 12:9–26. 2018.PubMed/NCBI View Article : Google Scholar
|
|
85
|
Tell G, Quadrifoglio F, Tiribelli C and
Kelley MR: The many functions of APE1/Ref-1: Not only a DNA repair
enzyme. Antioxid Redox Signal. 11:601–620. 2009.PubMed/NCBI View Article : Google Scholar
|
|
86
|
Sheng Q, Zhang Y, Wang R, Zhang J, Chen B,
Wang J, Zhang W and Xin X: Prognostic significance of APE1
cytoplasmic localization in human epithelial ovarian cancer. Med
Oncol. 29:1265–1271. 2012.PubMed/NCBI View Article : Google Scholar
|
|
87
|
Bazzani V, Barchiesi A, Radecka D,
Pravisani R, Guadagno A, Di Loreto C, Baccarani U and Vascotto C:
Mitochondrial apurinic/apyrimidinic endonuclease 1 enhances mtDNA
repair contributing to cell proliferation and mitochondrial
integrity in early stages of hepatocellular carcinoma. BMC Cancer.
20(969)2020.PubMed/NCBI View Article : Google Scholar
|
|
88
|
Wu HH, Chu YC, Wang L, Tsai LH, Lee MC,
Chen CY, Shieh SH, Cheng YW and Lee H: Cytoplasmic Ape1 Expression
Elevated by p53 aberration may predict survival and relapse in
resected non-small cell lung cancer. Ann Surg Oncol. 20 (Suppl
3):S336–S347. 2013.PubMed/NCBI View Article : Google Scholar
|
|
89
|
Abbotts R and Madhusudan S: Human AP
endonuclease 1 (APE1): From mechanistic insights to druggable
target in cancer. Cancer Treat Rev. 36:425–435. 2010.PubMed/NCBI View Article : Google Scholar
|
|
90
|
Malfatti MC, Bellina A, Antoniali G and
Tell G: Revisiting two decades of research focused on targeting
APE1 for cancer therapy: The pros and cons. Cells.
12(1895)2023.PubMed/NCBI View Article : Google Scholar
|
|
91
|
Kumar S, Zhao J, Talluri S, Buon L, Mu S,
Potluri LB, Liao C, Shi J, Chakraborty C, Gonzalez GB, et al:
Elevated APE1 dysregulates homologous recombination and cell cycle
driving genomic evolution, tumorigenesis, and chemoresistance in
esophageal adenocarcinoma. Gastroenterology. 165:357–373.
2023.PubMed/NCBI View Article : Google Scholar
|
|
92
|
Wang D, Xiang DB, Yang XQ, Chen LS, Li MX,
Zhong ZY and Zhang YS: APE1 overexpression is associated with
cisplatin resistance in non-small cell lung cancer and targeted
inhibition of APE1 enhances the activity of cisplatin in A549
cells. Lung Cancer. 66:298–304. 2009.PubMed/NCBI View Article : Google Scholar
|
|
93
|
Franchi LP, de Freitas Lima JEB, Piva HL
and Tedesco AC: The redox function of apurinic/apyrimidinic
endonuclease 1 as key modulator in photodynamic therapy. J
Photochem Photobiol B. 211(111992)2020.PubMed/NCBI View Article : Google Scholar
|
|
94
|
Zhou J, Wei Z, Yang C, Jia D, Pan B, Zeng
Y, Sun D and Yu Y: APE1 promotes radiation resistance against
radiation-induced pyroptosis by inhibiting the STING pathway in
lung adenocarcinoma. Transl Oncol. 36(101749)2023.PubMed/NCBI View Article : Google Scholar
|
|
95
|
Li Y, Zhao X, Xiao H, Yang B, Liu J, Rao
W, Dai X, Li M, Dai N, Yang Y and Wang D: APE1 may influence CD4+
naïve T cells on recurrence free survival in early stage NSCLC. BMC
Cancer. 21(233)2021.PubMed/NCBI View Article : Google Scholar
|
|
96
|
Woo J, Park H, Sung SH, Moon BI, Suh H and
Lim W: Prognostic Value of Human Apurinic/Apyrimidinic Endonuclease
1 (APE1) Expression in Breast Cancer. PLoS One.
9(e99528)2014.PubMed/NCBI View Article : Google Scholar
|
|
97
|
Lee SG, Lee DG, Joo YH and Chung N:
Synergistic inhibitory effects of the oxyresveratrol and
dacarbazine combination against melanoma cells. Oncol Lett.
22(667)2021.PubMed/NCBI View Article : Google Scholar
|
|
98
|
Gómez-Zorita S, González-Arceo M,
Fernández-Quintela A, Eseberri I, Trepiana J and Portillo MP:
Scientific evidence supporting the beneficial effects of
isoflavones on human health. Nutrients. 12(3853)2020.PubMed/NCBI View Article : Google Scholar
|
|
99
|
Sui J, Li M, Qian C, Wang S, Cheng Y, Chen
BP and Wang D: Functional analysis of tanshinone IIA that blocks
the redox function of human apurinic/apyrimidinic endonuclease
1/redox factor-1. Drug Des Devel Ther. 8:2147–2160. 2014.PubMed/NCBI View Article : Google Scholar
|
|
100
|
Cesaratto L, Codarin E, Vascotto C,
Leonardi A, Kelley MR, Tiribelli C and Tell G: Specific inhibition
of the redox activity of ape1/ref-1 by e3330 blocks tnf-α-induced
activation of IL-8 production in liver cancer cell lines. PLoS One.
8(e70909)2013.PubMed/NCBI View Article : Google Scholar
|
|
101
|
Kang S, Wang Z, Li B, Gao X, He W, Cao S,
Cai Y and Chen H: Anti-tumor effects of resveratrol on malignant
melanoma is associated with promoter demethylation of RUNX3 gene.
Pharmazie. 74:163–167. 2019.PubMed/NCBI View Article : Google Scholar
|
|
102
|
Laev SS, Salakhutdinov NF and Lavrik OI:
Inhibitors of nuclease and redox activity of apurinic/apyrimidinic
endonuclease 1/redox effector factor 1 (APE1/Ref-1). Bioorg Med
Chem. 25:2531–2544. 2017.PubMed/NCBI View Article : Google Scholar
|
|
103
|
Kim IS: Current perspectives on the
beneficial effects of soybean isoflavones and their metabolites for
humans. Antioxidants (Basel). 10(1064)2021.PubMed/NCBI View Article : Google Scholar
|
|
104
|
Hillman GG: Soy isoflavones protect normal
tissues while enhancing radiation responses. Semin Radiat Oncol.
29:62–71. 2019.PubMed/NCBI View Article : Google Scholar
|
|
105
|
Singh-Gupta V, Joiner MC, Runyan L, Yunker
CK, Sarkar FH, Miller S, Gadgeel SM, Konski AA and Hillman GG: Soy
isoflavones augment radiation effect by inhibiting APE1/Ref-1 DNA
repair activity in non-small cell lung cancer. J Thorac Oncol.
6:688–698. 2011.PubMed/NCBI View Article : Google Scholar
|
|
106
|
Su D, Delaplane S, Luo M, Rempel DL, Vu B,
Kelley MR, Gross ML and Georgiadis MM: Interactions of
apurinic/apyrimidinic endonuclease with a redox inhibitor: Evidence
for an alternate conformation of the enzyme. Biochemistry.
50:82–92. 2011.PubMed/NCBI View Article : Google Scholar
|
|
107
|
Luo M, Delaplane S, Jiang A, Reed A, He Y,
Fishel M, Nyland RL II, Borch RF, Qiao X, Georgiadis MM and Kelley
MR: Role of the multifunctional DNA repair and redox signaling
protein Ape1/Ref-1 in cancer and endothelial cells: Small-molecule
inhibition of the redox function of Ape1. Antioxid Redox Signal.
10:1853–1867. 2008.PubMed/NCBI View Article : Google Scholar
|
|
108
|
Zou GM, Karikari C, Kabe Y, Handa H,
Anders RA and Maitra A: The Ape-1/Ref-1 redox antagonist E3330
inhibits the growth of tumor endothelium and endothelial progenitor
cells: Therapeutic implications in tumor angiogenesis. J Cell
Physiol. 219:209–218. 2009.PubMed/NCBI View Article : Google Scholar
|
|
109
|
Sengupta S, Mantha AK, Mitra S and Bhakat
KK: Human AP endonuclease (APE1/Ref-1) and its acetylation regulate
YB-1-p300 recruitment and RNA polymerase II loading in the
drug-induced activation of multidrug resistance gene MDR1.
Oncogene. 30:482–493. 2011.PubMed/NCBI View Article : Google Scholar
|
|
110
|
Du Y, Zhou Y, Yan X, Pan F, He L, Guo Z
and Hu Z: APE1 inhibition enhances ferroptotic cell death and
contributes to hepatocellular carcinoma therapy. Cell Death Differ.
31:431–446. 2024.PubMed/NCBI View Article : Google Scholar
|
|
111
|
Sadek K, Abouzed T, Nasr S and Shoukry M:
Licochalcone B ameliorates liver cancer via targeting of apoptotic
genes, DNA repair systems, and cell cycle control. Iran J Pharm
Res. 19:372–386. 2020.PubMed/NCBI View Article : Google Scholar
|
|
112
|
Sadek K, Abouzeid T, Nasr S and Shukry M:
Role and potential targeting of hepatic apurinic/apyrimidinic
endonuclease-1 and cyclin-dependent kinase-4 in hepatocellular
carcinoma. Can J Physiol Pharmacol. 96(X)2018.PubMed/NCBI View Article : Google Scholar
|