Introduction
Hemangioblastomas are rare and vascular-rich benign
tumors of the central nervous system that account for 2% of all
intracranial tumors (1,2). Hemangioblastomas frequently occur in
the posterior cranial fossa, most commonly in the cerebellum
(3). Based on the radiological
features examined by magnetic resonance imaging (MRI), most
hemangioblastomas are classified into three categories: i) Large
cysts with reinforcing cystic walls or small nodules, ii) a
combination of solid and cystic features and iii) completely solid
tumors (4,5). Despite the progressive development of
neurosurgical techniques, surgical resection of solid
hemangioblastomas remains a great challenge (6). How to reduce the risk of
intraoperative hemorrhage, postoperative complications and high
mortality remains a concern.
Recent advancements in imaging techniques and
preoperative planning have improved the identification and
characterization of hemangioblastomas, allowing for more precise
surgical interventions (7,8). The integration of intraoperative MRI
and neuronavigation systems has enhanced the surgeon's ability to
achieve complete resection while minimizing damage to surrounding
brain tissue (9,10). Additionally, the use of functional
MRI and tractography has helped in preserving critical neurological
functions by mapping out vital brain structures and their
relationship to the tumor (11).
However, the highly vascular nature of these tumors
continues to pose significant challenges, particularly in managing
intraoperative bleeding (12).
Preoperative embolization has been employed as a strategy to reduce
intraoperative blood loss by decreasing the blood supply to the
tumor (13). This technique,
coupled with advanced anesthetic management and meticulous surgical
techniques, has contributed to improved surgical outcomes (14).
Moreover, understanding the molecular and genetic
underpinnings of hemangioblastomas has opened new avenues for
targeted therapies (15). For
instance, studies have identified mutations in the VHL gene
associated with sporadic hemangioblastomas, suggesting potential
therapeutic targets (16). The VHL
protein plays a crucial role in regulating hypoxia-inducible
factors (HIFs), and disruptions in this pathway can lead to tumor
development (17). Targeted
therapies aimed at this pathway are being explored, offering hope
for non-surgical treatment options (18).
These advancements underscore the importance of a
multidisciplinary approach, combining neurosurgery, neuro-oncology
and genetics, to optimize patient outcomes (19). Collaborative efforts involving
neurosurgeons, radiologists, pathologists and geneticists are
essential in developing personalized treatment plans that address
the unique characteristics of each tumor and patient (20).
Therefore, in the present study, the clinical data
of patients with sporadic cerebellar hemangioblastoma were
collected and retrospectively analyzed, including clinical
characteristics, radiographic features, postoperative recurrence
and prognosis, in order to determine the appropriate surgical
treatment strategy for sporadic cerebellar hemangioblastoma. This
comprehensive analysis aims to provide insight into improving
surgical techniques, reducing complications and enhancing long-term
prognosis for patients with this challenging condition. By
identifying factors associated with favorable outcomes and
potential complications, the study seeks to refine surgical
approaches and inform clinical decision-making, ultimately leading
to better management of sporadic cerebellar hemangioblastomas.
Patients and methods
Patients
The cases of patients with sporadic cerebellar
hemangioblastoma were collected. The study was approved by the
Ethics Committee of the General Hospital of Northern Theater
Command (Shenyang, China; approval no. 2021-135) on August 2nd,
2021. The patient selection criteria were as follows: i) All
patients had been diagnosed with sporadic cerebellar
hemangioblastoma after preoperative computed tomography (CT) and
MRI examinations of the brain, as well as postoperative pathologic
examinations; ii) detailed information about the patients'
hospitalization had been stored and multiple clinical
manifestations had been accurately recorded; iii) the patient had
no previous specific neurological disease or severe neurological
dysfunction.
Imaging examination
All patients underwent preoperative CT and MRI. A
total of 37 patients underwent CT angiography and 10 underwent
concurrent digital subtraction angiography (DSA). The preoperative
MRI data of the brains of the patients were analyzed in detail,
including T1 and T2 scanning sequences and T1 enhancement
sequences. The size, location and texture of the tumor were
measured and evaluated by MRI. Tumor sites were divided into the
left cerebellar hemisphere, right cerebellar hemisphere and
cerebellar vermis.
Tumors were classified as completely solid, a
combination of solid and cystic (<50% of the tumor mass was
cystic) or predominantly cystic (≥50% of the tumor mass was cystic)
(21). The size of the tumor was
indicated by the maximum diameter of the lesion, including the
cystic portion surrounding the tumor. The diagnosis of preoperative
hydrocephalus was determined by measurement of bilateral frontal
horn diameters or intracranial diameters (Evans index) after CT or
MRI. Postoperatively, patients were routinely followed up and
regularly underwent imaging examinations, and CT or MRI of the
brain could be performed at any time if the patient had any
particular conditions.
Surgical procedure
A total of 76 patients with sporadic cerebellar
hemangioblastoma underwent microsurgical resection of the tumor
under general anesthesia. Different surgical approaches were chosen
according to different parts of the tumor. The suboccipital
ipsilateral approach, suboccipital midline approach or suboccipital
supracerebellar approach were chosen mainly according to the
location of the solid part of the tumor. Ventricular drainage was
performed in 20 patients with obstructive hydrocephalus before
surgery.
For tumors located in the superior vermis or
inferior vermis, a prone position and suboccipital midline approach
was used. For tumors located in the cerebellar hemispheres and
extending toward the cerebellopontine angle, a lateral position and
suboccipital lateral approach were used. A total of 13 patients
were treated with a suboccipital midline approach and 63 patients
with a suboccipital lateral approach. The extent of surgery was
divided into gross total and partial excision. A total of 73
patients had gross total resection and 3 patients had partial
resections. Furthermore, 8 patients underwent vascular embolization
prior to tumor resection, and the others underwent direct
craniotomy.
Tumor recurrence and prognosis were determined by
the clinical presentation and imaging of the patients. The
patients' clinical status was assessed using the modified Rankin
scale (mRS) (22) (neurosurgeons
followed patients up by telephone or outpatient appointments). Poor
functional outcome was defined as mRS ≥2.
Statistical analysis
Statistical analyses were performed using SPSS
version 27 (IBM Corp.). Continuous variables were described as the
mean ± standard deviation and median with interquartile range, and
count variables as incidence (percentage).
Results
Patient characteristics
From July 2012 to April 2021, 76 patients with
sporadic cerebellar hemangioblastoma underwent one-stage surgical
treatment at the Department of Neurosurgery, General Hospital of
Northern Theater Command (Shenyang, China). From an initially
considered 86 patients, 7 patients with von Hippel-Lindau (VHL)
disease and 3 patients with incomplete medical records were
excluded. Finally, 76 patients diagnosed with sporadic cerebellar
hemangioblastoma were included in the present study. The clinical
characteristics and various imaging features of the 76 patients
with sporadic cerebellar hemangioblastoma are presented in Table I.
 | Table IClinical manifestations and a variety
of imaging features of the patients with sporadic cerebellar
hemangioblastomas (n=76). |
Table I
Clinical manifestations and a variety
of imaging features of the patients with sporadic cerebellar
hemangioblastomas (n=76).
| Item | Value |
|---|
| Mean symptom
duration, months | 3.1±4.4 |
| Symptomatic
presentations | |
|
Headache | 51 (67.1) |
|
Dizziness/vertigo | 50 (65.8) |
|
Cerebellar
ataxia | 45 (59.2) |
|
Nausea or
vomiting | 29 (38.2) |
|
Blurred
vision | 9 (11.8) |
|
Hearing
disturbances | 5 (6.6) |
|
Extremities
numbness | 4 (5.3) |
|
Diplopia | 3 (3.9) |
|
Movement
disorder | 1 (1.3) |
|
Extremity
paresis | 1 (1.3) |
|
Conscious
disturbance | 1 (1.3) |
| MRI features | |
|
Obstructive
hydrocephalus | 20 (26.3) |
|
Location of
tumor | |
|
Left
cerebellar hemisphere | 41 (53.9) |
|
Right
cerebellar hemisphere | 22 (28.9) |
|
Vermis | 13 (17.1) |
|
Composition
of tumor | |
|
Completely
solid | 11 (14.5) |
|
Combined
solid and cystic | 22 (28.9) |
|
Primarily
cystic | 44 (57.9) |
| Mean size of whole
tumor, mm | 37.9±11.1 |
| Mean size of solid
tumor, mm | 13.6±12.5 |
There were 76 patients in this study, including 42
males and 34 females. The age ranged from 23 to 72 years with a
mean age of 46.4±13.9 years. The median follow-up time was 52.5
(interquartile range, 20.0-74.5) months (range, 2-111 months). The
time from the date of microsurgery or interventional embolization
to the most recent clinical investigation was regarded as the
follow-up period. Detailed information of the 76 patients is
provided in Table SI.
The duration of clinical symptoms ranged from a
minimum of 0.3 months to a maximum of 24 months before admission to
hospital for diagnosis and the average was 3.1±4.4 months. The
difference in tumor size and location resulted in a variety of
clinical symptoms, mainly including increased intracranial
pressure, cerebellar dysfunction and cranial nerve damage. The most
common symptoms were headache in 51 patients (67.1%),
dizziness/vertigo in 50 patients (65.8%) and cerebellar ataxia in
45 patients (59.2%). Nausea or vomiting was the main complaint,
which was reported in 29 patients (38.2%), followed by blurred
vision (11.8%). Other neurologic deficits included hearing
disturbances (6.6%), numbness of extremities (5.3%), diplopia
(3.9%), movement disorder (1.3%), extremity paresis (1.3%) and
conscious disturbance (1.3%).
Imaging manifestations
Of the 76 patients, 41 had lesions located in the
left cerebellar hemisphere, 23 in the right cerebellar hemisphere
and 13 in the vermis. Among 57 patients with cystic tumors,
enhancement scans revealed small nodular enhancement at the margins
of the cyst wall in 53 cases and ring-like enhancement of the cyst
wall in 4 cases. A total of 11 patients had solid tumors, which
were markedly enhanced on enhancement scans. DSA was performed in
11 patients prior to surgery and the major arteries of blood supply
included the superior cerebellar artery, the anterior inferior
cerebellar artery, the posterior inferior cerebellar artery and the
cerebromedial branch of the occipital artery. The tumor size was
based on the maximum tumor diameter. In the present study, the
maximum tumor diameter was 62.0 mm, the minimum tumor diameter was
15.0 mm and the average diameter was 37.9±11.1 mm. Prior to
surgery, 20 of the 76 patients were found to have symptoms of
hydrocephalus, which were confirmed by imaging.
Surgery and adjuvant therapy
A total of 73 patients (96.1%) underwent gross total
resection. Furthermore, three patients underwent partial resection
for a variety of reasons, including i) uncontrolled intraoperative
hemorrhage, ii) to protect important neurologic function of the
brainstem, and iii) a deep lesion that was difficult to detect even
through a microscope. A comparison of preoperative and
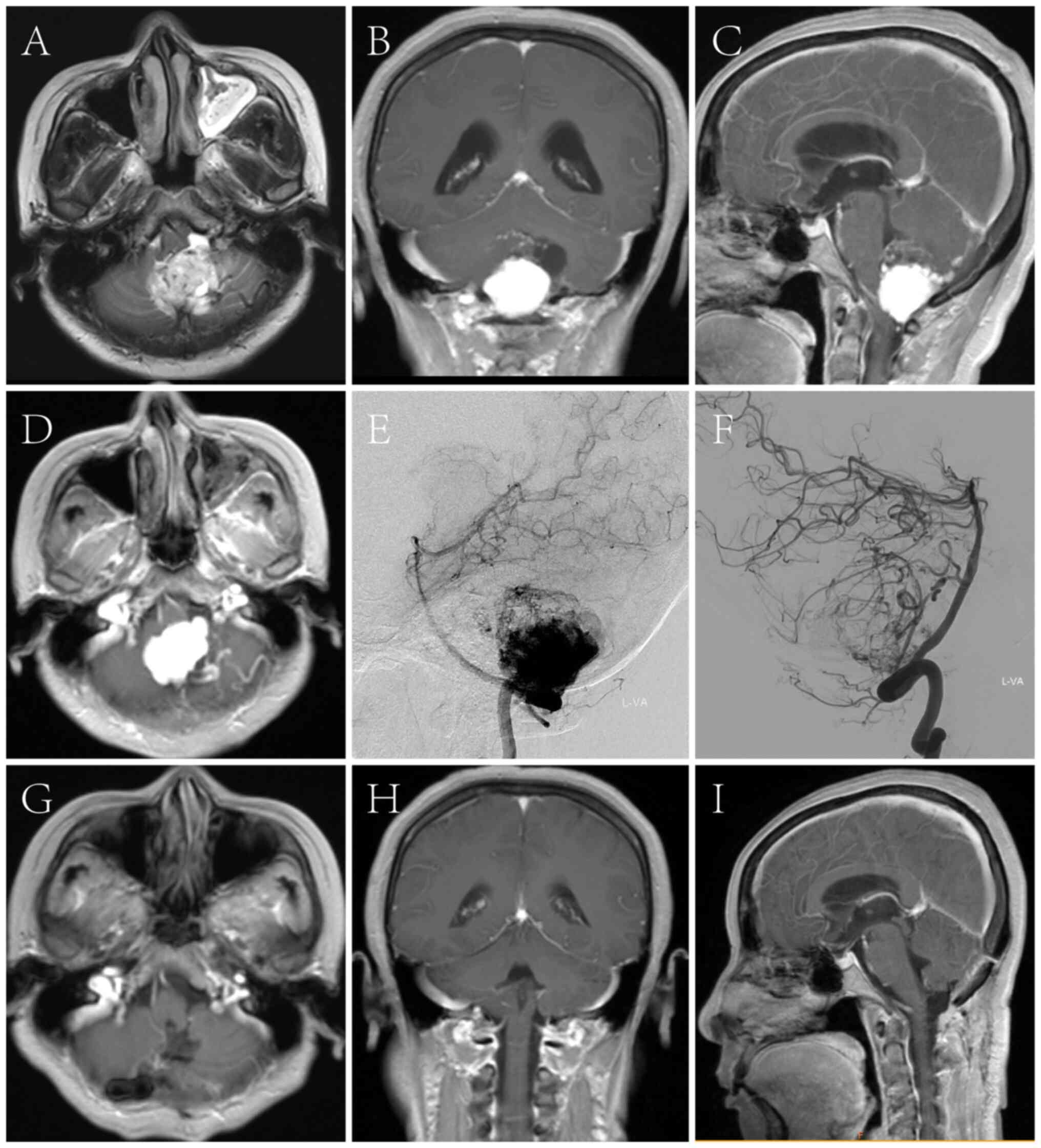
postoperative imaging in two cases is provided in Figs. 1 and 2. Of the 11 patients with cerebellar
solid hemangioblastoma, 8 patients were treated with endovascular
embolization followed by tumor resection 3 days later.
Follow-up
A total of 71 patients (83.1%) had a good prognosis
(mRS <2) after the first surgery. However, 5 patients (6.6%) had
significant neurological deficits (mRS ≥2) after surgery.
Furthermore, 3 partially resected patients underwent postoperative
radiotherapy and tumor recurrence in the original location was
observed at 24, 41 and 20 months after surgery, respectively. Of
these patients, 2 underwent a second surgery to completely remove
the recurrent tumor. After the second surgery, their symptoms
improved without permanent neurological deficits. The 2 patients
continued to receive radiotherapy, which resulted in a reduction in
tumor size and a resolution of symptoms, and therefore, observation
was recommended. A total of 8 patients with preoperative
embolization had no serious complications. Of these patients, two
developed mild postoperative headache, which gradually resolved the
next day (data not shown).
After several follow-ups, 8 cases were free of
complications and recurrence. However, in three solid cases without
preoperative endovascular embolization, postoperative cranial CT
examination after complete tumor resection showed peripheral
hemorrhage of 15-20 ml around the fourth ventricle (data not
shown). No other patient showed any recurrence during the follow-up
period.
Discussion
Hemangioblastomas are benign tumors of the central
nervous system that frequently occur in the posterior cranial
fossa, particularly the cerebellum. Hemangioblastomas tend to be
sporadic and the clinical presentation and treatment strategy
differ from those of patients with combined VLH disease (23-25).
In addition, the composition of the tumor has been reported to be
an important factor affecting the postoperative prognosis of
hemangioblastoma (5). Solid
hemangioblastomas have a higher risk of intraoperative hemorrhage
and postoperative complications compared to cystic
hemangioblastomas (26). Studies
have indicated that patients with solid hemangioblastoma have a
worse prognosis than patients with cystic hemangioblastoma
(26,27).
Due to the lack of characteristic clinical
manifestations, CT and MRI examinations have become the main
diagnostic methods for hemangioblastoma. According to the imaging
features of sporadic cerebellar hemangioblastomas, it can be
categorized into three types: Large cysts with reinforcing cystic
walls or small nodules, a combination of solid and cystic, and
completely solid tumors (5). Among
them, the type of cyst with small nodules was the most common. Of
the 76 cases included in the present study, 57 patients had cystic
tumors confirmed by MIR imaging, accounting for 75.0% of all
cases.
In the present study, the diameter of cystic
hemangioblastomas was measured by combining the respective lengths
of the cysts and nodules. Studies have indicated that the size of
the tumor correlates with the clinical presentation of the patient
(22,28). In the present study, the mean
diameter of the lesion was 37.9±11.1 mm and it produced a
significant mass effect in the posterior cranial fossa, leading to
preoperative hydrocephalus. Among the 57 patients with cystic
tumor, 16 patients presented with obstructive hydrocephalus, whose
clinical symptoms included headache, vertigo, cerebellar ataxia and
nausea or vomiting. In the present study, 54 cystic tumors were
completely resected and the clinical symptoms of these patients
improved significantly after surgery. A total of three cases of
cystic tumors were partially resected and underwent postoperative
radiotherapy; however, all of them recurred at different
time-points. Therefore, it was concluded that in cases of cystic
hemangioblastoma, complete resection significantly reduces the rate
of tumor recurrence. Drainage of cystic fluid and removal of
reinforcing tumor nodules have also been shown to be the most
appropriate treatment for cystic hemangioblastoma (22). As long as the tumor can be
completely removed, most patients' symptoms will remain stable or
ameliorative (29). Failure to
completely resect the tumor usually leads to tumor recurrence
(30). Therefore, the present
analysis suggests that complete resection of the tumor at the time
of initial surgery is essential to reduce the incidence of tumor
recurrence and complications.
In addition, the present study included four cystic
cases, which showed large non-enhancing cysts and surrounding
enhancing cystic walls on enhanced MRI scans. Since intraoperative
pathology confirmed that the reinforcing walls of the cystic tumors
also contained tumor tissue, it was ultimately decided to carefully
separate the tumor walls from the surrounding brain tissue and
resect them completely, while preserving the critical neurological
functional areas surrounding the cystic tumors as much as possible.
Previous studies have also reported that the resection strategy for
such cystic cases should be modified and refer to the surgical
access for solid tumors, and that all components of the tumor,
including the wall nodules and the cyst itself, must be removed
(4,31,32).
In addition, because the significantly enhanced cystic tumor wall
may be rich in capillaries, it has been suggested that preoperative
needle aspiration biopsy should be avoided due to concerns about
tumor hemorrhage (33,34). Therefore, it is vital in the
surgical management of such cystic tumors to perform intraoperative
pathology to determine clear tumor boundaries and thereby
completely resect the enhanced cystic wall.
Solid hemangioblastomas show marked enhancement on
MRI enhancement scans, which is the distinguishing imaging feature
of solid cases. Therefore, the diagnosis of solid hemangioblastoma
is not particularly difficult. However, the specific surgical
treatment strategy for this type of tumor is still
controversial.
Certain studies have argued that surgical outcomes
for patients with solid hemangioblastomas are poor regardless of
whether they have VHL or not (3,20).
However, other studies have shown that surgical outcomes in
patients with solid hemangioblastomas are not significantly
different from those in cystic patients (35,36).
In the present study, the majority of patients with solid
hemangioblastomas achieved favorable clinical outcomes without
serious complications or progression. Of the 11 patients with solid
hemangioblastomas, 8 underwent endovascular embolization prior to
surgical resection. During embolization, first, the primary
tumor-supplying artery was carefully identified by multi-angle
microangiography. Furthermore, it was ensured that the arteries
selected for embolization would not affect the blood supply to
important functional brain areas. The materials used for solid
tumor embolization included a variety of substances, such as
Onxy-18, flocculated sponge fragments and filament segments.
Consistent with previous reports, we have made preliminary
observations that the operative time for removal of solid
hemangioblastoma was significantly shortened and intraoperative
bleeding was more easily controlled after preoperative endovascular
embolization (37). After several
follow-ups, 8 cases were free of complications and recurrence.
However, in three solid cases without preoperative endovascular
embolization, postoperative cranial CT examination after complete
tumor resection showed peripheral hemorrhage of 15-20 ml around the
fourth ventricle. We believe that one of the most important reasons
for the poor prognosis of these three patients may have been
inadequate intraoperative hemostasis, which made intraoperative
bleeding difficult to control in the absence of preoperative
vascular embolization.
The safety and necessity of preoperative
interventional embolization of solid hemangioblastomas is still
controversial (35). Certain
studies have shown that preoperative embolization is associated
with high intracranial pressure and cerebral infarction (7,8).
However, in the present study, eight patients with preoperative
embolization had no serious complications. Of these patients, two
developed mild postoperative headache, which gradually resolved the
next day. During tumor resection, the numerous surgical techniques
available appear to influence patient prognosis. First, the
blood-supplying artery, the passing artery and the draining vein
must be correctly determined. In general, the supplying artery and
draining vein can be identified by looking at the vessel diameter,
color, pulse or intraoperative ultrasound. In difficult cases,
temporary closure clips are used to close the vessel to determine
the nature of the vessel. If there is swelling of the tumor, it is
a draining vein; otherwise, it is a supplying artery. In cases of
solid tumor adjacent to the brainstem, separation of the
tumor-brainstem interface is critical to the success of the
procedure. Since there is often a thin layer of edematous brain
tissue between the tumor and the brainstem, the tumor should be
cauterized along the brain tissue interface with low-power bipolar
electrocoagulation under neurophysiological monitoring and the
operative field should be continuously irrigated to minimize heat
conduction injury. Furthermore, the movements should be gentle so
as to not cause brainstem displacement by excessive intraoperative
pulling of the tumor. Most importantly, it was required to perform
complete tumor resection instead of removing the tumor piece by
piece, which could lead to intraoperative bleeding and
unpredictable consequences. The conclusion of the present study is
consistent with those of previous studies, namely that preoperative
embolization of solid hemangioblastomas is safe and necessary, and
complete tumor resection results in a good prognosis (37,38).
In the present study, there were only 76 patients.
Next, multicenter case reports should be collected and a detailed
prognostic analysis for each type of case should be performed.
In conclusion, total microsurgical resection is
essential to improve the health status of patients with sporadic
cerebellar cystic hemangioblastoma. In addition, as the safety of
vascular intervention techniques continues to improve, preoperative
embolization of arteries supplying solid hemangioblastomas can
reduce intraoperative bleeding and improve prognosis.
Supplementary Material
Characteristics of patients with
sporadic cerebellar hemangioblastomas.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
GH: Conceptualization, data curation,
editing/drafting/writing the manuscript, revising the manuscript
(for intellectual content), language editing; BZ and YL: Data
curation, software, visualization, obtaining materials, checking
and confirming the authenticity of raw data, language editing; YW:
Software, visualization, language editing, confirming the
authenticity of raw data, providing general supervision; YH:
Adjustment of conceptualization and study design, writing and
critical revision of the manuscript; XC: Data review, collation and
proofreading, data analysis and further processing, work
coordination. All of the authors have read and agreed to the final
version of the manuscript.
Ethics approval and consent to
participate
This study was approved by the Ethics Committee of
the General Hospital of Northern Theater Command (Shenyang, China;
approval no. 2021-135; August 2, 2021). All processes were
performed in accordance with the Declaration of Helsinki and local
ethical policies.
Patient consent for publication
Consent for publication was obtained from the two
patients whose images are shown in Figs. 1 and 2.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Ma D, Wang Y, Du G and Zhou L:
Neurosurgical management of brainstem hemangioblastomas: A
single-institution experience with 116 patients. World Neurosurg.
84:1030–1038. 2015.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Louis DN, Perry A, Reifenberger G, von
Deimling A, Figarella-Branger D, Cavenee WK, Ohgaki H, Wiestler OD,
Kleihues P and Ellison DW: The 2016 world health organization
classification of tumors of the central nervous system: A summary.
Acta Neuropathol. 131:803–820. 2016.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Lonser RR, Butman JA, Huntoon K, Asthagiri
AR, Wu T, Bakhtian KD, Chew EY, Zhuang Z, Linehan WM and Oldfield
EH: Prospective natural history study of central nervous system
hemangioblastomas in von Hippel-Lindau disease. J Neurosurg.
120:1055–1062. 2014.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Sun Z, Yuan D, Sun Y, Yan P and Zuo H:
Surgical resection of cerebellar hemangioblastoma with enhanced
wall thickness: A report of two cases. Oncol Lett. 9:1597–1599.
2015.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Fukuda M, Takao T, Hiraishi T, Yoshimura
J, Yajima N, Saito A and Fujii Y: Clinical factors predicting
outcomes after surgical resection for sporadic cerebellar
hemangioblastomas. World Neurosurg. 82:815–821. 2014.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Cheng J, Liu W, Hui X, Zhang S and Ju Y:
Pediatric central nervous system hemangioblastomas: Different from
adult forms? A retrospective series of 25 cases. Acta Neurochir
(Wien). 159:1603–1611. 2017.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Lonser RR, Glenn GM, Walther M, Chew EY,
Libutti SK, Linehan WM and Oldfield EH: von Hippel-Lindau disease.
Lancet. 361:2059–2067. 2003.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Ammerman JM, Lonser RR, Dambrosia J,
Butman JA and Oldfield EH: Long-term natural history of
hemangioblastomas in patients with von Hippel-Lindau disease:
Implications for treatment. J Neurosurg. 105:248–255.
2006.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Husain Q, Ghosh D, Vasdev A, Eloy JA and
Liu JK: Hemangioblastomas of the central nervous system in von
Hippel-Lindau disease: Review of the literature and a single
institution experience. Neurosurg Focus. 35(E8)2013.
|
|
10
|
Pichierri A, Di Bonaventura R, Spagnuolo
C, Ruggeri AG, Del Maestro M, Sturiale CL and Albanese A: Surgical
management of cerebellar hemangioblastomas: Report of 8 cases. J
Neurosurg Sci. 60:72–81. 2016.
|
|
11
|
Vortmeyer AO, Gläsker S, Lonser RR and
Lubensky IA: New insights into the pathogenesis of
hemangioblastomas: Cytogenetic and molecular genetic aspects.
Neurosurg Rev. 26:243–248. 2003.
|
|
12
|
Patel J, Rao VY and Lall RR: Innovations
in imaging techniques and neurosurgical navigation for brain tumor
surgery: Implications for the management of hemangioblastomas.
World Neurosurg. 123:292–303. 2019.
|
|
13
|
Wild-Bode C, Weller M and Wick W:
Molecular determinants of glioma cell migration and invasion. J
Neurosurg Sci. 45:73–80. 2001.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Ito E, Ichikawa T and Takahashi T:
Advances in the surgical treatment of hemangioblastomas: Current
perspectives and future directions. Neurosurg Rev. 34:211–219.
2011.
|
|
15
|
Vortmeyer AO and Lubensky IA: Molecular
biology of von Hippel-Lindau disease. Neurosurg Focus.
19(E2)2005.
|
|
16
|
Maher ER, Iselius L, Yates JR, Littler M,
Benjamin C, Harris R, Sampson J, Williams A, Ferguson-Smith MA and
Morton N: Von Hippel-Lindau disease: A genetic study. J Med Genet.
28:443–447. 1991.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Kaelin WG Jr: Molecular basis of the VHL
hereditary cancer syndrome. Nat Rev Cancer. 2:673–682.
2002.PubMed/NCBI View
Article : Google Scholar
|
|
18
|
Huntoon K, Willard S and Boulis N:
Advances in the understanding of the molecular pathogenesis of von
Hippel-Lindau disease: A review. J Neurosurg. 106:848–859.
2007.
|
|
19
|
Richard S, Campello C and Taillandier L:
Hemangioblastoma of the central nervous system. Neurochirurgie.
47:455–465. 2001.
|
|
20
|
Conway JE, Chou D, Clatterbuck RE, Brem H,
Long DM and Rigamonti D: Hemangioblastomas of the central nervous
system in von Hippel-Lindau syndrome and sporadic disease.
Neurosurgery. 48:55–62. 2001.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Liao CC and Huang YH: Clinical features
and surgical outcomes of sporadic cerebellar hemangioblastomas.
Clin Neurol Neurosurg. 125:160–165. 2014.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Nimbvikar AA, Panchawagh S, Chavan AP,
Ingole JR, Pargaonkar Y and Pai R: Modified rankin scale is a
reliable tool for the rapid assessment of stroke severity and
predicting disability outcomes. J Family Med Prim Care.
13:1085–1090. 2024.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Kasapas K, Malli A, Sfikas S and
Georgakoulias N: Sporadic pituitary stalk hemangioblastoma: A rare
case report and review of the literature. Cureus.
12(e9107)2020.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Kosty J, Staarman B, Zimmer LA and
Zuccarello M: Infundibular hemangioblastoma in a patient with
neurofibromatosis type 1: Case report and review of the literature.
World Neurosurg. 88:693.e697–e693.e612. 2016.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Fu H, Hao S, Wu Z, Zhang J and Zhang L:
Sporadic pituitary stalk hemangioblastoma. Neurol India.
59:937–938. 2011.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Moscovici S, Candanedo C, Spektor S, Cohen
JE and Kaye AH: Solid vs. cystic predominance in posterior fossa
hemangioblastomas: Implications for cerebrovascular risks and
patient outcome. Acta Neurochir (Wien). 164:1357–1364.
2022.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Xia H, Li J, Xia Y, Zhong D, Wu X, He D,
Shi D, Li J and Sun X: Sporadic solid/cystic hemangioblastomas in
the cerebellum: Retrospective study of more than ten years of
experience in a single center. World Neurosurg. 144:e908–e915.
2020.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Jang HJ, Moon BJ, Kim KH, Park JY, Chin
DK, Cho YE and Kim KS: Prognostic factors of spinal intramedullary
hemangioblastoma: Analysis of surgical outcomes and tumor
characteristics. J Korean Neurosurg Soc.
23(10.3340/jkns.2023.0221)2024.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Kuharic M, Jankovic D, Splavski B, Boop FA
and Arnautovic KI: Hemangioblastomas of the posterior cranial fossa
in adults: Demographics, clinical, morphologic, pathologic,
surgical features, and outcomes. A systematic review. World
Neurosurg. 110:e1049–e1062. 2018.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Lee GJ, Jung TY, Kim IY, Jung S, Jang WY,
Moon KS and Kim SK: The clinical experience of recurrent central
nervous system hemangioblastomas. Clin Neurol Neurosurg. 123:90–95.
2014.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Reecher HM, Hussain O, Treffy R and Nerva
JD: Preoperative embolization and microsurgical resection of a
cerebellar hemangioblastoma: Embolization goals and technical
nuances of the approaches. Illustrative case. J Neurosurg Case
Lessons. 7(CASE24100)2024.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Vargas-Urbina J, Crisanto-Silva JA,
Vasquez-Perez C, Davila-Adrianzen A, Alcas-Seminario D,
Lines-Aguilar W, Mamani-Choquepata R and Panta-Rojas G: Multimodal
management of giant solid hemangioblastomas in two patients with
preoperative embolization. Surg Neurol Int. 15(144)2024.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Wang Q, Zhang S, Cheng J, Liu W and Hui X:
Radiologic features and surgical strategy of hemangioblastomas with
enhanced cyst wall. World Neurosurg. 108:143–150. 2017.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Vergauwen E, Steiert C, Kruger MT, Jilg C,
Zschiedrich S, Klingler JH, Van Velthoven V and Gläsker S:
Cumulative surgical morbidity in patients with multiple cerebellar
and medullary hemangioblastomas. Clin Neurol Neurosurg.
197(106111)2020.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Niu L, Zhang Y, Li Q, Dai J, Yin H, Duan
L, Yang H, Liang W, Qin Z, Zhang J and Pan Y: The analysis of
correlative factors affecting long-term outcomes in patients with
solid cerebellar Hemangioblastomas. Clin Neurol Neurosurg.
150:59–66. 2016.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Wan JQ, Cui H and Wang Y: Surgical
management of large solid hemangioblastomas of the posterior fossa.
J Clin Neurosci. 18:39–42. 2011.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Saliou G, Giammattei L, Ozanne A and
Messerer M: Role of preoperative embolization of intramedullary
hemangioblastoma. Neurochirurgie. 63:372–375. 2017.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Sakamoto N, Ishikawa E, Nakai Y, Akutsu H,
Yamamoto T, Nakai K, Shiigai M, Tsurushima H, Isobe T, Takano S, et
al: Preoperative endovascular embolization for hemangioblastoma in
the posterior fossa. Neurol Med Chir (Tokyo). 52:878–884.
2012.PubMed/NCBI View Article : Google Scholar
|