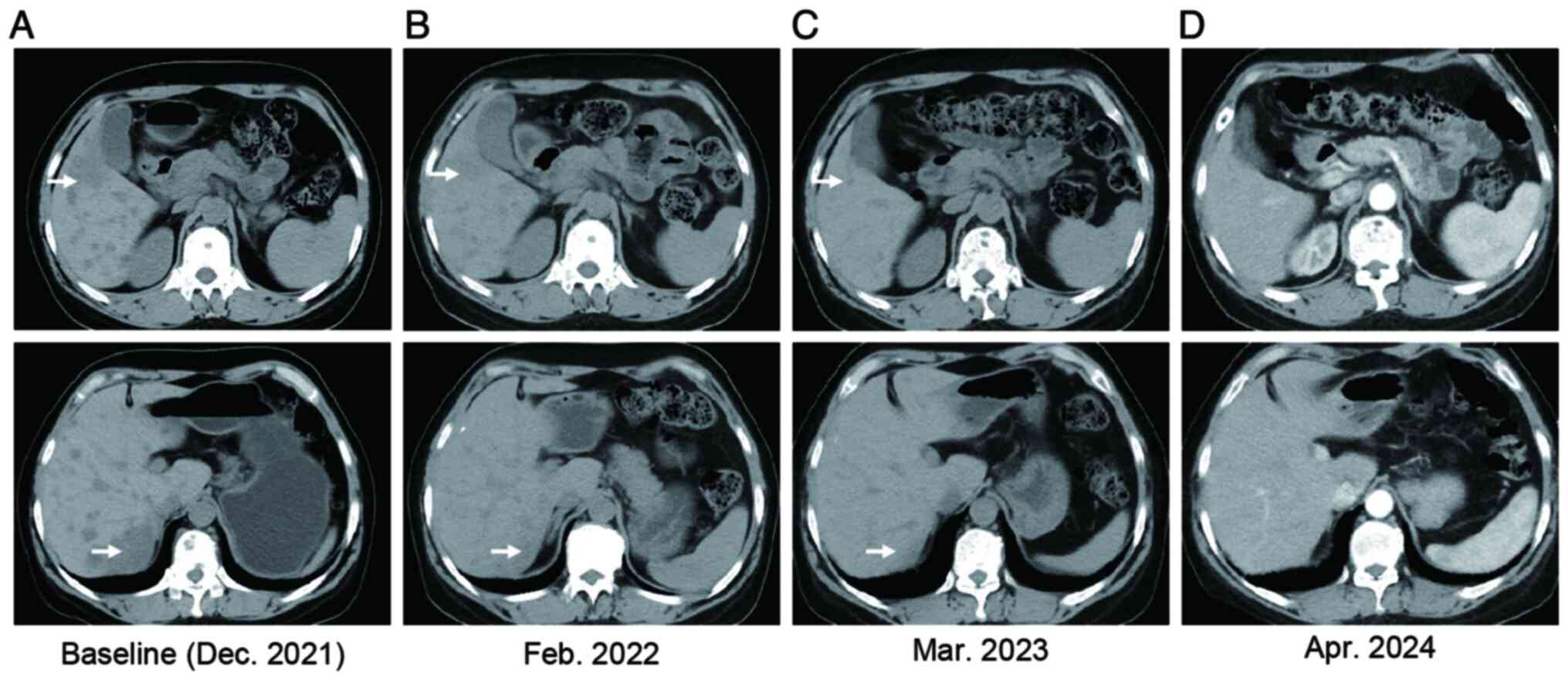
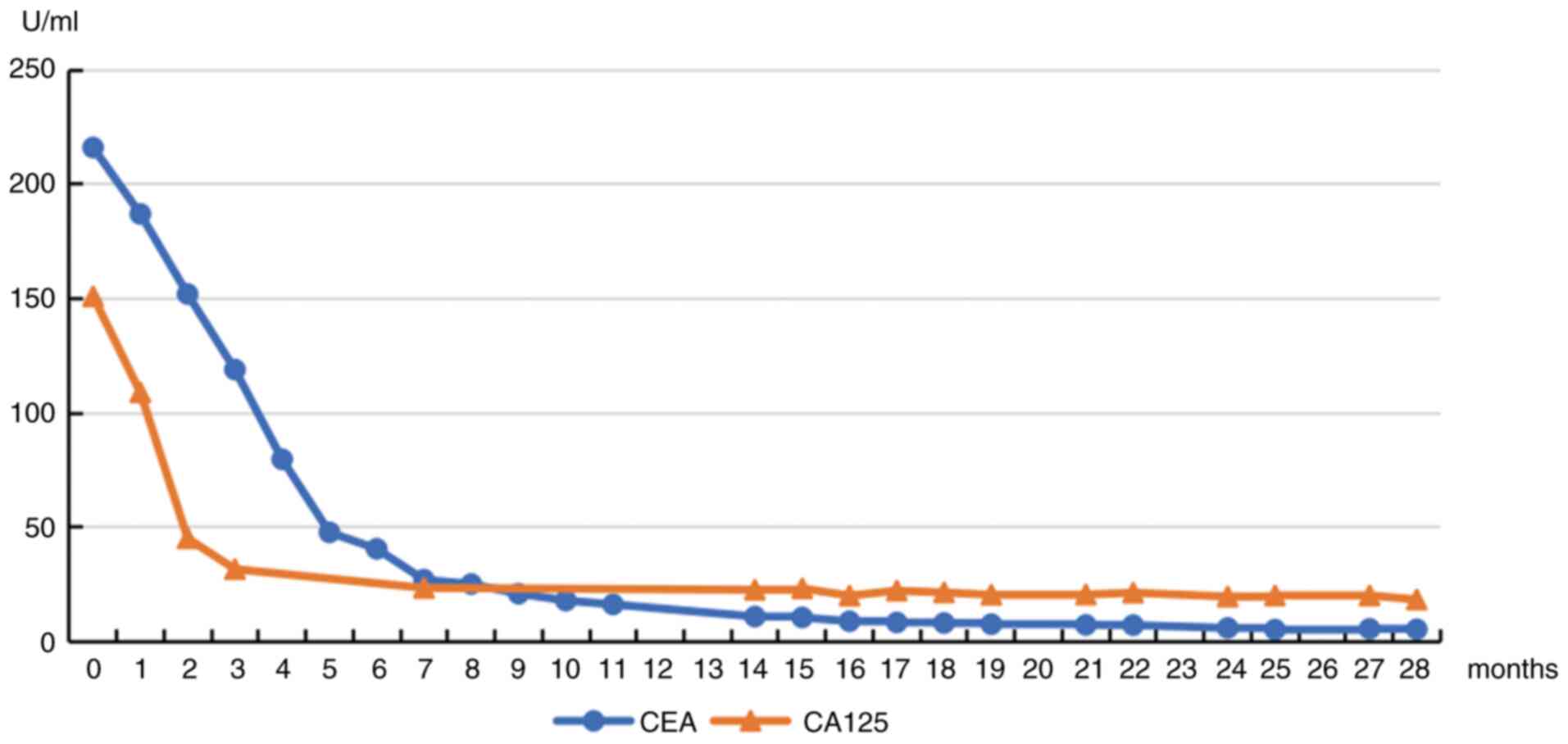
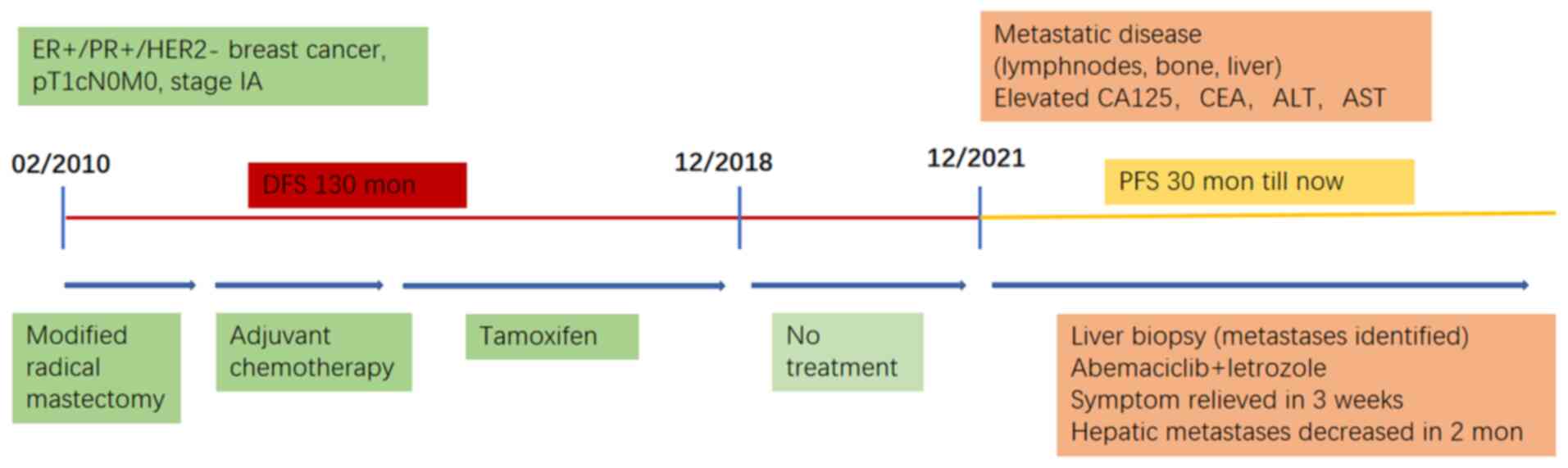
|
1
|
Prat A, Pineda E, Adamo B, Galvan P,
Fernandez A, Gaba L, Díez M, Viladot M, Arance A and Muñoz M:
Clinical implications of the intrinsic molecular subtypes of breast
cancer. Breast. 24 (Suppl 2):S26–S35. 2015.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Braal CL, Jongbloed EM, Wilting SM,
Mathijssen RHJ, Koolen SLW and Jager A: Inhibiting CDK4/6 in breast
cancer with palbociclib, ribociclib, and abemaciclib: Similarities
and differences. Drugs. 81:317–331. 2021.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Glaviano A, Wander SA, Baird RD, Yap KC,
Lam HY, Toi M, Carbone D, Geoerger B, Serra V, Jones RH, et al:
Mechanisms of sensitivity and resistance to CDK4/CDK6 inhibitors in
hormone receptor-positive breast cancer treatment. Drug Resist
Updat. 76(101103)2024.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Sherr CJ and Roberts JM: CDK inhibitors:
Positive and negative regulators of G1-phase progression. Genes
Dev. 13:1501–1512. 1999.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Spring LM, Wander SA, Andre F, Moy B,
Turner NC and Bardia A: Cyclin-dependent kinase 4 and 6 inhibitors
for hormone receptor-positive breast cancer: Past, present, and
future. Lancet. 395:817–827. 2020.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Im SA, Lu YS, Bardia A, Harbeck N,
Colleoni M, Franke F, Chow L, Sohn J, Lee KS, Campos-Gomez S, et
al: Overall survival with ribociclib plus endocrine therapy in
breast cancer. N Engl J Med. 381:307–316. 2019.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Slamon DJ, Neven P, Chia S, Fasching PA,
De Laurentiis M, Im SA, Petrakova K, Bianchi GV, Esteva FJ, Martín
M, et al: Overall survival with ribociclib plus fulvestrant in
advanced breast cancer. N Engl J Med. 382:514–524. 2020.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Slamon DJ, Neven P, Chia S, Fasching PA,
De Laurentiis M, Im SA, Petrakova K, Bianchi GV, Esteva FJ, Martín
M, et al: Phase III Randomized study of ribociclib and fulvestrant
in hormone receptor-positive, human epidermal growth factor
receptor 2-negative advanced breast cancer: MONALEESA-3. J Clin
Oncol. 36:2465–2472. 2018.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Sledge GW, Toi M, Neven P, Sohn J, Inoue
K, Pivot X, Burdaeva O, Okera M, Masuda N, Kaufman PA, et al: The
effect of abemaciclib plus fulvestrant on overall survival in
hormone receptor-positive, ERBB2-Negative breast cancer that
progressed on endocrine Therapy-MONARCH 2 A Randomized clinical
trial. JAMA Oncol. 6:116–124. 2020.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Tripathy D, Im SA, Colleoni M, Franke F,
Bardia A, Harbeck N, Hurvitz SA, Chow L, Sohn J, Lee KS, et al:
Ribociclib plus endocrine therapy for premenopausal women with
hormone-receptor-positive, advanced breast cancer (MONALEESA-7): A
randomised phase 3 trial. Lancet Oncol. 19:904–915. 2018.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Ribociclib as First-Line Therapy for
HR-Positive, advanced breast cancer. N Engl J Med.
379(2582)2018.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Rugo HS, Finn RS, Dieras V, Ettl J,
Lipatov O, Joy AA, Harbeck N, Castrellon A, Iyer S, Lu DR, et al:
Palbociclib plus letrozole as first-line therapy in estrogen
receptor-positive/human epidermal growth factor receptor 2-negative
advanced breast cancer with extended follow-up. Breast Cancer Res
Treat. 174:719–729. 2019.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Di Leo A, Toi M, Campone M, Sohn J,
Paluch-Shimon S, Huober J, Park IH, Trédan O, Chen SC, Manso L, et
al: MONARCH 3: Abemaciclib as initial therapy for patients with
HR+/HER2-advanced breast cancer. Ann Oncol. 28(1)2017.
|
|
14
|
Goetz MP, Toi M, Campone M, Sohn J,
Paluch-Shimon S, Huober J, Park IH, Trédan O, Chen SC, Manso L, et
al: MONARCH 3: Abemaciclib as initial therapy for advanced breast
cancer. J Clin Oncol. 35:3638–3646. 2017.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Cardoso F, Paluch-Shimon S, Senkus E,
Curigliano G, Aapro MS, Andre F, Barrios CH, Bergh J, Bhattacharyya
GS, Biganzoli L, et al: 5th ESO-ESMO international consensus
guidelines for advanced breast cancer (ABC 5). Ann Oncol.
31:1623–1649. 2020.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Gradishar WJ, Moran MS, Abraham J, Aft R,
Agnese D, Allison KH, Anderson B, Burstein HJ, Chew H, Dang C, et
al: Breast cancer, version 3.2022, NCCN clinical practice
guidelines in oncology. J Natl Compr Canc Netw. 20:691–722.
2022.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Gennari A, André F, Barrios CH, Cortés J,
de Azambuja E, DeMichele A, Dent R, Fenlon D, Gligorov J, Hurvitz
SA, et al: ESMO clinical practice guideline for the diagnosis,
staging and treatment of patients with metastatic breast cancer.
Ann Oncol. 32:1475–1495. 2021.PubMed/NCBI View Article : Google Scholar
|
|
18
|
O'Shaughnessy J: Extending survival with
chemotherapy in metastatic breast cancer. Oncologist. 10 (Suppl
3):S20–S29. 2005.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Zhu H and Doğan BE: American Joint
Committee on Cancer's Staging System for Breast Cancer, Eighth
Edition: Summary for Clinicians. Eur J Breast Health. 17:234–238.
2021.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Oken MM, Creech RH, Tormey DC, Horton J,
Davis TE, McFadden ET and Carbone PP: Toxicity and response
criteria of the Eastern Cooperative Oncology Group. Am J Clin
Oncol. 5:649–655. 1982.PubMed/NCBI
|
|
21
|
Burstein HJ, Somerfield MR, Barton DL,
Dorris A, Fallowfield LJ, Jain D, Johnston SRD, Korde LA, Litton
JK, Macrae ER, et al: Endocrine treatment and targeted therapy for
hormone receptor-positive, human epidermal growth factor receptor
2-negative metastatic breast cancer: ASCO guideline update. J Clin
Oncol. 39:3959–3977. 2021.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Giuliano M, Schettini F, Rognoni C, Milani
M, Jerusalem G, Bachelot T, De Laurentiis M, Thomas G, De Placido
P, Arpino G, et al: Endocrine treatment versus chemotherapy in
postmenopausal women with hormone receptor-positive, HER2-negative,
metastatic breast cancer: A systematic review and network
meta-analysis. Lancet Oncol. 20:1360–1369. 2019.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Rugo HS, Finn RS, Gelmon K, Joy AA,
Harbeck N, Castrellon A, Mukai H, Walshe JM, Mori A, Gauthier E, et
al: Progression-free survival outcome is independent of objective
response in patients with estrogen receptor-positive, human
epidermal growth factor receptor 2-negative advanced breast cancer
treated with palbociclib plus letrozole compared with letrozole:
Analysis from PALOMA-2. Clin Breast Cancer. 20:e173–e180.
2020.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Janni W, Alba E, Bachelot T, Diab S,
Gil-Gil M, Beck TJ, Ryvo L, Lopez R, Tsai M, Esteva FJ, et al:
First-line ribociclib plus letrozole in postmenopausal women with
HR+, HER2-advanced breast cancer: Tumor response and pain reduction
in the phase 3 MONALEESA-2 trial. Breast Cancer Res Treat.
169:469–479. 2018.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Johnston SRD, Harbeck N, Hegg R, Toi M,
Martin M, Shao ZM, Zhang QY, Martinez Rodriguez JL, Campone M,
Hamilton E, et al: Abemaciclib combined with endocrine therapy for
the adjuvant treatment of HR+, HER2-, node-positive, high-risk,
early breast cancer (monarchE). J Clin Oncol. 38:3987–3998.
2020.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Hafner M, Mills CE, Subramanian K, Chen C,
Chung M, Boswell SA, Everley RA, Liu C, Walmsley CS, Juric D and
Sorger PK: Multiomics profiling establishes the polypharmacology of
FDA-Approved CDK4/6 inhibitors and the potential for differential
clinical activity. Cell Chem Biol. 26:1067–1080.e8. 2019.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Goetz MP, Toi M, Huober J, Sohn J, Trédan
O, Park IH, Campone M, Chen SC, Manso LM, Paluch-Shimon S, et al:
Abemaciclib plus a nonsteroidal aromatase inhibitor as initial
therapy for HR+, HER2-advanced breast cancer: Final overall
survival results of MONARCH 3. Ann Oncol. 35:718–727.
2024.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Johnston S, O'Shaughnessy J, Martin M,
Huober J, Toi M, Sohn J, André VAM, Martin HR, Hardebeck MC and
Goetz MP: Abemaciclib as initial therapy for advanced breast
cancer: MONARCH 3 updated results in prognostic subgroups. NPJ
Breast Cancer. 7(80)2021.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Lu YS, Mahidin EIBM, Azim H, Eralp Y, Yap
YS, Im SA, Rihani J, Bowles J, Alfaro TD, Wu J, et al: Abstract
GS1-10: Primary Results From the Randomized Phase II RIGHT Choice
Trial of Premenopausal Patients With Aggressive HR+/HER2− Advanced
Breast Cancer Treated With Ribociclib + Endocrine Therapy vs
Physician's Choice Combination Chemotherapy. Cancer Res. 83 (Suppl
5):GS1–GS10. 2023.
|
|
30
|
Lu YS, Mahidin EIBM, Azim H, Eralp Y, Yap
YS, Im SA, Rihani J, Gokmen E, El Bastawisy A, Karadurmus N, et al:
Final results of RIGHT Choice: Ribociclib plus endocrine therapy vs
combination chemotherapy in premenopausal women with clinically
aggressive Hormone Receptor-Positive/Human Epidermal Growth Factor
Receptor 2-Negative advanced breast cancer. J Clin Oncol.
42:2812–2821. 2024.PubMed/NCBI View Article : Google Scholar
|