Introduction
Acute leukemia (AL) is a malignancy of hematopoietic
progenitor cells and is characterized by excessive numbers of
immature cells in the bone marrow (1,2). AL
can be classified into two subtypes, namely acute myeloid leukemia
(AML) and acute lymphocytic leukemia (ALL). AML is more frequently
reported in adults aged >40 years old, whilst ALL occurs mainly
in children (3,4). The incidence of AML had been higher
compared with that of ALL (5,6). The
age-adjusted incidence of AML was 4.3 per 100,000 people per year,
whilst that of ALL was 1.7, in the United States (5,6).
Although the 5-year overall survival (OS) reached 90% in pediatric
patients with ALL, the 5-year OS in patients with ALL (≥50 years)
and AML were only 25% and 24%, respectively (6-9).
Each subtype of AL can be further subdivided into categories as
defined by the French-American-British (FAB) system or the World
Health Organization (WHO) system (10-12).
Specifically, according to FAB system, AML and ALL is divided into
eight (M0-M7) and three (L1-L3) subtypes, respectively (10,11).
A commonality of AML and ALL is that the multiplication of immature
hematopoietic cells leads to the decreased production of normal
hematopoietic cells, resulting in a number of pathological
conditions, such as anemia, thrombocytopenia and leukopenia
(13). However, AML and ALL have
differing pathophysiological processes. Myeloid cells proliferate
into their mature end cells within the bone marrow, whereas the
lymphoid precursors migrate to the lymphoid organs to complete
maturation (1). Therefore, the
mechanisms underlying the inhibition of normal hematopoiesis will
likely differ between these two subtypes of AL, especially the
forms of hypocytosis. In particular, to the best of our knowledge,
studies investigating the ability of complete blood count (CBC)
parameters to distinguish between patients with AML and ALL remain
scarce in the literature. Currently, AML and ALL are distinguished
mainly by cytological analysis of a bone marrow puncture, based on
different cytological features of lymphoblasts and myeloblasts
(11,14,15).
Lymphoblasts are characterized by small to large sized blasts,
moderately condensed to dispersed chromatin, inconspicuous or
prominent nucleoli, scant or moderately abundant cytoplasm with
variable basophilia and vacuolation, whilst myeloblasts are
characteristically large-sized blasts, fine nuclear chromatin,
presence of one or more prominent nucleoli, and varying amounts of
cytoplasm with azurophilic granules (11,14,15).
Moreover, the presence of ‘Auer rods’ or ‘Phi bodies’ is the
myeloblast characteristic (11,14,15).
Peripheral blood routine examination is simpler, more convenient
and safer than bone marrow puncture. Therefore, the present study
was undertaken to compare the CBC results between Chinese patients
with AML and those with ALL. In addition, CBC parameters between
patients with acute promyelocytic leukemia (APL, also named M3), a
unique subtype of AML and those with non-M3 AML, were compared.
Subsequently, the influence of factors, such as hemocytopenia, sex
and age, on the OS of patient were further analyzed.
Patients and methods
Patients
The patients were selected from the Department of
Hematology, Zhongshan Hospital of Xiamen University (Xiamen,
China), between January 2015 and May 2019. AL was diagnosed
according to the 2016 WHO criteria (12). The diagnostic procedures included
cytomorphology, cytogenetics, molecular genetics and
immunophenotyping of the bone marrow. Clinical follow-up
information was obtained by retrospective review of the electronic
charts and telephone follow-up. OS was measured from the date of
diagnosis to mortality from any cause or the last follow-up, which
was used to compare the clinical outcomes. The follow-up of all
patients ended in April 2024.
The inclusion criteria were as follows: i) Newly
diagnosed with AL (age, 6-90 years); ii) CBC analysis performed
before treatment; and iii) availability of all clinical data. The
exclusion criteria were as follows: i) Presence of any type of
cancer, hyperthyroidism, hemorrhoids, splenectomy, coagulation
disorders, chronic cardiopulmonary diseases, combined immune system
diseases, infectious diseases, severe organic lesions, mental
illness and severe liver or kidney dysfunction; ii) coronary heart
disease controlled by oral drugs for 3 years after percutaneous
coronary intervention; iii) long-term anemia before AL; iv)
treatment with red blood cell (RBC) or platelet (PLT) transfusion
before diagnosis; and v) use of any drug that could affect CBC
results, such as corticosteroids, antibiotics and diuretics.
Methods
CBC analysis of EDTA-anticoagulated blood samples
was performed. CBC counts [numbers of white blood cells (WBCs),
neutrophils, eosinophils, basophils, monocytes, lymphocytes, RBCs,
PLTs and hemoglobin levels] were measured using an automated blood
cell counter (Sysmex XN-9000; Sysmex Corporation). The following
data were obtained and analyzed: Age, sex, leukemia subtype,
initial CBC counts, CNS involvement and treatments.
In the present study, leukocytosis was defined as
any WBC count >10x109/l (16). Leukopenia was defined as a WBC
count of <3.5x109/l (17). Thrombocytopenia was defined as a
PLT count of <100x109/l (16). In addition, <110 g/l (for women)
and <120 g/l (for men) normal hemoglobin levels were used to
define anemia (18).
Statistical analysis
All data analyses were performed using SPSS version
26.0 (IBM Corp.). Data for quantitative variables were reported as
medians and (interquartile) ranges, whereas data for qualitative
variables were reported as numbers and percentages. A Shapiro-Wilk
normality test was performed to check if the data were normally
distributed. Between-group comparisons of quantitative data were
performed using the Mann-Whitney U test if the data were found to
be non-normally distributed, and the unpaired independent-samples
t-test for normally distributed data. The χ2 test was
used for comparing categorical data. OS curves were drawn by the
Kaplan-Meier method and a log-rank test was used to compare OS
between the two groups. Univariable Cox proportional hazards models
were used to analyze OS-related factors. Among the factors in
univariable models, those significant at P≤0.1 were used in a
limited backward selection procedure to build multivariable models.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Baseline characteristics
In the present study, the CBC results of 147
patients with de novo AL were investigated, which included
105 patients with AML and 42 patients with ALL. AML patients was
predominant (71.4%) over the ALL patients (28.6%). The median age
in patients with ALL (38 years) was lower compared with that of AML
patients (54 years). The similar sex ratio was observed in patients
with AML and ALL. The AML subgroups included 24 (22.8%) cases of
M3, 70 (66.7%) patients with non-M3, and 11 (10.5%) patients with
unknown type. Among patients with ALL, there were 35 cases of B-ALL
(83.3%), 5 cases of T-ALL (11.9%) and 2 cases of unknown cell type
(4.8%). In addition, only 1 patient with ALL at diagnosis suffered
from central nervous system (CNS) metastasis. The main clinical
characteristics of AL patients are described in Table SI.
CBC results analysis
The results of CBC analysis for patients with AL are
presented in Table I. Patients
with ALL were found to have significantly higher lymphocyte
(P=0.021) and RBC counts (P=0.001) compared with those in patients
with AML (Table I). P<0.05 were
obtained in both eosinophil count (P=0.022) and eosinophil
percentage (P=0.035) between the AML group and the ALL group,
however, the same the median value of eosinophil count and
eosinophil percentage was observed in the two groups (Table I). In addition, the mean
corpuscular volume and mean corpuscular hemoglobin values were
significantly increased in AML patients compared with those in
patients with ALL (both P<0.001; Table I).
 | Table IComplete blood count analysis of
patients with AL. |
Table I
Complete blood count analysis of
patients with AL.
| Parameter | Reference
value | AL (n=147) | Acute myeloid
leukemia (n=105) | Acute lymphocytic
leukemia (n=42) | P-value |
|---|
| White blood cell
count, x109/l | 3.50-9.50 | 5.86
(2.21-43.25) | 5.39
(1.81-44.19) | 5.89
(3.73-21.64) | 0.423 |
| Neutrophil count,
x109/l | 1.80-6.30 | 1.47
(0.42-4.79) | 1.26
(0.33-5.02) | 1.52
(0.55-3.91) | 0.728 |
| Neutrophil
percentage, % | 40.00-75.00 | 17.20
(8.00-37.85) | 17.90
(8.20-36.90) | 16.75
(5.80-47.70) | 0.947 |
| Eosinophil count,
x109/l | 0.02-0.52 | 0.00
(0.00-0.01) | 0.00
(0.00-0.00) | 0.00
(0.00-0.05) | 0.022 |
| Eosinophil
percentage, % | 0.40-8.00 | 0.00
(0.00-0.10) | 0.00
(0.00-0.00) | 0.00
(0.00-0.40) | 0.035 |
| Basophil count,
x109/l | 0.00-0.06 | 0.00
(0.00-0.01) | 0.00
(0.00-0.00) | 0.00
(0.00-0.01) | 0.117 |
| Basophil
percentage, % | 0.00-1.00 | 0.00
(0.00-0.10) | 0.00
(0.00-0.00) | 0.00
(0.00-0.20) | 0.073 |
| Lymphocyte count,
x109/l | 1.10-3.20 | 1.98
(1.02-4.91) |
1.57(0.87-4.46) | 2.35
(1.53-6.15) | 0.021 |
| Lymphocyte
percentage, % | 20.00-50.00 | 31.0
(13.00-54.30) | 26.00
(10.00-60.00) | 36.75
(27.30-52.00) | 0.077 |
| Monocyte count,
x109/l | 0.10-0.60 | 0.19
(0.04-0.92) | 0.19
(0.03-1.58) | 0.14
(0.05-0.69) | 0.386 |
| Monocyte
percentage, % | 3.00-10.00 | 4.00
(1.00-14.85) | 4.40
(1.00-15.10) | 3.25
(1.00-10.00) | 0.323 |
| RBC count,
x1012/l | 4.30-5.80 | 2.49
(1.97-3.09) | 2.38
(1.89-2.92) | 2.83
(2.26-3.59) | 0.001 |
| Hemoglobin,
g/l | 130.00-175.00 | 75.00
(64.50-90.00) | 74.00
(62.00-88.00) | 78.50
(66.00-104.00) | 0.087 |
| Hematocrit, % | 40.00-50.00 | 23.20
(19.65-27.95) | 22.80
(18.70-27.60) | 23.75
(20.80-30.20) | 0.054 |
| Mean corpuscular
volume, fl | 82.00-100.00 | 93.90
(87.20-101.05) | 96.20
(90.30-102.40) | 87.90
(83.80-93.80) | <0.001 |
| Mean corpuscular
hemoglobin, pg | 27.00-34.00 | 31.20
(28.95-33.50) | 31.90
(30.30-34.60) | 29.50
(27.00-30.60) | <0.001 |
| Mean corpuscular
hemoglobin concentration, g/l | 316.00-354.00 | 333.00
(319.00-343.00) | 332.00
(319.00-344.00) | 334.00
(318.00-340.00) | 0.722 |
| Red blood cell
distribution width, % | 0.00-15.00 | 15.80
(14.25-17.90) | 15.80
(14.30-17.60) | 15.80
(13.70-19.00) | 0.938 |
| Platelet count,
x109/l | 125.00-350.00 | 40.00
(0.40-500.00) | 37.00
(14.00-70.00) | 44
(19.00-94.00) | 0.301 |
Different forms of hypocytosis
analysis
The frequencies of different forms of hypocytosis
were next compared between the AML group and the ALL group
(Table II). The percentage of
patients with leukopenia (WBC count <3.5x109/l) was
found to be significantly higher in the AML group compared with
that in the ALL group (P=0.015). In addition, the percentage of
patients with both leukopenia and anemia was significantly higher
in the AML group compared with that in the ALL group (P=0.016).
Similarly, the percentage of patients with both leukopenia and
thrombocytopenia was significantly higher in the AML group compared
with that in the ALL group (P=0.015). Statistically significant
rates of pancytopenia were observed in the AML and ALL groups
(P=0.019), with the higher percentage of patients with pancytopenia
in the AML group.
 | Table IIComparison of frequencies of
different forms of hypocytosis between patients with AML and
ALL. |
Table II
Comparison of frequencies of
different forms of hypocytosis between patients with AML and
ALL.
| Parameters | AML (n=105) | ALL (n=42) | P-value |
|---|
| Leukopenia
a, N (%) | | | 0.015 |
|
Yes | 42 (40.0) | 8 (19.0) | |
|
No | 63 (60.0) | 34 (81.0) | |
| Leukocytosis, N
(%) | | | 0.101 |
|
Yes | 48 (45.7) | 13 (31.0) | |
|
No | 57 (54.3) | 29 (69.0) | |
| Anemia, N (%) | | | 0.479 |
|
Yes | 100 (95.2) | 38 (90.5) | |
|
No | 5 (4.8) | 4 (9.5) | |
| Thrombocytopenia, N
(%) | | | 0.219 |
|
Yes | 89 (84.8) | 32 (76.2) | |
|
No | 16 (15.2) | 10 (23.8) | |
|
Leukopeniaa and anemia, N (%) | | | 0.016 |
|
Yes | 39 (37.1) | 7 (16.7) | |
|
No | 66 (62.9) | 35 (83.3) | |
|
Leukopeniaa and thrombocytopenia, N (%) | | | 0.015 |
|
Yes | 33 (31.4) | 5 (11.9) | |
|
No | 72 (68.6) | 37 (88.1) | |
| Anemia and
thrombocytopenia, N (%) | | | 0.064 |
|
Yes | 87 (82.9) | 29 (27.6) | |
|
No | 18 (17.1) | 13 (12.4) | |
| Pancytopenia, N
(%)b | | | 0.019 |
|
Yes | 32 (30.5) | 5 (11.9) | |
|
No | 73 (69.5) | 37 (88.1) | |
CBC results analysis in patients with
and without M3 AML
Subsequently, the CBC results of patients with de
novo AML (n=94) were investigated further, including 24
patients with M3 AML and 70 patients with non-M3 AML (Tables SII and SIII). Patients with M3 AML had a lower
median age (47 vs. 55 years), compared with that in patients with
non-M3 AML. The same gender ratio was observed in patients with and
without M3 AML. Notably, WBC count was found to be significantly
lower in the M3 group compared with that in the non-M3 group
(P=0.003). Both the neutrophil count (P=0.020) and lymphocyte count
(P<0.001) were also observed to be significantly lower in the M3
group compared with those in the non-M3 AML group. In addition, the
platelet count was lower in the M3 group compared with that in the
non-M3 AML group (P=0.031).
Hypocytosis in patients with and
without M3 AML
The frequencies of different forms of hypocytosis
were also compared between patients with M3 and non-M3 AML
(Table SIV). The percentage of
patients with leukopenia was found to be significantly higher in
the M3 AML group compared with that in the non-M3 AML group
(P=0.007). Conversely, the percentage of patients with leukocytosis
was significantly higher in the non-M3 AML group compared with that
in the M3 AML group (P=0.034). Furthermore, the percentage of
patients with both leukopenia and anemia was significantly higher
in the M3 AML group compared with that in the non-M3 AML group
(P=0.033).
OS analysis
To further understand the influence of various
factors, such as hemocytopenia, on the prognosis of patients, their
OS was next analyzed. Notably, 84.4% (n=124, including 91 patients
with AML and 33 patients with ALL) of patients with AL received
chemotherapy treatments, whilst 15.6% (n=23, including 14 patients
with AML and 9 patients with ALL) received supportive care
(Table SI). In particular,
patients with non-M3 AML received fludarabine, cytarabine and
mitoxantrone, the standard ‘3 + 7’ regimens or the reduced ‘3 +
7’-based regimens (19,20). By contrast, patients with M3 AML
received treatment regimens containing all-trans retinoic acid
(ATRA) + anthracyclines or arsenic trioxide (ATO) + ATRA (21-23).
Patients with ALL received corticosteroids alone or in combination
with another drug (such as vincristine or cyclophosphamide)
(24). Of all patients, 10.9%
underwent hematopoietic stem cell transplantation, including 7
patients with AML and 9 patients with ALL (Table SI).
In patients with AL, the median survival was 30
months and 5-year OS was 25.2% (patients with AML: Median survival,
24 months; 5-year survival, 26.7%; ALL patients: Median survival,
33 months; 5-year survival, 21.4%). Among patients with AML, the
5-year survival rates for patients with M3 and non-M3 AML were
50.0% and 18.6%, respectively.
The two common prognostic factors associated with
shorter survival in patients with AL (Table III), with AML (Table IV) and non-M3 AML (Table V) were found to be age >60 years
and WBC count >10x109/l. However, OS did not
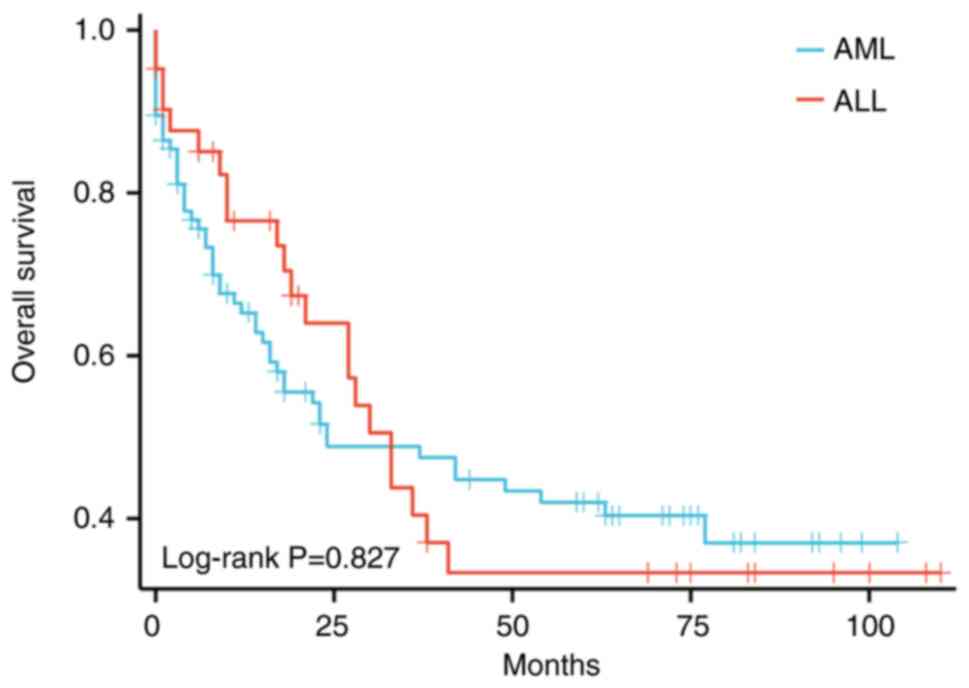
significantly differ between patients with AML and ALL (Fig. 1 and Table III). Among patients with AML, the
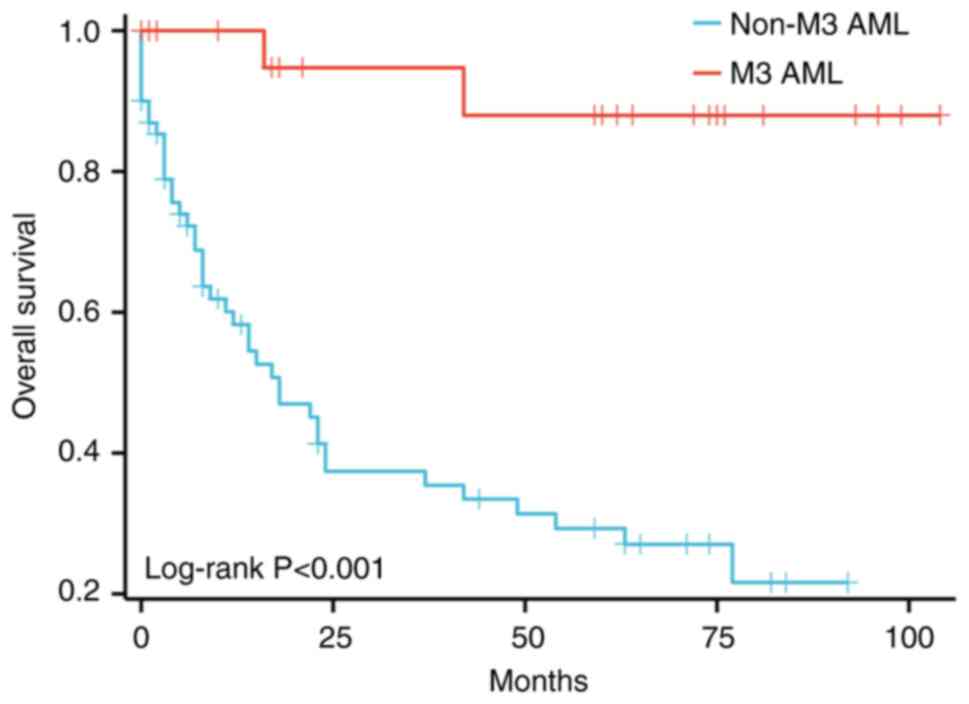
median OS of patients with M3 AML were not achieved, whilst those
with non-M3 AML were 18 months. Patients with M3 AML had
significantly longer OS compared with those with non-M3 AML
(P<0.05; Fig. 2 and Table IV). For patients with ALL, the
prognostic factors for survival duration were age and hemoglobin
level (Table VI). In addition,
patients with ALL aged <60 years and anemia were found to be
associated with a favorable outcome (for age ≥60 years: HR, 2.715;
95% CI, 1.069-6.894; P=0.036; for anemia: HR, 0.185; 95% CI,
0.046-0.747; P=0.018).
 | Table IIIUnivariate and multivariable analyses
of variables predicting overall survival in the patients with acute
leukemia. |
Table III
Univariate and multivariable analyses
of variables predicting overall survival in the patients with acute
leukemia.
| | Univariate
analysis | Multivariable
analysis |
|---|
| Variable | Comparison
groups | Wald | HR (95% CI) | P-value | Wald | HR (95% CI) | P-value |
|---|
|
French-American-British system
classification | Acute lymphocytic
leukemia vs. Acute myeloid leukemia | 0.047 | 0.947
(0.575-1.558) | 0.829 | | | |
| Sex | Male vs.
Female | 0.021 | 0.967
(0.615-1.521) | 0.884 | | | |
| Age, years | ≥60 vs. <60 | 18.612 | 2.725
(1.728-4.297) | <0.001 | 25.483 | 3.522
(2.160-5.741) | <0.001 |
| White blood cell
count, x109/l | <3.5 vs.
≥3.5 | 0.130 | 0.914
(0.559-1.493) | 0.718 | | | |
| | >10.0 vs.
≤10.0 | 8.129 | 1.937
(1.230-3.053) | 0.004 | 17.731 | 2.826
(1.742-4.583) | <0.001 |
| Hemoglobin,
g/l | Male, <120 vs.
≥120; female, <110 vs. ≥110 | 0.438 | 1.406
(0.513-3.852) | 0.508 | | | |
| Platelet count,
x109/l | <100 vs.
≥100 | 1.482 | 1.452
(0.797-2.648) | 0.223 | | | |
|
Pancytopeniaa | Yes vs. no | 0.130 | 0.914
(0.559-1.493) | 0.718 | | | |
| Received a
transplant | Yes vs. no | 3.946 | 0.428
(0.185-0.989) | 0.047 | 2.808 | 0.485
(0.208-1.130) | 0.094 |
 | Table IVUnivariate and multivariable analyses
of variables predicting overall survival in patients with AML. |
Table IV
Univariate and multivariable analyses
of variables predicting overall survival in patients with AML.
| | Univariate
analysis | Multivariable
analysis |
|---|
| Variable | Comparison
groups | Wald | HR (95% CI) | P-value | Wald | HR (95% CI) | P-value |
|---|
|
French-American-British classification | M3 AML vs. Non-M3
AML | 11.470 | 0.087
(0.021-0.357) | 0.001 | 8.333 | 0.122
(0.029-0.509) | 0.004 |
| Sex | Male vs.
Female | 0.182 | 0.889
(0.518-1.525) | 0.669 | | | |
| Age, years | ≥60 vs. <60 | 13.271 | 2.749
(1.595-4.736) | <0.001 | 15.441 | 3.486
(1.870-6.499) | <0.001 |
| White blood cell
count, x109/l | <3.5 vs.
≥3.5 | 0.178 | 0.886
(0.505-1.555) | 0.673 | | | |
| | >10.0 vs.
≤10.0 | 4.965 | 1.858
(1.077-3.204) | 0.026 | 10.540 | 2.811
(1.506-5.245) | 0.001 |
| Hemoglobin,
g/l | Male: <120 vs.
≥120 | 2.018 | 4.203
(0.580-30.469) | 0.155 | | | |
| | Female: <110 vs.
≥110 | | | | | | |
| Platelet count,
x109/l | <100 vs.
≥100 | 0.205 | 1.181
(0.575-2.429) | 0.651 | | | |
|
Pancytopeniaa | Yes vs. no | 0.914 | 1.334
(0.739-2.410) | 0.339 | | | |
| Received a
transplant | Yes vs. no | 1.231 | 0.516
(0.161-1.660) | 0.267 | | | |
 | Table VUnivariate and multivariable analyses
of variables predicting overall survival in patients with non-M3
acute myeloid leukemia. |
Table V
Univariate and multivariable analyses
of variables predicting overall survival in patients with non-M3
acute myeloid leukemia.
| | Univariate
analysis | Multivariable
analysis |
|---|
| Variable | Comparison
groups | Wald | HR (95% CI) | P-value | Wald | HR (95% CI) | P-value |
|---|
| Sex | Male vs.
female | 0.030 | 0.948
(0.520-1.728) | 0.862 | | | |
| Age, years | ≥60 vs. <60 | 4.331 | 1.894
(1.038-3.455) | 0.037 | 8.341 | 2.680
(1.373-5.233) | 0.004 |
| White blood cells
count, x109/l | <3.5 vs.
≥3.5 | 0.232 | 1.170
(0.617-2.220) | 0.630 | | | |
| | >10.0 vs.
≤10.0 | 4.323 | 1.937
(1.039-3.612) | 0.038 | 11.018 | 3.213
(1.613-6.402) | 0.001 |
| Hemoglobin,
g/l | Male, <120 vs.
≥120; female: <110 vs. ≥110 | 1.726 | 3.792
(0.519-27.700) | 0.189 | | | |
| Platelet count,
x109/l | <100 vs.
≥100 | 0.001 | 1.014
(0.483-2.130) | 0.971 | | | |
|
Pancytopeniaa | Yes vs. no | 1.326 | 1.503
(0.751-3.008) | 0.250 | | | |
| Received a
transplant | Yes vs. no | 3.343 | 0.332
(0.102-1.083) | 0.068 | 3.711 | 0.306
(0.092-1.021) | 0.054 |
 | Table VIUnivariate and multivariable analyses
of variables predicting overall survival in patients with acute
lymphocytic leukemia. |
Table VI
Univariate and multivariable analyses
of variables predicting overall survival in patients with acute
lymphocytic leukemia.
| | Univariate
analysis | Multivariable
analysis |
|---|
| Variable | Comparison
groups | Wald | HR (95% CI) | P-value | Wald | HR (95% CI) | P-value |
|---|
| Classification | B vs. T | 0.810 | 0.601
(0.199-1.820) | 0.368 | | | |
| Sex | Male vs.
Female | 0.227 | 1.226
(0.531-2.832) | 0.634 | | | |
| Age, years | ≥60 vs. <60 | 4.211 | 2.503
(1.042-6.012) | 0.040 | 4.415 | 2.715
(1.069-6.894) | 0.036 |
| White blood cells
count, x109/l | <3.5 vs.
≥3.5 | 0.016 | 0.933
(0.316-2.759) | 0.900 | | | |
| | >10.0 vs.
≤10.0 | 4.010 | 2.413
(1.019-5.716) | 0.045 | 3.757 | 2.451
(0.990-6.068) | 0.053 |
| Hemoglobin,
g/l | Male, <120 vs.
≥120; female, <110 vs. ≥110 | 3.737 | 0.284
(0.080-1.018) | 0.053 | 5.607 | 0.185
(0.046-0.747) | 0.018 |
| Platelet count,
x109/l | <100 vs.
≥100 | 1.783 | 2.099
(0.707-6.233) | 0.182 | | | |
|
Pancytopeniaa | Yes vs. no | 0.103 | 0.788
(0.184-3.376) | 0.748 | | | |
| Received a
transplant | Yes vs. no | 2.756 | 0.354
(0.104-1.206) | 0.097 | 1.258 | 0.484
(0.136-1.719) | 0.262 |
Discussion
AML is a leukemia subtype that targets the myeloid
lineage, whereas ALL occurs when immature lymphoblasts amplify in
the bone marrow and inhibit the generation of normal blood cells
(25). Regardless of the AL
subtype, the immature leukemic cells invade the bone marrow where
normal hematopoietic cells are produced, leading to the decreased
production of healthy cells in the bone marrow, resulting in
decrease of normal mature blood cells in the peripheral blood
(13). Therefore, the frequent
presentation involves peripheral blood and results in hypocytosis,
such as anemia, leukopenia and thrombocytopenia (13). Results from the present study
showed that normocytic normochromic anemia and thrombocytopenia are
frequent occurrences in both AML and ALL. These findings are
consistent with those from Schumacher et al (26), which reported that in AML, anemia
(normocytic normochromic anemia is the grand majority of cases)
occurrence is common whereas thrombocytopenia is also common. In
addition, this previous study (26) also reported that in ALL, normocytic
normochromic anemia is commonly present and thrombocytopenia is
generally severe. However, the difference between AML and ALL in
terms of hypocytosis could not be derived from Schumacher et
al (26) and other previous
reports (1,27-30).
In order to solve this issue, the present study performed CBC
results analysis between patients with AML and ALL.
ALL patients were found to have significantly higher
lymphocyte and RBC counts compared with those in patients with AML
in the present study. In addition, the percentage of patients with
hypocytosis (including only leukopenia, both leukopenia and anemia,
both leukopenia and thrombocytopenia, and pancytopenia) was
significantly higher in the AML group compared with that in the ALL
group. These data suggest that normal hematopoiesis is more
frequently inhibited in AML compared with that in ALL. Therefore,
patients with AL with peripheral blood findings of leukopenia,
pancytopenia, or both leukopenia and anemia or both leukopenia and
thrombocytopenia are likely to have AML. Clinically, patients with
AML are more likely to require blood transfusion compared with
patients with ALL.
The mechanisms underlying the higher incidence of
myelosuppression in AML compared with ALL require further study.
However, this difference may be due to different expression of a
number of critical regulators of hematopoiesis between AML and ALL.
In particular, GATA-1 factor, which is a hematopoietic
transcription factor, is required for the proliferation and
survival of erythroid precursors and mature cells (31), where it also serves a role in
megakaryocytic differentiation (32). Ayala et al (33) previously identified GATA-1
expression in 43.9% patients with AML and 66.7% ALL patients,
indicating markedly different expression profile between the two
diseases. Similarly, the expression of erythroid Krüppel-like
factor (EKLF), which is involved in erythroid proliferation and
hemoglobinization (34), was noted
in 39% patients with AML and 50% patients with ALL (33). These findings indicate that the
higher expression of GATA-1 and EKLF in ALL may be associated with
a lower probability of myelosuppression.
Consistent with previous studies (35-38),
the present findings showed that age <60 years and WBC count
<10x109/l were prognostic factors for longer survival
in patients with AML or patients with non-M3 AML, but leukopenia at
the time of diagnosis was not associated with the prognosis in AML.
Creutzig et al (35)
previously reported that a WBC count of <2x109/l was
associated with a superior prognosis. Although cut-offs for WBC
were set, with counts of <2x109/l indicating
leukopenia, no association between leukopenia and OS was observed
in the present study. Patients with M3 AML had significantly longer
OS compared with those with non-M3 AML, which is consistent with
previous studies (39,40). Sasaki et al (39) previously reported that after the
introduction of ATRA and ATO in the 1990s, the 5-year survival
increased from 20% during the 1980-1989 period to 75% during the
2010-2017 period in patients with M3 AML. However, the 5-year
survival rate only increased from 9 to 21% during the same period
in patients with non-M3 AML (39).
Older age and high WBC counts have been frequently considered to be
viable predictors of poor outcome in ALL (41). In the present study, for patients
with ALL, the prognostic factors for survival duration were found
to be age and hemoglobin level. A WBC count of
>10x109/l was not associated with an unfavorable
outcome in patients with ALL. This result may be due to the small
sample size. In addition, the present findings are not consistent
with that of previous study, which reported no association between
hemoglobin levels and OS in patients with ALL (42). However, other reports found that
the lower hemoglobin levels are associated with a superior outcome
in patients with ALL (43,44).
There are some limitations in the present study.
Firstly, it was a retrospective study and the data was derived from
a single center. Furthermore, only one patient with ALL at
diagnosis suffered from CNS metastasis in the present study.
Therefore, a comparative analysis of CNS metastasis incidence
between patients with AML and ALL could not be performed.
In conclusion, data from the present study suggest
that normal hematopoiesis is more frequently inhibited in patients
with AML compared with that in patients with ALL. Similarly,
amongst patients with AML, the incidence of myelosuppression is
higher in patients with M3 AML compared with non-M3 AML patients.
To the best of our knowledge, the present study was the first to
offer a peripheral blood feature set for distinguishing AML and
ALL, where patients with AL with peripheral blood findings
indicative of leukopenia, pancytopenia, or both leukopenia and
anemia or both leukopenia and thrombocytopenia are likely to have
AML. Nevertheless, this finding needs to be confirmed by studies
with a larger cohort in the future. In addition, further research
is needed to elucidate the potential mechanisms. It is hoped that
these findings can provide clinicians with future research ideas
for assessing the difference between AML and ALL, leading to more
accurate diagnoses.
Supplementary Material
Characteristics of patients with AL
according to the type of leukemia.
Characteristics of patients with M3
and non-M3 AML.
Results of complete blood count
analysis of patients with M3 and non-M3 AML.
Comparison of frequencies of different
forms of hypocytosis between patients with M3 and non-M3 AML.
Acknowledgements
Not applicable.
Funding
Funding: The present study was supported by Natural Science
Foundation of Xiamen, China (grant no. 3502Z202372058), the
Middle-aged and Young Teachers Foundation of Fujian Educational
Committee in China (grant no. JAT220407) and the Research Project
of Xiamen Medical College in China (grant no. K2023-39).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
WC and JH designed the project. HW collected
clinical data. WC and JH analyzed the data obtained in the present
study and generated the tables. WC wrote the manuscript. All the
authors have read and approved the final manuscript. WC and HW
confirm the authenticity of all the raw data.
Ethics approval and consent to
participate
The present study was approved by the Medical Ethics
Committees of Zhongshan Hospital of Xiamen University, Xiamen,
China (approval no. xmzsyyky2021151) and was conducted in
accordance with the guidelines of the institution. The need for
informed consent was waived due to the retrospective nature of the
study.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Rose-Inman H and Kuehl D: Acute leukemia.
Emerg Med Clin North Am. 32:579–596. 2014.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Terwilliger T and Abdul-Hay M: Acute
lymphoblastic leukemia: A comprehensive review and 2017 update.
Blood Cancer J. 7(e577)2017.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Daver NG, Iqbal S, Renard C, Chan RJ,
Hasegawa K, Hu H, Tse P, Yan J, Zoratti MJ, Xie F and Ramsingh G:
Treatment outcomes for newly diagnosed, treatment-naïve
TP53-mutated acute myeloid leukemia: A systematic review and
meta-analysis. J Hematol Oncol. 16(19)2023.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Malard F and Mohty M: Acute lymphoblastic
leukaemia. Lancet. 395:1146–1162. 2020.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2019. CA Cancer J Clin. 69:7–34. 2019.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Shallis RM, Wang R, Davidoff A, Ma X and
Zeidan AM: Epidemiology of acute myeloid leukemia: Recent progress
and enduring challenges. Blood Rev. 36:70–87. 2019.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Pulte D, Gondos A and Brenner H:
Improvement in survival in younger patients with acute
lymphoblastic leukemia from the 1980s to the early 21st century.
Blood. 113:1408–1411. 2009.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Pulte D, Jansen L, Gondos A, Katalinic A,
Barnes B, Ressing M, Holleczek B, Eberle A and Brenner H: GEKID
Cancer Survival Working Group. Survival of adults with acute
lymphoblastic leukemia in Germany and the United States. PLoS One.
9(e85554)2014.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Hunger SP, Lu X, Devidas M, Camitta BM,
Gaynon PS, Winick NJ, Reaman GH and Carroll WL: Improved survival
for children and adolescents with acute lymphoblastic leukemia
between 1990 and 2005: A report from the children's oncology group.
J Clin Oncol. 30:1663–1669. 2012.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Suguna E, Farhana R, Kanimozhi E, Kumar
PS, Kumaramanickavel G and Kumar CS: Acute myeloid leukemia:
Diagnosis and management based on current molecular genetics
approach. Cardiovasc Hematol Disord Drug Targets. 18:199–207.
2018.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Bennett JM, Catovsky D, Daniel MT,
Flandrin G, Galton DA, Gralnick HR and Sultan C: Proposals for the
classification of the acute leukaemias. French-American-British
(FAB) co-operative group. Br J Haematol. 33:451–458.
1976.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Arber DA, Orazi A, Hasserjian R, Thiele J,
Borowitz MJ, Le Beau MM, Bloomfield CD, Cazzola M and Vardiman JW:
The 2016 revision to the World Health Organization classification
of myeloid neoplasms and acute leukemia. Blood. 127:2391–2405.
2016.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Blackburn LM, Bender S and Brown S: Acute
leukemia: Diagnosis and treatment. Semin Oncol Nurs.
35(150950)2019.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Sekar MD, Raj M and Manivannan P: Role of
morphology in the diagnosis of acute leukemias: Systematic review.
Indian J Med Paediatr Oncol. 44:464–473. 2023.
|
|
15
|
Ladines-Castro W, Barragán-Ibañez G,
Luna-Pérez MA, Santoyo-Sánchez A, Collazo-Jaloma J, Mendoza-García
E and Ramos-Peñafiel CO: Morphology of leukaemias. Rev Méd Hosp Gen
Méx. 79:107–113. 2016.
|
|
16
|
Haznedaroğlu İC, Kuzu I and İlhan O: WHO
2016 definition of chronic myeloid leukemia and tyrosine kinase
inhibitors. Turk J Haematol. 37:42–47. 2020.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Shi M, Qin Y, Chen S, Wei W, Meng S, Chen
X, Li J, Li Y, Chen R, Su J, et al: Characteristics and risk
factors for readmission in HIV-infected patients with Talaromyces
marneffei infection. PLoS Negl Trop Dis.
17(e0011622)2023.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Liu CA, Zhang Q, Ruan GT, Shen LY, Xie HL,
Liu T, Tang M, Zhang X, Yang M, Hu CL, et al: Novel diagnostic and
prognostic tools for lung cancer cachexia: Based on nutritional and
inflammatory status. Front Oncol. 12(890745)2022.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Ma TT, Lin XJ, Cheng WY, Xue Q, Wang SY,
Liu FJ, Yan H, Zhu YM and Shen Y: Development and validation of a
prognostic model for adult patients with acute myeloid leukaemia.
EBioMedicine. 62(103126)2020.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Koller CA, Kantarjian HM, Feldman EJ,
O'Brien S, Rios MB, Estey E and Keating M: A phase I-II trial of
escalating doses of mitoxantrone with fixed doses of cytarabine
plus fludarabine as salvage therapy for patients with acute
leukemia and the blastic phase of chronic myelogenous leukemia.
Cancer. 86:2246–2251. 1999.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Lo-Coco F, Avvisati G, Vignetti M, Thiede
C, Orlando SM, Iacobelli S, Ferrara F, Fazi P, Cicconi L, Di Bona
E, et al: Retinoic acid and arsenic trioxide for acute
promyelocytic leukemia. N Engl J Med. 369:111–121. 2013.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Rego EM, Kim HT, Ruiz-Argüelles GJ,
Undurraga MS, Uriarte Mdel R, Jacomo RH, Gutiérrez-Aguirre H, Melo
RA, Bittencourt R, Pasquini R, et al: Improving acute promyelocytic
leukemia (APL) outcome in developing countries through networking,
results of the international consortium on APL. Blood.
121:1935–1943. 2013.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Dayama A, Dass J, Seth T, Mahapatra M,
Mishra PC and Saxena R: Clinico-hematological profile and outcome
of acute promyelocytic leukemia patients at a tertiary care center
in North India. Indian J Cancer. 52:309–312. 2015.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Hoelzer D, Bassan R, Dombret H, Fielding
A, Ribera JM and Buske C: ESMO Guidelines Committee. Acute
lymphoblastic leukaemia in adult patients: ESMO clinical practice
guidelines for diagnosis, treatment and follow-up. Ann Oncol. 27
(Suppl 5):v69–v82. 2016.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Pui CH, Robison LL and Look AT: Acute
lymphoblastic leukaemia. Lancet. 371:1030–1043. 2008.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Schumacher HR, Alvares CJ, Blough RI and
Mazzella F: Acute leukemia. Clin Lab Med. 22:153–192.
2002.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Cornell RF and Palmer J: Adult acute
leukemia. Dis Mon. 58:219–238. 2012.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Cripe LD: Adult acute leukemia. Curr Probl
Cancer. 21:1–64. 1997.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Meenaghan T, Dowling M and Kelly M: Acute
leukaemia: Making sense of a complex blood cancer. Br J Nurs.
21:76. 78–83. 2012.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Gralnick HR, Galton DAG, Catovsky D,
Sultan C and Bennett JM: Classification of acute leukemia. Ann
Intern Med. 87:740–753. 1977.
|
|
31
|
Weiss MJ and Orkin SH: Transcription
factor GATA-1 permits survival and maturation of erythroid
precursors by preventing apoptosis. Proc Natl Acad Sci USA.
92:9623–9627. 1995.PubMed/NCBI View Article : Google Scholar
|
|
32
|
McDevitt MA, Fujiwara Y, Shivdasani RA and
Orkin SH: An upstream, DNase I hypersensitive region of the
hematopoietic-expressed transcription factor GATA-1 gene confers
developmental specificity in transgenic mice. Proc Natl Acad Sci
USA. 94:7976–7981. 1997.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Ayala RM, Martínez-López J, Albízua E,
Diez A and Gilsanz F: Clinical significance of Gata-1, Gata-2,
EKLF, asnd c-MPL expression in acute myeloid leukemia. Am J
Hematol. 84:79–86. 2009.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Perkins AC, Sharpe AH and Orkin SH: Lethal
beta-thalassaemia in mice lacking the erythroid CACCC-transcription
factor EKLF. Nature. 375:318–322. 1995.PubMed/NCBI View
Article : Google Scholar
|
|
35
|
Creutzig U, Zimmermann M, Ritter J, Henze
G, Graf N, Löffler H and Schellong G: Definition of a standard-risk
group in children with AML. Br J Haematol. 104:630–639.
1999.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Redaelli A, Lee JM, Stephens JM and Pashos
CL: Epidemiology and clinical burden of acute myeloid leukemia.
Expert Rev Anticancer Ther. 3:695–710. 2003.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Ganzel C and Rowe JM: Prognostic factors
in adult acute leukemia. Hematol Oncol Clin North Am. 25:1163–1187.
2011.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Arellano M, Bernal-Mizrachi L, Pan L,
Tighiouart M, Souza L, Guo X, McLemore M, Lima L, Sunay S, Heffner
LT, et al: Prognostic significance of leukopenia at the time of
diagnosis in acute myeloid leukemia. Clin Lymphoma Myeloma Leuk.
11:427–432. 2011.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Sasaki K, Ravandi F, Kadia TM, DiNardo CD,
Short NJ, Borthakur G, Jabbour E and Kantarjian HM: De novo acute
myeloid leukemia: A population-based study of outcome in the United
States based on the surveillance, epidemiology, and end results
(SEER) database, 1980 to 2017. Cancer. 127:2049–2061.
2021.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Hu J, Liu YF, Wu CF, Xu F, Shen ZX, Zhu
YM, Li JM, Tang W, Zhao WL, Wu W, et al: Long-term efficacy and
safety of all-trans retinoic acid/arsenic trioxide-based therapy in
newly diagnosed acute promyelocytic leukemia. Proc Natl Acad Sci
USA. 106:3342–3347. 2009.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Bassan R and Hoelzer D: Modern therapy of
acute lymphoblastic leukemia. J Clin Oncol. 29:532–543.
2011.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Baccarani M, Corbelli G, Amadori S,
Drenthe-Schonk A, Willemze R, Meloni G, Cardozo PL, Haanen C,
Mandelli F and Tura S: Adolescent and adult acute lymphoblastic
leukemia: Prognostic features and outcome of therapy. A study of
293 patients. Blood. 60:677–684. 1982.PubMed/NCBI
|
|
43
|
Ng SM, Lin HP, Ariffin WA, Zainab AK, Lam
SK and Chan LL: Age, sex, haemoglobin level, and white cell count
at diagnosis are important prognostic factors in children with
acute lymphoblastic leukemia treated with BFM-type protocol. J Trop
Pediatr. 46:338–343. 2000.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Settin A, Al Haggar M, Al Dosoky T, Al Baz
R, Abdelrazik N, Fouda M, Aref S and Al-Tonbary Y: Prognostic
cytogenetic markers in childhood acute lymphoblastic leukemia.
Indian J Pediatr. 74:255–263. 2007.PubMed/NCBI View Article : Google Scholar
|