Introduction
As of 2022, liver cancer ranked as the 8th most
common cancer worldwide. According to Global Cancer Statistics
2022, there were 865,269 new cases of liver cancer diagnosed and
757,948 deaths were reported. Mongolia demonstrates the highest
occurrence of hepatocellular carcinoma (HCC) globally, with a rate
of 86 cases per 100,000 people. This prevalence greatly surpasses
that of neighboring nations, being 4 times higher compared with
China, and >20 times higher compared with Russia, as well as
surpassing the incidence rates observed in any other country
worldwide (1).
In the diagnosis of HCC, alpha-fetoprotein (AFP) is
widely used as a biomarker. However, problems associated with its
poor specificity and sensitivity have been reported in certain
studies (2,3). According to international standards,
AFP test results are typically confirmed with the assistance of
computerized tomography scans and magnetic resonance imaging
analyses (4-6).
However, in Mongolia, there are fewer opportunities to perform
these scans at present. A recent study has shown that the use of
biomarkers such as glypican-3 (GPC3), Golgi protein 73 and
des-gamma carboxyprothrombin, in addition to AFP, has practical
benefits for the early diagnosis of liver cancer (7). Among these markers, GPC3 has
attracted significant attention, primarily due to the fact that it
is specifically expressed in tumors. Moreover, it is potentially
useful as a marker for cases of HCC where AFP levels are either low
or absent, and in combination with AFP (8-11).
GPC3 is a heparan sulfate proteoglycan consisting of
580 amino acids, with a molecular weight of 70 kDa, which is
attached to the glycosyl-phosphatidylinositol region of cell
membranes (12,13). The GPC3 gene is located on the
human X chromosome (in the Xq26 chromosomal region) and has
important roles in cell proliferation and division during
embryogenesis. The expression of GPC3 has been identified in fetal
placenta, liver, lung and kidney tissues, although it is rarely
found in the tissues of healthy adults (14). During embryonic development, GPC3
interacts with signaling pathways and proteins, including the Wnt,
fibroblast growth factor and bone morphogenetic proteins signaling
pathways, to regulate cell division, proliferation and apoptosis
(15-18).
In addition, studies have shown that activated GPC3 protein
increases the rates of cell proliferation and growth by increasing
the synthesis of heparan sulfate growth factors from tumor cells
through the sulfatase-2 enzyme (19,20).
A number of studies have also shown that the levels
of GPC3 are increased in the serum and tissues of patients with
HCC, but not in cases of liver injury, cirrhosis or viral hepatitis
(21,22). Moreover, several studies have
performed immunohistochemical (IHC) evaluations of GPC3 protein
expression in HCC tissue samples, wherein it was noted that GPC3
may be useful as a marker for tumor diagnosis, staging, treatment
outcome, disease progression and recurrence (23-26).
To the best of the authors' knowledge, the tissue
expression of GPC3 protein has not been reported in Mongolian
patients with HCC. Therefore, the aim of the present study was to
assess the association between GPC3 protein and the clinical
characteristics of Mongolian patients with HCC.
Materials and methods
Study subjects and samples
Laboratory experiments were performed in
collaboration with the Central Scientific Research Laboratory of
the Institute of Medical Sciences (Ulaanbaatar, Mongolia) and the
Hepato-Biliary-Pancreatic Surgical Department of the National
Cancer Center of Mongolia (Ulaanbaatar, Mongolia). Liver tissue and
serum samples from patients with HCC were collected between October
2022 and March 2023 at the National Cancer Center in Ulaanbaatar,
Mongolia. Serum samples from the control and risk groups were
collected during the same period at The Third Central Hospital in
Ulaanbaatar, Mongolia.
Serum samples were collected from a total of 270
participants, comprising the HCC group (n=90), the risk group (RG)
(n=90) and the healthy control group (n=90). The RG included
patients with chronic hepatitis, toxic hepatitis,
alcohol-associated liver disease and other liver disorders. The
average age of the participants was 61.0±9.5 years. A total of 107
(39.7%) of the subjects were males and 163 (60.3%) were
females.
A total of 64 people who were diagnosed with HCC and
underwent surgical treatment were included in the tissue analysis.
Following surgery, 14 participants were excluded from the study, as
pathological examination of the cancer tissues extracted during
surgery revealed that their diagnoses were other than HCC. GPC3
protein expression was evaluated in both the cancerous tissue and
the surrounding tissue of 50 patients with HCC using an IHC
staining method. The average age of the participants was 64.06±9.1
years. A total of 21 (42%) of the subjects were males and 29 (58%)
were females.
Tumor staging was conducted based on the 8th
tumor-node-metastasis (TNM) classification system for liver cancer
[The National Comprehensive Cancer Network®
(NCCN®), version 5.2020(27): AJCC, 8th edn, 2017(28)]. Tumors were classified according to
the predominant histological subtype proposed by the 2017 American
Joint Committee on Cancer classification system.
The present study was approved by the Ethics
Committee of Mongolian National University of Medical Sciences
(approval nos. 2022/3-05 and 2022.05.20; Ulaanbaatar, Mongolia),
and the various experimental procedures were performed according to
the Declaration of Helsinki (2013). All patients provided written
informed consent.
Detection of the serum GPC3 (sGPC3)
level
GPC3 in serum of patients was detected using a Human
Glypican 3 Quantikine® QuicKit™ enzyme-linked
immunosorbent assay (ELISA) Kit (cat. no. DGLY30) from R&D
Systems, Inc. ELISA analysis was performed according to the
manufacturer's protocol using a BIOBASE-EL10A ELISA microplate
reader. The optical density was determined at 450 nm. All
procedures were performed at room temperature and all samples were
measured in triplicate.
IHC analysis
The protein expression of GPC3 was assessed on
extracted HCC tissues through IHC staining using an anti-GPC3
antibody (200:1; cat. no. SC-65443; Santa Cruz Biotechnology, Inc.
https://www.scbt.com/home). Liver tissues were
fixed in 10% neutral buffered formalin for 24 h at room
temperature. Paraffin embedded tissue blocks were cut into 4-µm
sections for IHC staining. The prepared tissues were deparaffinized
in xylene at room temperature (18-22˚C) for 10 min, followed by
rehydration in a 100, 100, 95, 80 and 70% ethanol series at room
temperature (2 min each dilution of ethanol). Subsequently, the
tissue slices were subjected to antigen retrieval at 120˚C for 10
min in 10 mmol/l citrate buffer (pH 6.0).
Subsequently, endogenous peroxidase activity was
quenched by treatment with 3% hydrogen peroxide dissolved in
methanol, followed by treatment with the anti-GPC3 primary
antibody. The antibody was incubated with the tissues at room
temperature for 1 h. Subsequently, a secondary antibody
[broad-spectrum secondary antibody solution; cat. no. D01-110;
Golden Bridge International (GBI) (Labs) Ltd. https://www.gbiinc.com/] was added, and incubated with
the tissue at room temperature for 15 min according to the
manufacturer's protocols. Streptavidin-HRP was added, and incubated
at room temperature for a further 15 min. Finally, a drop of
aminoethyl carbazole solution [cat. no. C01-12; GBI (Labs) Ltd.
https://www.gbiinc.com/] was applied to the
tissue, which was observed under a microscope (Olympus BX41;
Olympus Corporation) until a red color developed.
Tissue microarray analysis (TMA)
The paraffin-embedded liver cancer tissue was
observed under a light microscope (Olympus CHA; Olympus
Corporation), and a suitable area was marked out. A 2-mm diameter
punch was inserted into the cancer tissue to a depth of 5 mm, which
was then placed into a newly prepared paraffin block. After
inserting all the tissues, 1-µm thick tissue sections were cut from
the prepared tissue microarray block using a microtome prior to IHC
analysis as aforementioned.
Evaluation of GPC3 in tissue
samples
When evaluating the results, the expression of GPC3
protein was categorized as negative, weakly positive or strongly
positive based on the percentage of cells exhibiting red-brown
staining, and the intensity of the staining within the cell
membrane, cytoplasm and nucleus. The following criteria were used
to assign scores, which were determined according to the average
scores calculated by histopathologists and researchers: Score, 0:
Percentage of stained cells <5%; score, 1: Percentage of stained
cells 5-25%; score, 2: Percentage of stained cells 26-50%; and
score, 3: Percentage of stained cells >50%. In terms of the
color intensity, the following values were assigned: Score, 0: No
staining; score, 1: Weak staining; score, 2: Moderate; and score,
3: Strong staining. To calculate the final score, the percentage of
stained cells score was multiplied by the intensity of staining
score (range 0-9) as follows: Negative, 0-1; weakly positive, 2-4;
moderate staining, 5-7; strongly positive, 8-9.
Statistical analysis
Statistical analysis for the tables was performed
using Chi-square (χ2) test or Fisher's exact test [to
test the association between the sGPC3 level and the patient's
clinical and histopathological characteristics] with SPSS software
(version 24.0; IBM Corp.). One way ANOVA test was applied and
followed by Tukey's test to analyze the differences in sGPC3 levels
among the groups. P<0.05 was considered to indicate a
statistically significant difference. Receiver operating
characteristic (ROC) curves were performed to define the optimal
cut-off values, and to assess sensitivity, specificity and
respective areas under the curve (AUCs). Data are expressed as the
mean ± SD, or n (%). All experiments were conducted in
triplicate.
Results
sGPC3 is a diagnostic marker for
HCC
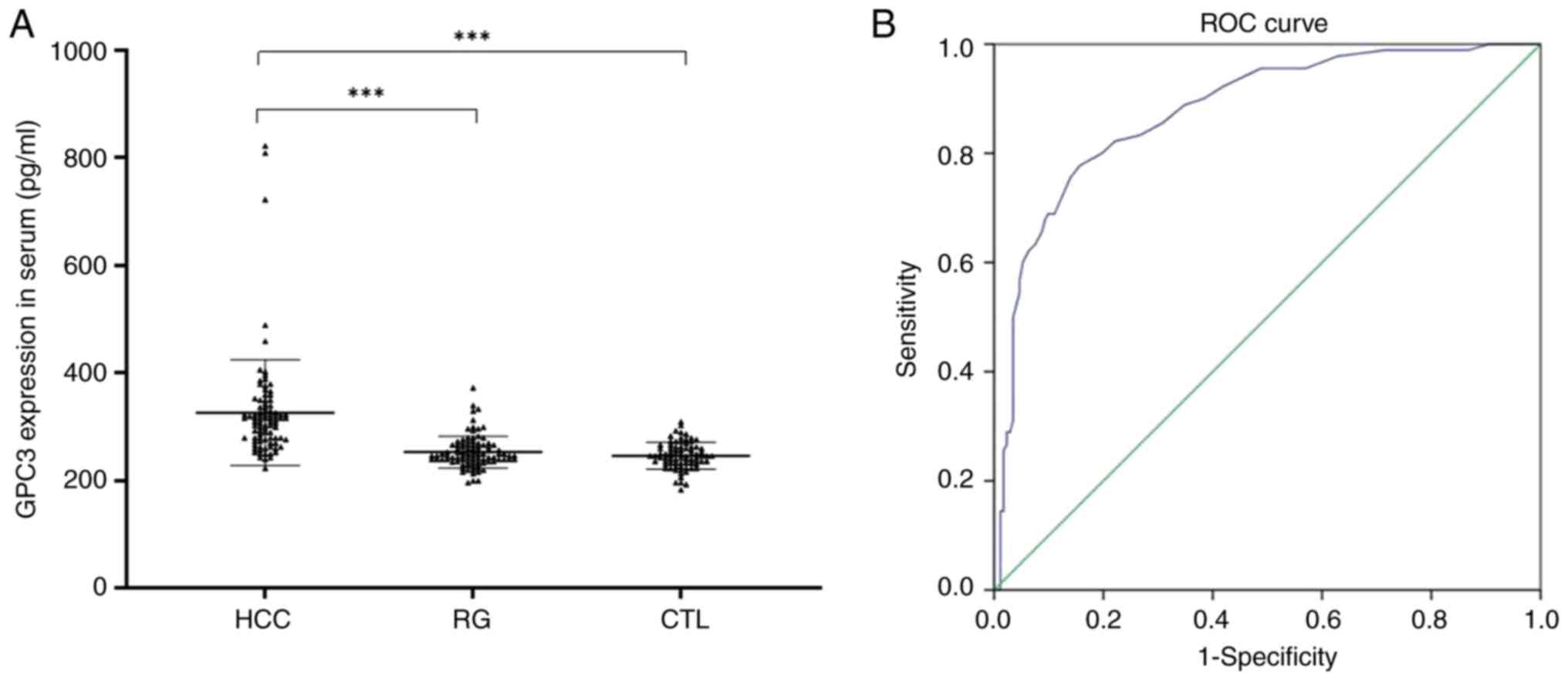
The sGPC3 levels in the 3 different experimental
groups (HCC group, RG and control group) are shown in Fig. 1. The median sGPC3 level was found
to be 327.25±98.22 pg/ml in the HCC group (n=90), whereas the
median values ± range were 253.21±29.53 pg/ml in the RG (n=90) and
245.31±23.38 pg/ml in the control group (n=90).
Significant differences in the sGPC3 level were
noted among these 3 groups (P=0.001); however, the sGPC3 levels in
the RG were not significantly different from those in the control
group (P>0.05; Fig. 1A).
The ROC curve of sGPC3 is presented in Fig. 1B. Based on the ROC curve, the
cut-off value was set to 270 pg/ml. The sensitivity and specificity
percentage values were 83.3 and 84.4% respectively, with AUC=0.892.
The positive predictive value was 83%, whereas the negative
predictive value was 91.02%.
The χ2 test and Fisher's exact test were
performed on the HCC group (n=90) to test the association between
the sGPC3 level and the patients' clinical and histopathological
characteristics (Table I).
Patients were grouped into two groups with respect to the sGPC3
level, namely >270 pg/ml (n=17) or <270 pg/ml (n=73). A
statistically significant association was observed between sGPC3
levels and cirrhosis, as well as between sGPC3 levels and hepatitis
C virus (HCV) infection. However, no significant associations were
observed between sGPC3 and the other clinical characteristics of
the patients. Statistically significant values are shown in bold
(Table I).
 | Table ISerum GPC3 level and baseline
characteristics of the patients with hepatocellular carcinoma. |
Table I
Serum GPC3 level and baseline
characteristics of the patients with hepatocellular carcinoma.
| | GPC3 (pg/ml) | |
|---|
| No. |
Characteristics | | ≥270 (n=73) | <270 (n=17) | P-value |
|---|
| 1 | Age | ≤60 | 29 | 10 | 0.152 |
| | | >60 | 44 | 7 | |
| 2 | Sex | Male | 36 | 9 | 0.788 |
| | | Female | 37 | 8 | |
| 3 | HBV | Yes | 34 | 11 | 0.178 |
| | | No | 39 | 6 | |
| 4 | HCV | Yes | 38 | 4 | 0.034a |
| | | No | 35 | 13 | |
| 5 | Cirrhosis | Yes | 58 | 8 | 0.013a |
| | | No | 15 | 9 | |
| 6 | TNM stage | T1 | 12 | 3 | 0.541 |
| | | T2 | 26 | 6 | |
| | | T3 | 30 | 4 | |
| | | T4 | 5 | 4 | |
| 7 | Tumor number | Single | 43 | 13 | 0.338 |
| | | Multiple | 30 | 4 | |
| 8 | Tumor size | ≤5 cm | 46 | 12 | 0.711 |
| | | >5 cm | 27 | 5 | |
| 9 | AFP | ≤20 ng/ml | 31 | 7 | 0.923 |
| | | >20 ng/ml | 42 | 10 | |
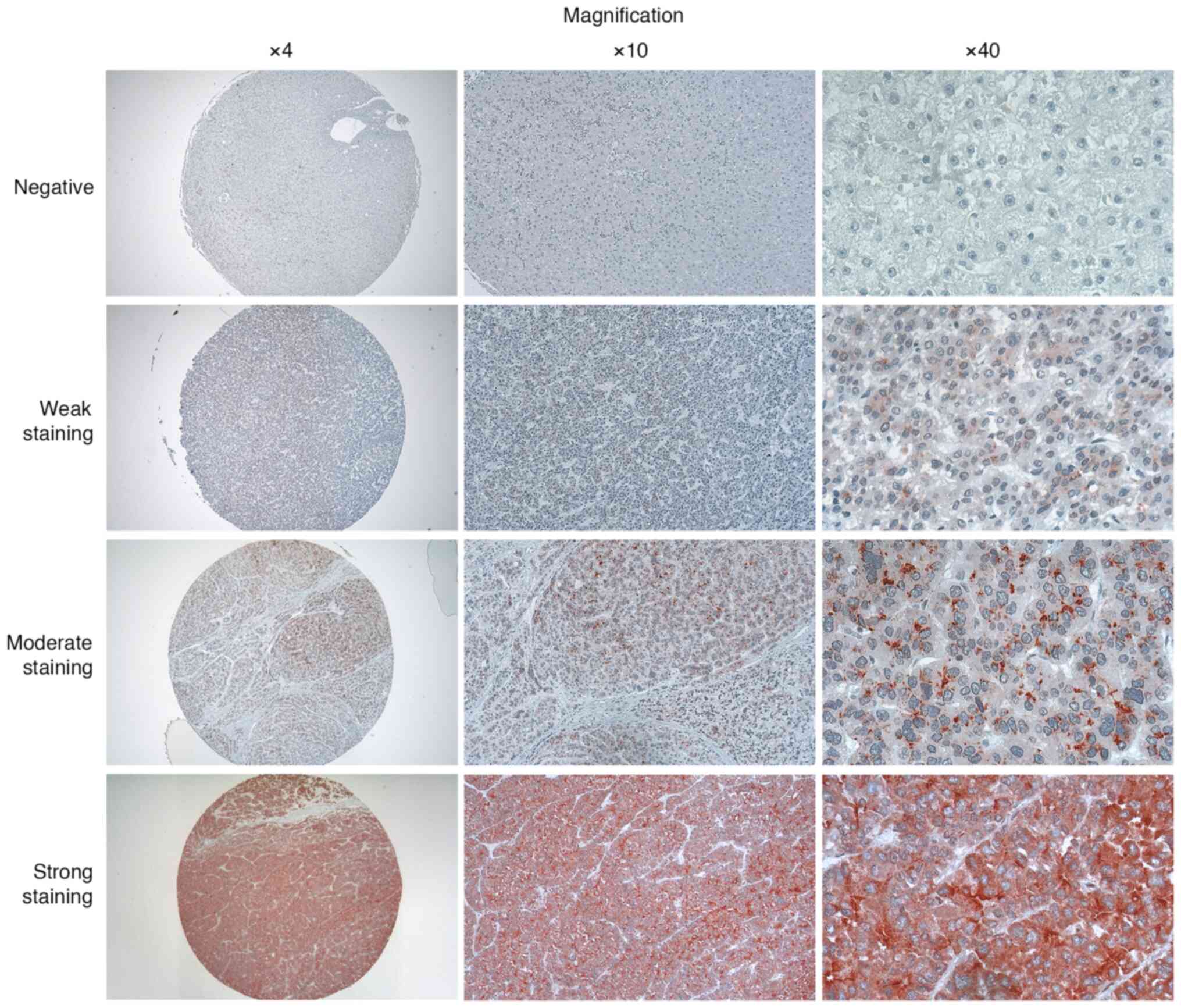
A total of 50 participants from the HCC group (21
men and 29 women aged 33-79 years, with an average age of 64.06±9.1
years) were evaluated for GPC3 expression with IHC analysis.
Through IHC staining, GPC3 protein was found to be positively
stained in the cytoplasm, membrane and canaliculi of cells in 38
out of 50 (76%) participants. Representative images of IHC staining
are demonstrated in Fig. 2. Among
these, 16 of 38 (42.1%) participants exhibited weak positive
staining, whereas the remaining 22 (57.9%) displayed strong
positive staining. TMA construction and representative images in
different magnifications are shown in Fig. 3.
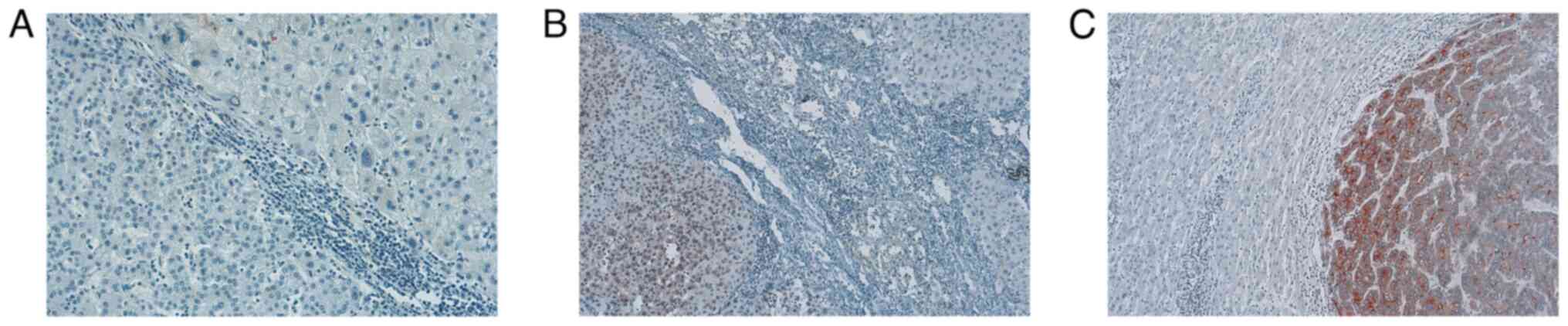 | Figure 2IHC staining of GPC3 in HCC tissue
samples. Representative images of IHC slides including both tumor
and tumor adjacent normal tissue are shown. (A) A liver tissue
stained negatively for GPC3 with poorly differentiated
hepatocellular carcinoma, marked by disorganized and bizarre cells.
The tissue section shows irregular and distorted tumor cells
against the adjacent liver tissue. The cancerous regions exhibit
highly abnormal cell shapes and sizes, contrasting with the
surrounding normal hepatocytes. (B) A liver tissue stained
moderately for GPC3 with moderately differentiated hepatocellular
carcinoma, characterized by a more irregular cellular arrangement.
The GPC3 staining is patchy, highlighting areas of tumor cells
against the adjacent liver tissue. The cancerous regions exhibit
less organized trabeculae, contrasting with the surrounding normal
hepatocyte. (C) A liver tissue stained strongly for GPC3 with a
micro-trabecular pattern of well-differentiated hepatocellular
carcinoma. The cancerous cells, marked by intense GPC3 staining,
contrast with the adjacent normal liver tissue, highlighting the
irregular trabeculae of the carcinoma against the orderly structure
of the healthy hepatocytes. Magnification, х100. IHC,
immunohistochemical; GPC3, glypican 3; HCC, hepatocellular
carcinoma. |
In early-stage cancers, GPC3 protein expression was
found to be absent in 8 out of 32 cases (25%), weakly positive in
10 out of 32 cases (31.3%), and strongly positive in 14 out of 32
cases (43.8%). Similarly, the expression of GPC3 was absent in 4
out of 18 cases (22.2%), weakly positive in 6/18 cases (33.3%), and
strongly positive in 8/18 cases (44.4%) (P>0.05) in late-stage
cancers.
In Table II, the
patients are grouped according to the tissue expression of GPC3.
After having performed the χ2 test and Fisher's exact
test, clinical characteristics such as age, sex, hepatitis B virus
(HBV) infection, HCV infection, cirrhosis, tumor number and tumor
size did not show statistically significant association with GPC3
protein expression (P>0.05).
 | Table IITissue expression of GPC3 protein and
clinical characteristics of the patients. |
Table II
Tissue expression of GPC3 protein and
clinical characteristics of the patients.
| | GPC3 | |
|---|
| No. |
Characteristics | Classification | Positive | Negative | P-value |
|---|
| 1 | Age (years) | ≤60 | 12 | 5 | 0.728 |
| | | >61 | 26 | 7 | |
| 2 | Sex | Male | 17 | 4 | 0.485 |
| | | Female | 21 | 8 | |
| 3 | HBV | Yes | 16 | 5 | 0.979 |
| | | No | 22 | 7 | |
| 4 | HCV | Yes | 18 | 8 | 0.243 |
| | | No | 20 | 4 | |
| 5 | Cirrhosis | Yes | 24 | 11 | 0.079 |
| | | No | 14 | 1 | |
| 6 | Fibrosis | Stage 2 | 6 | 2 | 0.989 |
| | (Ishak score) | Stage 3 | 9 | 2 | |
| | | Stage 4 | 15 | 5 | |
| | | Stage 5 | 5 | 2 | |
| | | Stage 6 | 3 | 1 | |
| 7 | Steatosis | Grade 0 | 26 | 4 | 0.058 |
| | | Grade 1 | 11 | 7 | |
| | | Grade 2 | 1 | 0 | |
| | | Grade 3 | 0 | 1 | |
| 8 | Histological
grade | Low grade | 18 | 5 | 0.730 |
| | | High grade | 20 | 7 | |
| 9 | Histological
cell | Classic | 32 | 10 | 1.000 |
| | type | Clear cell | 6 | 2 | |
| 10 |
Differentiation | Poorly
differentiated | 14 | 2 | 0.622 |
| | | Moderately
differentiated | 20 | 10 | |
| | | Well
differentiated | 4 | 0 | |
| 11 | TNM stage (pT) | T1 | 6 | 0 | 0.974 |
| | | T2 | 18 | 8 | |
| | | T3 | 9 | 4 | |
| | | T4 | 5 | 0 | |
| 12 | Tumor number | Single | 28 | 10 | 0.705 |
| | | Multiple | 10 | 2 | |
| 13 | Tumor size | ≤5 cm | 27 | 10 | 0.480 |
| | | >5 сm | 11 | 2 | |
| 14 | Vascular
invasion | Yes | 32 | 12 | 0.314 |
| | | No | 6 | 0 | |
| 15 | AFP | ≤20 ng/ml | 13 | 8 | 0.047 |
| | | >20 ng/ml | 25 | 4 | |
However, a statistically significant association was
observed between GPC3 protein expression and serum AFP (sAFP)
levels: In particular, when the sAFP level was either normal or
<20 ng/ml, the GPC3 protein was positively stained in 13/21
(61.9%) of cases. On the other hand, when the sAFP level was >20
ng/ml, the GPC3 protein was positively stained in 25/29 (86.2%) of
cases (P=0.047).
In addition, histopathological characteristics,
including fibrosis, steatosis, histological grade, histological
cell type, differentiation, TNM stage and vascular invasion, did
not show statistically significant association with GPC3 protein
expression (P>0.05) (Table
II).
Discussion
In the present study, the GPC3 levels in the serum
of 270 patients were initially evaluated to assess differences
between the HCC, the RG and the control group. The HCC group was
found to have significantly higher levels of sGPC3 compared with
the RG and the control group. By contrast, no significant
differences were noted between the RG and the control group.
Similarly, other research groups have shown that the sGPC3 level
was significantly elevated in patients with HCC, but not in the
healthy control group (29). In
the aforementioned study, the average sGPC3 level was found to be
99.94±267.2 ng/ml, whereas the HCC group in the present study
showed an sGPC3 level of 327.25±98.22 pg/ml (30). This difference may have been due to
differences in the assay kit sensitivity or population genetic
characteristics. On the other hand, Baatarkhuu et al
(31) investigated the difference
in sGPC3 expression levels between Mongoloids and Caucasians. They
reported no significant differences in sGPC3 between these ethnic
groups, and an increased sGPC3 level was detected in 50-55% of the
patients with HCC.
Moreover, the ROC curve of sGPC3 showed AUC=0.892,
with 83.3% sensitivity and 84.07% specificity, to distinguish HCC
from the control group. These results indicated that the sGPC3
level may effectively be used to detect HCC. Qiao et al
(32) found that the sGPC3 level
was the best marker, with an AUC of 0.892 and a cut-off value of
26.8 ng/ml, and a sensitivity of 51.5% and specificity of 92.8%
compared with AFP and human cervical cancer oncogene. In addition,
the study by Liu et al (10) revealed that sGPC3 levels were
>300 ng/l in 50% of patients with HCC with sAFP levels <100
mcg/l.
The χ2 and Fisher's exact test results in
the present study did not show any significant association between
sGPC3 and the sAFP; however, the sGPC3 level was found to be
associated with HCV infection and cirrhosis.
In several previous studies (33-42),
the range of percentages of positive GPC3 expression in the tissues
of HCC was found to be 52.5-85%, and GPC3 was not detected in
healthy liver tissue, liver injury, cirrhosis or viral hepatitis,
suggesting the possibility of this marker being used as a
diagnostic biomarker for HCC. In the present study, GPC3 protein
was shown to be positive in 76% (38/50) of the participants
diagnosed with HCC, which was similar to that reported in previous
studies. In addition, no direct associations were identified
between GPC3 protein expression and other clinical and
histopathological characteristics, including age, sex, tumor size,
tumor number, TNM stage, differentiation, histological grade,
histological cell type and vascular invasion, in the present study
(P>0.05).
However, GPC3 protein was found to be positive in
61.9% (13/21) of all participants with normal sAFP levels (≤20
ng/ml), which was consistent with the results of other studies.
Therefore, GPC3 may have more diagnostic value compared with AFP,
the traditional biomarker for the diagnosis of liver cancer
(43). Similar to this finding,
Liu et al (11) reported
that, when GPC3 was combined with AFP, the AUC and sensitivity
values were increased from 0.879 and 79.52% to 0.925 and 88.10%,
respectively. In addition, 43/68 AFP-negative patients had elevated
sGPC3 levels. These findings indicated that GPC3 may serve as a
valuable marker alongside AFP in HCC diagnosis.
The present study did, however, had certain
limitations. First, the number of samples was small and, it was not
possible to collect survival and recurrence data due to the limited
source. Therefore, larger samples are required in future studies
with survival analysis. Furthermore, benign liver diseases could
not be included for making comparisons with the HCC tissue samples
due to the small sample size. Lastly, the experiments were
conducted in a limited amount of time. In a future study, the
authors plan to employ in vitro assays using an expression
vector to further evaluate the potential of the marker.
In conclusion, in the present study, a high level of
sGPC3 was observed in the HCC group, and this was found to be
associated with the HCV status and cirrhosis. In the tissue
analysis, GPC3 protein was specifically expressed in the cytoplasm,
membrane and canaliculi of HCC. The expression of GPC3 was not
found to be associated with other clinical features, such as age,
sex, viral hepatitis, cirrhosis, tumor, size, histological cell
type and vascular invasion. However, the tissue expression of GPC3
was directly correlated with the AFP level in the serum. Hence, it
is considered that the potential importance of GPC3 in HCC
diagnosis should be further studied by determining the amount of
GPC3 protein in the serum of participants, and comparing this with
the results of the IHC analysis.
Acknowledgements
Not applicable.
Funding
Funding: The present study was supported by the Mongolian
National University of Medical Sciences, Supporting Foundation for
Science and Technology (grant no. 2022/24).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
BBat, MS, OT, NN and BK analyzed and interpreted
patient data on ELISA, IHC and TMA. MS, BBat, OT and BM designed
and conducted the present study. BBat, OT and NEN collected serum
samples from the subjects. UG, GK, EB, MR, DEO, MBo, MBy, YA, MC,
NG, AB, TD, LNO and BBay collected tissue samples and performed
histological examinations of hepatocellular carcinoma and combined
the clinical data of patients. BBat, BM and MS were major
contributors to the writing of the manuscript. SJ, MBat and TL
designed the present study, confirm the authenticity of all the raw
data, revised the manuscript and gave the final approval for
publishing the manuscript. All authors read and approved the final
version of the manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of the Mongolian National University of Medical Sciences
(approval nos. 2022/3-05 and 2022.05.20; Ulaanbaatar, Mongolia),
and all procedures were conducted according to the Declaration of
Helsinki. All patients provided written informed consent.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Sung H, Ferlay J, Siegel RL, Laversanne M,
Soerjomataram I, Jemal A and Bray F: Global Cancer Statistics 2020:
GLOBOCAN estimates of incidence and mortality worldwide for 36
cancers in 185 countries. CA Cancer J Clin. 71:209–249.
2021.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Wang T and Zhang KH: New blood biomarkers
for the diagnosis of AFP-Negative hepatocellular carcinoma. Front
Oncol. 10(1316)2020.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Gupta S, Bent S and Kohlwes J: Test
characteristics of α-fetoprotein for detecting hepatocellular
carcinoma in patients with hepatitis C: A systematic review and
critical analysis. Ann Intern Med. 139:46–50. 2003.PubMed/NCBI View Article : Google Scholar
|
|
4
|
European Association for the Study of the
Liver. Electronic address; easloffice@easloffice.eu; European
Association for the Study of the Liver. EASL clinical practice
guidelines: Management of hepatocellular carcinoma. J Hepatol.
69:182–236. 2018.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Omata M, Cheng AL, Kokudo N, Kudo M, Lee
JM, Jia J, Tateishi R, Han KH, Chawla YK, Shiina S, et al:
Asia-Pacific clinical practice guidelines on the management of
hepatocellular carcinoma: A 2017 update. Hepatol Int. 11:317–370.
2017.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Zhou J, Sun HC, Wang Z, Cong WM, Wang JH,
Zeng MS, Yang JM, Bie P, Liu LX, Wen TF, et al: Guidelines for
diagnosis and treatment of primary liver cancer in China (2017
Edition). Liver Cancer. 7:235–260. 2018.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Mansouri V, Razzaghi M, Nikzamir A,
Ahmadzadeh A, Iranshahi M, Haghazali M and Hamdieh M: Assessment of
liver cancer biomarkers. Gastroenterol Hepatol Bed Bench. 13 (Suppl
1):S29–S39. 2020.PubMed/NCBI
|
|
8
|
Ye L, Li D, Chen Y and Yu X: Evaluation
for clinical and prognostic implications of glypican-3 and
α-fetoprotein in hepatocellular carcinoma: A new subtype? Transl
Cancer Res. 9:3443–3452. 2020.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Xu D, Su C, Sun L, Gao Y and Li Y:
Performance of serum glypican 3 in diagnosis of hepatocellular
carcinoma: A meta-analysis. Ann Hepatol. 18:58–67. 2019.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Liu H, Li P, Zhai Y, Qu CF, Zhang LJ, Tan
YF, Li N and Ding HG: Diagnostic value of glypican-3 in serum and
liver for primary hepatocellular carcinoma. World J Gastroenterol.
16:4410–4415. 2010.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Liu S, Wang M, Zheng C, Zhong Q, Shi Y and
Han X: Diagnostic value of serum glypican-3 alone and in
combination with AFP as an aid in the diagnosis of liver cancer.
Clin Biochem. 79:54–60. 2020.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Filmus J and Capurro M: Glypican-3: A
marker and a therapeutic target in hepatocellular carcinoma. FEBS
J. 280:2471–2476. 2013.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Filmus J, Capurro M and Rast J: Glypicans.
Genome Biol. 9(224)2008.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Iglesias BV, Centeno G, Pascuccelli H,
Ward F, Peters MG, Filmus J, Puricelli L and de Kier Joffé EB:
Expression pattern of glypican-3 (GPC3) during human embryonic and
fetal development. Histol Histopathol. 23:1333–1340.
2008.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Paine-Saunders S, Viviano BL, Zupicich J,
Skarnes WC and Saunders S: Glypican-3 controls cellular responses
to Bmp4 in limb patterning and skeletal development. Dev Biol.
225:179–187. 2000.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Capurro MI, Xu P, Shi W, Li F, Jia A and
Filmus J: Glypican-3 inhibits Hedgehog signaling during development
by competing with patched for Hedgehog binding. Dev Cell.
14:700–711. 2008.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Midorikawa Y, Ishikawa S, Iwanari H,
Imamura T, Sakamoto H, Miyazono K, Kodama T, Makuuchi M and
Aburatani H: Glypican-3, overexpressed in hepatocellular carcinoma,
modulates FGF2 and BMP-7 signaling. Int J Cancer. 103:455–465.
2003.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Zhu ZW, Friess H, Wang L, Abou-Shady M,
Zimmermann A, Lander AD, Korc M, Kleeff J and Büchler MW: Enhanced
glypican-3 expression differentiates the majority of hepatocellular
carcinomas from benign hepatic disorders. Gut. 48:558–564.
2001.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Lin Q, Xiong LW, Pan XF, Gen JF, Bao GL,
Sha HF, Feng JX, Ji CY and Chen M: Expression of GPC3 protein and
its significance in lung squamous cell carcinoma. Med Oncol.
29:663–669. 2012.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Ushiku T, Uozaki H, Shinozaki A, Ota S,
Matsuzaka K, Nomura S, Kaminishi M, Aburatani H, Kodama T and
Fukayama M: Glypican 3-expressing gastric carcinoma: Distinct
subgroup unifying hepatoid, clear-cell, and
alpha-fetoprotein-producing gastric carcinomas. Cancer Sci.
100:626–632. 2009.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Ofuji K, Saito K, Suzuki S, Shimomura M,
Shirakawa H, Nobuoka D, Sawada Y, Yoshimura M, Tsuchiya N,
Takahashi M, et al: Perioperative plasma glypican-3 level may
enable prediction of the risk of recurrence after surgery in
patients with stage I hepatocellular carcinoma. Oncotarget.
8:37835–37844. 2017.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Haruyama Y and Kataoka H: Glypican-3 is a
prognostic factor and an immunotherapeutic target in hepatocellular
carcinoma. World J Gastroenterol. 22:275–283. 2016.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Kaseb AO, Hassan M, Lacin S, Abdel-Wahab
R, Amin HM, Shalaby A, Wolff RA, Yao J, Rashid A, Vennapusa B, et
al: Evaluating clinical and prognostic implications of Glypican-3
in hepatocellular carcinoma. Oncotarget. 7:69916–69926.
2016.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Zhang J, Zhang M, Ma H, Song X, He L, Ye X
and Li X: Overexpression of glypican-3 is a predictor of poor
prognosis in hepatocellular carcinoma: An updated meta-analysis.
Medicine (Baltimore). 97(e11130)2018.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Kawaida M, Yamazaki K, Tsujikawa H, Fukuma
M, Abe Y, Kitago M, Shinoda M, Kitagawa Y and Sakamoto M: Diffuse
and canalicular patterns of glypican-3 expression reflect
malignancy of hepatocellular carcinoma. Pathol Int. 69:125–134.
2019.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Okuda H, Nakanishi T, Takatsu K, Saito A,
Hayashi N, Takasaki K, Takenami K, Yamamoto M and Nakano M: Serum
levels of des-gamma-carboxy prothrombin measured using the revised
enzyme immunoassay kit with increased sensitivity in relation to
clinicopathologic features of solitary hepatocellular carcinoma.
Cancer. 88:544–549. 2000.PubMed/NCBI
|
|
27
|
National Comprehensive Cancer Network
(NCCN) Hepatobiliary Cancers v05.2020. Available from: https://www.nccn.org/professionals/physician_gls/pdf/hepatobiliary.pdf.
Accessed December 21, 2020.
|
|
28
|
Amin MB, Greene FL, Edge SB, Compton CC,
Gershenwald JE, Brookland RK, Meyer L, Gress DM, Byrd DR and
Winchester DP: The Eighth Edition AJCC Cancer Staging Manual:
Continuing to build a bridge from a population-based to a more
‘personalized’ approach to cancer staging. CA Cancer J Clin.
67:93–99. 2017.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Capurro M, Wanless IR, Sherman M, Deboer
G, Shi W, Miyoshi E and Filmus J: Glypican-3: A novel serum and
histochemical marker for hepatocellular carcinoma.
Gastroenterology. 125:89–97. 2003.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Chen M, Li G, Yan J, Lu X, Cui J, Ni Z,
Cheng W, Qian G, Zhang J and Tu H: Reevaluation of glypican-3 as a
serological marker for hepatocellular carcinoma. Clin Chim Acta.
423:105–111. 2013.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Baatarkhuu O1, Malov SI, Rasulov RI,
Dvornichenko VV, Savilov ED, Malov IV and Yushchuk ND:
Hepatocellular carcinoma associated with hepatitis B and C in
mongoloids and caucasians of North-East Asia. Infectious Diseases:
News, Opinions, Training. 4:38–44. 2021.
|
|
32
|
Qiao SS, Cui ZQ, Gong L, Han H, Chen PC,
Guo LM, Yu X, Wei YH, Ha SA, Kim JW, et al: Simultaneous
measurements of serum AFP, GPC-3 and HCCR for diagnosing
hepatocellular carcinoma. Hepatogastroenterology. 58:1718–1724.
2011.PubMed/NCBI View
Article : Google Scholar
|
|
33
|
Hippo Y, Watanabe K, Watanabe A,
Midorikawa Y, Yamamoto S, Ihara S, Tokita S, Iwanari H, Ito Y,
Nakano K, et al: Identification of soluble NH2-terminal fragment of
glypican-3 as a serological marker for early-stage hepatocellular
carcinoma. Cancer Res. 64:2418–2423. 2004.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Libbrecht L, Severi T, Cassiman D, Vander
Borght S, Pirenne J, Nevens F, Verslype C, van Pelt J and Roskams
T: Glypican-3 expression distinguishes small hepatocellular
carcinomas from cirrhosis, dysplastic nodules, and focal nodular
hyperplasia-like nodules. Am J Surg Pathol. 30:1405–1411.
2006.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Shafizadeh N, Ferrell LD and Kakar S:
Utility and limitations of glypican-3 expression for the diagnosis
of hepatocellular carcinoma at both ends of the differentiation
spectrum. Mod Pathol. 21:1011–1018. 2008.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Ligato S, Mandich D and Cartun RW: Utility
of glypican-3 in differentiating hepatocellular carcinoma from
other primary and metastatic lesions in FNA of the liver: An
immunocytochemical study. Mod Pathol. 21:626–631. 2008.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Coston WM, Loera S, Lau SK, Ishizawa S,
Jiang Z, Wu CL, Yen Y, Weiss LM and Chu PG: Distinction of
hepatocellular carcinoma from benign hepatic mimickers using
Glypican-3 and CD34 immunohistochemistry. Am J Surg Pathol.
32:433–444. 2008.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Baumhoer D, Tornillo L, Stadlmann S,
Roncalli M, Diamantis EK and Terracciano LM: Glypican 3 expression
in human nonneoplastic, preneoplastic, and neoplastic tissues: A
tissue microarray analysis of 4,387 tissue samples. Am J Clin
Pathol. 129:899–906. 2018.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Tátrai P, Somorácz Á, Batmunkh E,
Schirmacher P, Kiss A, Schaff Z, Nagy P and Kovalszky I: Agrin and
CD34 immunohistochemistry for the discrimination of benign versus
malignant hepatocellular lesions. Am J Surg Pathol. 33:874–885.
2009.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Anatelli F, Chuang ST, Yang XJ and Wang
HL: Value of glypican 3 immunostaining in the diagnosis of
hepatocellular carcinoma on needle biopsy. Am J Clin Pathol.
130:219–223. 2008.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Yan B, Wei JJ, Qian YM, Zhao XL, Zhang WW,
Xu AM and Zhang SH: Expression and clinicopathologic significance
of glypican 3 in hepatocellular carcinoma. Ann Diagn Pathol.
15:162–169. 2011.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Ning S, Bin C, Na H, Peng S, Yi D,
Xiang-hua Y, Fang-yin Z, Da-yong Z and Rong-cheng L: Glypican-3, a
novel prognostic marker of hepatocellular cancer, is related with
postoperative metastasis and recurrence in hepatocellular cancer
patients. Mol Biol Rep. 39:351–357. 2012.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Li B, Liu H, Shang HW, Li P, Li N and Ding
HG: Diagnostic value of glypican-3 in alpha fetoprotein negative
hepatocellular carcinoma patients. Afr Health Sci. 13:703–709.
2013.PubMed/NCBI View Article : Google Scholar
|