Introduction
Radioactive particles are small rods composed of
sealed radioactive isotopes encased within metal shells, measuring
0.8 mm in diameter and 4.5 mm in length, with a half-life of 59.6
days (1). Close-range radiotherapy
using radioactive 125I particles is a form of radiation
therapy in which these particles are implanted into tumors or
surrounding tissues infiltrated by cancer cells, guided by imaging
modalities such as computed tomography (CT) and positron emission
tomography-computed tomography (PET-CT) (2). This technique is considered one of
the emerging treatment modalities in oncologic radiotherapy.
Radioactive 125I particles are directly implanted at the
lesion site, where they continuously emit beta particles and gamma
rays during their radioactive decay, disrupting the DNA structure
of tumor cells and achieving precise targeted therapy (3). A study by Wang et al (4) demonstrated that CT-guided close-range
radiotherapy with radioactive 125I particles for locally
advanced non-small cell lung cancer, following first-line
chemotherapy failure, yielded favorable local control rates,
effectively alleviating symptoms and improving patients' quality of
life. Additionally, research by Ji et al (5) confirmed that CT-guided
125I seed implantation is both effective and safe for
treating recurrent and/or metastatic malignant tumors in the
chest.
Radiation-induced pneumonia (RP) is a common
complication associated with radiotherapy. When surrounding normal
tissues are exposed to close-range radiation from 125I
particles, damage can occur to type II alveolar cells, leading to
alveolar collapse and atelectasis, Additionally, endothelial cell
damage can result in altered pulmonary blood flow, increased
vascular permeability, and capillary obstruction (6). Cytokines such as Interleukin-1 alpha,
Tumor Necrosis Factor-alpha (TNF-α) and TNF-β also play a role in
the inflammatory response (7).
Pathological examinations revealed changes such as alveolar septal
edema, endothelial cell swelling and thickening of the vessel wall.
Typical clinical symptoms of RP include dyspnea, dry cough,
hypoxemia and low-grade fever (8).
There is no specific time interval for the onset of RP following
close-range radiotherapy. Acute RP typically occurs 4-12 weeks
after treatment, while symptoms of delayed or fibrotic RP may
appear 6-12 months later. The severity of inflammation is
influenced by the volume and dose of radiation received by
surrounding normal tissues (9).
Currently, various predictive models have been proposed to assess
the risk of RP; however, most of these models are based on singular
or limited variables and often fail to comprehensively consider
individual patient characteristics and biological mechanisms. Due
to the complexity and multifactorial nature of RP, there is no
definitive model that accurately predicts its occurrence following
close-range radiotherapy with radioactive 125I
particles. The present study aimed to combine relevant data,
including basic patient information, clinical symptoms, tumor
characteristics, preoperative laboratory tests, intraoperative data
and dose-related parameters of close-range radiotherapy, to predict
RP and validate the effectiveness of the model.
A nomogram is a visual statistical tool used to
integrate the effects of multiple predictors into a simple and
understandable graphic, assisting clinicians in assessing the risk
of a specific disease or complication. By assigning a score to each
variable, a nomogram allows users to calculate the probability of a
particular event (such as the occurrence of RP) based on the
patient's specific characteristics.
In medicine, the application of nomograms is
becoming increasingly widespread, as they can transform complex
multivariable models into intuitive and convenient tools, enhancing
the efficiency of clinical decision-making. The present study
employed a nomogram to develop a risk prediction model for RP that
incorporates multiple relevant variables, thereby providing
reliable risk assessment and decision support for clinical
practice.
Materials and methods
Study population
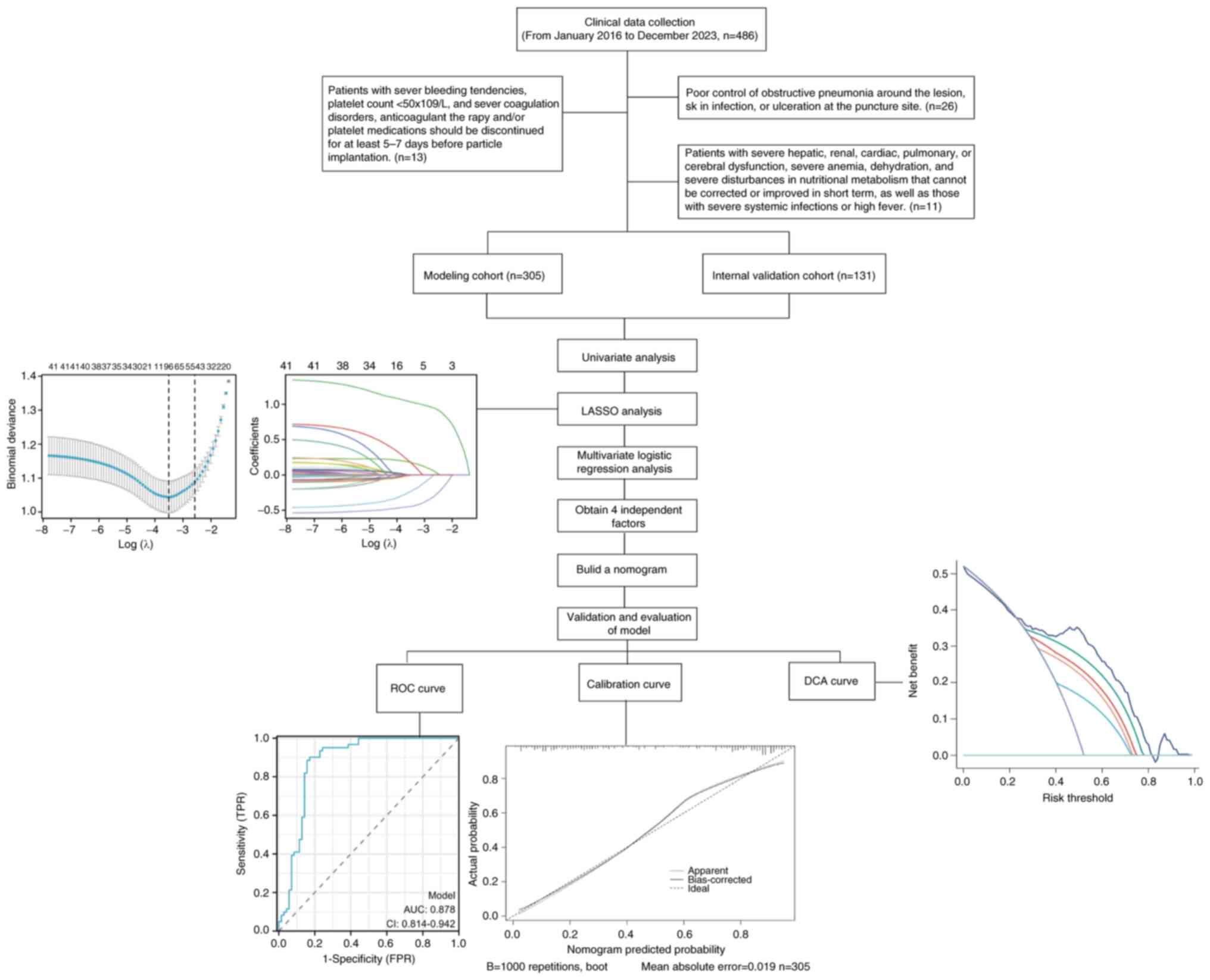
The present study included data from 436 patients
with advanced lung cancer who underwent close-range radiotherapy
with radioactive 125I particles at General Hospital of
Northern Theater Command from January 2016 to December 2023
(Shenyang, China). The patients were randomly assigned in a 7:3
ratio, with 305 patients allocated to the modeling cohort and 131
patients included in the internal validation cohort. The inclusion
criteria were as follows: i) Poor cardiopulmonary function or
advanced age preventing surgical intervention; ii) refusal of
surgical intervention; iii) ineligibility for repeat surgery due to
postoperative recurrence; iv) patients with residual or progressive
tumors after radiotherapy or chemotherapy; v) patients with disease
progression following other anti-tumor treatments; and vi) an
expected survival of at least 3 months. The exclusion criteria were
as follows: i) Poor control of obstructive RP around the lesion,
skin infection, or ulceration at the puncture site; ii) patients
with severe bleeding tendencies, a platelet count
<50x109/l, or severe coagulation disorders.
Anticoagulant therapy and/or platelet medications should be
discontinued for at least 5-7 days prior to particle implantation;
iii) patients with severe hepatic, renal, cardiac, pulmonary, or
cerebral dysfunction, severe anemia, dehydration, and severe
nutritional metabolism disturbances that cannot be corrected or
improved in the short term, as well as those with severe systemic
infections or high fever. All patients included in the present
study provided signed informed consent prior to the procedure. The
present study was approved (approval no. YL2021-07) by the Ethics
Review Committee of the General Hospital of Northern Theater
Command in China (Shenyang, China).
Data collection
The present study collected a comprehensive set of
perioperative data, including patients' basic information, clinical
symptoms, tumor characteristics and preoperative laboratory test
results. Patient baseline information comprised: Age, sex, smoking
status, Zymosan-Activated Serum (ZPS) scale, key performance
indicator system (KPS) score and Numeric Rating Scale (NRS) score.
Clinical symptoms include cough, sputum production, chest tightness
and shortness of breath. Tumor characteristics encompassed:
Preoperative lung cancer diameter, preoperative TNM staging, tumor
location, preoperative lung atelectasis, obstructive pneumonia,
superior vena cava obstruction syndrome. Preoperative laboratory
tests included: Carcinoembryonic antigen (CEA), neuron-specific
enolase (Nse), cytokeratin 19 fragment, squamous cell carcinoma
(SCC) antigen, and preoperative white blood cell count.
Additionally, intraoperative data and dosimetric parameters related
to close-range radiotherapy dose were collected: Surgical time,
planned target volume (PTV), maximum dose, average dose, single
particle dose, preoperative diameter at 90% cumulative volume
(D90) and volume at 100% cumulative volume
(V100), D90 and V100 of the 1-cm
and 2-cm irradiated areas around the lesion (represented as X1
cmD90, X2 cmD90, X1
cmV100, X2 cmV100), number
of particles, puncture needle path and puncture distance.
Close-range radiotherapy method using
radioactive 125I particles
Preoperative planning: Imaging studies (CT, enhanced
CT, PET-CT) are transferred to a three-dimensional treatment
planning system. Treatment plans are designed within this system,
establishing pre-treatment protocols that determine the number of
implanted needles, their positions, and the number and placement of
particles. Individual particle activities are selected, total
activity in the target area is calculated, and the anticipated dose
distribution in both tumor and normal tissues. Intraoperative
procedure: Based on the patient's condition, an appropriate
position was chosen and was secured. Local anesthesia is
administered for the implantation of radioactive particles. The
implantation is performed under CT guidance, with a routine scan of
0.5-cm slice thickness to local the tumor and mark the
corresponding range on the body surface. According to the TPS
treatment plan, the appropriate intercostal space is selected as
the puncture implantation plane, and the needle insertion position,
angle and depth are determined. Under CT guidance, the particle
needle is inserted into the predetermined position within the
tumor, and particles are implanted according to the TPS plan. The
particle needle is inserted in a single motion to minimize the dose
received by the operator during implantation and to reduce the risk
of postoperative pneumothorax. A pen-style implantation gun is used
to implant the particles in a retractable manner, with spacing of
0.5-1.5 cm between particles. During the procedure, the patient's
heart rate, blood pressure and blood oxygen saturation are
continuously monitored. The patient's level of consciousness,
breathing, pain, coughing, and any hemoptysis are also observed and
symptomatic treatment is provided as needed. Following particle
implantation, postoperative CT images are uploaded into the TPS for
quality assessment, focusing on particle and dose reconstruction.
After the procedure, the patient is monitored with
electrocardiogram and oxygen therapy until their condition
stabilizes. A follow-up chest CT scan is conducted 24 h
postoperatively to check for any secondary pneumothorax,
hemothorax, or particle displacement.
The grading of radiation pneumonitis
and symptomatic treatment
Radiation pneumonitis refers to a series of
pathological and physiological changes induced by the irradiation
of a certain volume of normal lung tissue by particles. This
condition can lead to acute exudative or tissue fibrotic changes,
ultimately impairing the patient's respiratory function. Grade 0:
No abnormalities. Grade 1: Mild dry cough or shortness of breath
after exertion. Grade 2: Persistent cough requiring antitussive
medication and mild exertional dyspnea with no dyspnea at rest.
Symptomatic support and antibiotics (consider corticosteroids) are
required for fever, acute exudative changes on chest CT, or
elevated neutrophil percentage require. Grade 3: Severe cough that
is unresponsive to antitussive medication, or dyspnea at rest.
Intermittent oxygen therapy is necessary if there is clinical or
radiographic evidence of acute pneumonia, and steroid therapy may
be required. Grade 4: Severe respiratory failure necessitating
continuous oxygen therapy and mechanical ventilation.
Statistical analysis
R software (Version 4.1.2; https://www.R-project.org) was utilized for
statistical analysis, while Graphpad Prism (Version 6.0; Dotmatics)
was employed to create forest plots. The data were randomly divided
into a training set and a validation set in a 7:3 ratio. All data
were assessed for normality using the Kolmogorov-Smirnov test. For
continuous variables with normal distribution, the mean and
standard deviation were calculated, and unpaired Student's t test
was applied. The Mann-Whitney U test was employed to evaluate
non-normally distributed data, which are expressed as the median
(interquartile range). Comparative analysis of categorical
variables was performed using Pearson chi-square test or Fisher's
exact test, with results presented as P-values and percentages.
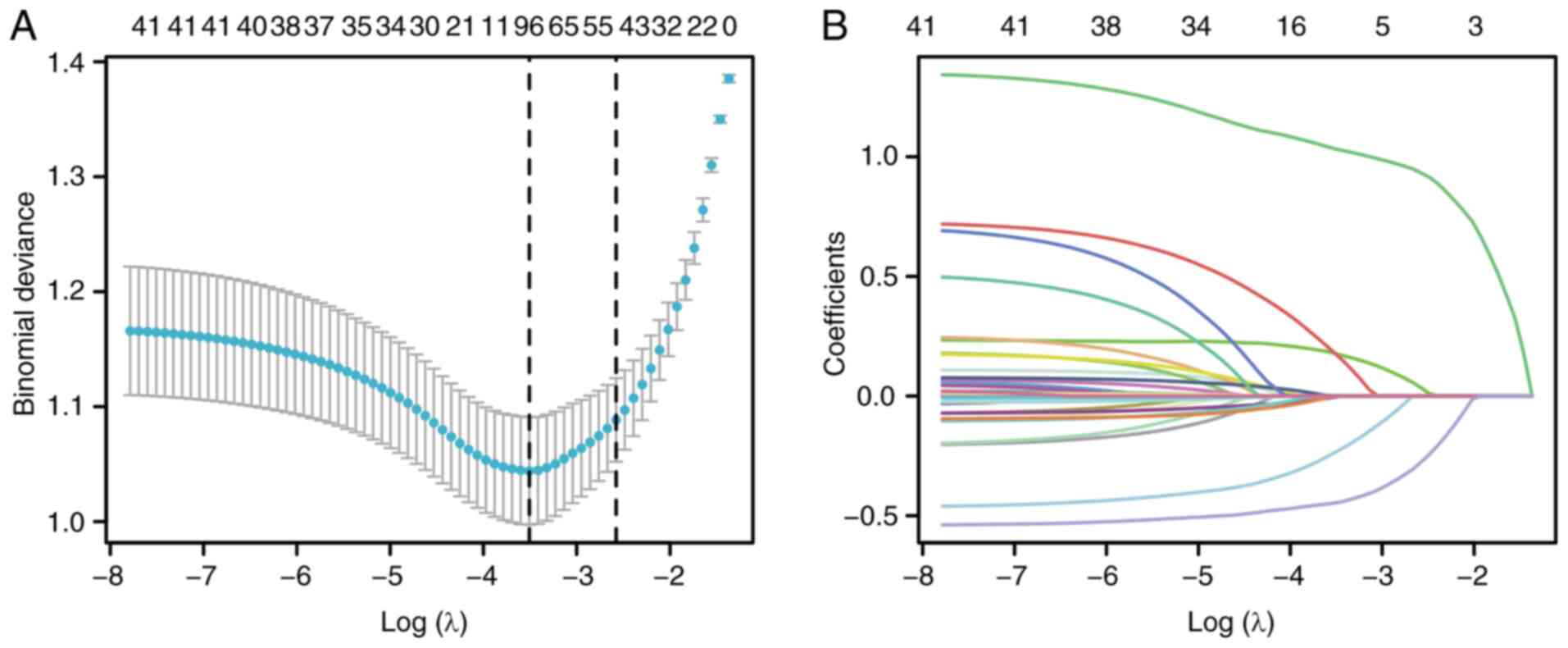
Subsequently, the least absolute shrinkage and selection operator
(LASSO) method was applied to identify potential predictive
features. Multivariable logistic regression analysis was conducted
on variables with P<0.05 from the univariate analysis. And a
line chart prediction model for radiation pneumonitis due to
late-stage lung cancer treated with radioactive 125I
particle brachytherapy was developed using the four selected
variables. A calibration curve was plotted to assess the
calibration of the line chart and the discriminatory performance
was measured using receiver operating characteristic (ROC) curve.
The clinical utility of the line chart was evaluated through
decision curve analysis (DCA) by measuring the net benefit at
different probability thresholds. The incidence rates of RP at
different grades were estimated using the Kaplan-Meier method, and
differences in RP incidence rates between groups were compared
using the log-rank test. The flowchart detailing these procedures
is presented in Fig. 1.
Results
The baseline data
The present study included 436 patients with
late-stage lung cancer who underwent radioactive 125I
particle brachytherapy. The patients were randomly allocated in a
7:3 ratio, with 305 patients assigned to the modeling cohort and
131 patients included in the internal validation cohort. The
modeling cohort consisted of 134 men with a mean age of 62.37±10.37
years, while the internal validation cohort included 67 males with
a mean age of 64.29±10.35 years. Statistical analysis was performed
on patient demographics, clinical symptoms, general tumor
characteristics, and preoperative laboratory tests in the both
cohorts. These variables included: Age, sex, smoking status, ZPS
scale, KPS score, NRS score, clinical symptoms (cough, sputum,
chest tightness, dyspnea), preoperative lung cancer diameter,
preoperative TNM staging, tumor location, preoperative lung
collapse, obstructive pneumonia, superior vena cava obstruction
syndrome and laboratory test results (preoperative CEA,
preoperative Nse, preoperative cytokeratin 19 fragment,
preoperative SCC and white blood cell count). In addition,
intraoperative data and relevant dosimetric parameters for
brachytherapy were collected, including: operation time, PTV
volume, maximum dose, average dose, single particle dose,
preoperative D90 and V100, X1
cmD90, X2 cmD90, X1
cmV100, X2 cmV100, number of
particles, puncture needle path and puncture distance. There were
no significant statistical differences in any of these data between
the two cohorts (all P>0.05, Table
I).
 | Table IBaseline data. |
Table I
Baseline data.
| Variable | Total (n=436) | train_set
(n=305) | valid_set
(n=131) | Statistic | P-value |
|---|
| Year, Mean ±
SD | 62.95±10.39 | 62.37±10.37 | 64.29±10.35 | t=-1.773 | 0.077 |
| Preoperative
longitudinal diameter, Mean ± SD | 4.49±1.08 | 4.51±1.10 | 4.44±1.04 | t=0.565 | 0.572 |
| Zubrod ECOG WHO
Performance Status zymosan-activated serum scale five-point scale,
Mean ± SD | 2.05±0.78 | 2.05±0.77 | 2.05±0.80 | t=-0.093 | 0.926 |
| Karnofsky
Performance Status key performance indicator system score, Mean ±
SD | 76.06±10.57 | 75.90±10.63 | 76.41±10.46 | t=-0.462 | 0.644 |
| Numeric Rating
Scale score, Mean ± SD | 3.89±1.53 | 3.90±1.59 | 3.84±1.37 | t=0.434 | 0.665 |
| Preoperative
carcinoembryonic antigen, Mean ± SD | 5.15±4.40 | 5.21±4.82 | 5.02±3.26 | t=0.410 | 0.682 |
| Preoperative neuron
specific enolase, Mean ± SD | 10.98±10.19 | 10.78±9.76 | 11.42±11.16 | t=-0.602 | 0.547 |
| Preoperative
cytokeratin 19 fragment, Mean ± SD | 5.64±6.16 | 5.95±6.37 | 4.92±5.59 | t=1.615 | 0.107 |
| Preoperative
squamous cell carcinoma antigen, Mean ± SD | 3.97±3.12 | 3.99±3.03 | 3.91±3.33 | t=0.248 | 0.804 |
| Preoperative white
blood cells, Mean ± SD | 6.14±2.20 | 6.11±2.27 | 6.20±2.04 | t=-0.377 | 0.707 |
| Planning target
volume, Mean ± SD | 14.82±7.26 | 14.71±7.31 | 15.05±7.17 | t=-0.450 | 0.653 |
| Maximum dose, Mean
± SD |
141016.46±42196.25 |
143900.33±40194.77 |
134302.10±45988.02 | t=2.187 | 0.059 |
| Mean dose, Mean ±
SD |
43000.70±5607.01 |
42755.98±5823.37 |
43570.47±5042.76 | t=-1.392 | 0.165 |
| Preoperative D90,
Mean ± SD |
16059.98±3333.95 |
16068.28±3314.52 |
16040.66±3391.49 | t=0.079 | 0.937 |
| Preoperativev100,
Mean ± SD | 94.08±1.45 | 94.01±1.43 | 94.24±1.47 | t=-1.507 | 0.133 |
| X1
cmD90, Mean ± SD | 12228.99±
2622.87 | 12160.97±
2663.68 | 12387.36±
2528.21 | t=-0.826 | 0.409 |
| X1
cmV100, Mean ± SD | 93.49±1.85 | 93.44 ± 1.84 | 93.62 ± 1.87 | t=-0.952 | 0.342 |
| X2
cmD90, Mean ± SD |
9624.29±1755.06 |
9628.42±1799.53 |
9614.66±1653.52 | t=0.075 | 0.940 |
| X2
cmV100, Mean ± SD | 91.26±2.29 | 91.28±2.38 | 91.22±2.08 | t=0.227 | 0.821 |
| Number of implanted
particles, Mean ± SD | 82.15±25.62 | 81.66±26.00 | 83.27±24.77 | t=-0.602 | 0.547 |
| Surgical duration,
Mean ± SD | 47.55±11.86 | 47.16±11.74 | 48.46±12.14 | t=-1.050 | 0.294 |
| Puncture needle
tract, Mean ± SD | 14.15±7.63 | 14.13±7.78 | 14.19±7.29 | t=-0.079 | 0.937 |
| Puncture distance,
Mean ± SD | 0.68±0.35 | 0.68±0.36 | 0.66±0.35 | t=0.622 | 0.534 |
| Sex, n (%) | | | | χ²=1.918 | 0.166 |
|
Male | 201 (46.1) | 134 (43.93) | 67 (51.15) | | |
|
Female | 235 (53.9) | 171 (56.07) | 64 (48.85) | | |
| Smoke, n (%) | | | | χ²=0.779 | 0.377 |
|
Yes | 160 (36.7) | 116 (38.03) | 44 (33.59) | | |
|
No | 276 (63.3) | 189 (61.97) | 87 (66.41) | | |
| Primary tumor
pathology type, n (%) | | | | - | 0.445 |
|
Adenocarcinoma
of the lung | 217 (49.77) | 157 (51.48) | 60 (45.80) | | |
|
Squamous
cell carcinoma of the lung | 111 (25.46) | 74 (24.26) | 37 (28.24) | | |
|
Small cell
lung cancer | 98 (22.48) | 69 (22.62) | 29 (22.14) | | |
|
Neuroendocrine
tumor | 5 (1.15) | 2 (0.66) | 3 (2.29) | | |
|
Cancer with
SMARCA4 deficiency | 5 (1.15) | 3 (0.98) | 2 (1.53) | | |
| Preoperative T, n
(%) | | | | χ²=2.983 | 0.225 |
|
2 | 144 (33.03) | 93 (30.49) | 51 (38.93) | | |
|
3 | 180 (41.28) | 130 (42.62) | 50 (38.17) | | |
|
4 | 112 (25.69) | 82 (26.89) | 30 (22.90) | | |
| Preoperative N, n
(%) | | | | χ²=5.531 | 0.063 |
|
1 | 35 (8.03) | 24 (7.87) | 11 (8.40) | | |
|
2 | 188 (43.12) | 121 (39.67) | 67 (51.15) | | |
|
3 | 213 (48.85) | 160 (52.46) | 53 (40.46) | | |
| Preoperative M, n
(%) | | | | χ²=0.011 | 0.917 |
|
0 | 218(50) | 153 (50.16) | 65 (49.62) | | |
|
1 | 218(50) | 152 (49.84) | 66 (50.38) | | |
| Pulmonary lobe, n
(%) | | | | χ²=1.005 | 0.316 |
|
Left
lung | 298 (68.35) | 204 (66.89) | 94 (71.76) | | |
|
Right
lung | 138 (31.65) | 101 (33.11) | 37 (28.24) | | |
| Pulmonary segment,
n (%) | | | | χ²=1.258 | 0.533 |
|
Upper lobe
of the lung | 157 (36.01) | 109 (35.74) | 48 (36.64) | | |
|
Middle lobe
of the lung | 44 (10.09) | 34 (11.15) | 10 (7.63) | | |
|
Lower lobe
of the lung | 235 (53.9) | 162 (53.11) | 73 (55.73) | | |
| Atelectasis, n
(%) | | | | χ²=0.150 | 0.699 |
|
Yes | 177 (40.6) | 122 (40.00) | 55 (41.98) | | |
|
No | 259 (59.4) | 183 (60.00) | 76 (58.02) | | |
| Obstructive
pneumonia, n (%) | | | | χ²=0.053 | 0.819 |
|
Yes | 210 (48.17) | 148 (48.52) | 62 (47.33) | | |
|
No | 226 (51.83) | 157 (51.48) | 69 (52.67) | | |
| Superior vena cava
obstruction, n (%) | | | | χ²=2.141 | 0.143 |
|
Yes | 213 (48.85) | 142 (46.56) | 71 (54.20) | | |
|
No | 223 (51.15) | 163 (53.44) | 60 (45.80) | | |
| Cough, n (%) | | | | χ²=0.022 | 0.881 |
|
Yes | 194 (44.5) | 135 (44.26) | 59 (45.04) | | |
|
No | 242 (55.5) | 170 (55.74) | 72 (54.96) | | |
| Expectoration, n
(%) | | | | χ²=0.475 | 0.491 |
|
Yes | 214 (49.08) | 153 (50.16) | 61 (46.56) | | |
|
No | 222 (50.92) | 152 (49.84) | 70 (53.44) | | |
| Chest tightness and
shortness of breath, n (%) | | | | χ²=2.391 | 0.122 |
|
Yes | 211 (48.39) | 155 (50.82) | 56 (42.75) | | |
|
No | 225 (51.61) | 150 (49.18) | 75 (57.25) | | |
| Asthma, n (%) | | | | χ²=0.158 | 0.691 |
|
Yes | 210 (48.17) | 145 (47.54) | 65 (49.62) | | |
|
No | 226 (51.83) | 160 (52.46) | 66 (50.38) | | |
| Particle spacing
(mCi), n (%) | | | | χ²=5.193 | 0.268 |
|
0.5 | 81 (18.58) | 52 (17.05) | 29 (22.14) | | |
|
0.6 | 96 (22.02) | 75 (24.59) | 21 (16.03) | | |
|
0.7 | 66 (15.14) | 48 (15.74) | 18 (13.74) | | |
|
0.9 | 25 (5.73) | 17 (5.57) | 8 (6.11) | | |
|
1 | 168 (38.53) | 113 (37.05) | 55 (41.98) | | |
| Radiotherapy, n
(%) | | | | χ²=1.646 | 0.199 |
|
Yes | 196 (44.95) | 131 (42.95) | 65 (49.62) | | |
|
No | 240 (55.05) | 174 (57.05) | 66 (50.38) | | |
| Chemotherapy, n
(%) | | | | χ²=1.418 | 0.234 |
|
Yes | 212 (48.62) | 154 (50.49) | 58 (44.27) | | |
|
No | 224 (51.38) | 151 (49.51) | 73 (55.73) | | |
Factors influencing RP following
brachytherapy for late-stage lung cancer
Based on LASSO Logistic regression, the independent
variables in the dataset were screened, and those corresponding to
non-zero coefficients at Lambda.min were selected for subsequent
multivariate analysis. As illustrated in Fig. 2, the selected variables include:
Smoking status, preoperative N and M staging, superior vena cava
obstruction syndrome, preoperative white blood cells, maximum dose
and chemotherapy. To further evaluate seven potential predictive
factors and optimize the predictive model, a multivariable logistic
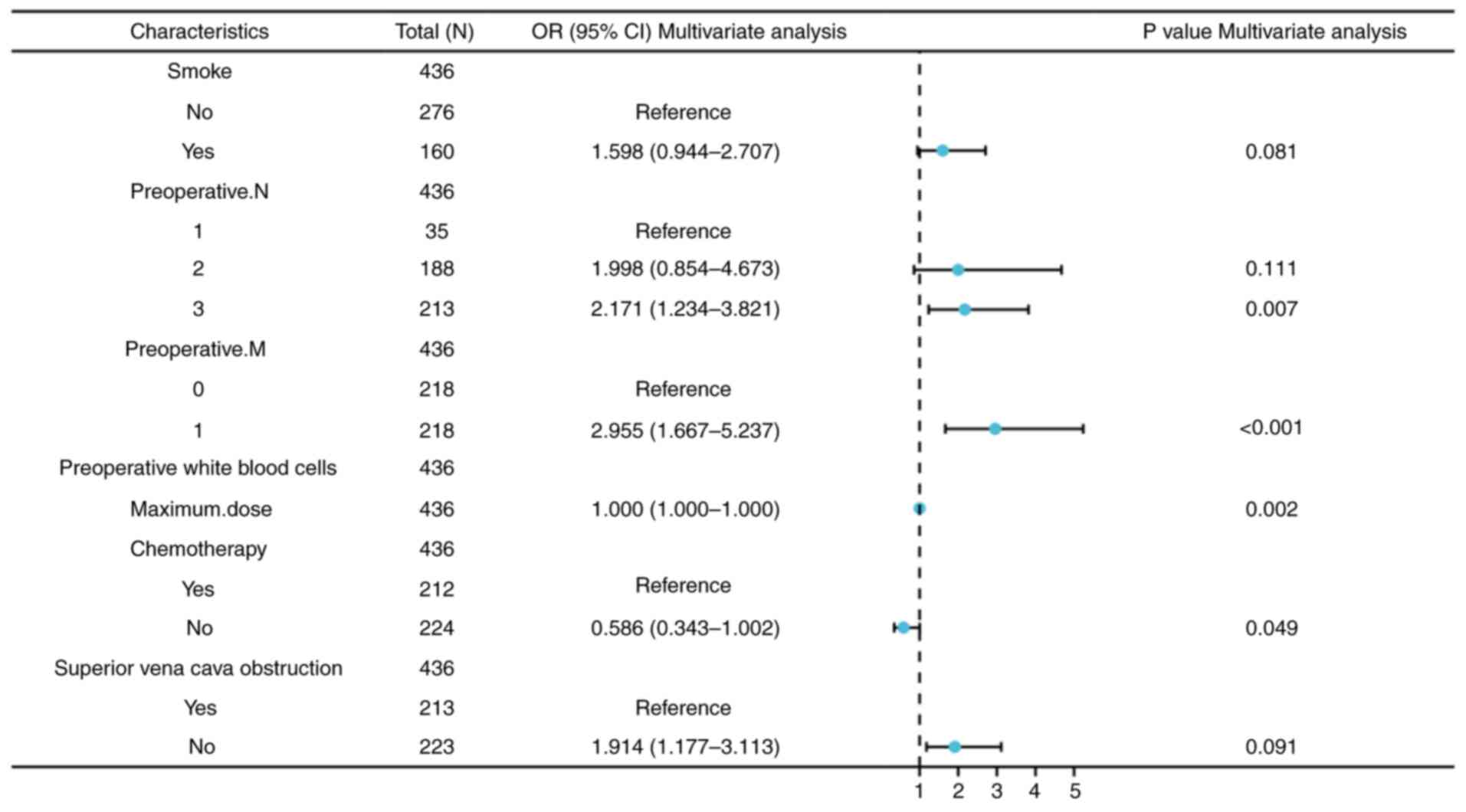
regression analysis was conducted. The results indicated that
preoperative N3 stage [95% CI, 2.171 (1.234-3.821),
P=0.007], preoperative M1 stage [95% CI, 2,955
(1.667-5.237), P<0.001], maximum dose [95% CI 1.000,
(1.000-1.000), P=0.002] and chemotherapy [95% CI, 0.586
(0.343-1.002), P=0.049] were independent risk factors influencing
RP in patients with late-stage lung cancer treated with
brachytherapy, as summarized in Table
II and depicted in Fig. 3.
 | Table IILogistic univariate and multivariate
regression analysis of risk factors for radiation pneumonia
following brachytherapy for late-stage lung cancer. |
Table II
Logistic univariate and multivariate
regression analysis of risk factors for radiation pneumonia
following brachytherapy for late-stage lung cancer.
| | Univariate
analysis | Multivariate
analysis |
|---|
|
Characteristics | Total (N) | Odds ratio (95%
CI) | P-value | Odds ratio (95%
CI) | P-value |
|---|
| Smoke | 436 | | | | |
|
No | 276 | Reference | | Reference | |
|
Yes | 160 | 3.919
(2.569-5.978) | <0.001 | 1.598
(0.944-2.707) | 0.081 |
| Preoperative N | 436 | | | | |
|
1 | 35 | Reference | | Reference | |
|
2 | 188 | 1.654
(0.784-3.490) | 0.186 | 1.998 (0.854
4.673) | 0.111 |
|
3 | 213 | 7.307
(4.698-11.364) | <0.001 | 2.171
(1.234-3.821) | 0.007 |
| Preoperative M | 436 | | | | |
|
0 | 218 | Reference | | Reference | |
|
1 | 218 | 9.514
(6.143-14.736) | <0.001 | 2.955
(1.667-5.237) | <0.001 |
| preoperative white
blood cells | 436 | 0.942
(0.864-1.027) | 0.177 | | |
| Maximum dose | 436 | 1.000
(1.000-1.000) | <0.001 | 1.000
(1.000-1.000) | 0.002 |
| Chemotherapy | 436 | | | | |
|
Yes | 212 | Reference | | Reference | |
|
No | 224 | 0.174
(0.115-0.263) | <0.001 | 0.586
(0.343-1.002) | 0.049 |
| Superior vena cava
obstruction | 436 | | | | |
| Yes | 213 | Reference | | | |
| No | 223 | 1.388
(0.952-2.024) | 0.088 | | 0.091 |
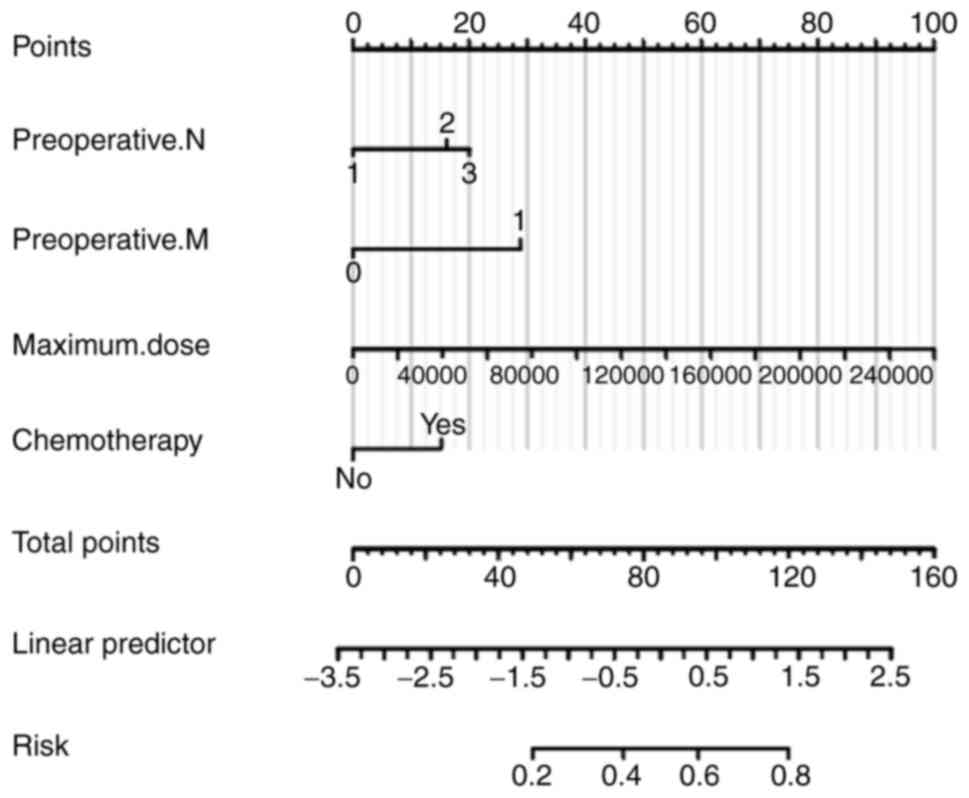
Development and validation of the
predictive model
A nomogram plot (Fig.
4) was constructed based on four independent risk factors:
Preoperative N and M staging, maximum dose and whether chemotherapy
was administered. To use the nomogram, a vertical line is drawn for
each variable to determine its respective score. By summing those
scores, the total score, which indicates the risk probability of
RP, can be calculated. For example, a patient with a preoperative
T3N2M1 stage, a maximum dose of
136510 Gy, and who received preoperative chemotherapy would have a
total score of 114 points, corresponding to a 77% risk of RP.
As illustrated in Fig.
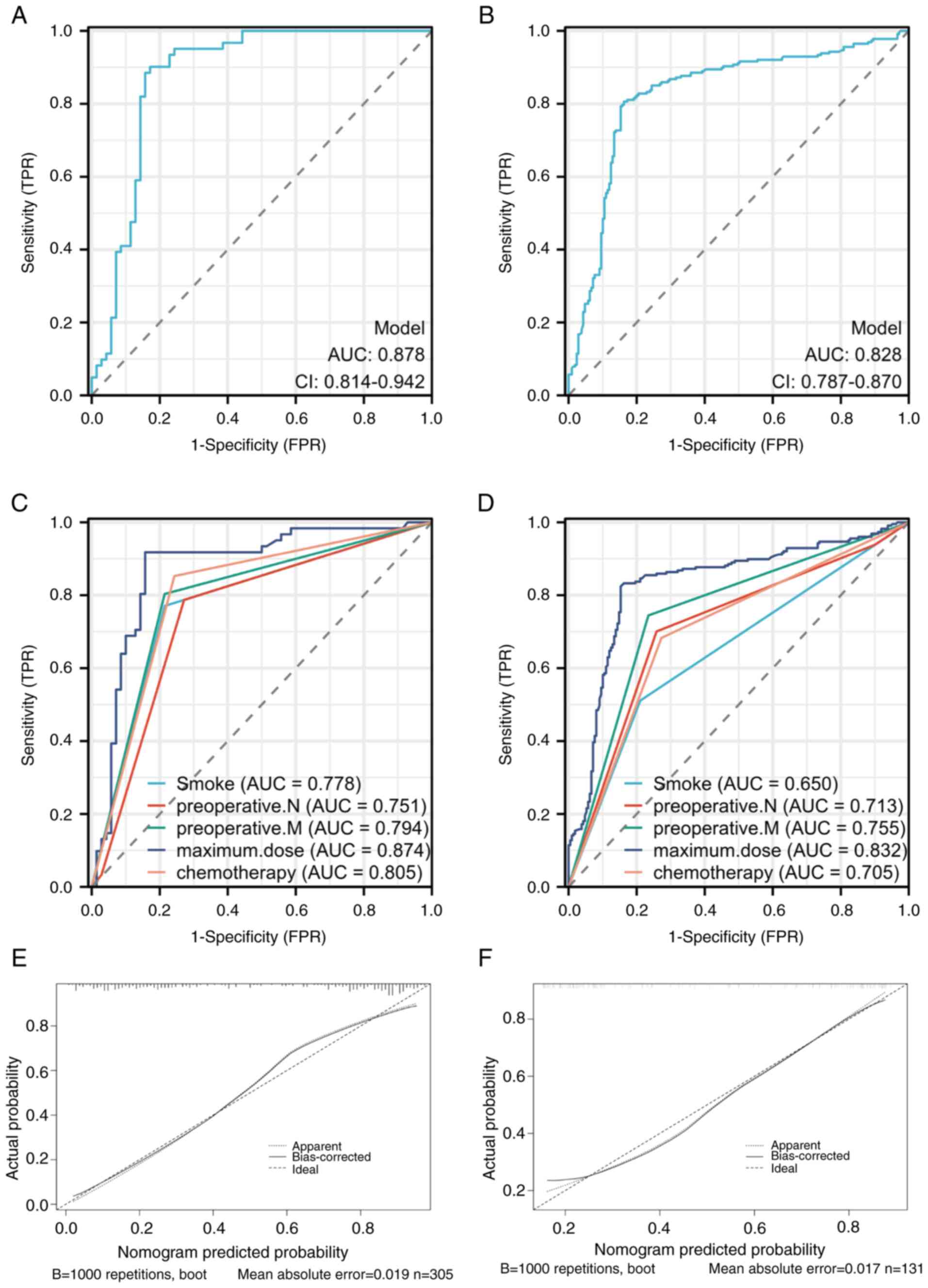
5, the area under the ROC curve in the training set (Fig. 5A) was 0.878 (95% CI, 0.814-0.942).
The calibration curve and ROC curve for the validation set yielded
results similar to those in the training set, with the area under
the ROC curve in the validation set (Fig. 5B) being 0.828 (95% CI,
0.787-0.870). For individual predictors in the training set
(Fig. 5C), the area under the ROC
curve was 0.778 (95% CI, 0.706-0.850) for smoking, 0.751 (95% CI,
0.674-0.828) for preoperative N staging, 0.794 (95% CI,
0.725-0.864) for preoperative M staging, 0.874 (95% CI,
0.808-0.940) for maximum dose, and 0.805 (95% CI, 0.737-0.872) for
preoperative chemotherapy. In the validation set (Fig. 5D), the areas under the ROC curve
were 0.650 (95% CI, 0.607-0.693) for smoking, 0.713 (95% CI,
0.669-0.758) for preoperative N staging, 0.755 (95% CI,
0.715-0.795) for preoperative M staging, 0.832 (95% CI,
0.791-0.873) for maximum dose, and 0.705 (95% CI, 0.662-0.748) for
preoperative chemotherapy. The calibration curves indicate that
both the training (Fig. 5E) and
validation (Fig. 5F) set were
close to the 45-degree line, indicating that the model accurately
predicts actual events.
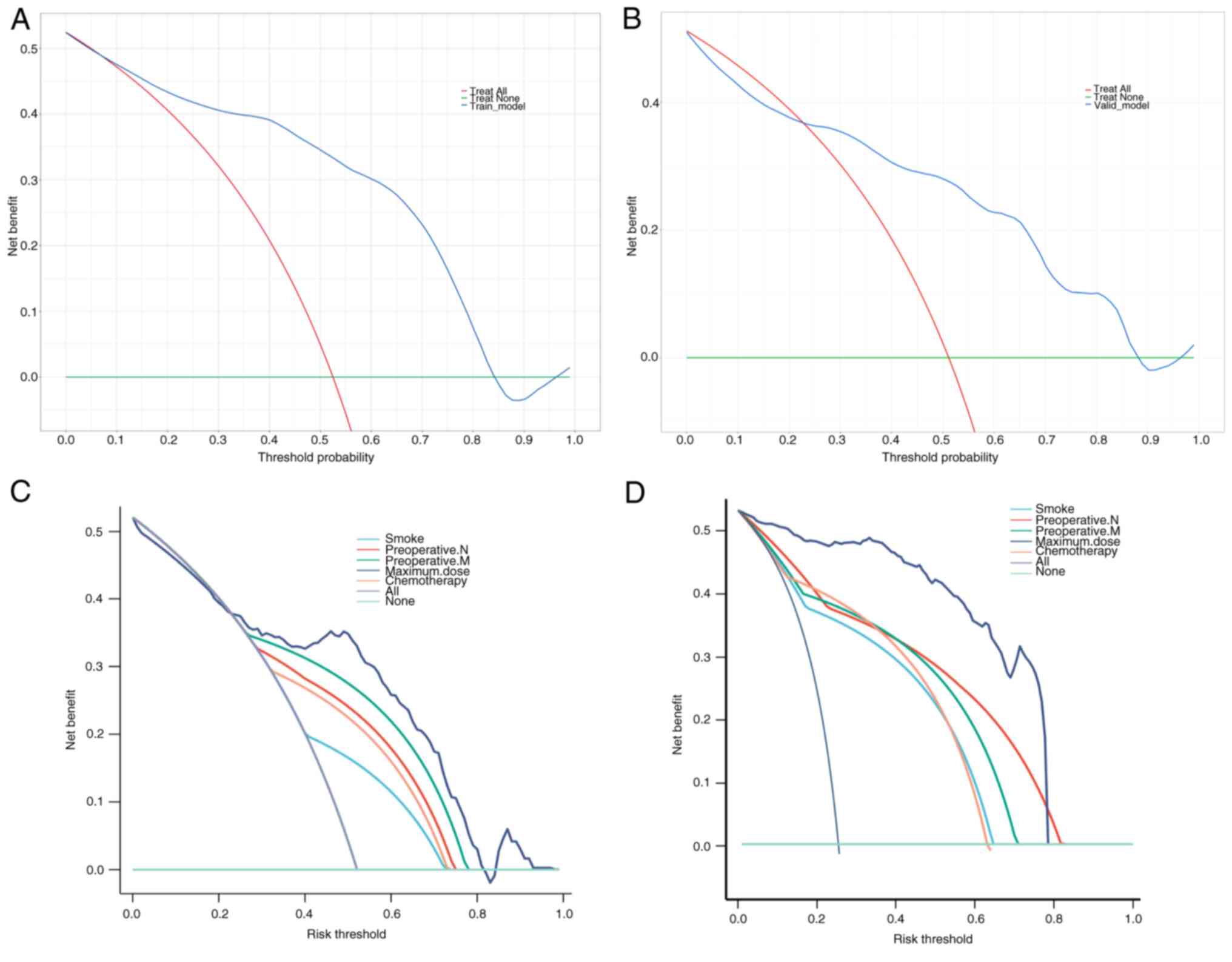
DCA demonstrates that clinical decisions based on
the predictive model are beneficial, highlighting the practical
clinical application and feasibility of the model in both the
training set (Fig. 6A) and
validation set (Fig. 6B). Among
the variables, maximum dose achieves the most significant clinical
benefits in practice, showing promising prospects for clinical
application. The maximum dose provides the highest benefits in both
the training and validation sets (Fig.
6C and D).
Grading of RP and Kaplan-Meier
analysis
Based on the grading of RP, the data were divided
into two RP of grade 2 or below and grade 3 or higher. In the group
with RP of grade 3 or higher, the average maximum dose is
139,187.27 mCi, while in the group with grade 2 or below, it is
129,864.09 mCi.
Patients with preoperative T1 and T3 staging
(P<0.001) (Fig. 7A),
preoperative M1 staging [P<0.01, HR=8.26 (4.66-14.64)] (Fig. 7C), a maximum dose exceeding
139,187.27 mCi [P<0.001, HR=9.63 (4.61-20.12)] (Fig. 7E), and those who underwent
preoperative chemotherapy [P<0.001, HR=0.12 (0.07-0.22)]
(Fig. 7G) were found to be more
likely to develop RP of grade 3 or higher (P<0.001).
Specifically, for patients with T3 staging, the probability of
developing pneumonia of grade 3 or higher at 6 and 12 weeks was 15
and 24%, respectively. For patients with M1 staging developing
pneumonia of grade 3 or higher at 6 weeks and 12 weeks is 12 and
29%, respectively. Patients who underwent chemotherapy have a 10%
probability at 6 weeks and 22% probability at 12 weeks of
developing pneumonia of grade 3 or higher.
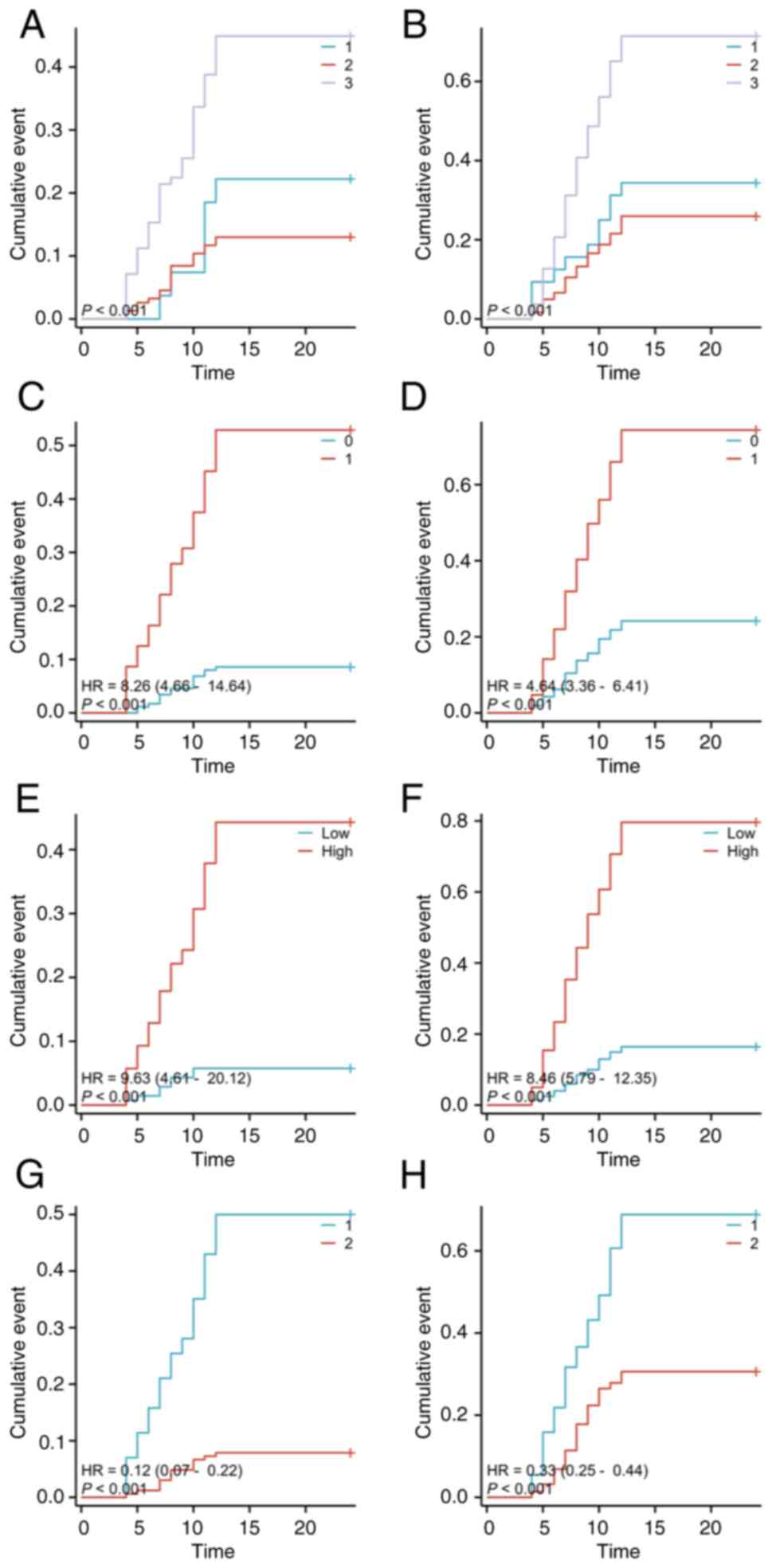 | Figure 7Kaplan-Meier curves of patients staged
preoperatively as T1, T2 and T3:
(A) for grade 3+ pneumonia, (B) for grade 2-pneumonia. Preoperative
staging as M0, M1: (C) for grade 3+
pneumonia, (D) for grade 2-pneumonia. Patients divided into high
and low groups based on maximum dose >139,187.27 mCi,
<139,187.27 mCi: (E) for grade 3+ pneumonia. Patients divided
into high and low groups based on maximum dose >129,864.09 mCi,
<129,864.09 mCi: (F) for grade 2-pneumonia. Grouping based on
whether preoperative chemotherapy was administered (1 for yes, 2
for no): (G) for grade 3+ pneumonia, (H) for grade 2-pneumonia. |
Conversely, for RP of grade 2 or lower, patients
with preoperative T1 and T3 staging (P<0.001) (Fig. 7B), preoperative M1 staging
[P<0.01, HR=4.64 (3.36-6.41)] (Fig.
7D), and a maximum dose exceeding 139,187.27 mCi [P<0.001,
HR=8.46 (5.79-12.35)] (Fig. 7F),
and those who underwent preoperative chemotherapy [P<0.001,
HR=0.33 (0.25-0.44)] (Fig. 7H)
were found to be more likely to develop RP of grade 2 or below
(P<0.001). Specifically, for patients with T3 staging, the
probability of developing pneumonia of grade 2 or below at 6 and 12
weeks was 20 and 41%, respectively. For patients with M1 staging,
these probabilities were 18% at 6 weeks and 33% at 12 weeks.
Patients who underwent chemotherapy had an 18% probability at 6
weeks and 36% probability at 12 weeks of developing pneumonia of
grade 2 or below.
Discussion
In 2022, lung cancer ranked first among all new
cases of malignant tumors in China, accounting for 18.06% of the
total cancer cases. Similarly, lung cancer accounted for 23.9% of
all deaths from malignant tumors in China, also ranking first
(10). Early-stage lung cancer
often presents with no obvious symptoms, and in clinical practice,
most patients seek medical attention when symptoms appear, by which
time it is already in the advanced stage, leading to a loss of the
opportunity for surgery. The overall 5-year survival rate for
patients with advanced-stage lung cancer is ~15% (11). Radioactive 125I particle
brachytherapy, radio-chemotherapy and targeted immunotherapy
techniques are widely used in the treatment of advanced lung cancer
(12). The radioactive
125I particle brachytherapy has the advantages of
minimal complications and significant efficacy, leading to a rapid
increase in the number of patients with advanced lung cancer
receiving this treatment annually. In epidermal growth factor
receptor tyrosine kinase inhibitor treatment failure, 58.6% of
patients experience progression of the original lesion, while 20.7%
of patients have both progression of the original lesion and the
emergence of new lesions. Therefore, radioactive 125I
particle brachytherapy can effectively provide local control of the
original lesion, thereby extending the patients' progression-free
survival and overall survival (OS) (13). A study by Zhang et al
(14) reported that in patients
with oligo-recurrence after first-line chemotherapy failure in
non-small cell lung cancer, the short-term efficacy and quality of
life of those receiving radioactive 125I particle
implantation therapy were superior to those continuing
chemotherapy, while the OS was comparable. Compared with external
beam radiation therapy, radioactive 125I particle
brachytherapy avoids the disadvantage of expanding the irradiation
range due to respiratory motion, thereby effectively reducing
damage to surrounding normal tissues (15). The incidence of radiation
pneumonitis after combined radio chemotherapy for non-small cell
lung cancer is 43.33%, while the incidence of radiation pneumonitis
with radioactive 125I particle implantation therapy is
6.25%. Both approaches have similar short-term efficacy rates and 1
and 2-year OS rates (16).
Brachytherapy is particularly effective in treating certain types
of tumors, but it still faces numerous challenges in predicting and
managing RP, which underscores the significance of the present
study. Uneven dose distribution: Brachytherapy typically uses
high-dose rate or low-dose rate radiation sources, resulting in
complex dose distributions that may lead to some healthy lung
tissue receiving excessively high radiation doses. This
heterogeneity in dosing complicates risk assessment, as different
patients and treatment protocols can lead to variable dose
exposures. Biological heterogeneity: There may be significant
differences in the biological characteristics of lung tissue among
patients, including lung function, tissue response and individual
sensitivity to radiation. This heterogeneity makes predictions
based on a single marker or risk factor less accurate, increasing
the complexity of managing RP. Complexity of clinical assessment:
The symptoms of RP can be similar to those of other complications
(such as infections or tumor progression), making it challenging
for clinicians to recognize and diagnose RP early on. This
misdiagnosis or late diagnosis may adversely affect patient
management and treatment outcomes. Lack of standardized predictive
models: Currently, while there are some risk assessment tools
available, there are still limited standardized predictive models
that specifically address the unique risk factors associated with
brachytherapy. This results in a lack of effective tools in
clinical practice to predict and manage the risk of RP, thereby
limiting the implementation of personalized treatment. The
significance of the present study lies in filling these gaps by
developing a predictive model that can more accurately identify RP
risk factors associated with brachytherapy and providing clinicians
with practical management strategies. In doing so, it was aimed to
enhance the accuracy of RP predictions, deliver improved treatment
outcomes for patients, and improve their quality of life.
The present study collected data from 436 patients
with advanced lung cancer who underwent radioactive 125I
particle brachytherapy. These patients were randomly assigned in a
7:3 ratio, with 305 patients allocated to the modeling cohort and
131 patients included in the internal validation cohort. Since the
research was conducted in a military hospital, collaboration with
other medical institutions poses certain challenges. In the future,
the authors plan to gradually initiate collaborative projects with
local hospitals to expand their sample size, thereby improving the
statistical accuracy. Currently in the present study, combining
patient demographics, clinical symptoms, tumor characteristics,
preoperative laboratory tests, intraoperative data and
brachytherapy dosage for screening, LASSO logistic variable
selection identified non-zero coefficient variables including:
Smoking, preoperative N and M staging, superior vena cava syndrome,
preoperative white blood cell count, maximum dose and chemotherapy.
Further evaluation using a multiple logistic regression model
revealed that preoperative N3 stage [95% CI, 2.171
(1.234-3.821), P=0.007], preoperative M1 stage [95% CI,
2.955 (1.667-5.237), P<0.001], maximum dose (95% CI, 1.000
(1.000-1.000), P=0.002) and chemotherapy [95% CI, 0.586
(0.343-1.002), P=0.049] were independent risk factors influencing
radiation pneumonitis in patients with advanced lung cancer
undergoing brachytherapy. The correlation between tumor volume,
tumor staging and radiation pneumonitis is controversial.
Some studies suggest that higher tumor TNM staging,
larger volume, closer proximity to the hilum or lower lung, and a
larger irradiated lung volume are associated with an increased risk
of radiation pneumonitis. This is consistent with the findings of
De Petris et al (17).
However, other studies have revealed that tumor volume is not
related to the occurrence of radiation pneumonitis (18). This is mainly related to other
confounding factors such as patient lung volume size and volume of
lung tissue irradiated (19). When
combined radiotherapy is used, it leads to exudative and
inflammatory changes in the local lung tissue, a reduction in type
II alveolar cells and surfactant, and a series of
pathophysiological changes in the blood vessel wall and lung
tissue. The combined toxic effects exacerbate lung damage, making
radiation pneumonitis more likely to occur and difficult to recover
from when combined with chemotherapy. The study by Zha et al
(16) confirmed that chemotherapy
is an important factor in accurately predicting symptomatic
pneumonitis. Together with age, smoking index and whole lung volume
at 5 Gy/mean lung dose, they constructed a nomogram prediction
model with an area under the ROC curve of 0.89, demonstrating
favorable calibration. Chemotherapy, as a significant risk factor
for predicting radiation pneumonitis, is consistent with the
results of the aforementioned study. The authors understand the
potential value of conducting stratified analyses based on
different chemotherapy regimens or timing (for example, combination
therapy vs. sequential therapy), as this could indeed provide more
specific guidance for clinical decision-making. However, due to the
sample size of the present study and the availability of data,
categorizing chemotherapy regimens and timing at this stage may
lead to instability in the statistical results and complexity in
interpretation. Therefore, stratified analyses were decided not to
be performed on chemotherapy regimens and timing in order to
maintain the rigor of the present study and the reliability of the
results. This direction will be considered in future research.
There is still controversy regarding the correlation
between dosimetric parameters and the occurrence of radiation
pneumonitis. The study by Bi et al (20) concluded that the MLD is the most
critical dose-volume parameter influencing radiation pneumonitis,
and it can improve prevention of the occurrence of radiation
pneumonitis in patients undergoing combined immunotherapy and
radiotherapy. On the other hand, Ji et al (21) hypothesized that there is no clear
correlation between dosimetric parameters and the occurrence of
postoperative radiation pneumonitis. The correlation between
radiotherapy dose and radiation pneumonitis cannot be directly
applied to the prediction of radiation pneumonitis in brachytherapy
using radioactive 125I particles. The conclusion of the
present study is that among dosimetric parameters, only the maximum
dose is a predictor of radiation pneumonitis, which is consistent
with the findings of Flakus et al (22). Moreover, it is noteworthy that the
maximum dose as a predictor of radiation pneumonitis in
brachytherapy with radioactive 125I particles is a
significant finding that adds to the understanding of the complex
relationship between radiotherapy and lung toxicity. While
dosimetric parameters may not always correlate directly with the
occurrence of radiation pneumonitis, the maximum dose appears to be
a critical factor in brachytherapy, possibly due to the localized
high-dose delivery characteristics of this treatment modality.
Regarding the implications of this finding for treatment planning
and dose limitations, reassessment of current dosing standards and
treatment protocols may be needed to ensure that the selection of
the maximum dose effectively targets the tumor while not
excessively increasing the risk of RP. This may involve
individualized dose adjustment, assessment of pulmonary sensitivity
and a comprehensive application of other therapeutic modalities to
mitigate potential side effects.
Furthermore, the present study underscores the need
for a comprehensive approach in predicting and managing radiation
pneumonitis. Age, smoking history and other patient-specific
factors must be considered along with dosimetric parameters to
develop accurate prediction models. Additionally, the combined
effects of radiotherapy and chemotherapy on lung tissue need to be
carefully evaluated to optimize treatment outcomes and minimize
toxicity. Time-to-event analysis was considered to be included in
the predictive model and the possibility of developing a nomogram
to update risk predictions over time as well as creating an online
calculator was explored. However, the current understanding of this
approach is limited, which prevented the authors from implementing
it in the present study. Additionally, it was observed that some
relevant literature also did not include dynamic nomograms or
online calculators but instead opted for static risk prediction
models. Nevertheless, this did not hinder the present study from
obtaining valuable predictive results (23).
The predictors identified in the present study
primarily emphasize the individual characteristics of patients
(such as age, underlying diseases and lung function) as well as the
specific characteristics of the tumor (such as N and M
classifications). These factors reflect the biological
characteristics and treatment suitability of the patients when
undergoing radiation therapy, providing a basis for personalized
treatment. By contrast, the predictors for EBRT typically place
greater emphasis on the radiation dose, irradiated area, radiation
technique and specific implementation details of the treatment.
These factors directly affect the extent of radiation's impact on
lung tissue and are essential components in assessing the risk of
RP. By comparing the two, it was found that while the factors
recognized in the present study's model focus more on the patient's
individual biological characteristics, integrating these factors
with the dosage and technical factors of EBRT could provide
clinicians with a more comprehensive risk assessment tool. This
multidimensional analysis can improve support of clinical
decision-making and assist physicians in developing more
personalized radiation therapy plans, thereby reducing the
incidence of RP. Therefore, further exploring the relationships
among these predictive factors will help expand the understanding
of the mechanisms underlying RP and guide future research
directions.
In conclusion, the findings of the present study
contribute to the ongoing efforts to improve the prediction and
management of radiation pneumonitis in patients undergoing
brachytherapy with radioactive 125I particles. Compared
with existing models or guidelines, the present study's approach
emphasizes multivariable analysis, which can consider the subtle
differences in patients' individual characteristics and treatment
plans. This personalized risk assessment method can assist
clinicians in making more precise decisions when formulating
treatment plans. In comparing with existing models, it was also
noted that some commonly used risk assessment tools may be based
solely on a single or few clinical characteristics, lacking a
comprehensive consideration of multiple factors. The present study
aimed to address this limitation by introducing more variables,
thereby enhancing the predictive capability for the occurrence of
RP. Through these comparisons, it was aimed to be demonstrated that
the nomogram of the present study did not only improve accuracy but
also assisted physicians in improving identification of high-risk
patients in clinical applications, allowing for the formulation of
more rational treatment plans.
The limitations of the present study include: i)
Data completeness and accuracy: Retrospective studies rely on
existing medical records and databases, the accuracy and
completeness of which can be affected by various factors, such as
data entry errors, missing information, or inconsistencies in
recording treatment protocols. This may lead to some key variables
being excluded from the analysis, potentially impacting the
accuracy of the present study's predictive model. ii) Time effects:
With advances in medical technology, the methods and standards of
radiation therapy have evolved. In light of new treatment
strategies and techniques, the data from retrospective studies may
not reflect the true impact of radiation pneumonia risk in current
clinical practice. This needs to be considered when applying the
present study's findings to avoid generalizing outdated standards
and results to modern treatments. iii) Limitations in inferring
causality: Retrospective studies typically struggle to establish
clear causal relationships. In the present study research, although
several risk factors were identified to be associated with
radiation pneumonia, this did not imply that these factors
necessarily cause the occurrence of RP. The present study's
findings need to be cautiously interpreted and the predictive
nature of this mod emphasized rather than making causal judgments.
iv) Inherent limitations of retrospective studies: The present
study was a retrospective analysis and there may be biases in
patient selection criteria and data collection. For instance, the
selected patients may significantly differ from the general
population (for example, age and tumor staging), which can affect
the model's applicability. v) Limitations in variable selection:
Even when using advanced methods such as LASSO for variable
selection, important influencing factors may still be omitted, or
unrelated variables may be included, thereby affecting the model's
accuracy. vi) Lack of external validation: Ideally, validation
should be conducted on external datasets to ensure the model's
generalizability. However, the present study may lack sufficient
external validation data.
Future research should continue to explore the
interaction of various risk factors and dosimetric parameters to
develop more effective strategies for preventing and treating this
potentially debilitating complication of radiotherapy. In the
present study, it was demonstrated that four independent risk
factors (preoperative N and M staging, maximum dose and receipt of
chemotherapy) can predict the occurrence of postoperative radiation
pneumonitis in patients undergoing 125I brachytherapy
for cancer. The training cohort's area under the ROC curve for the
nomogram construction was 0.878 (95% CI, 0.814-0.942), while the
validation cohort's area under the ROC curve was 0.828 (95% CI,
0.787-0.870). The calibration curve indicates that the model can
perfectly predict actual events. The DCA suggests that clinical
decisions based on the predictive model are beneficial, implying
practical clinical applicability and operability of the model. The
maximum dose in both the training and validation cohorts revealed
the most ideal clinical benefits in clinical practice, indicating
promising clinical utility.
Integrating the nomogram into the existing clinical
workflow can provide clinicians with a practical tool to aid in
making more accurate patient care and treatment adjustment
decisions. By showcasing real case studies that demonstrate the
application of the nomogram in patient management, its value and
effectiveness can be highlighted in clinical decision-making,
helping physicians understand its importance. Effectively
incorporating the nomogram into the existing clinical workflow will
enhance clinicians' decision-making capabilities, offering patients
more precise and personalized care and treatment adjustments.
Combining educational training, system integration, standardized
processes and multidisciplinary collaboration will be beneficial in
achieving this goal, ultimately improving patient health outcomes.
The feasible initiatives mainly include integrating it with
Electronic Health Records for direct data access, developing a
user-friendly interface for easy data entry, providing training for
clinicians, embedding it into Clinical Decision Support Systems for
risk-based recommendations, establishing feedback channels for
continuous improvement, standardizing risk communication through
clinical documentation, promoting collaboration among healthcare
professionals, and regularly updating the nomogram based on new
research. These efforts will ultimately enhance the effectiveness
and application of the nomogram in clinical practice, leading to
improved patient care outcomes.
Acknowledgements
Not applicable.
Funding
Funding: The present study was supported by Joint Plan of
Liaoning Provincial People's Livelihood Science and Technology
(grant no. 2022JH2/101500021).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
GZ, SH and ZW contributed to the study conception
and design. Material preparation, data collection and analysis were
performed by TD and WZ. The first draft of the manuscript was
written by TD. All authors read and approved the final version of
the manuscript. TD and WZ confirm the authenticity of all the raw
data.
Ethics approval and consent to
participate
The study was reviewed and approved [approval no.
YLS No. (2019) 69] by the Medical Ethics Committee of the General
Hospital of Northern Theater Command in China (Shenyang, China).
All patients provided signed informed consent.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Huang M, Lin Q, Wang H, Chen J, Bai M,
Wang L, Zhu K, Jiang Z, Guan S, Li Z, et al: Survival benefit of
chemoembolization plus Iodine125 seed implantation in unresectable
hepatitis B-related hepatocellular carcinoma with PVTT: A
retrospective matched cohort study. Eur Radiol. 26:3428–3436.
2016.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Song J, Fan X, Zhao Z, Chen M, Chen W, Wu
F, Zhang D, Chen L, Tu J and Ji J: 125I brachytherapy of
locally advanced non-small-cell lung cancer after one cycle of
first-line chemotherapy: A comparison with best supportive care.
Onco Targets Ther. 10:1345–1352. 2017.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Yang DY, Lin YP, Xue C, Fan JM, Wang Y,
Cai C, Song DH and Zeng YM: CT-guided percutaneous implantation of
125I particles in treatment of early lung cancer. J
Thorac Dis. 12:5996–6009. 2020.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Wang H, Lu J, Zheng XT, Zha JH, Jing WD,
Wang Y, Zhu GY, Zeng CH, Chen L and Guo JH: Oligorecurrence
non-small cell lung cancer after failure of first-line
chemotherapy: Computed tomography-guided 125I seed
implantation vs second-line chemotherapy. Front Oncol.
10(470)2020.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Ji Z, Jiang Y, Guo F, Peng R, Sun H, Wang
P, Fan J and Wang J: Radiation-related adverse effects of CT-guided
implantation of 125I seeds for thoracic recurrent and/or
metastatic malignancy. Sci Rep. 9(14803)2019.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Wang G, Zhang F, Yang B, Xue J, Peng S,
Zhong Z, Zhang T, Lu M and Gao F: Feasibility and clinical value of
CT-guided (125)I brachytherapy for bilateral lung recurrences from
colorectal carcinoma. Radiology. 278:897–905. 2016.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Su L, Dong Y, Wang Y, Wang Y, Guan B, Lu
Y, Wu J, Wang X, Li D, Meng A and Fan F: Potential role of
senescent macrophages in radiation-induced pulmonary fibrosis. Cell
Death Dis. 12(527)2021.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Cai G, Liang S, Li C, Meng X and Yu J:
Left ventricular systolic dysfunction is a possible independent
risk factor of radiation pneumonitis in locally advanced lung
cancer patients. Front Oncol. 9(1511)2020.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Simeonova AO, Fleckenstein K, Wertz H,
Frauenfeld A, Boda-Heggemann J, Lohr F and Wenz F: Are three doses
of stereotactic ablative radiotherapy (SABR) more effective than 30
doses of conventional radiotherapy? Transl Lung Cancer Res.
1:45–53. 2012.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Zheng RS, Chen R, Han BF, Wang SM, Li L,
Sun KX, Zeng HM, Wei WW and He J: Cancer incidence and mortality in
China, 2022. Zhonghua Zhong Liu Za Zhi. 46:221–231. 2024.PubMed/NCBI View Article : Google Scholar : (In Chinese).
|
|
11
|
Münsterberg J, Loreth D, Brylka L, Werner
S, Karbanova J, Gandrass M, Schneegans S, Besler K, Hamester F,
Robador JR, et al: ALCAM contributes to brain metastasis formation
in non-small-cell lung cancer through interaction with the vascular
endothelium. Neuro Oncol. 22:955–966. 2020.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Wang Y, Zhu L, Lin X, He C, An Z, Tang J,
Lv W and Hu J: Therapeutic effect of CT-guided 125I seed
implantation on advanced lung cancer and pulmonary metastatic
carcinoma. Zhongguo Fei Ai Za Zhi. 23:424–428. 2020.PubMed/NCBI View Article : Google Scholar : (In Chinese).
|
|
13
|
Wang X and Wang D: Clinical analysis of
125I seed implantation combined with epidermal growth factor
receptor-tyrosine kinase inhibitors in advanced non-small cell lung
cancer. J BUON. 26:1879–1886. 2021.PubMed/NCBI
|
|
14
|
Zhang T, Lu M, Peng S, Zhang W, Yang G,
Liu Z, Singh S, Yang Y, Zhang F and Gao F: CT-guided implantation
of radioactive 125I seed in advanced non-small-cell lung cancer
after failure of first-line chemotherapy. J Cancer Res Clin Oncol.
140:1383–1390. 2014.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Cao X, Fang L, Cui CY, Gao S and Wang TW:
DTI and pathological changes in a rabbit model of radiation injury
to the spinal cord after 125I radioactive seed
implantation. Neural Regen Res. 13:528–535. 2018.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Zha Y, Zhang J, Yan X, Yang C, Wen L and
Li M: A dynamic nomogram predicting symptomatic pneumonia in
patients with lung cancer receiving thoracic radiation. BMC Pulm
Med. 24(99)2024.PubMed/NCBI View Article : Google Scholar
|
|
17
|
De Petris L, Lax I, Sirzén F and Friesland
S: Role of gross tumor volume on outcome and of dose parameters on
toxicity of patients undergoing chemoradiotherapy for locally
advanced non-small cell lung cancer. Med Oncol. 22:375–381.
2005.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Yu X, Li J, Zhong X and He J: Combination
of Iodine-125 brachytherapy and chemotherapy for locally recurrent
stage III non-small cell lung cancer after concurrent
chemoradiotherapy. BMC Cancer. 15(656)2015.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Takeda A, Tsurugai Y, Sanuki N, Enomoto T,
Shinkai M, Mizuno T, Aoki Y, Oku Y, Akiba T, Hara Y and Kunieda E:
Clarithromycin mitigates radiation pneumonitis in patients with
lung cancer treated with stereotactic body radiotherapy. J Thorac
Dis. 10:247–261. 2018.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Bi J, Meng R, Yang D, Li Y, Cai J, Zhang
L, Qian J, Xue X, Hu S, Yuan Z, et al: Dosimetric predictors of
radiation pneumonitis in patients with prior immunotherapy
exposure: A multi-institutional analysis. Radiother Oncol.
190(110040)2024.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Ji Z, Ni Y, He C, Huo B, Liu S, Ma Y, Song
Y, Hu M, Zhang K, Wang Z, et al: Clinical outcomes of radioactive
seed brachytherapy and microwave ablation in inoperable stage I
non-small cell lung cancer. Am J Cancer Res. 13:3753–3762.
2023.PubMed/NCBI
|
|
22
|
Flakus MJ, Kent SP, Wallat EM, Wuschner
AE, Tennant E, Yadav P, Burr A, Yu M, Christensen GE, Reinhardt JM,
et al: Metrics of dose to highly ventilated lung are predictive of
radiation-induced pneumonitis in lung cancer patients. Radiother
Oncol. 182(109553)2023.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Huang P and Yi X: Risk factors and a model
for prognosis prediction after intravenous thrombolysis with
alteplase in acute ischemic stroke based on propensity score
matching. Int J Immunopathol Pharmacol.
38(3946320241274231)2024.PubMed/NCBI View Article : Google Scholar
|