1. Introduction
The immune checkpoint inhibitors (ICIs), represented
by programmed cell death protein-1 (PD-1) and programmed
death-ligand 1 (PD-L1), as well as cytotoxic T
lymphocyte-associated antigen 4 (CTLA-4), have greatly improved
clinical the outcomes in patients with cancer in comparison to
cytokine-based immunotherapies of more than a decade ago (1-3),
but can cause a series of irAEs (4). In all patients who take ICIs, irAEs
can affect almost every organ, with an overall incidence of 70-90%
(5), while the incidence of
immune-related severe adverse events (ir-SAEs) is between 10-43%
(6,7). irAEs are classified according to the
Common Terminology Criteria for Adverse Events version 5.0(5), and grades 3-5 are considered severe.
Anti-PD-1 and -PD-L1 therapies cause 10 and 30.1% of ir-SAEs,
manifested as pneumonitis, hepatitis and neurotoxicity, while the
31% of severe ir-SAEs caused by anti-CTLA-4 therapy manifest as
colitis and diarrhea (6-9).
ir-SAEs, including immune myocarditis, can lead to treatment
interruption, poor prognosis and patient death.
The identification of ir-SAEs is further complicated
by the fact that ICIs are often chosen in combination with
chemotherapy, targeted therapy, radiotherapy and other therapies in
current tumor treatment modalities (10-13).
Determining that AEs are caused by ICIs rather than concurrent
treatment modalities is challenging. In addition, the lack of
distinct clinical manifestations of ir-SAEs in the early stages of
treatment makes identification difficult for healthcare
professionals, and early judgment can affect decisions about
treatment approaches. For example, immune checkpoint-related
pneumonia is similar to infectious pneumonia in terms of clinical
symptoms and imaging, and immune-associated pneumonia has a
mortality rate of 35% (9). If
irAEs cannot be diagnosed accurately, it will delay the treatment
of adverse reactions and antitumor therapy for patients, and in
addition, the use of other incorrect agents will increase the
toxicity of the drugs for patients, so that they will lose the
chance of prolonged survival and a cure, which will lead to
irreversible consequences.
Risk factor studies are critical to improving
patient safety by helping to identify ir-SAEs early, monitoring
changes in irAEs and clarifying treatment regimens that lead to
adverse events in patients on multiple lines and multi-drug
therapy. However, fewer current studies have reported the risk
factors associated with ir-SAEs than the predictors of ICI efficacy
(14). There is a dearth of
information on factors associated with ir-SAEs, and additional
research is required to identify contributing factors. Therefore,
the present review examines the factors that affect patients with
ir-SAEs, including demographic characteristics, disease-related
information, blood indices and biomarkers. The aim of the present
review is to assist medical professionals in assessing and treating
ir-SAEs, alleviating patients' physical and emotional strain, and
ensuring treatment safety.
2. Factors associated with ir-SAEs
Risk factors associated with ir-SAEs include the
demographic characteristics of age, body mass index (BMI), smoking,
ethnicity and family history of cancer (FHC).
Age
Baldini et al (15) investigated 603 patients treated
with anti-PD-1/PD-L1 and found a higher incidence of ir-SAEs in
patients aged ≥70 years than in those aged <70 years (33 vs.
25%; P<0.05) (15). Elderly
patients are a group of concern for ir-SAEs, as the immune system
changes with age, exhibiting reduced infiltration of B cells, CD8 T
cells and myeloid dendritic cells. When treated with ICIs, this
change may activate more T cells, leading to increased immune
responses in various organ systems and causing ir-SAEs (16,17).
Therefore, when using immunotherapy in the elderly in the clinic,
it is still necessary to assess the benefit, strictly monitor the
process of use and be alert to the occurrence of ir-SAEs to ensure
the safety of the drug. However, there have been inconsistent
results with regard to age. Ksienski et al (18) retrospectively analyzed 302 patients
with melanoma treated with anti-PD-1, comparing the incidence of
irAEs between groups aged <75 and ≥75 years, and showed that the
incidence of discontinuation due to immunotoxicity was similar
(31.8 vs. 40.0%; P=0.50) (18).
The reasons for the different findings might be attributed to
differences in the study population or research methodology.
Currently, there is still a lack of data on elderly patients with
cancer in ICI clinical trials and real-world studies, and more
elderly patients need to be included in future in-depth
studies.
BMI
A meta-analysis confirmed that high BMI (≥25
kg/m2) increased the risk of ir-SAEs [odds ratio (OR),
2.62] (19). It was suggested that
obesity may play a key role in the induction of immunotherapeutic
toxicity. Obese patients can produce a variety of adipocyte-derived
molecules that are responsible for altering the inflammatory and
immune landscapes, and this makes patients more susceptible to
triggering ir-SAEs, as the immune response of the body is
exacerbated by treatment with ICIs (20). Based on these results, the study by
Cortellini et al (21)
hypothesized that patients with a high BMI have a pro-inflammatory
state, which affects the regulation of immune and inflammatory
responses and can lead to ir-SAEs. Lowering the BMI appears to be
one of the effective ways to reduce the incidence of ir-SAEs.
Therefore, healthcare professionals need to be educated on this
before patients are ready to start immunotherapy. However, De
Filippi et al (22)
investigated 133 patients with Hodgkin's lymphoma treated with
nivolumab and found no association between BMI and ir-SAEs.
Currently, aside from the aforementioned studies, there are still
few studies on BMI with ir-SAEs and the results are inconsistent,
which may be related to the fact that thresholds for BMI have been
defined differently in different studies. Therefore, in the future,
it is necessary to define the BMI threshold and use more
large-sample, full-cancer population studies to explore the
relationship between BMI and ir-SAEs to further explore the
intrinsic connection.
Smoking
A study by Wood et al (23) on 153 patients with advanced
non-small cell lung cancer (NSCLC) treated with pembrolizumab found
that failure to quit smoking early in treatment was also associated
with ir-SAEs (OR, 2.27) (23).
This condition may be related to the negative effects on the immune
system of the chemicals in tobacco, such as nicotine, which not
only increase the risk of inflammation and infection in the body,
but also cause hyperactivation of the inflammatory system during
treatment with ICIs, increasing the incidence of ir-SAEs (24). Therefore, medical staff need to
advise and supervise patients who start immunotherapy but still
have smoking habits, and observe them closely to detect signs of
ir-SAEs as early as possible and deal with them promptly.
Ethnicity
Abdelrahim et al (25) found that compared with Caucasian
populations, the Asian population was more susceptible to
ICI-related acute kidney injury (ICI-AKI) [hazard ratio (HR),
5.970]. Consistent with the ethnic differences in their study,
there were differences between ethnicities in the global prevalence
of kidney injury-like diseases (such as chronic kidney disease and
uremia), with Asian populations having a higher prevalence
(26). The underlying mechanisms
linking ethnicity to prior ir-SAEs are unclear due to a lack of
research, and the fact that relevant studies included samples of
<1% of non-caucasian patients makes the conclusions much less
credible. Larger sample studies are needed to explore this finding
in the future.
FHC
FHC was categorized into three different types by
direct and collateral branches, namely FHC-high (in cases where
cancer was diagnosed in both the immediate and lateral line),
FHC-low (in cases where cancer was diagnosed in only one family
line) and FHC-negative. A multicenter study investigating 822
patients found that FHC-high patients were more likely to develop
ir-SAEs compared with FHC-negative patients (P=0.012) (27). This result provided a link between
FHC burden and the occurrence of ir-SAEs. This may be related to
the higher immunosensitivity and more active immune system when
treated with ICIs. Therefore, medical professionals need to pay
specific attention to FHC-high patients when administering
immunotherapy to patients with cancer. However, only the
aforementioned study discussed the association between FHC and
ir-SAEs (27), which is an area
that future studies could focus on.
3. Disease-related information associated
with ir-SAEs
Risk factors associated with ir-SAEs include the
disease-related information of disease history, treatment regimen
and cancer type.
Disease history
Risk factors associated with ir-SAEs include a
disease history of autoimmune diseases (ADs) and cardiovascular
disease.
AD
In early clinical trials of ICIs, patients with AD
were mostly excluded, but they are not excluded from treatment by
the U.S. Food and Drug Administration With the widespread use of
ICIs, there is a growing need for more reliable data for patients
with AD combined with cancer who require ICI therapy. Several
studies have confirmed increased ICI-associated risk of ir-SAEs in
patients with AD due to abnormal immune function. Sorah et
al (28) investigated 14
patients and found that a history of AD was a significant predictor
of ICI-AKI (14%). Akturk et al (29) reported 2 cases of ir-SAEs in
patients with AD treated with anti-PD-1 therapy, suggesting that
physicians should be cautious in treating such patients with ICIs.
This may occur as pre-existing AD in the context of immunotherapy
causes T cells and other immune cells to be activated, which can
lead to a high susceptibility to ir-SAEs (30). However, Tang et al (31) investigated patients with cancer
treated with PD-1/PD-L1 and found that patients with a history of
AD had no increased risk of death from ir-SAEs (HR, 1.03). Although
this is excellent news for patients with cancer suffering from AD,
the large variations in sample size and tumor type between studies
are still not eliminated based on the safety of the life of the
patient.
Cardiovascular disease. By reviewing 3,326
oncology patients undergoing ICIs in the Mayo Clinic from March
2010 to July 2019 to analyze the clinical relationship between
cardiovascular disease and patients receiving immunotherapy, Oren
et al (32) demonstrated
the association of hypertension with ir-SAEs (OR, 4.3; HR, 1.32)
(32). Noseda et al
(33), using the VigiBase
database, found that drugs labeled for the treatment of
cardiovascular disease were risk factors for severe ICI-associated
myocarditis when selecting 108 cases of ICI-associated myocarditis
and 108 non-myocarditis irAEs controls (Cramer's coefficient of
effect size: φ=0.214) (33). Many
electronic databases, such as VigiBase, are unable to provide
comprehensive information on whether patients had comorbidities
before treatment. The databases only provide information on
concomitant medications and their indications for use. Accordingly,
it can be hypothesized that patients with cardiovascular disease
have a higher risk of developing ir-SAEs, which is also associated
with an increased inflammatory response in the body that is
exacerbated by the stimulation of the immune system by ICIs during
immunotherapy. Despite the paucity of information related to the
disease history, considering the clinical complexity surrounding
disease history development, healthcare professionals should do a
thorough job of asking questions and fully understanding the
history of the disease to achieve early prevention. By constructing
a large database of adverse events to assist medical personnel in
making accurate judgments about medication choices for this
population, and by strengthening close observation before, during
and after the treatment process, it is believed that ICIs are
equally promising for oncology populations with AD, hypertension
and cardiovascular disease history.
Treatment regimen
Risk factors associated with ir-SAEs include a
treatment regimen of combination therapy. Kim et al
(34) employed a systematic review
and meta-analysis to explore whether treatment regimens for
patients with melanoma receiving ICIs had an impact on irAEs and
found that a higher incidence of ir-SAEs occurred in combination
therapy than in monotherapy (34).
In the study, the incidence of ir-SAEs was 24.5% when patients were
treated with a single ICI drug, while the incidence was 41.0% with
the combination of two ICIs. Regardless of the type of cancer,
including melanoma, lung cancer, gastric cancer and colorectal
cancer, patients treated with a CTLA-4 inhibitor in combination
with PD-1/PD-L1 had a higher risk of ir-SAEs than those treated
with just PD-1/PD-L1 (14,35,36).
Regarding the combination of chemotherapy with ICIs, a
meta-analysis by Huang et al (37) observed that CTLA-4 inhibition
combined with chemotherapy had the highest risk of ir-SAEs, with
the most common being severe diarrhea (37). Furthermore, the study by Zheng
et al (38) revealed that
ICIs in combination with dacarbazine, paclitaxel or carboplatin
would increase the incidence of ir-SAEs (38). This increased incidence of ir-SAEs
was the same in patients who were administered ICIs combined with
radiotherapy (13 vs. 1%) (39).
Another type of cancer treatment is targeted therapy including
tyrosine kinase inhibitors (TKIs) and macromolecular monoclonal
antibodies. A number of widely known drugs, such as osimertinib,
anlotinib and lenvatinib, belong to the TKI family. In a
meta-analysis of pancreatic immunotherapy for renal cell carcinoma,
the combination of lenvatinib plus pembrolizumab was associated
with a significantly higher likelihood of ir-SAEs (40). In a retrospective study of 126
patients with NSCLC treated with osimertinib and ICIs inhibitors,
Schoenfeld et al (41)
found that the use of osimertinib was associated with an increased
incidence of ir-SAEs. Similarly, in patients with SCLC, combination
therapy of anlotinib with ICIs has been found to be associated with
severe immune-related pneumonia and thyroiditis (42). Macromolecular monoclonal
antibodies, such as bevacizumab, were likewise found by Zhang and
Xu (43) to increase the risk of
ir-SAEs in patients with ovarian cancer when used in combination
with PD-1/PD-L1 inhibitors. A new therapy, phototherapy, including
photodynamic therapy and photothermal therapy, has also
increasingly been used in various patients with cancer, such as
patients with breast cancer and melanoma, with an increased
efficacy as well as an increased incidence of ir-SAEs when combined
with ICIs (44). A possible reason
for the association of combination treatments with an increased
incidence of ir-SAEs is that these treatments differentially
enhance T-cell-mediated tumor cell killing, which, when combined
with ICIs, leads to an increased immune status and susceptibility
of the organism to ir-SAEs (44-48).
In summary, the high level of difficulty in
antitumor therapy and the low immunity of patients with tumors put
patients on combination regimens at higher risk for SAEs.
Therefore, health education and disease observation should be
strengthened for patients on combination therapy.
Cancer type
Risk factors associated with ir-SAEs include the
cancer types of superficial spreading melanoma, lung cancer
(especially NSCLC), breast cancer and renal cell carcinoma.
L'Orphelin et al (49) conducted a study using data from the
RicMel database and linked the previous history of cancer and
treatment with anticancer drugs to the late onset (>1 year) of
ir-SAEs in patients with superficial spreading melanoma (OR, 5.23)
(49). Using data from the
REISAMIC database of 1,187 patients receiving ICIs, Ruste et
al (50) found lung tumors to
be a risk factor for developing ICI in patients with ir-SAEs. With
regard to lung cancer histological types, according to the study by
Brumberger et al (51),
patients with NSCLC are more likely to develop ir-SAEs than those
with SCLC. A meta-analysis study comprising 11 trials showed that
among patients with cancer using pembrolizumab, patients with
breast cancer were most likely to develop ir-SAEs (52). After further analysis of the
different types of ir-SAEs, Khoja et al (6) observed that patients with renal cell
carcinoma were more susceptible to ir-SAEs in the lungs at the time
of immunotherapy compared with patients with melanoma, whereas
patients with melanoma were more susceptible to skin and
gastrointestinal tract ir-SAEs (6). Although the exact mechanistic role of
histology remains elusive, these findings may be interpreted as the
differences in the immune microenvironment between different cancer
types driving tissue-specific ir-SAEs (6). Regarding common cancer types, while
20-50% of patients with liver or gastric cancer have been reported
to develop ir-SAEs following immunotherapy, whether these cancers
are associated with a higher overall frequency of ir-SAEs has been
rarely explored (53). Only one
study by Lou et al (54)
investigated the relationship between hepatocellular carcinoma
subtypes and the occurrence of ir-SAEs. However, the study found no
difference in the incidence of ir-SAEs when analyzing the toxicity
profiles of ICIs in patients with different types of hepatocellular
carcinoma (54). The current
analysis primarily included the association between lung cancer and
melanoma patients and the incidence of ir-SAEs, and rarely
addressed other populations. As patients with a wide range of
cancer types are enrolled in ICI treatments, future studies will
need to add analyses of more cancer types. Based on the widespread
use of ICIs in various solid cancer types, multiple subgroup
analyses could be conducted in future studies with large samples to
explore whether cancer type can be used as a risk factor for
ir-SAEs.
4. Laboratory examinations associated with
ir-SAEs
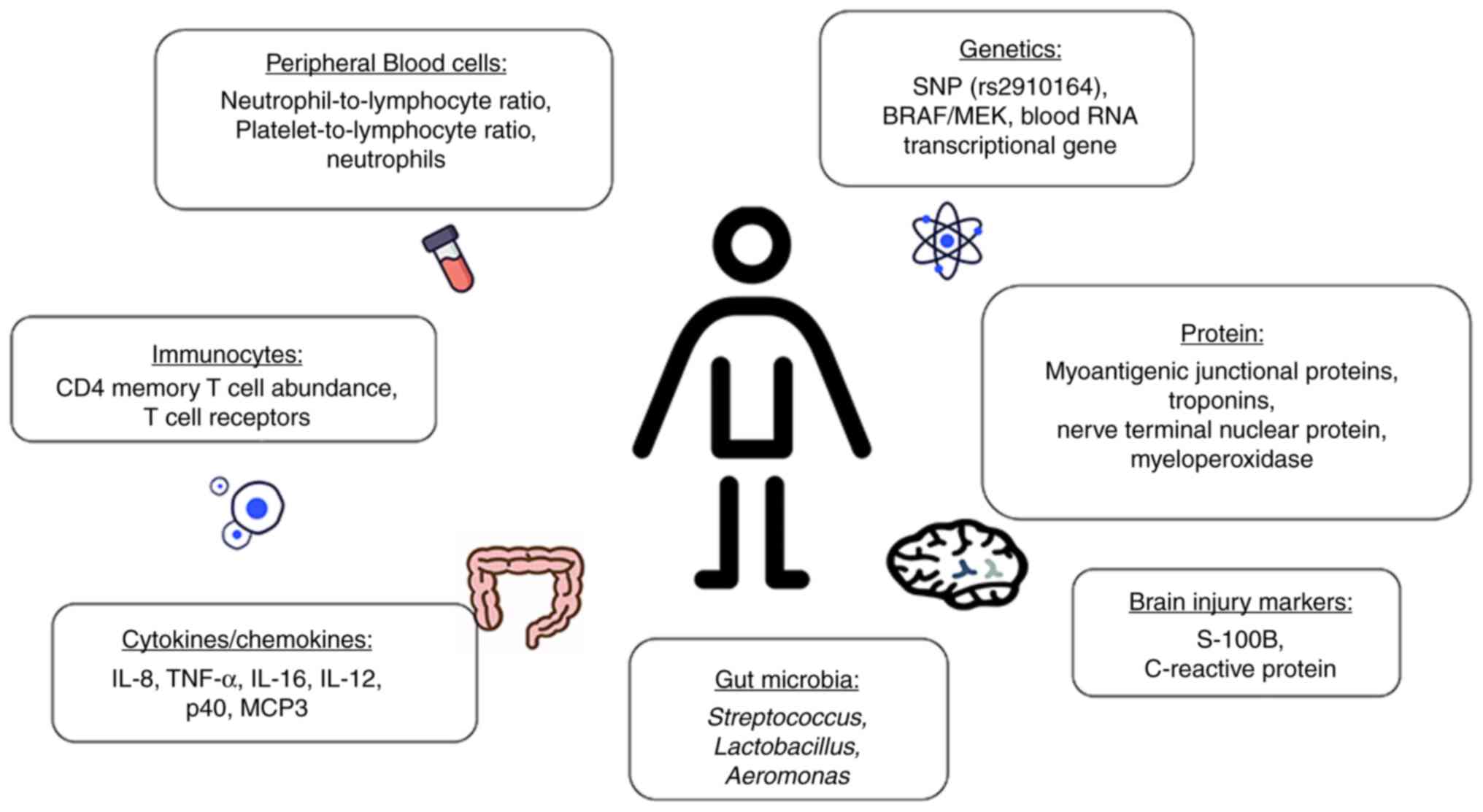
Risk factors associated with ir-SAEs include the
laboratory examination parameters of peripheral blood cells,
immunocytes, cytokines/chemokines, genetics, gut microbia, proteins
and brain injury markers (Fig.
1).
Peripheral blood cells
Studies have confirmed the association between
baseline neutrophil-to-lymphocyte ratio (NLR) levels and the
occurrence of ir-SAEs. Liu et al (55) investigated 150 patients with NSCLC
treated with anti-PD-1 therapy and found that low baseline NLR was
associated with ir-SAEs. Zhao et al (56) observed the association of ir-SAEs
with a baseline NLR of >6 in 832 patients treated with PD-1 (OR,
1.16). However, the NLR values were not consistent. Takada et
al (57) observed that, in 73
patients with gastric and renal cancer treated with nivolumab, the
risk of developing ir-SAEs was reduced when baseline NLR was
<4.3. Regardless of the threshold, various studies have shown
that imbalances in the NLR are associated with a higher incidence
of ir-SAEs. The NLR is a general immune response marker to various
stress stimuli, and an imbalance in the NLR directly reduces the
antitumor immune response, which can lead to an overactive body
immune response when treated with ICIs, thereby increasing the
incidence of ir-SAEs (58).
Besides the NLR, there are other blood cell
parameters that have been reported to be associated with ir-SAEs,
such as the platelet-to-lymphocyte ratio (PLR) and neutrophil
levels. The study by Liu et al (55) included 150 patients with NSCLC
receiving PD-1 inhibitors and analyzed the association of
peripheral blood markers with ir-SAEs. It was concluded that low
PLR and low baseline neutrophil levels were significantly
associated with the development of ir-SAEs (P-values of 0.0016 and
0.009, respectively) (55). As
first-line cells responding to inflammatory reactions, neutrophils
play an important role in the defense against invading pathogens
during infectious inflammation in the body (59). The increase in PLR also reflects a
relative increase in platelet count, and studies have shown that
platelets can release anti-inflammatory factors in addition to
being involved in hemostasis. In an inflammatory state, platelets
balance the body's inflammatory response by releasing
anti-inflammatory factors (60).
However, the complexity of the relationships in hematological
indices will require future large-scale, well-designed randomized
controlled studies to analyze the relationship between blood cells
and ir-SAEs. Peripheral blood cells have the advantages of easy
access and low cost to assess, especially NLR and PLR, which can
reflect the inflammatory state and have gained the attention of
physicians. It is believed that more related studies will be
reported in the future.
Immunocytes
Activated CD4 memory T-cell abundance and more
diverse T-cell receptors in the peripheral blood before treatment
were associated with ir-SAEs in 164 patients with melanoma treated
with ICIs [area under the curve (AUC)=0.90 and AUC=0.80] (61). Meanwhile, enrichment of regulatory
T cells in colitic lesions showed a close association with severe
immune-associated colitis. A study involving 209 patients with
cancer from global pharmacovigilance databases found an association
between increased CD4+ T cells and immune-associated
encephalitis (62). By including a
comparison of 28 healthy people and 87 advanced patients with NSCLC
treated with ICIs, Zamora et al (63) concluded that patients in the
CD4+PLT+ low and
CD14+PLT+ high percentage groups presented
with a higher rate of ir-SAE (63). Das et al (64) analyzed changes in circulating B
cells before and after the first cycle of treatment in 39 patients
with advanced melanoma who underwent immune checkpoint blockade,
concluding that patients with early B-cell alterations experienced
a higher incidence of ir-SAEs (P<0.001). The increased levels of
inflammatory factors are mainly related to the mechanism of irAEs.
The application of ICIs enhances the activity of T cells against
antigens present in tumors and healthy tissue, and increases the
levels of pre-existing autoantibodies and inflammatory factors
(4). This indicates the
involvement of inflammatory factors in the development of irAEs.
Since CTLA-4 and PD-1 are involved in the regulation of B-cell and
T-cell tolerance (64),
anti-CTLA-4 and anti-PD-1 cause B-cell alterations and increase the
risk of autoimmunity. Although the mechanism of irAEs is not clear
yet, immune cells may be potential predictors of ir-SAEs and can
help healthcare professionals gain a deeper understanding of the
mechanisms.
Cytokines/chemokines
The study by Chao et al (65), which included 164 patients with
NSCLC undergoing ICIs, revealed that higher baseline levels of IL-8
were associated with a lower incidence of checkpoint inhibitor
pneumonitis in patients with NSCLC (OR, 0.758) (65). The study conducted by Costantini
et al (66) analyzed the
plasma of 35 patients with NSCLC and found that higher levels of
TNF-α (P=0.036), IL-16 (P=0.040), IL-12p40 (P=0.015)
and MCP3 (P=0.025) were all predictive biomarkers of
ir-SAEs. Cytokines are a class of small molecule proteins that have
a wide range of biological activities and play a key role in life
activities by regulating intrinsic immunity, adaptive immunity and
repairing damaged tissues (67). A
previous study revealed that cytokines are associated with a
variety of ADs, including thyroiditis, inflammatory bowel disease
and systemic sclerosis (68).
Therefore, cytokines are recognized as risk factors that are
strongly associated with ir-SAEs. However, the diversity of ir-SAEs
with a wide range of cytokines also provides challenges for future
studies. In addition, with the progressive development of cytokine
inhibitors in the treatment of ir-SAEs, exploring which cytokine
inhibitors are effective in overcoming tissue-specific ir-SAEs is a
key to future research.
Genetics
Patient genetic background is a crucial predictor of
irAE susceptibility. A single nucleotide polymorphism (rs2910164)
responsible for reduced miR-146a expression was associated with
ir-SAEs in 167 patients with cancer (69). Huang et al (37) included 25 randomized controlled
trials (12,925 patients with advanced melanoma) and found that
ir-SAEs were associated with a high incidence of overall BRAF/MEK
expression (32.11%) (37).
Analysis of 360 patients with melanoma revealed that whole blood
RNA transcriptional gene markers were associated with severe
immune-related diarrhea (AUC=0.785) (70). Expression of these genes may be
associated with hypersensitivity of the immune system. In addition,
genes that are simultaneously expressed on the surface of specific
organs and tumor cells contribute to the high incidence of ir-SAEs
(71). The identified genetic
variants can be used to construct polygenic risk scores, thus
providing patients and clinicians with personalized scores that
measure the risk of ir-SAEs. In the future, more genomic prediction
models should be developed that are more accurate and suitable for
clinical use and measurement to facilitate the timely
identification of populations at risk for ir-SAEs.
Gut microbia
Liu et al (72) found that high concentrations of
Streptococcus, Lactobacillus and narrow-feeding
Aeromonas in baseline stool samples from 150 patients
receiving anti-PD-1 therapy were associated with ir-SAEs (AUC=0.66)
(72). The use of ICIs may disrupt
gut microbial homeostasis and dysregulate the pre-existing
intestinal ecology, which is typically characterized by a reduction
in microbial diversity and/or substantial changes in resident
species. In turn, ecological dysregulation in the gut may trigger
inflammatory signaling pathways and affect overall immune function
(73). This gives medical
professionals hope that gut microbes can be used as predictive
markers for ir-SAEs. Future work is needed to assess whether the
effects of gut microbes are consistent across tumors and across
drugs.
Protein
Okazaki et al (74) found that in PD-1-deficient mice
that developed dilated cardiomyopathy, the cause of the
cardiomyopathy was the production of anti-cardiac troponin I. The
study also found myoantigenic junctional proteins and troponins
expressed in primary tumors in myocardial tissues of
immune-associated cardiomyopathies, concluding that these antigens
may trigger immune responses to normal myocardial tissues (74). Elevated levels of troponin, nerve
terminal nuclear protein (NT-proBNP) and myeloperoxidase are
predictive factors of the development of ir-SAEs. The reason for
this is that elevated levels of these proteins lead to a mechanism
of inflammatory cell infiltration associated with cardiomyocyte
degeneration/necrosis (75).
However, studies on proteins in this context are still rare and
more, large-sample, well-designed studies are needed to determine
their role in increasing the incidence of ir-SAEs.
Brain injury markers
By analyzing 1 patient with melanoma who developed
encephalitis after using ICIs, Bjursten et al (76) observed that brain injury markers
S-100B and C-reactive protein increased before the appearance of
signs or symptoms of encephalomyelitis; this suggests a potential
role for the costimulatory receptor inducible T-cell costimulatory
receptor on CD4+ and CD8+ T cells in
mediating encephalomyelitis and other severe irAEs. In addition,
brain damage markers in the blood could facilitate the early
diagnosis of encephalitis (76).
There is a very small number of studies on proteins and markers of
brain injury, but it has been reported that such markers reappear
after the onset of irAEs (76).
Thus, these disease-related markers are not predictive but help
medical personnel to observe and analyze ir-SAEs.
5. Conclusion
Immunotherapy increases the incidence of ir-SAEs,
which can be fatal to patients receiving ICIs. Thus, the early
detection of patients at high risk for ir-SAEs is essential for
effective prevention of adverse outcomes and improvement of the
safety of the treatment. The present review summarizes the factors
associated with ir-SAEs in terms of demographic characteristics,
disease-related information and laboratory examinations by
consolidating data from various studies. Inconsistency in the
results of studies related to risk factors such as age, BMI,
disease history and cancer type may be due to differences in the
types of cancer studied, the small sample sizes or the differences
in the methodology of the studies. Future prospective studies
should be conducted to validate these findings using larger cohorts
and standardized methods. Collectively, the present review
emphasizes the immense need for research on the determinant of
ir-SAEs. Risk factors for predicting and tracking ir-SAEs in
patients receiving immunotherapy could be used to facilitate
tailored monitoring, early identification and intervention, and
customized treatment. Healthcare professionals must be trained to
recognize these risk factors, and high-risk individuals with risk
factors must be closely monitored for ir-SAEs. For risk factors
that can undergo intervention, such as smoking and abnormal BMI,
medical professionals need to inform patients in good time and
guide them to make adjustments in order to reduce the occurrence of
ir-SAEs.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
Not applicable.
Authors' contributions
ZS MG, LZ and XL made contributions to the
conception and design of this manuscript. ZS and MG carried out the
literature searches and composed the initial draft of the
manuscript. The manuscript was reviewed and revised by LZ and XL.
All authors read and approved the final version of the manuscript.
Data authentication is not applicable.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Lee JB, Kim HR and Ha SJ: Immune
checkpoint inhibitors in 10 years: Contribution of basic research
and clinical application in cancer immunotherapy. Immune Netw.
22(e2)2022.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Carlino MS, Larkin J and Long GV: Immune
checkpoint inhibitors in melanoma. Lancet. 398:1002–1014.
2021.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Xia L, Liu Y and Wang Y: PD-1/PD-L1
blockade therapy in advanced non-small-cell lung cancer: Current
status and future directions. Oncologist. 24 (Suppl 1):S31–S41.
2019.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Postow MA, Sidlow R and Hellmann MD:
Immune-related adverse events associated with immune checkpoint
blockade. N Engl J Med. 378:158–168. 2018.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Zhang L, Reynolds KL, Lyon AR, Palaskas N
and Neilan TG: The evolving immunotherapy landscape and the
epidemiology, diagnosis, and management of cardiotoxicity: JACC:
CardioOncology primer. JACC CardioOncol. 3:35–47. 2021.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Khoja L, Day D, Wei-Wu Chen T, Siu LL and
Hansen AR: Tumour- and class-specific patterns of immune-related
adverse events of immune checkpoint inhibitors: A systematic
review. Ann Oncol. 28:2377–2385. 2017.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Herbst RS, Giaccone G, de Marinis F,
Reinmuth N, Vergnenegre A, Barrios CH, Morise M, Felip E, Andric Z,
Geater S, et al: Atezolizumab for first-line treatment of
PD-L1-selected patients with NSCLC. N Engl J Med. 383:1328–1339.
2020.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Zhou C, Li M, Wang Z, An D and Li B:
Adverse events of immunotherapy in non-small cell lung cancer: A
systematic review and network meta-analysis. Int Immunopharmacol.
102(108353)2022.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Wang DY, Salem JE, Cohen JV, Chandra S,
Menzer C, Ye F, Zhao S, Das S, Beckermann KE, Ha L, et al: Fatal
toxic effects associated with immune checkpoint inhibitors: A
systematic review and meta-analysis. JAMA Oncol. 4:1721–1728.
2018.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Heymach JV, Mitsudomi T, Harpole D,
Aperghis M, Jones S, Mann H, Fouad TM and Reck M: Design and
rationale for a phase III, double-blind, placebo-controlled study
of neoadjuvant durvalumab + chemotherapy followed by adjuvant
durvalumab for the treatment of patients with resectable stages II
and III non-small-cell lung cancer: The AEGEAN trial. Clin Lung
Cancer. 23:e247–e251. 2022.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Rodríguez-Abreu D, Powell SF, Hochmair MJ,
Gadgeel S, Esteban E, Felip E, Speranza G, De Angelis F, Dómine M,
Cheng SY, et al: Pemetrexed plus platinum with or without
pembrolizumab in patients with previously untreated metastatic
nonsquamous NSCLC: Protocol-specified final analysis from
KEYNOTE-189. Ann Oncol. 32:881–895. 2021.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Bernstein MB, Krishnan S, Hodge JW and
Chang JY: Immunotherapy and stereotactic ablative radiotherapy
(ISABR): A curative approach? Nat Rev Clin Oncol. 13:516–524.
2016.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Grant MJ, Herbst RS and Goldberg SB:
Selecting the optimal immunotherapy regimen in driver-negative
metastatic NSCLC. Nat Rev Clin Oncol. 18:625–644. 2021.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Rubio-Infante N, Ramírez-Flores YA,
Castillo EC, Lozano O, García-Rivas G and Torre-Amione G:
Cardiotoxicity associated with immune checkpoint inhibitor therapy:
A meta-analysis. Eur J Heart Fail. 23:1739–1747. 2021.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Baldini C, Martin Romano P, Voisin AL,
Danlos FX, Champiat S, Laghouati S, Kfoury M, Vincent H,
Postel-Vinay S, Varga A, et al: Impact of aging on immune-related
adverse events generated by anti-programmed death (ligand)PD-(L)1
therapies. Eur J Cancer. 129:71–79. 2020.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Shah KP, Song H, Ye F, Moslehi JJ, Balko
JM, Salem JE and Johnson DB: Demographic factors associated with
toxicity in patients treated with anti-programmed cell death-1
therapy. Cancer Immunol Res. 8:851–855. 2020.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Kanjanapan Y and Yip D: Characteristics
and risk factors for microbial infections during cancer immune
checkpoint therapy. Cancer Med. 9:9027–9035. 2020.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Ksienski D, Truong PT, Croteau NS, Chan A,
Sonke E, Patterson T, Clarkson M, Hackett S and Lesperance M:
Immune related adverse events and treatment discontinuation in
patients older and younger than 75 years with advanced melanoma
receiving nivolumab or pembrolizumab. J Geriatr Oncol. 13:220–227.
2022.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Guzman-Prado Y, Ben Shimol J and Samson O:
Body mass index and immune-related adverse events in patients on
immune checkpoint inhibitor therapies: A systematic review and
meta-analysis. Cancer Immunol Immunother. 70:89–100.
2021.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Assumpção JAF, Pasquarelli-do-Nascimento
G, Duarte MSV, Bonamino MH and Magalhães KG: The ambiguous role of
obesity in oncology by promoting cancer but boosting antitumor
immunotherapy. J Biomed Sci. 29(12)2022.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Cortellini A, Bersanelli M, Santini D,
Buti S, Tiseo M, Cannita K, Perrone F, Giusti R, De Tursi M,
Zoratto F, et al: Another side of the association between body mass
index (BMI) and clinical outcomes of cancer patients receiving
programmed cell death protein-1 (PD-1)/programmed cell death-ligand
1 (PD-L1) checkpoint inhibitors: A multicentre analysis of
immune-related adverse events. Eur J Cancer. 128:17–26.
2020.PubMed/NCBI View Article : Google Scholar
|
|
22
|
De Filippi R, Morabito F, Santoro A,
Tripepi G, D'Alò F, Rigacci L, Ricci F, Morelli E, Zinzani PL and
Pinto A: Body mass index is not associated with survival outcomes
and immune-related adverse events in patients with Hodgkin lymphoma
treated with the immune checkpoint inhibitor nivolumab. J Transl
Med. 19(489)2021.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Wood C, Lopez G, Zhao L, Li M, Surya N,
Patel S, Grogan M, Bertino E, Shields P, He K, et al: P78.05
Patterns of irAE during first line pembrolizumab for NSCLC:
Incidence, risk factors, and impact on clinical outcome. J Thorac
Oncol. 16 (Suppl 1):S639–S640. 2021.
|
|
24
|
Muthumalage T and Rahman I: Pulmonary
immune response regulation, genotoxicity, and metabolic
reprogramming by menthol- and tobacco-flavored e-cigarette
exposures in mice. Toxicol Sci. 193:146–165. 2023.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Abdelrahim M, Mamlouk O, Lin H, Lin J,
Page V, Abdel-Wahab N, Swan J, Selamet U, Yee C, Diab A, et al:
Incidence, predictors, and survival impact of acute kidney injury
in patients with melanoma treated with immune checkpoint
inhibitors: A 10-year single-institution analysis. Oncoimmunology.
10(1927313)2021.PubMed/NCBI View Article : Google Scholar
|
|
26
|
GBD Chronic Kidney Disease Collaboration.
Global, regional, and national burden of chronic kidney disease,
1990-2017: A systematic analysis for the global burden of disease
study 2017. Lancet. 395:709–733. 2020.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Cortellini A, Buti S, Bersanelli M, Giusti
R, Perrone F, Di Marino P, Tinari N, De Tursi M, Grassadonia A,
Cannita K, et al: Evaluating the role of FAMIly history of cancer
and diagnosis of multiple neoplasms in cancer patients receiving
PD-1/PD-L1 checkpoint inhibitors: The multicenter FAMI-L1 study.
Oncoimmunology. 9(1710389)2020.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Sorah JD, Rose TL, Radhakrishna R,
Derebail VK and Milowsky MI: Incidence and prediction of immune
checkpoint inhibitor-related nephrotoxicity. J Immunother.
44:127–131. 2021.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Akturk HK, Alkanani A, Zhao Z, Yu L and
Michels AW: PD-1 inhibitor immune-related adverse events in
patients with preexisting endocrine autoimmunity. J Clin Endocrinol
Metab. 103:3589–3592. 2018.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Haanen J, Ernstoff MS, Wang Y, Menzies AM,
Puzanov I, Grivas P, Larkin J, Peters S, Thompson JA and Obeid M:
Autoimmune diseases and immune-checkpoint inhibitors for cancer
therapy: Review of the literature and personalized risk-based
prevention strategy. Ann Oncol. 31:724–744. 2020.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Tang K, Tiu BC, Wan G, Zhang S, Nguyen N,
Leung B, Gusev A, Reynolds KL, Kwatra SG and Semenov YR:
Pre-existing autoimmune disease and mortality in patients treated
with anti-PD-1 and Anti-PD-L1 therapy. J Natl Cancer Inst.
114:1200–1202. 2022.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Oren O, Yang EH, Molina JR, Bailey KR,
Blumenthal RS and Kopecky SL: Cardiovascular health and outcomes in
cancer patients receiving immune checkpoint inhibitors. Am J
Cardiol. 125:1920–1926. 2020.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Noseda R, Ruinelli L, Gaag LCV and Ceschi
A: Pre-existing cardiovascular conditions as clinical predictors of
myocarditis reporting with immune checkpoint inhibitors: A vigibase
study. Cancers (Basel). 12(3480)2020.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Kim PH, Suh CH, Kim HS, Kim KW, Kim DY,
Lee EQ, Aizer AA, Guenette JP and Huang RY: Immune checkpoint
inhibitor with or without radiotherapy in melanoma patients with
brain metastases: A systematic review and meta-analysis. Korean J
Radiol. 22:584–595. 2021.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Miyashita H, Mikami T, Satoi S, Cruz C and
Galsky MD: Incidence and risk of colitis with programmed death 1
versus programmed death ligand 1 inhibitors for the treatment of
cancer. J Immunother. 43:291–298. 2020.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Luoma AM, Suo S, Williams HL, Sharova T,
Sullivan K, Manos M, Bowling P, Hodi FS, Rahma O, Sullivan RJ, et
al: Molecular pathways of colon inflammation induced by cancer
immunotherapy. Cell. 182:655–671.e22. 2020.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Huang YF, Xie WJ, Fan HY and Du J:
Comparative risks of high-grade adverse events among FDA-approved
systemic therapies in advanced melanoma: Systematic review and
network meta-analysis. Front Oncol. 10(571135)2020.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Zheng J, Huang B, Xiao L, Wu M and Li J:
Treatment- and immune-related adverse events of immune checkpoint
inhibitors in esophageal or gastroesophageal junction cancer: A
network meta-analysis of randomized controlled trials. Front Oncol.
12(821626)2022.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Shaverdian N, Lisberg AE, Bornazyan K,
Veruttipong D, Goldman JW, Formenti SC, Garon EB and Lee P:
Previous radiotherapy and the clinical activity and toxicity of
pembrolizumab in the treatment of non-small-cell lung cancer: A
secondary analysis of the KEYNOTE-001 phase 1 trial. Lancet Oncol.
18:895–903. 2017.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Quhal F, Mori K, Bruchbacher A, Resch I,
Mostafaei H, Pradere B, Schuettfort VM, Laukhtina E, Egawa S,
Fajkovic H, et al: First-line Immunotherapy-based combinations for
metastatic renal cell carcinoma: A systematic review and network
meta-analysis. Eur Urol Oncol. 4:755–765. 2021.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Schoenfeld AJ, Arbour KC, Rizvi H, Iqbal
AN, Gadgeel SM, Girshman J, Kris MG, Riely GJ, Yu HA and Hellmann
MD: Severe immune-related adverse events are common with sequential
PD-(L)1 blockade and osimertinib. Ann Oncol. 30:839–844.
2019.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Que S, Feng W and Xu Y: Progress on
immunotherapy combined with chemotherapy, radiotherapy and targeted
therapy for brain metastasis of small cell lung cancer. J Chin
Oncol. 30:177–185. 2024.
|
|
43
|
Zhang Q and Xu H: Meta-analysis of
efficacy and adverse effects of different combination schemes of
PD-1/PD-L1 inhibitors in the treatment of ovarian cancer. Chin J
Clin Obstet Gynecol. 25:292–297. 2024.
|
|
44
|
Cheng Y, Yang F and Zhang Y: Research
progress of phototherapy combined with immune checkpoint inhibitors
in the treatment of tumors. Chin J Cancer Biother. 31:626–631.
2024.
|
|
45
|
Lizotte PH, Hong RL, Luster TA, Cavanaugh
ME, Taus LJ, Wang S, Dhaneshwar A, Mayman N, Yang A, Kulkarni M, et
al: A high-throughput immune-oncology screen identifies EGFR
inhibitors as potent enhancers of antigen-specific cytotoxic
T-lymphocyte tumor cell killing. Cancer Immunol Res. 6:1511–1523.
2018.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Mazzola R, Jereczek-Fossa BA, Franceschini
D, Tubin S, Filippi AR, Tolia M, Lancia A, Minniti G, Corradini S,
Arcangeli S, et al: Oligometastasis and local ablation in the era
of systemic targeted and immunotherapy. Radiat Oncol.
15(92)2020.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Galluzzi L, Spranger S, Fuchs E and
López-Soto A: WNT signaling in cancer immunosurveillance. Trends
Cell Biol. 29:44–65. 2019.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Du S, Zhou L, Alexander GS, Park K, Yang
L, Wang N, Zaorsky NG, Ma X, Wang Y, Dicker AP and Lu B: PD-1
modulates radiation-induced cardiac toxicity through cytotoxic T
lymphocytes. J Thorac Oncol. 13:510–520. 2018.PubMed/NCBI View Article : Google Scholar
|
|
49
|
L'Orphelin JM, Varey E, Khammari A, Dreno
B and Dompmartin A: Severe late-onset grade III-IV adverse events
under immunotherapy: A retrospective study of 79 cases. Cancers
(Basel). 13(4928)2021.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Ruste V, Goldschmidt V, Laparra A,
Messayke S, Danlos FX, Romano-Martin P, Champiat S, Voisin AL,
Baldini C, Massard C, et al: The determinants of very severe
immune-related adverse events associated with immune checkpoint
inhibitors: A prospective study of the French REISAMIC registry.
Eur J Cancer. 158:217–224. 2021.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Brumberger ZL, Branch ME, Klein MW, Seals
A, Shapiro MD and Vasu S: Cardiotoxicity risk factors with immune
checkpoint inhibitors. Cardiooncology. 8(3)2022.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Sher AF, Golshani GM and Wu S: Fatal
adverse events associated with pembrolizumab in cancer patients: A
meta-analysis. Cancer Invest. 38:130–138. 2020.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Llovet JM, Castet F, Heikenwalder M, Maini
MK, Mazzaferro V, Pinato DJ, Pikarsky E, Zhu AX and Finn RS:
Immunotherapies for hepatocellular carcinoma. Nat Rev Clin Oncol.
19:151–172. 2022.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Lou S, Cao Z, Chi W, Wang X, Feng M, Lin
L, Ding Y, Liu K, Qu L, Zhao G, et al: The safety concerns
regarding immune checkpoint inhibitors in liver cancer patients
rising mainly from CHB. Front Pharmacol. 14(1164309)2023.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Liu W, Liu Y, Ma F, Sun B, Wang Y, Luo J,
Liu M and Luo Z: Peripheral blood markers associated with
immune-related adverse effects in patients who had advanced
non-small cell lung cancer treated with PD-1 inhibitors. Cancer
Manag Res. 13:765–771. 2021.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Zhao L, Li Y, Jiang N, Song X, Xu J, Zhu
X, Chen C, Kong C, Wang X, Zong D, et al: Association of blood
biochemical indexes and antibiotic exposure with severe
immune-related adverse events in patients with advanced cancers
receiving PD-1 inhibitors. J Immunother. 45:210–216.
2022.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Takada S, Murooka H, Tahatsu K, Yanase M,
Umehara K, Hashishita H, Toru H, Satoru M, Sagawa T, Fujikawa K, et
al: Identifying early predictive markers for immune-related adverse
events in nivolumab-treated patients with renal cell carcinoma and
gastric cancer. Asian Pac J Cancer Prev. 23:695–701.
2022.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Zhang W, Tan Y, Li Y and Liu J: Neutrophil
to Lymphocyte ratio as a predictor for immune-related adverse
events in cancer patients treated with immune checkpoint
inhibitors: A systematic review and meta-analysis. Front Immunol.
14(1234142)2023.PubMed/NCBI View Article : Google Scholar
|
|
59
|
Drobni ZD, Zafar A, Zubiri L, Zlotoff DA,
Alvi RM, Lee C, Hartmann S, Gilman HK, Villani AC, Nohria A, et al:
Decreased absolute lymphocyte count and increased
neutrophil/lymphocyte ratio with immune checkpoint
inhibitor-associated myocarditis. J Am Heart Assoc.
9(e018306)2020.PubMed/NCBI View Article : Google Scholar
|
|
60
|
Zhou JG, Wong AH, Wang H, Tan F, Chen X,
Jin SH, He SS, Shen G, Wang YJ, Frey B, et al: Elucidation of the
application of blood test biomarkers to predict immune-related
adverse events in atezolizumab-treated NSCLC patients using machine
learning methods. Front Immunol. 13(862752)2022.PubMed/NCBI View Article : Google Scholar
|
|
61
|
Lozano AX, Chaudhuri AA, Nene A,
Bacchiocchi A, Earland N, Vesely MD, Usmani A, Turner BE, Steen CB,
Luca BA, et al: T cell characteristics associated with toxicity to
immune checkpoint blockade in patients with melanoma. Nat Med.
28:353–362. 2022.PubMed/NCBI View Article : Google Scholar
|
|
62
|
Johnson DB, McDonnell WJ,
Gonzalez-Ericsson PI, Al-Rohil RN, Mobley BC, Salem JE, Wang DY,
Sanchez V, Wang Y, Chastain CA, et al: A case report of clonal
EBV-like memory CD4+ T cell activation in fatal
checkpoint inhibitor-induced encephalitis. Nat Med. 25:1243–1250.
2019.PubMed/NCBI View Article : Google Scholar
|
|
63
|
Zamora C, Riudavets M, Anguera G,
Alserawan L, Sullivan I, Barba A, Serra J, Ortiz MA, Gallardo P,
Perea L, et al: Circulating leukocyte-platelet complexes as a
predictive biomarker for the development of immune-related adverse
events in advanced non-small cell lung cancer patients receiving
anti-PD-(L)1 blocking agents. Cancer Immunol Immunother.
70:1691–1704. 2021.PubMed/NCBI View Article : Google Scholar
|
|
64
|
Das R, Bar N, Ferreira M, Newman AM, Zhang
L, Bailur JK, Bacchiocchi A, Kluger H, Wei W, Halaban R, et al:
Early B cell changes predict autoimmunity following combination
immune checkpoint blockade. J Clin Invest. 128:715–720.
2018.PubMed/NCBI View Article : Google Scholar
|
|
65
|
Chao Y, Zhou J, Hsu S, Ding N, Li J, Zhang
Y, Xu X, Tang X, Wei T, Zhu Z, et al: Risk factors for immune
checkpoint inhibitor-related pneumonitis in non-small cell lung
cancer. Transl Lung Cancer Res. 11:295–306. 2022.PubMed/NCBI View Article : Google Scholar
|
|
66
|
Costantini A, Takam Kamga P, Julie C,
Corjon A, Dumenil C, Dumoulin J, Ouaknine J, Giraud V, Chinet T,
Rottman M, et al: Plasma biomarkers screening by multiplex ELISA
assay in patients with advanced non-small cell lung cancer treated
with immune checkpoint inhibitors. Cancers (Basel).
13(97)2020.PubMed/NCBI View Article : Google Scholar
|
|
67
|
Kaminska P, Tempes A, Scholz E and Malik
AR: Cytokines on the way to secretion. Cytokine Growth Factor Rev.
79:52–65. 2024.PubMed/NCBI View Article : Google Scholar
|
|
68
|
Liu C, Chu D, Kalantar-Zadeh K, George J,
Young HA and Liu G: Cytokines: From clinical significance to
quantification. Adv Sci (Weinh). 8(e2004433)2021.PubMed/NCBI View Article : Google Scholar
|
|
69
|
Marschner D, Falk M, Javorniczky NR,
Hanke-Müller K, Rawluk J, Schmitt-Graeff A, Simonetta F, Haring E,
Dicks S, Ku M, et al: MicroRNA-146a regulates immune-related
adverse events caused by immune checkpoint inhibitors. JCI Insight.
5(e132334)2020.PubMed/NCBI View Article : Google Scholar
|
|
70
|
Friedlander P, Wood K, Wassmann K,
Christenfeld AM, Bhardwaj N and Oh WK: A whole-blood RNA
transcript-based gene signature is associated with the development
of CTLA-4 blockade-related diarrhea in patients with advanced
melanoma treated with the checkpoint inhibitor tremelimumab. J
Immunother Cancer. 6(90)2018.PubMed/NCBI View Article : Google Scholar
|
|
71
|
Refae S, Gal J, Ebran N, Otto J,
Borchiellini D, Peyrade F, Chamorey E, Brest P, Milano G and
Saada-Bouzid E: Correction to: Germinal Immunogenetics predict
treatment outcome for PD-1/PD-L1 checkpoint inhibitors. Invest New
Drugs. 39:287–292. 2021.PubMed/NCBI View Article : Google Scholar
|
|
72
|
Liu W, Ma F, Sun B, Liu Y, Tang H, Luo J,
Chen H and Luo Z: Intestinal microbiome associated with
immune-related adverse events for patients treated with anti-PD-1
inhibitors, a real-world study. Front Immunol.
12(756872)2021.PubMed/NCBI View Article : Google Scholar
|
|
73
|
Levy M, Kolodziejczyk AA, Thaiss CA and
Elinav E: Dysbiosis and the immune system. Nat Rev Immunol.
17:219–232. 2017.PubMed/NCBI View Article : Google Scholar
|
|
74
|
Okazaki T, Tanaka Y, Nishio R, Mitsuiye T,
Mizoguchi A, Wang J, Ishida M, Hiai H, Matsumori A, Minato N and
Honjo T: Autoantibodies against cardiac troponin I are responsible
for dilated cardiomyopathy in PD-1-deficient mice. Nat Med.
9:1477–1483. 2003.PubMed/NCBI View
Article : Google Scholar
|
|
75
|
Ji C, Roy MD, Golas J, Vitsky A, Ram S,
Kumpf SW, Martin M, Barletta F, Meier WA, Hooper AT, et al:
Myocarditis in cynomolgus monkeys following treatment with immune
checkpoint inhibitors. Clin Cancer Res. 25:4735–4748.
2019.PubMed/NCBI View Article : Google Scholar
|
|
76
|
Bjursten S, Pandita A, Zhao Z, Fröjd C, Ny
L, Jensen C, Ullerstam T, Jespersen H, Borén J, Levin M, et al:
Early rise in brain damage markers and high ICOS expression in CD4+
and CD8+ T cells during checkpoint inhibitor-induced
encephalomyelitis. J Immunother Cancer. 9(e002732)2021.PubMed/NCBI View Article : Google Scholar
|