1. Introduction
Immunotherapy utilizes the body's immune system to
act as a natural defense against cancer cells, which is notably
different to traditional cancer treatments, including chemotherapy
and radiotherapy. Advancements in immunotherapy have revolutionized
disease management and cancer immunology. Results of clinical
trials demonstrated that several types of immunotherapies, such as
adoptive cellular therapy and immune checkpoint inhibitors (ICIs),
exhibit potential in the treatment of cancer; however, efficacy may
vary between patients and benefits may only be observed in certain
groups (1).
Receptors, such as Toll-like receptors (TLRs),
bridge the gap between innate and adaptive immunity, highlighting
them as potential targets for therapeutic interventions. TLRs
contribute to the first line of defense against pathogens,
triggering signaling pathways that initiate immune and inflammatory
responses (2).
The limited immunogenicity of cancer cells in
combination with an immunosuppressive microenvironment is a pivotal
protective factor that allows evasion of immune surveillance.
Immune cells do not distinctly recognize tumor cells, and the tumor
microenvironment (TME) notably hinders the infiltration or survival
of immune cells (3).
TLRs are evolutionarily-conserved receptors that
play a vital role in immune responses, particularly in recognizing
microbes. TLRs belong to the family of pattern recognition
receptors and are directly involved in regulating inflammatory
responses, as well as activating innate or adaptive immune
responses to eliminate infectious microorganisms and debris derived
from cancer cells.
TLRs expressed on the cell membrane form an
effective group with those located on the endosomal plasma
membrane. These include TLR1, TLR2, TLR4, TLR5, TLR6, TLR10, TLR3,
TLR7, TLR8 and TLR9. In cancer immunotherapy, the modulation of
TLRs is employed to enhance the immune response against cancer
cells. Thus, specific drugs or agents are designed to activate
specific TLRs, thereby initiating an immune response that targets
cancer. This strategy is commonly referred to as TLR agonism.
2. An overview of R848 and R837
R848 and R837 are synthetic compounds that modulate
the immune response against cancer cells, acting as agonists for
TLRs. R848, also known as Resiquimod, activates TLR7/8 receptors in
various immune cells. R848 induces the anti-tumor response; thus,
exhibiting potential as an immunotherapeutic agent. Notably,
dendritic cells are actively involved in initiating and regulating
immune responses, and these are stimulated by R848. However,
immunotherapies are only effective in a specific TME, and R848
remodels the TME to aid in immunotherapy (4,5).
R837, also known as Imiquimod, also activates TLR7/8
receptors in various immune cells. R837 is well-established as a
combination therapy with chemotherapy and photochemical therapy,
and is used to improve immunotherapeutic efficacy (6).
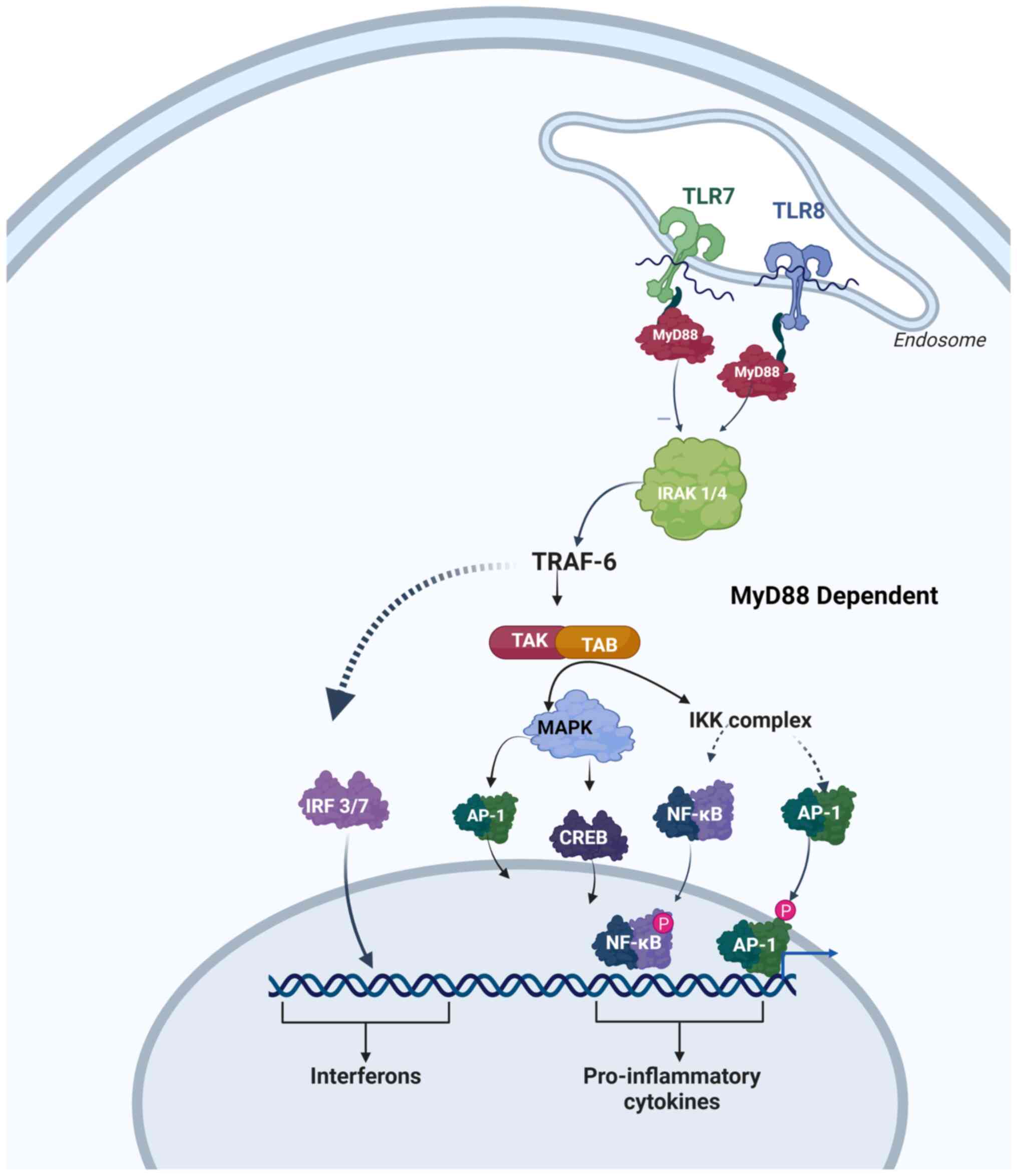
3. Mechanisms of action
R848 and R837 are imidazoquinolines that act as
agonists for TLR7 or TLR8. Their drug-receptor interaction
activates immune cells and creates an environment that stimulates T
helper cell (Th1) immune responses. In humans, R848 functions as an
agonist for both TLR7 and TLR8; however, in mice, it acts as a
preferential agonist to TLR7. R837 is a selective antagonist of
TLR7 only. The Myd88-dependent signaling pathway is utilized by
R848 and R837 to activate TLR7/TLR8, which in turn activates
transcription factors; namely, NF-κB and JNK. Ultimately, this
pathway leads to the production of Th1 cytokines, such as type I
interferon (IFN I), interleukin (IL)-6, IL-12, IFN-γ and tumor
necrosis factor-α (TNF-α), while suppressing the expression and
secretion of Th2 cytokine, IL-4. Moreover, Th1 cytokines trigger
the release of several cytokines that facilitate innate and
acquired immunity, through activation of monocytes, macrophages,
and plasmacytoid dendritic cells via TLR7/8. In addition, the
activation of TLRs stimulates the activity of antigen-presenting
cells (APCs). APCs present antigens to T-cells, thereby initiating
an immune response. This, in turn, triggers tumor-associated
macrophages (TAMs) that modulate the TME through activating innate
immune responses. Thus, tumor growth is inhibited and the TME is
disrupted via R848- and R837-mediated activation of TAMs (Fig. 1) (7).
4. Preclinical studies in cancer
immunotherapy using R848 and R837
Several preclinical studies have previously been
conducted to study the role and effects of R848 and R837 in cancer
immunotherapy. Ye et al (8)
evaluated the impact of intravenous R848 and stereotactic body
radiation therapy (SBRT) on tumor growth and immune response in
primary pancreatic tumors, using murine models of orthotopic
pancreatic ductal adenocarcinoma (PDAC). Results of this previous
study demonstrated that the combination of R848 and SBRT
significantly activated the pancreatic TME, increased levels of
IFN-γ, granzyme B and chemokine ligand 5, and decreased levels of
IL-4, IL-6 and IL-10. In addition, R848 and SBRT increased tumor
antigen-specific CD8+ T-cells, reduced the number of regulatory
T-cells and improved antigen-presenting cell maturation (8). Moreover, Mottas et al
(9) utilized amphiphilic polymer
and monoblock peptide nanoparticles to encapsulate and deliver R848
and R837 to human and murine cancer cell lines. Results of this
previous study revealed that R848 and R837 targeted blood-borne
macrophages and promoted tumor regression in experimental gliomas
without adaptive immunity. In a further study, nanoparticles
consisting of polyglycolic acid (PLGA) combined with
immunostimulant granulocyte-colony stimulating factor (ICG) were
used as a carrier for R848 via near-infrared (NIR). In both human
and murine cell lines for prostate cancer, a favorable
immunohistochemical response was observed. The combination of
nanoparticles containing cancer cell membranes derived from
surgically excised cancer cells and mesoporous polydopamine
carrying R848 were also studied, and the results revealed that this
nano vaccine exhibited potent phagocytosis, isotype targeting and
tumor-specific immune responses when exposed to NIR. In addition,
this combination promoted photothermal immunotherapy at the tumor
site (10). Results of a further
previous study revealed that R837 in combination with
hematoporphyrin monomethyl ether arrested primary tumor progression
and prevented lung metastasis, when administered via biomedical
ultrasound (7,11-13).
Thus, the results of preclinical studies have led to the
development of clinical trials that aimed to determine the efficacy
and safety of R837 and R848 in healthy human volunteers.
5. Clinical trials
R837 and R848, initially explored for their
potential in the treatment of herpes simplex virus (HSV), exhibited
limitations in clinical trials due to insufficient efficacy and
notable side effects. Despite evidence suggesting success in the
treatment of acute and chronic HSV lesions, off-label usage became
common (14-16).
For example, R837 demonstrated potential in the treatment of
vaginal or cervical human papillomavirus (HPV) lesions, and the
effects were more apparent in combination with photodynamic therapy
or HPV therapeutic vaccines for HPV-16-mediated vulvar
intraepithelial neoplasia (17).
However, significant side effects, such as severe vulval erythema,
and unsuccessful trials in patients with cervical intraepithelial
neoplasia were reported (18).
In a clinical trial, R837 demonstrated disease
clearance rates exceeding 50%, highlighted by increased levels of
immune cells in biopsied lesions. Approval from the Food and Drug
Administration (FDA) was granted for the treatment of basal cell
carcinoma in 2004, with high disease clearance rates observed in
Phase III clinical trials (19).
Notably, Th1 polarization, enhanced IFN I signaling and immune cell
recruitment were observed in skin biopsies. R848 also demonstrated
high levels of efficacy in cutaneous T-cell lymphoma treatment,
inducing significant improvements in patients (20). Notably, clinical trials that aim to
explore novel combinations and delivery strategies of R837 and R848
are ongoing (Table I).
 | Table IRegistered clinical trials that aimed
to determine the safety and efficacy of Imiquimod and Resiquimod in
the treatment of various cancers. |
Table I
Registered clinical trials that aimed
to determine the safety and efficacy of Imiquimod and Resiquimod in
the treatment of various cancers.
| No. | Date of
registration | Status of
recruitment | Scientific
title | Study type | Study design | Phase of trial | Sample size | Trial ID for
reference |
|---|
| 1 | 08-03-2002 | Complete | Phase II topical
immunomodulatory therapy with Imiquimod for the chemoprevention of
recurrent and high- grade cervical intraepithelial neoplasia
(CIN) | Interventional | Open label
study | Phase 2 | 57 | NCT00031759 |
| 2 | 06-08-2003 | Complete | A randomized
controlled trial of excisional surgery vs. Imiquimod 5% cream for
nodular and superficial basal cell carcinoma | Interventional | Randomized, open
label study | Phase 3 | 500 | NCT00066872 |
| 3 | 08-03-2004 | Complete | Double-blind,
vehicle- controlled study to evaluate apoptosis in basal cell
carcinoma treated with Aldara™ (Imiquimod) cream, 5% applied once
or twice a day | Interventional | Randomized,
double-blind study | Phase 1 | 48 | NCT00079300 |
| 4 | 07-01-2006 | Completed | Evaluation of the
impact of adjuvants accompanying peptide immunization in high- risk
melanoma | Interventional | Randomized,
parallelly assigned, open label study | Phase 2 | 104 | NCT00273910 |
| 5 | 27-03-2007 | Completed | Laser and
TLR-agonist immunotherapy: a novel autologous melanoma vaccine
study | Interventional | Open label
study | Phase 1 | 11 | NCT00453050 |
| 6 | 26-07-2007 | Recruiting | Three non-invasive
treatment options for superficial basal cell carcinoma: PDT vs.
Imiquimod vs. 5-fluorouracil-treatment of SBCC | Interventional | Single blinded
masking used, uncontrolled, treatment | Phase 4 | 600 | NL-OMON31670 |
| 7 | 25-09-2007 | Not recruiting | A pilot study of
topical imiquimod therapy for the treatment of recurrent
extramammary Paget's disease | Interventional | Single allocation,
open label study | N/A | 8 | NCT00504023 |
| 8 | 25-09-2007 | Completed | A randomised phase
II multi-centre trial of topical treatment in women with vulvar
intraepithelial neoplasia (tr3-vin) | Interventional | A randomized phase
II multi-center trial (treatment) | 2 | 204 | ISRCTN34420460 |
| 9 | 25-06-2008 | Not recruiting | Combination Therapy
with Imiquimod Cream 5% and Tazarotene Cream 0.1% for the treatment
of Lentigo Maligna | Interventional | Randomized,
parallelly assigned, open label study | N/A | 90 | NCT00707174 |
| 10 | 01-09-2009 | Recruiting | Phase I study to
determining toxicity and immunity of the p53 synthetic long
peptides vaccine combined with interferon-alfa in patients treated
for colorectal cancer-p53-slp vaccine in combination with IFNA | Interventional | Open uncontrolled
TRIAL | 1 | 10 | NL-OMON33981 |
| 11 | 07-11-2008 | Completed | A phase I efficacy
and safety study of hpv16- specific therapeutic DNA-vaccinia
vaccination in combination with topical Imiquimod, in patients with
hpv16+ high grade cervical dysplasia (CIN3) | Interventional | Non- randomized,
parallelly assigned. open label study | Phase 1 | 75 | NCT00788164 |
| 12 | 26-11-2008 | Active, not
recruiting | Vaccination of
patients with ovarian cancer with dendritic cell/tumor fusions with
GM-CSF and Imiquimod | Interventional | Randomized,
parallelly assigned, open label study | Phase 2 | 23 | NCT00799110 |
| 13 | 05-01-2009 | Authorized | Clinical and
immunological effects of Imiquimod and HPV- vaccination compared to
Imiquimod alone in female patients with usual type vin | Interventional | Controlled,
randomized, double blind: parallelly assigned study | Phase 2 | N/A |
EUCTR2008-008251-42-NL |
| 14 | 13-01-2009 | Completed | Phase II study of
topical Imiquimod and weekly Abraxane for the treatment of breast
cancer cutaneous metastases | Interventional | Single group
assignment, open label STUDY | Phase 2 | 15 | NCT00821964 |
| 15 | 11-05-2009 | Completed | Phase II evaluation
of Imiquimod, a topical toll-like receptor 7 (tlr7) agonist in
breast cancer patients with chest wall recurrence or skin
metastases | Interventional | Open label, single
group assignment | Phase 2 | 10 | NCT00899574 |
| 16 | 01-10-2009 | Not recruiting | A pilot study of
pngvl4a-crt/e7 (detox) for the treatment of patients with hpv16+
cervical intraepithelial neoplasia 2/3 (cin2/3) | Interventional | Non- randomized.
Intervention model, parallel assignment, open label | Phase 1 | 132 | NCT00988559 |
| 17 | 28-04-2010 | Not recruiting | Topical Imiquimod
in treating patients with persistent HPV-infection after surgical
or radiation treatment of cervical cancer-tactiq (treatment after
cervical cancer with topical Imiquimod)-trial | Interventional | Controlled,
randomized, open labelled, parallel assigned study | Phase 3 | Na |
EUCTR2010-019657-18-AT |
| 18 | 09-06-2010 | Not recruiting | Phase I/II study of
peptide vaccination associated with tumoral immunomodulation with
proinflammatory cytokines and Imiquimod in patients with advanced
metastatic melanoma | Interventional | Open label, single
group assignment | Phase 2 | 21 |
EUCTR2010-020435-40-BE |
| 19 | 19-07-2011 | Not recruiting | Imiquimod/BTIC
lysate- based vaccine immunotherapy for diffuse intrinsic pontine
glioma in children and young adults | Interventional | Single group
assignment, open label STUDY | Phase 1 | 8 | NCT01400672 |
| 20 | 17-08-2011 | Not recruiting | Phase I/II study of
tlr7 agonist Imiquimod, cyclophosphamide, and radiotherapy in
breast cancer patients with chest wall recurrence or skin
metastases | Interventional | Non- randomized,
parallelly assigned, open label study | Phase 1/ phase
2 | 32 | NCT01421017 |
| 21 | 30-04-2013 | Authorized-
recruitment may be ongoing or finished | Risk of skin cancer
on skin areas treated with Ingenol mebutate gel, 0.015% and
Imiquimod cream, 5% | Interventional | Randomized, open
label, parallelly assigned study | Phase 4 | 480 |
EUCTR2012-003112-31-GB |
| 22 | 19-08-2013 | Complete | A phase 4 trial
comparing the cumulative incidence of SCC after treatment with
Ingenol mebutate and Imiquimod for multiple actinic keratoses on
face and scalp. A multi-centre, randomised, two-arm, open label,
active- controlled, parallel group, 36-month trial | Interventional | Randomized,
parallelly assigned, open label study | Phase 4 | 485 | NCT01926496 |
| 23 | 28-08-2013 | Complete | Phase II clinical
trial of combination of personalized peptide vaccination,
cyclophosphamide and Imiquimod for advanced pancreatic cancer
patients who failed standard therapy-Phase II study of peptide
vaccination for advanced pancreaticcancer patients | Interventional | Parallel,
randomized study | Phase 2 | 66 |
JPRN-UMIN000011593 |
| 24 | 11-08-2014 | Terminated | Phase I/II study of
low- dose cyclophosphamide, tumor associated peptide antigen-
pulsed dendritic cell therapyand Imiquimod, in patients with
progressive and/or refractory solid malignancies | Interventional | Single group
assignment, open label STUDY | Phase 1/ phase
2 | 3 | NCT02224599 |
| 25 | 15-09-2014 | Active: not
recruiting | Surgical excision
vs. combined treatment with curettage and Imiquimod for nodular
basal cell carcinoma: an open, non-inferiority, randomized
controlled trial | Interventional | Randomized,
parallelly assigned, open label study | Phase 3 | 145 | NCT02242929 |
| 26 | 10-03-2015 | Completed | A randomised
controlled multicentre trial of Imiquimod vs. radiotherapy for
lentigo maligna (LM) when staged surgical excision with 5 mm
margins is not possible, is refused, or fails | Interventional | Randomized,
parallelly assigned, open label study | Phase 3 | 126 | NCT02394132 |
| 27 | 20-03-2015 | Active, not
recruiting | A randomised
controlled multicentre trial to evaluate the effect of Imiquimod
vs. radiotherapy on treatment failure at 6 months in patients with
lentigo maligna (LM) for which staged surgical excision with 5 mm
margins is not possible, is refused, or fails | Interventional | Randomized,
controlled trial, open masking parallelly assigned study | Phase 3 | 266 |
ACTRN12615000266561 |
| 28 | 01-01-2016 | Recruiting | Randomized trial of
treatment of vaginal intraepithelial neoplasia (vain): laser
vaporization and Imiquimod | Interventional | Single centric,
randomized interventional trial | N/a | 60 | ISRCTN23349576 |
| 29 | 21-06-2017 | Active, not
recruiting | A feasibility trial
of alternating intravaginal application of 5- fluorouracil and
Imiquimod for treatment of high- grade cervical squamous
intraepithelial lesions | Interventional | Pilot prospective
study | N/a | 13 | NCT03196180 |
| 30 | 06-07-2017 | Completed | Randomized clinical
trial evaluating the efficacy of topical Imiquimod in high grade
cervical intraepithelial lesions | Interventional | Randomized.
parallelly assigned, single blinded study | Phase 2 | 90 | NCT03233412 |
| 31 | 20-07-2022 | Open to
recruitment | Assessing
effectiveness of combined therapy of antioxidant and topical 5%
Imiquimod in management of oral leucoplakia-A randomised clinical
trial | Interventional | Randomized,
parallel group, active controlled trial | Phase 3/ phase
4 | 60 |
CTRI/2022/07/044182 |
| 32 | 04-03-2024 | Not yet
recruiting | Immunophenotyping,
microbiome, clinical outcome and biomarkers for predicting
immunological response in patients with high-grade cervical
intraepithelial lesions treated with Imiquimod | Interventional | Randomized, open
labelled, parallelly assigned | Phase 4 | 96 | NCT06356012 |
| 33 | 16-09-2010 | Not recruiting | A phase II clinical
trial evaluating autologous dendritic cells pulsed with tumor
lysate antigen +/- toll-like receptor agonists for the treatment of
malignant glioma | Interventional | Randomized,
parallelly assigned, open label study | Phase 2 | 60 | NCT01204684 |
| 34 | 15-07-2012 | Completed | A phase I/IIA,
dose- ranging safety and efficacy study of topical Resiquimod for
the treatment of early stage cutaneous t cell lymphoma | Interventional | Non- randomized,
single group assignment, open label study | Phase 1/ phase
2 | 13 | NCT01676831 |
| 35 | 29-10-2020 | Not recruiting | Clinical trial exit
interview study in cutaneous t-cell lymphoma (CTCL) to capture
meaningful treatment benefit from a patient's perspective | Interventional | Randomized, single
blinded study | Phase 3/ phase
4 | 30 |
EUCTR2020-001992-34-DE |
The SINS study (trial no. NCT00066872) was a
multi-centric, randomized study including 500 patients with basal
skin cell cancer, who participated for 18 months. Patients were
categorized depending on the presence of superficial or nodular
lesions and the participating center. Patients were randomly
assigned to two treatment arms, and those with superficial lesions
were treated for 6 weeks, while patients with nodular lesions were
treated for 12 weeks. Patients in treatment arm one received
topical Imiquimod, and this was applied to a single lesion once
daily. Surgical excision was offered to patients who exhibited
recurrence or failed early treatment. Patients in treatment arm two
underwent surgical excision. Follow-up was carried out at 6, 12 and
18 weeks, every 6 months for two years, and every 5 years. Results
of this previous study revealed that Imiquimod was effective in
~90% of patients with large superficial basal cell carcinoma
(diameter, >4 cm). Notably, Imiquimod was considered the
preferred course of treatment; however, it was only marginally less
effective than excisional surgery due to the associated cosmetic
outcomes (21).
Females who are yet to reach menopause are primarily
affected by vulval intraepithelial neoplasia, a pre-malignant
disease. This condition is symptomatic and invasive, leading to
challenges in clinical practice. Treatment goals include symptom
relief and inhibition of disease spread. Surgery is frequently used
to cure this malignant condition; however, it may be deformative
and exhibits a high recurrence rate. Recent pilot and small-scale
studies using novel topical therapies with Imiquimod as a
non-surgical alternative have demonstrated positive outcomes.
A randomized Phase II multi-centric clinical trial
(trial no. ISRCTN34420460) included 204 patients. Patients received
topical treatment of Imiquimod or Cidofovir for up to 24 weeks.
Every 6 weeks, patients were reviewed and evaluated, and treatment
was administered for up to 24 weeks if there was no full response.
Notably, a full response was established histologically at 30 weeks
following therapy initiation. In total, 156 patients (87%) followed
the treatment plan for >6 weeks (78 in each group). Prior to the
initial 6-week safety evaluation, 5 patients in the Imiquimod group
and 7 patients in the Cidofovir group stopped responding to
follow-up or withdrew from the study. Of the 84 patients assigned
to the Cidofovir treatment group, 31 (37%) experienced adverse
events of grade 3 or above, whereas 39 (46%) of the 84 patients
assigned to the Imiquimod treatment group experienced adverse
events of grade 3 or above. Notably, the most common grade 3 and 4
events were headache, pruritus, pain in the vulva and exhaustion.
Further investigations into the use of Cidofovir and Imiquimod are
required, as these reagents demonstrate high levels of efficacy and
safety, and may exhibit potential as an alternative to surgical
intervention in the treatment of vulval intraepithelial neoplasia
(22).
An open, blinded, non-controlled clinical trial
(trial no. NL-OMON3398) aimed to determine whether Imiquimod,
granulocyte-macrophage colony-stimulating factor, IL-2 and IFN-α
induce peritumoral responses when used in conjunction with a
peptide vaccination. The type of human leukocyte antigen and gene
expression of the tumor determined whether the MAGE-3.A1 peptide,
NA17.A2 peptide or both peptide vaccines would be used. If both
antigens were expressed, the patient was treated with both peptide
vaccines. This combination triggers cytolytic T-cell (CTL)
responses, which were evaluated through comparing the frequency of
either anti-MAGE-3. A1 or anti-NA17.A2 CTLp in the pre- and
post-immune blood of patients. This trial exhibited limitations, as
there were a small number of subjects, and technical problems led
to unreliable data. Thus, the trial was subsequently terminated
(23).
NCT03196180 was an early Phase I, open-label
clinical trial involving 13 patients with high-grade cervical
intraepithelial neoplasia, who were treated with topical
fluorouracil and Imiquimod. In patients with precancerous cervical
lesions, topical fluorouracil and Imiquimod ointment may lead to
positive outcomes due to fewer associated adverse effects. For the
treatment of high-grade cervical squamous intraepithelial lesions,
a once-weekly intravaginal application of 5-fluorouracil was used,
and this was alternated with a once-weekly application of
Imiquimod. For 8-16 weeks, 5-fluorouracil and Imiquimod treatments
were alternated. The median age of participants was 27±2.9 years.
There were no grade 3/4 adverse events, six (46.15%) grade 2
adverse events and all individuals reported grade 1 adverse events.
In total, 3 patients (23.08%) reported specific adverse events.
Notably, 10 patients (90.91%) exhibited histologic regression to
cervical intraepithelial neoplasia (CIN), and 7 patients (63.63%)
tested negative for hr-HPV following the study. Further
investigations are required for the use of topical treatments in
CIN 2/3 as alternatives or adjuncts to surgical therapy (24).
The NCT01676831 Phase I clinical trial was an
open-label, dose-ranging trial including patients with early-stage
(IA-IIA) cutaneous T-cell lymphoma (CTCL). This study investigated
the safety and efficacy of Resiquimod gel at concentrations of 0.06
and 0.03%, administered to individuals with early-stage cutaneous
T-cell lymphoma lesions. In 75% of patients, considerable
improvements were observed in treated lesions, and 30% of patients
experienced total clearance of lesions. In addition, Resiquimod
also induced the regression of untreated lesions. According to the
modified Severity-Weighted Assessment Tool, 92% of patients
exhibited a ≥50% improvement in body surface area involved, and 2
patients experienced full clearance of the disease. In total, 4 out
of 5 patients with follicotropic disease demonstrated improvements.
In addition, 90% of patients exhibited reduced clonal malignant
T-cells, and 30% exhibited completely eradicated malignant T-cells,
according to T-cell receptor sequencing and flow cytometry analysis
of treated lesions. Elevated responses were associated with
enhanced cutaneous T-cell effector functions, enhanced natural
killer cell functions, and the recruitment and growth of benign
T-cell clones in treated skin. Cytokines were also associated with
persistent clinical inflammation in patients with malignant T-cells
that had been almost completely eliminated. A systemic response to
medication was associated with the enhanced activation of
circulating dendritic cells in 50% of patients. Further Phase II
trials using topical Resiquimod in early-stage CTCL are required
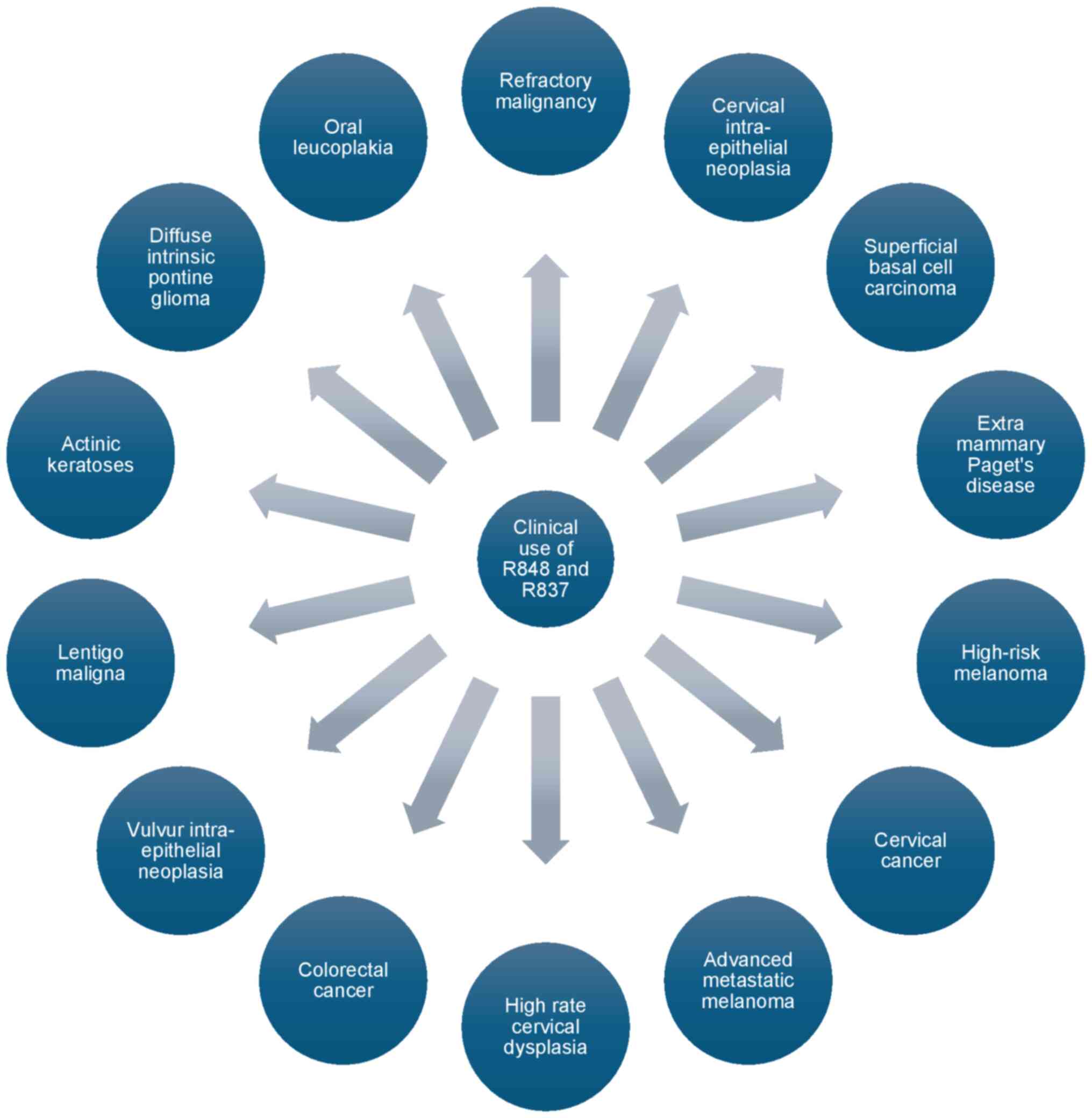
(23). Diseases that may be
targeted by R837 and R848 are summarized in Fig. 2.
6. Combination therapy as an advanced
therapeutic approach
ICIs
Previous research has focused on the combination of
the TLR7/8 agonist, R848, with ICIs in cancer cells. Hänel et
al (24) demonstrated that the
combination of R848 with poly(I: C) was more effective in eliciting
an immunostimulatory response than when R848 was used alone. This
combination induces the reprogramming of macrophages into M1
hot-antitumor effectors through signal transducer and activator of
transcription 1 activation (9).
This study included patients with lung cancer resistant to immune
checkpoint blockade (ICB) (25).
TLRs
In a preclinical study using a humanized mouse
model, Poly(I: C) combined with R848 increased the expression of
various cytokines, compared with Poly(I: C) or R848 administered
alone (26). In addition, the
synergistic use of Poly(I: C) and R848 with E7 DNA promoted the
regression of tumors, and increased the levels of antigen-specific
IFN-γ and non-specific intertumoral IL-12(27). Results of a previous study also
demonstrated that the combination of TLR agonists with
prostaglandins and TLR3 Poly(I: C)/R848/PGE2 aided dendritic cells
in the mobilization and secretion of cytokines, including IL-12
(p70) (28).
In a murine model, treatment with biocompatible
anchors for the membrane (BAM), R848, Poly(I: C) and lipoteichoic
acid led to an 83% eradication of advanced progressive melanoma and
reduced tumor recurrence, when in contact with the surface of tumor
cells (29).
Chemotherapy and radiotherapy
TLR7/8 agonist, R848, plays a crucial role in the
action of oxaliplatin. Oxaliplatin is a chemotherapeutic drug that
promotes the maturation of myeloid-derived suppressor cells (MDSCs)
into M1-like macrophages, which play key roles in various immune
reactions (30). A combination of
Resiquimod with chemotherapeutic agents exhibits potential in the
treatment of cancer, as dosage can be altered depending on patient
outcomes. At present, research is focused on the use of
chemotherapeutic drugs in combination with TLR-mediated
immunotherapy (31). Notably,
reduced tumor growth was observed following treatment with R837 in
combination with γ-ionizing radiation (IR). This combination of
treatment increased autophagy and the number of CD8+ T-cells, and
decreased Tregs and MDSCs (31).
Adjuvant therapy
In the presence of R837-containing nanoparticles as
adjuvants, PLGA-ICG-R837 nanoparticles function as vaccines.
PLGA-ICG-R837 nanoparticles may trigger NIR laser-induced
photothermal ablation of primary tumors and tumor-associated
antigens. In mice, PLGA-ICG-R837 nanoparticles target tumor cells;
thus, limiting metastasis. This strategy led to the establishment
of a robust immune memory effect, potentially protecting against
tumor recurrence following initial elimination (32).
Dual-functional nanoparticles combining partial
thromboplastin time and immunotherapy components (PLGA-ICG-R848
NPs) promoted the maturation of dendritic cells, as evidenced by
increased proportions of CD11c+CD86+ and CD11c+CD80+ cells. This
strategy exhibits potential for improving the immune response
against tumor cells (33).
TLR7/8 agonist-containing nanoparticles have also
been developed for systemic administration. Results of a previous
study described semiconducting polymer nano adjuvant (SPNIIR) for
second near-infrared (NIR-II) photothermal immunotherapy. When
exposed to NIR-II light, SPNIIR generated heat to erode tumors,
induced immunogenic cell death and released R848, enhancing the
maturation of dendritic cells. This approach effectively inhibited
the growth of primary and distant tumors, and eliminated lung
metastasis in a murine tumor model (34).
Zhang et al (35) explored intravenous TLR7/8 agonist
administration using thermosensitive liposomes (TSLs) to enhance
CD8+ T-cell infiltration. Mice treated with R848-loaded TSLs
combined with local hyperthermia and αPD-1 exhibited a 3-fold
increase in median survival, compared with controls. Mice treated
with R848-TSLs and αPD-1 developed immunity to NDL cells, and
remained tumor-free when NDL tumors were reintroduced. Results of a
previous study demonstrated that polydopamine-based PDA-PEG-R848-CD
nanoparticles impacted 4T1 breast tumors under NIR laser
irradiation, generated tumor-associated antigens and significantly
improved the efficacy of PD-L1 checkpoint blockade therapy,
activating innate and adaptive immune systems in vivo
(36,37).
7. Limitations of R837 and R848
Applications of R837 and R848 may be limited due to
low levels of efficacy and associated levels of toxicity in cells.
R837 and R848 may exhibit potential in promoting immune responses;
however, this may lead to the development of specific antibodies.
Notably, this process is known as an antibody-drug reaction (ADR).
ADRs may reduce the efficacy of the drug and cause undesirable
adverse effects. Thus, to moderate the risk of ADRs, further in
vitro investigations are required to establish and eliminate
immunogenic epitopes (38).
Results of previous studies have demonstrated the
potential of these drugs as cancer therapies. However, systemic
administration of these TLR7/8 agonists remains challenging.
Although TLR7 tolerance has been previously demonstrated, the
tolerance induction mechanism remains unclear. Thus, further
investigations are required to determine the efficacy of R837 and
R848 in cancer. In addition, the association between TLR
expression, agonist activity and circadian rhythm are yet to be
explored in the context of TLR7/8 agonism.
TLRs play a complex, multifaceted role in the TME.
Immune cell-expressed TLRs may support immunosurveillance through
promoting and activating both innate and adaptive immune effectors.
However, myeloid-derived suppressor cells are recruited by TLR7
expressed by malignant cells; thus, promoting the growth and spread
of tumors. The pharmacokinetic characteristics of the
small-molecule adjuvants cause substantial systemic inflammation,
which may impact their use in clinical practice. However, results
of a previous study revealed that the covalent attachment of TLR7/8
agonists to polymer scaffolds may modify the associated safety
profile and increase levels of clearance. When encapsulated in
nanoparticles, R837 promoted the development of DCs. However, this
previous study did not investigate drug reactions or ADRs (39,40).
Moreover, TLRs produced by tumor cells may interact
with stimuli to promote the growth of tumors.
Imidazoquinolines-based small molecules stimulate immune responses;
however, their pharmacokinetic profile leads to systemic
inflammation, ultimately impacting their use in clinical practice.
Tumor immunotherapy exhibits limitations in practice due to the
absence of biomarkers. Existing biomarkers, such as microsatellite
instability, PD-L1, genetic mutations, and tumor mutational burden
also exhibit limitations, and these are impacted by the type of
cancer and cancer heterogeneity (41). The aforementioned biomarkers may
not be present in all patients; thus, further investigations are
required to establish reliable biomarkers of tumor immunotherapy
(42). Integrative biomarker
frameworks consolidating safety profiling, clinical history and
prescient calculations may aid in the development of effective
treatment options (10).
Patient selection and stratification are crucial in
clinical trials. Notably, patients may be grouped according to age,
cancer type and stage. Clinical trials including patients that are
well-suited to treatments may lead to the development of customized
treatment plans, and further demonstrate the integrity of the trial
(43).
Clinical trials involving R848 and R837 should focus
on stratifying patients according to cancer type, cancer stage,
patient well-being and the presence of any comorbid conditions
(44). Patients may also be
stratified according to the presence of specific biomarkers,
medical history or previous adverse events that may impact
treatment outcomes (45).
8. Future directions of the R848 and R837
immunotherapy strategy
Drug delivery systems, such as nanoparticles or
liposomes, may be optimized to enhance the targeted delivery of
R848 and R837, and to control off-target effects. Administering a
sustainable release system of drugs may improve therapeutic
outcomes via the reduction in the frequency of treatments and
improved convenience for patients. In addition, these factors may
enhance compliance within immunotherapeutic practice. Developing
systems that are FDA-approved will also lead to increased uptake in
clinical practice. At present, systems that use FDA-approved lipids
to deliver mRNA to dendritic cells are ongoing in clinical trials
(46,47). Immunotherapies specific to patient
profiles may be managed through biomarker-driven approaches. These
approaches include genetic profiling and immune system analysis. In
addition, personalized cancer vaccines may be established via
neoantigen identification advancements. Numerous immunotherapeutic
approaches, such as ICIs, agonistic antibodies, cancer vaccines,
lymphocyte-activating cytokines, CAR T-cells and bispecific
antibodies exhibit potential in the treatment of cancer (48). R848 and R837 may also exhibit
potential as an immunotherapy, with precise specifications and a
targeted approach. Moreover, the combination of R848 and R837 with
chemotherapy, radiation therapy, nanoparticle-mediated
immunoadjuvant therapy or phototherapy may exhibit potential in the
treatment of cancer (6,49).
In the preliminary stage of malignancy, the TME is
more susceptible to treatment, and cancer cells may not develop
immunosuppressive mechanisms. Thus, introducing agonists in the
early stages of disease may lead to earlier activation of immune
cells, such as dendritic cells, and the initiation of an adaptive
immune response. Notably, tumors may be recognized by immune cells
during remission, demonstrating the potential of TLR agonists in
the early stages of disease (50,51).
9. Conclusions
Numerous previous studies have focused on TLR7/8
signaling in immune cells and progenitor cells, and demonstrated
that agonists exhibit high levels of efficacy in tumor cells, in
which TLR7/8 signaling either promotes or inhibits tumor
growth.
R848 and R837 are considered innate immunity
activators that target tumor growth and metastases, and these
exhibit potential as a novel therapeutic option for the treatment
of cancer. Clinical trials focused on the safety and efficacy of
these molecules are ongoing. Notably, Imiquimod and Resiquimod
exhibit increased levels of anti-tumor activity when used in
combination therapies; thus, R848 and R837 may exhibit improved
outcomes when used in combination. Therapeutic drug delivery using
nanoparticles may be limited due to the off-target effects of R848
and R837, and improvements in targeted delivery and control are
required. In conclusion, further investigations into drug delivery,
personalized approaches and tumor resistance are required for the
development of R848 and R837 as an immunotherapy.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
Not applicable.
Authors' contributions
KB was responsible for study design and writing the
manuscript. MA was responsible for editing the manuscript. MW and
AM was responsible for final revision of the manuscript. Data
authentication is not applicable. All authors read and approved the
final version of the manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Zhang Y and Zhang Z: The history and
advances in cancer immunotherapy: Understanding the characteristics
of tumor-infiltrating immune cells and their therapeutic
implications. Cell Mol Immunol. 17:807–821. 2020.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Pahlavanneshan S, Sayadmanesh A,
Ebrahimiyan H and Basiri M: Toll-like receptor-based strategies for
cancer immunotherapy. J Immunol Res. 2021(9912188)2021.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Chen X, Zhang Y and Fu Y: The critical
role of Toll-like receptor-mediated signaling in cancer
immunotherapy. Med Drug Disc. 14(100122)2022.
|
|
4
|
Spyvee M, Hawkins LD and Ishizaka ST:
Chapter 12 - Modulators of Toll-Like Receptor (TLR) Signaling. In:
Annual Reports in Medicinal Chemistry. Vol 45. Academic Press,
pp191-207, 2010.
|
|
5
|
Ryu KA, Stutts L, Tom JK, Mancini RJ and
Esser-Kahn AP: Stimulation of innate immune cells by
light-activated TLR7/8 agonists. J Am Chem Soc. 136:10823–10825.
2014.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Bhagchandani S, Johnson JA and Irvine DJ:
Evolution of Toll-like receptor 7/8 agonist therapeutics and their
delivery approaches: From antiviral formulations to vaccine
adjuvants. Adv Drug Deliv Rev. 175(113803)2021.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Tie Y, Tang F, Wei YQ and Wei XW:
Immunosuppressive cells in cancer: Mechanisms and potential
therapeutic targets. J Hematol Oncol. 15(61)2022.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Ye J, Mills BN, Qin SS, Garrett-Larsen J,
Murphy JD, Uccello TP, Han BJ, Vrooman TG, Johnston CJ, Lord EM,
Belt BA, et al: Toll-like receptor 7/8 agonist R848 alters the
immune tumor microenvironment and enhances SBRT-induced antitumor
efficacy in murine models of pancreatic cancer. J Immunother
Cancer. 10(e004784)2022.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Mottas I, Bekdemir A, Cereghetti A,
Spagnuolo L, Yang YS, Müller M, Irvine DJ, Stellacci F and Bourquin
C: Amphiphilic nanoparticle delivery enhances the anticancer
efficacy of a TLR7 ligand via local immune activation.
Biomaterials. 190:111–120. 2019.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Wang DR, Wu XL and Sun YL: Therapeutic
targets and biomarkers of tumor immunotherapy: Response versus
non-response. Sig Transduct Target Ther. 7(331)2022.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Leśniak M, Lipniarska J, Majka P, Kopyt W,
Lejman M and Zawitkowska J: The Role of TRL7/8 agonists in cancer
therapy, with special emphasis on hematologic malignancies.
Vaccines (Basel). 11(277)2023.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Sun H, Li Y, Zhang P, Xing H, Zhao S, Song
Y, Wan D and Yu J: Targeting toll-like receptor 7/8 for
immunotherapy: Recent advances and prospectives. Biomark Res.
10(89)2022.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Karnik I, Her Z, Neo SH, Liu WN and Chen
Q: Emerging preclinical applications of humanized mouse models in
the discovery and validation of novel immunotherapeutics and their
mechanisms of action for improved cancer treatment. Pharmaceutics.
15(1600)2023.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Deza G, Martin-Ezquerra G, Curto-Barredo
L, García JV and Pujol RM: Successful treatment of hypertrophic
herpes simplex genitalis in an HIV-infected patient with topical
Imiquimod. J Dermatol. 42:1176–1178. 2015.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Miller RL, Imbertson LM, Reiter MJ and
Gerster JF: Treatment of primary herpes simplex virus infection in
guinea pigs by imiquimod. Antiviral Res. 44:31–42. 1999.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Schacker TW, Conant M, Thoming C, Stanczak
T, Wang Z and Smith M: Imiquimod 5-Percent cream does not alter the
natural history of recurrent herpes genitalis: A phase II,
Randomized, double-blind, placebo-controlled study. Antimicrob
Agents Chemother. 46:3243–3248. 2002.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Winters U, Daayana S, Lear JT, Tomlinson
AE, Elkord E, Stern PL and Kitchener HC: Clinical and immunologic
results of a phase II trial of sequential imiquimod and
photodynamic therapy for vulval intraepithelial neoplasia. Clin
Cancer Res. 14:5292–5299. 2008.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Wouters T, Hendriks N, Koeneman M, Kruse
AJ, van de Sande A, van Beekhuizen HJ, Gerestein KG, Bekkers RLM
and Piek JMJ: Systemic adverse events in Imiquimod use for cervical
intraepithelial neoplasia-A case series. Case Rep Womens Health.
21(e00105)2019.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Lebwohl M, Dinehart S, Whiting D, Lee PK,
Tawfik N, Jorizzo J, Lee JH and Fox TL: Imiquimod 5% cream for the
treatment of actinic keratosis: Results from two phase III,
randomized, double-blind, parallel group, vehicle-controlled
trials. J Am Acad Dermatol. 50:714–721. 2004.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Geisse J, Caro I, Lindholm J, Golitz L,
Stampone P and Owens M: Imiquimod 5% cream for the treatment of
superficial basal cell carcinoma: Results from two phase III,
randomized, vehicle-controlled studies. J Am Acad Dermatol.
50:722–733. 2004.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Ackerman SE, Gonzalez JC, Gregorio JD,
Paik JC, Hartmann FJ, Kenkel JA, Lee A, Luo A, Pearson CI, Nguyen
ML, et al: Abstract 1559: TLR7/8 immune-stimulating antibody
conjugates elicit robust myeloid activation leading to enhanced
effector function and anti-tumorimmunity in pre-clinical models.
Cancer Res. 79 (13 Suppl)(S1559)2019.
|
|
22
|
Ackerman SE, Pearson CI, Gregorio JD,
Gonzalez JC, Kenkel JA, Hartmann FJ, Luo A, Ho PY, LeBlanc H, Blum
LK, et al: Immune-stimulating antibody conjugates elicit robust
myeloid activation and durable antitumor immunity. Nat Cancer.
2:18–33. 2021.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Dudek AZ, Yunis C, Harrison LI, Kumar S,
Hawkinson R, Cooley S, Vasilakos JP, Gorski KS and Miller JS: First
in human phase I trial of 852A, a novel systemic toll-like receptor
7 agonist, to activate innate immune responses in patients with
advanced cancer. Clin Cancer Res. 13:7119–7125. 2007.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Hänel G, Angerer C, Petry K, Lichtenegger
FS and Subklewe M: Blood DCs activated with R848 and poly (I: C)
induce antigen-specific immune responses against viral and
tumor-associated antigens. Cancer Immunol Immunother. 71:1705–1718.
2022.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Anfray C, Mainini F, Digifico E, Maeda A,
Sironi M, Erreni M, Anselmo A, Ummarino A, Gandoy S, Expósito F, et
al: Intratumoral combination therapy with poly(I:C) and Resiquimod
synergistically triggers tumor-associated macrophages for effective
systemic antitumoral immunity. J Immunother Cancer.
9(e002408)2021.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Pearson FE, Chang K, Minoda Y, Rojas IML,
Haigh OL, Daraj G, Tullett KM and Radford KJ: Activation of human
CD141(+) and CD1c(+) dendritic cells in vivo with combined TLR3 and
TLR7/8 ligation. Immunol Cell Biol. 96:390–400. 2018.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Sajadian A, Tabarraei A, Soleimanjahi H,
Fotouhi F, Gorji A and Ghaemi A: Comparing the effect of Toll-like
receptor agonist adjuvants on the efficiency of a DNA vaccine. Arch
Virol. 159:1951–1960. 2014.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Gierlich P, Lex V, Technau A, Keupp A,
Morper L, Glunz A, Sennholz H, Rachor J, Sauer S, Marcu A, et al:
Prostaglandin E2 in a TLR3- and 7/8-agonist-based DC maturation
cocktail generates mature, cytokine-producing, migratory DCs but
impairs antigen cross-presentation to CD8(+) T-cells. Cancer
Immunol Immunother. 69:1029–1042. 2020.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Caisova V, Uher O, Nedbalova P, Jochmanova
I, Kvardova K, Masakova K, Krejcova G, Padoukova L, Chmelar J,
Kopecky J and Ženka J: Effective cancer immunotherapy based on the
combination of TLR agonists with stimulation of phagocytosis. Int
Immunopharmacol. 59:86–96. 2018.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Liu Z, Xie Y, Xiong Y, Liu S, Qiu C, Zhu
Z, Mao H, Yu M and Wang X: TLR 7/8 agonist reverses oxaliplatin
resistance in colorectal cancer via directing the myeloid-derived
suppressor cells to tumoricidal M1-macrophages. Cancer Lett.
469:173–185. 2020.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Liu C, Han C and Liu J: The role of
toll-like receptors in oncotherapy. Oncol Res. 27:965–978.
2019.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Cho JH, Lee HJ, Ko HJ, Yoon BI, Choe J,
Kim KC, Hahn TW, Han JA, Choi SS, Jung YM, et al: The TLR7 agonist
Imiquimod induces anti-cancer effects via autophagic cell death and
enhances anti-tumoral and systemic immunity during radiotherapy for
melanoma. Oncotarget. 8:24932–24948. 2017.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Chen Q, Xu L, Liang C, Wang C, Peng R and
Liu Z: Photothermal therapy with immune-adjuvant nanoparticles
together with checkpoint blockade for effective cancer
immunotherapy. Nat Commun. 7(13193)2016.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Lin W, Li C, Xu N, Watanabe M, Xue R, Xu
A, Araki M, Sun R, Liu C, Nasu Y and Huang P: Dual-Functional PLGA
nanoparticles co-loaded with indocyanine green and resiquimod for
prostate cancer treatment. Int J Nanomed. 16:2775–2787.
2021.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Zhang H, Tang WL, Kheirolomoom A, Fite BZ,
Wu B, Lau K, Baikoghli M, Raie MN, Tumbale SK, Foiret J, et al:
Development of thermosensitive resiquimod-loaded liposomes for
enhanced cancer immunotherapy. J Control Release. 330:1080–1094.
2021.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Li J, Yu X, Jiang Y, He S, Zhang Y, Luo Y
and Pu K: Second near-infrared photothermal semiconducting polymer
nanoadjuvant for enhanced cancer immunotherapy. Adv Mater.
33(e2003458)2021.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Noman MZ, Parpal S, Van Moer K, Xiao ML,
Yu Y, Viklund J, De Milito A, Hasmim M, Andersson M, Amaravadi RK,
et al: Inhibition of Vps34 reprograms cold into hot inflamed tumors
and improves anti-PD-1/PD-L1 immunotherapy. Sci Adv.
6(eaax7881)2020.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Shakhnovich V, Meibohm B, Rosenberg A,
Kierzek AM, Hasenkamp R, Funk RS, Thalhauser CJ, van der Graaf PH,
Wang YC and Hamuro L: Immunogenicity in clinical practice and drug
development: When is it significant? Clin Transl Sci. 13:219–223.
2020.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Zhang R, Jia M, Lv H, Li M, Ding G, Cheng
G and Li J: Assembling Au8 clusters on surfaces of bifunctional
nanoimmunomodulators for synergistically enhanced low dose
radiotherapy of metastatic tumor. J Nanobiotechnology.
22(20)2024.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Zahm CD, Colluru VT, McIlwain SJ, Ong IM
and McNeel DG: TLR stimulation during T-cell activation lowers PD-1
expression on CD8(+) T-cells. Cancer Immunol Res. 6:1364–1374.
2018.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Costa Svedman F, Jalsenius M, Höiom V,
Grozman V, Bergqvist M, Söderdahl F, Eriksson H, Rotstein S, Ny L,
Ascierto PA, et al: Plasma thymidine kinase activity as a novel
biomarker in metastatic melanoma patients treated with immune
checkpoint inhibitors. Cancers (Basel). 14(702)2022.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Rizzo A, Ricci AD and Brandi G: Pd-L1,
TMB, MSI, and other predictors of response to immune checkpoint
inhibitors in biliary tract cancer. Cancers (Basel).
13(553)2021.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Shen X and Zhao B: Efficacy of PD-1 or
PD-L1 inhibitors and PD-L1 expression status in cancer:
Meta-analysis. BMJ. 362(k3529)2018.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Siegel RL, Miller KD, Fuchs HE and Jemal
A: Cancer statistics, 2021. CA Cancer J Clin. 71:7–33.
2021.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Wang X, Piantadosi S, Le-Rademacher J and
Mandrekar SJ: Statistical considerations for subgroup analyses. J
Thorac Oncol. 16:375–380. 2021.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Riley RS, June CH, Langer R and Mitchell
MJ: Delivery technologies for cancer immunotherapy. Nat Rev Drug
Discov. 18:175–196. 2019.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Jin SM, Lee SN, Kim JE, Yoo YJ, Song C,
Shin HS, Phuengkham H, Lee CH, Um SH and Lim YT: Overcoming
chemoimmunotherapy-induced immunosuppression by assemblable and
depot forming immune modulating nanosuspension. Adv Sci (Weinh).
8(2102043)2021.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Fang X, Lan H, Jin K, Gong D and Qian J:
Nanovaccines for cancer prevention and immunotherapy: An update
review. Cancers (Basel). 14(3842)2022.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Wolchok JD, Kluger H, Callahan MK, Postow
MA, Rizvi NA, Lesokhin AM, Segal NH, Ariyan CE, Gordon RA, Reed K,
et al: Nivolumab plus ipilimumab in advanced melanoma. N Engl J
Med. 369:122–133. 2013.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Muraoka D, Seo N, Hayashi T, Tahara Y,
Fujii K, Tawara I, Miyahara Y, Okamori K, Yagita H, Imoto S, et al:
Antigen delivery targeted to tumor-associated macrophages overcomes
tumor immune resistance. J Clin Invest. 129:1278–1294.
2019.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Garrido-Martin EM, Mellows TWP, Clarke J,
Ganesan AP, Wood O, Cazaly A, Seumois G, Chee SJ, Alzetani A, King
EV, et al: M1hot tumor-associated macrophages boost
tissue-resident memory T cells infiltration and survival in human
lung cancer. J Immunother Cancer. 8(e000778)2020.PubMed/NCBI View Article : Google Scholar
|