1. Introduction
Cancer incidence. Globally, cancer is amongst
the most prevalent diseases and the second most common cause of
mortality after cardiovascular diseases likely due to changing
lifestyles, genetics and population ageing. In 2008, 12.7 million
cancer cases and 7.6 million cancer deaths were reported worldwide,
of which 56 and 64% were reported in developing countries,
respectively (1). Between 2007 and
2008, 23% of the 500,000 deaths reported in the United States were
cancer-related (2). Leukaemia is
the most common cause of death amongst those <40 years old,
whereas lung cancer is the most common cause of death amongst those
aged ≥40(3). The most common
cancer amongst women is breast cancer, which is most prevalent
amongst women in the age group of 20-59 years-old, whereas
leukaemia and lung cancers are common in age groups <20 and
>60 years old, respectively. Globally, breast cancer accounts
for 23% of all reported cancer cases and is responsible for 14% of
all cancer-related deaths in women worldwide (the highest amongst
cancer-related deaths) (4).
Several risk factors for breast cancer have been
extensively investigated and commonly include early menarche age,
late menopausal age, short breastfeeding sessions,
late-early-full-term pregnancy, nulliparity and low parity
(5). However, studies have been
conducted mainly in developed countries in the Western world.
Although a handful of studies in Asia have reported similar
findings as those in the West, they were primarily conducted in
cities and urban areas wherein lifestyles are somewhat comparable
to Western lifestyles; therefore, they not represent a considerable
part of the Asian population (6,7).
Hence, an extensive and all-inclusive study with a large sample
size is warranted to determine whether risk factors play the same
role amongst Asian populations in third-world countries as those in
the West. Such data will substantially contribute to a suitable
strategic approach for improving breast cancer awareness and
management in Asia (8).
Cancer is an unregulated proliferation of cells
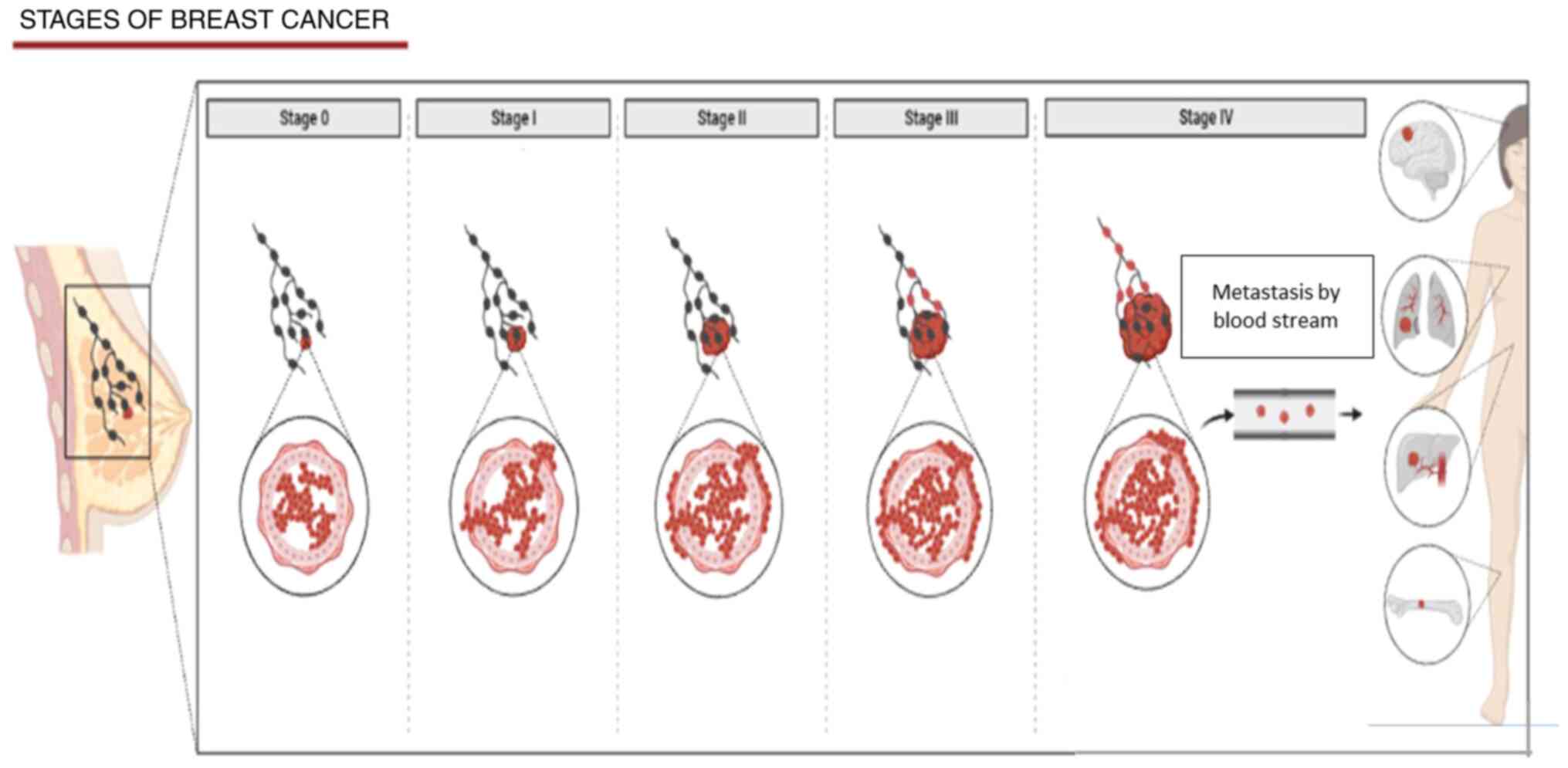
leading to excessive cell division (9). The growth stages of cancer cells from
normal to malignant are demonstrated in Fig. 1. Cancer cells exhibit six essential
changes in their physiology that lead to progression and
metastasis: i) Insensitivity to growth-inhibiting signals; ii)
self-sufficiency in the presence of various growth signals; iii)
limitless replicative potential; iv) tissue invasion and
metastasis; v) sustained angiogenesis; and vi) evasion of
programmed cell death (apoptosis) (10,11).
Cancer can be divided into carcinomas, lymphomas, sarcomas and
leukaemia. Carcinomas are the most commonly diagnosed cancers in
the skin, lungs, pancreas, breasts and in other different organs or
glands (12,13). Lymphomas are related to the
lymphocytes of the body's immune system, and sarcomas arise from
muscle, bone, cartilage, or fat and are uncommon cancer types
(14). Leukaemia is the cancer of
the blood, bone marrow and bone; metastasis is the distribution and
growth of cancer in different locations or organs in the body
(15). The stages of development
common to almost all cancer types are illustrated in Fig. 1. Firstly, the cancer starts in the
innermost lining (stage 1). Later, the cancer moves to the inner
wall of tissue and then distributes outside tissue to the nearest
tissues (extravasation) but not to the lymph nodes (stage 2). It
then spreads specifically to the closest lymph nodes; however, not
to other parts of the body (stage 3). Finally, the cancer
metastasises to other vital organs of the body, such as the lungs
and liver (stage 4) (16).
Breast cancer
Breast cancer is the most common cancer in women
worldwide (17). The age-adjusted
breast cancer incidence in Malaysia is 47.4/100,000, which is ~50%
of that in North America. The Chinese have the highest incidence of
breast cancer (59.9/100,000), followed by Indians (54.2/100,000)
and Malays (34.9/100,000) (18).
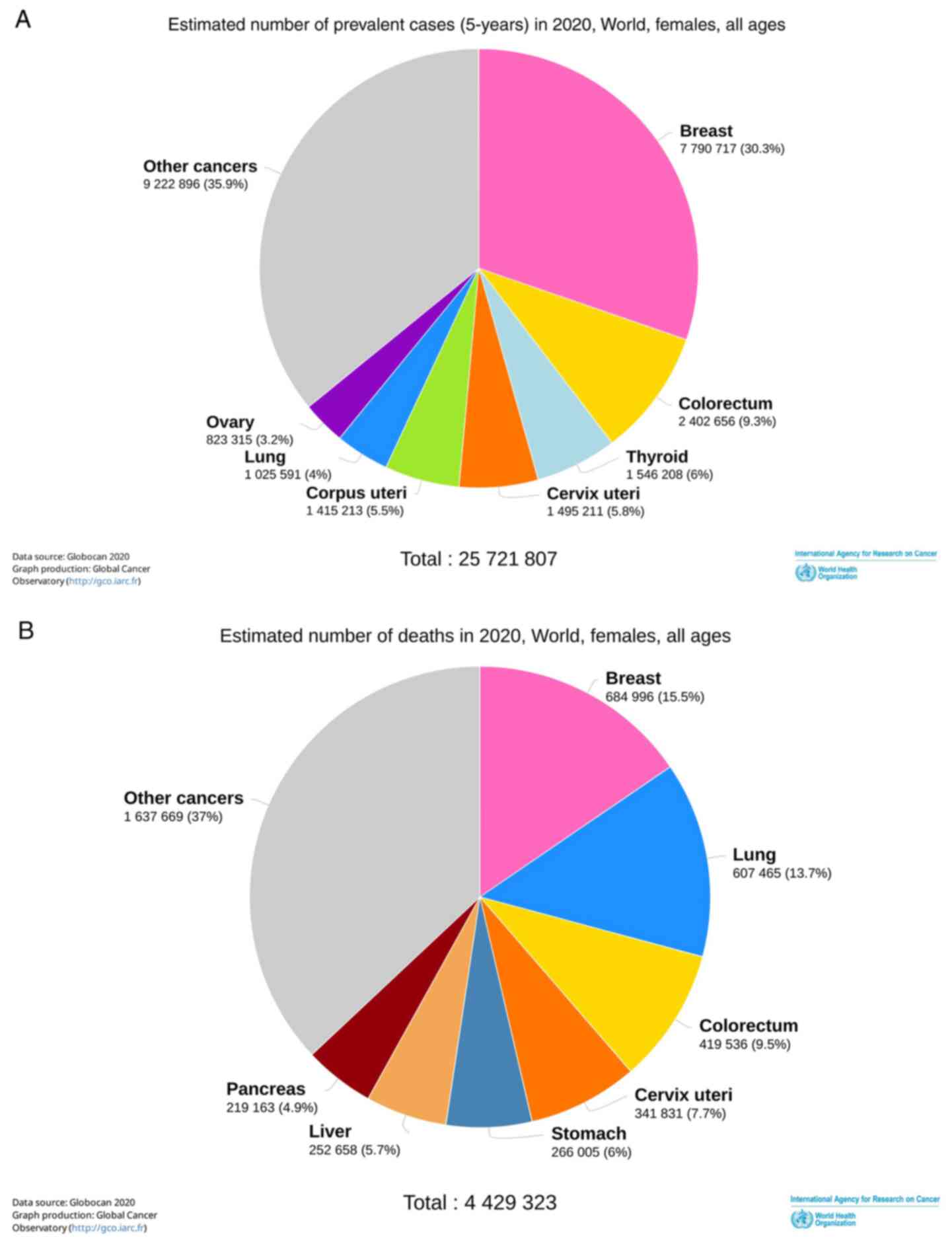
The estimated incidence and death rates of all cancer types and
breast cancer worldwide in 2020 as reported by the WHO is
demonstrated in Fig. 2.
According to GLOBOCAN 2012, breast cancer is the
second most prevalent cancer and fifth most common cause of
cancer-related deaths worldwide, with an age-standardised incidence
rate of 43.3 per 100,000 women per year and a worldwide mortality
rate of 12.9% in 2012 (19,20).
In the USA, the cancer mortality rate decreased by 1.8% per year in
male patients and by 1.6% in female patients between 2004 and 2008,
and the age-standardised cancer death rate reduced by 1.5% (175.8
per 100,000). The cancer incidence rates in men (by 0.6% per year)
are declining as a result of medical development and health care,
whereas those in women have remained the same. Between 1990 and
2008, the cancer-related death rate decreased by ~22.9% in male
patients and 15% in female patients (21,22).
In 2016, the global cancer prevalence ranged from
0.2-2% and was roughly similar as reported in numerous previous
studies, which revealed that breast cancer had the highest
incidence amongst all cancers in the world (~0.12%) with a total of
8.16 million cases (23,24). Between 2008 and 2017, breast cancer
prevalence rates worldwide increased by 20% (25). In 2002, the AVON Breast Cancer
Foundation reported that >39,600 breast cancer-related deaths
occurred in a population of American women (26). Furthermore, in 2018, breast cancer
was reported to be the second most widespread cancer in the world,
accounting for 2 million cases, and the most predominant cancer in
women (27).
In Malaysia, breast cancer is an important cause of
death amongst the population regardless of the sex; however, is
more prominent in women than in men (28). Colorectal cancer is more dominant
in male patients than in female patients (29). Lung cancer is the third most common
cancer in the Malaysian population and second most common cancer
amongst male patients; it is responsible for 29% of cancer-related
deaths in men and 26% of all cancer-related deaths in women
worldwide (30). The National
Cancer Registry (NCR) reported data upholding the latter finding.
Specifically, data from the NCR revealed that >2,000 reported
cases of lung cancer exhibited male predisposition with the M:F
ratio of 1,445:603. Lung, prostate, bronchus and colorectal cancers
accounted for the highest proportion of cancer-related deaths
amongst both sexes. In terms of predisposition, the most prominent
cancers amongst female patients are breast, lung, bronchus and
colorectal cancers, whereas those amongst male patients are lung,
prostate, bronchus and colorectal cancers (31).
Cancer treatment pathways
Chemotherapy and surgery are the most common
therapies for cancer. However, chemotherapeutic agents are
associated with several side effects, including digestive problems,
DNA damage, hair loss, non-specific targets and leukopenia.
Moreover, the development of drug resistance decreases the
efficiency of drugs in chemotherapy (32-34).
Anticancer therapeutic pathways work through numerous mechanisms of
action. They include invasion, metastasis, induction of apoptosis
in cancer cells and inhibition of cancer cell growth (35). Apoptosis may be initiated by a
mitochondrial-mediated (intrinsic) pathway or by a death
receptor-mediated (extrinsic) pathway (36,37);
each of these strategies involves the activation of caspases
(38).
Numerous cancer therapies that have been developed
have been associated with intrinsic drug resistance, which reduces
the likelihood that patients with cancer will have promising
survival times. Amongst all cancer mechanisms, genetic alterations
or aberrations have been designated as the primary culprit in
cancer development and metastasis (39). Radiotherapy and anticancer
agents/drugs may target various signalling pathways in the cell
cycle. This effect activates the apoptotic machinery (programmed
cell death) of cancer cells towards cell survival pathways
(40). The ratios and percentages
of survival and apoptotic pathways result in the overall efficacy
of anticancer therapy and are all activated by therapeutic agents
(41). In cancer cells, caspases
result in apoptosis by inducing caspase-cascade signals.
Proapoptotic proteins/enzymes, such as caspases 8, 9 and 3, may be
downregulated in various malignancies (42).
A protein known as TP53 contains tumour protein P53,
a tumour-suppressor gene. Its primary function in cell cycle
transitions involves regulating and managing damage reactions (DNA
damage detection) and apoptosis (43). The association between P53 and drug
resistance has been thoroughly investigated in several clinical
trials and studies, and most works have established strong
associations between P53 variants and chemoresistance, especially
in ovarian and lung carcinomas (44). However, a solution to these issues
has yet to be found due to the multifactorial characteristics of
symptoms.
Protein kinase B (PKB), also known as AKT, is
present in a broad range of cancers and plays a vital role in
cancer progression along with downstream receptors that control
cancer cell progression (45,46).
AKT prevents apoptosis by inactivating numerous proapoptotic
factors, such as mammalian rapamycin target, the Bcl-2-associated
death promoter Poor and caspase-9, hence contributing to cancer
cell survival and progression (47). Tumour metastasis is another
complicated process involving: i) The detachment of cells from the
primary tumour, (ii) invasion into the surrounding normal tissue,
(iii) further intravasation into the blood circulatory system and
(iv) extravasation and growth in other organs. The cytoskeleton
plays a crucial role in promoting the colonisation of metastatic
tumour cells. Rho GTPases are one of the important families of
GTPases, which regulate the actin cytoskeleton 12; Rho enhances
tumour cell metastasis by increasing cell movement (16).
Natural products from microalgae
Algae are organisms that can perform photosynthetic
activity; worldwide, they naturally grow in freshwater and
wastewater environments that are mainly located near the sea
(48). They have the potential for
advanced life applications, at least in the coming five decades,
because they are one of the most diverse groups of organisms in the
ecosystem; their roles depend on their supply and demand, as well
as technology (49). Although
algae and their extracts are being extensively used in the
pharmaceutical industry as bioactive compounds and new drugs and in
the nutraceutical industry as probiotics and antioxidants, they
have not yet not been explored entirely and may have several
applications that require investigation (48).
Algae can be classified into different sizes on the
basis of their natural visualisation. Macroalgae can be visualised
naturally (for example, seaweeds), whereas microalgae require
microscopy aids to be visualised (48). Microalgae contain vitamins, amino
acids, proteins, pigments, polyunsaturated fatty acids, minerals
and antioxidants. They possess a wide range of biological
activities, including anti-inflammatory, antimicrobial,
antioxidant, antiviral, antiallergy and antitumoral activities
(50,51). They have also been found to exhibit
antibacterial, antifungal and antineoplastic properties (52). Marine algae can decrease the risk
of cancer because they may exert potential antioxidant and
anti-inflammatory effects and are rich in dietary fibre (53). The role of marine microalgae in
anticancerous activity may reduce the dangerous side effects of
radiation therapy and chemotherapeutic drugs (51).
As aforementioned, microalgae are photosynthetic and
can produce hydrocarbons and lipids through the consumption of
carbon dioxide and solar energy, thus behaving as a source of
energy for the environmental ecosystem (54). They contain various components that
could be utilised for human health and provide protection against
chronic diseases (55). Microalgae
have been described as a rich source of carbon. Therefore, they
have a wide range of applications, including pharmaceuticals,
health supplements, cosmetics and biofuels (56). They have also been employed in
wastewater remediation (57).
Microalgae produce several high-value products (HVPs), such as
food, agar, alginates, carotenoids, fatty acids and pigments, which
are beneficial for wild and marine creatures and have several
applications in the pharmaceutical, industrial and nutraceutical
sectors. They contain long-chain polyunsaturated fatty acids, such
as omega-3 and omega-6, which are beneficial for the cardiac and
neurological development of the human body (58). Spirulina and
Chlorella species are leading the worldwide microalgae
market because they are gaining acceptance in food and
health-related supermarkets and stores (48). Compared with traditional protein
sources, such as fish, eggs and soybean, microalgae provide more
substantial contributions to protein supply, making them an
improved source of natural protein in terms of quality and quantity
(48).
Microalgae are classified into four main groups on
the basis of their colours: Green (chlorophytes), red
(rhodophytes), blue green (cyanobacteria) and other colours
(chromophytes) (48). Each group
consists of hundreds of species, and every species has thousands of
strains (59). The carotenoids,
polysaccharides and sterols obtained from marine microalgae are
natural sources of drugs. They exhibit potential anticancer
activities against different cancer types, as illustrated in
Table I (60). Microalgae are the primary source of
various valuable products, such as fibre, enzymes, protein, oil,
carbohydrates and minerals (calcium, magnesium, iron, potassium and
iodine) and are considered as nutritional supplements and health
products for humans (61). They
also have a remarkable vitamin content. Land-grown vegetables lack
vitamin B12, whereas microalgae are a valuable source of this
vitamin, along with vitamins C, B1 and B2(62).
 | Table IMicroalgal pharmaceutical
significance. |
Table I
Microalgal pharmaceutical
significance.
| First author/s,
year' | Disease | Type of
microalgae | Composition | Significance | (Refs.) |
|---|
| Cha et al,
2008 | Colon
carcinoma | Chlorella
ellipsoidea | Carotenoid
extract | Significant | (66) |
| Kusaikin et
al, 2010 | Colorectal
adenocarcinoma | Synedra
acus | Polysaccharide | Revealed a
dose-time dependent trend | (67) |
| Pasquet et
al, 2011 | Breast cancer | Dunaliella
tertiolecta | Violaxanthin | Apoptosis induction
in MCF-7 cells | (68) |
| Erfani et
al, 2015 | Breast
adenocarcinoma | Cladophoropsis
sp. | Ethanol
extract | Has high cytotoxic
effects | (69) |
| Srivastava et
al, 1988 | Skin melanoma | Amphidinium
carterae | Hexane
fraction | Prognostic
significance in intermediate thickness melanomas | (70) |
| Kim et al,
2014 | Liver
hepatocellular carcinoma | Navicula
incerta | Stigmasterol
(phytosterol) | Revealed potent
apoptosis inductive effects | (71) |
Asia, Australia and the USA were the first to start
the production of microalgae in the 1980s (63). Previous technological advancements
and progress in biotechnology have enabled the production of
high-value nutrients, such as fatty acids, phycobilin, carotenoids,
polysaccharides, poly-hydroxy-alkanoates and sterols, from
microalgae through the management of culture systems (64). Microalgae could survive in aquatic
and non-aquatic ecosystems (65)
and tolerate a wide range of parameters; for example, temperature,
salinity and pH. Microalgal bioactive components play an essential
role in disease-inhibiting and health-promoting products. The
pharmaceutical value of microalgae is revealed in Table I.
2. Applications of microalgae
Algae have been used as a source of food and
therapeutics for different illnesses in Japan, Taiwan, China and
Australia for ≥2,000 years (48).
Historically, microalgae have supplied nutrients to humans; for
example, Nostoc, a genus of cyanobacteria, has been consumed
by the Chinese for 20 centuries (66). The microalgal environment is
surrounded by various factors that contribute to the production of
numerous compounds, such as carbohydrates, lipids, proteins and
other secondary metabolites of medicinal value (67). The most common algal products
include hydrocolloids, carotenoids and polyunsaturated fatty acids
and have wide applications in food, feed, pharmaceuticals,
cosmetics and chemicals (48).
The global application of alga-based products in
different sectors (for example, pharmaceuticals) has increased
(68). Various products in the
pharmaceutical industry are derived from microalgae and used as
antiviral, antimicrobial and antifungal drugs and therapeutic
proteins. The pharmaceutical products derived from microalgae
include omega-3 fatty acids, eicosapentaenoic acid (EPA),
docosahexaenoic acid (DHA), β-carotene and astaxanthin (69). Algal products are categorised into
three types on the basis of their market value, namely, high-,
medium- and low-value products (48). Microalgae and cyanobacteria are
marketable resources of HVPs (for example, β-carotene, astaxanthin,
pigments and algal extracts), which are used in cosmetics and
pharmaceutical industries (48).
EPA and DHA derived from algae participate in the
inhibition of numerous diseases, including thrombosis,
atherosclerosis and arthritis (48). Omega-3 and omega-6 are the two most
important fatty acids extracted from microalgae (70), wherein they are found in high
concentrations (48). In contrast
to humans, the microalgal species Crypthecodinium,
Thraustochytrium and Schizochytrium contain the
omega-3 fatty acid DHA, and Phaeodactylum, Chlorella
and Monodus contain EPA (48).
Astaxanthin is one of the most prominent and active
carotenoid molecules present in various types of microalgae. It is
the most abundant pigment in nature and isolated from microalgae
through extraction (71). The
global astaxanthin market has been estimated to be US $257 million
(48). Aquaculture utilises the
majority of astaxanthin produced from microalgae specifically in
fish coloration. The market size of astaxanthin was last assessed
in 2016 and found to be US $555.4 million (48). Astaxanthin is used in the salmon
feed industry due to its antioxidant activity (48). It is produced commercially;
however, it is distributed naturally in several different species
of microalgae. It is most abundant in Hematococcus
pluvialis. Astaxanthin has been used widely in the
nutraceutical and pharmaceutical industries due to its antioxidant
and fortification activities (72). β-Carotene is another molecule that
is abundant in microalgae. It provides health benefits to humans
because it is a favourable source of vitamin A and has antioxidant
effects (59). The carotenoid
content of most algae is 0.1-2%. However, Dunaliella, if
cultured under the appropriate conditions of high salinity and
light intensity, could yield up to 14% of β-carotene (73). The prospective product areas of
algae include nutrient-rich food, bioenergy, bioactive medicine,
novel enzymes, special chemicals, bio-fertilisers and
bioremediation.
3. Microalgae types in the literature
Chlorella species are non-flagellate
autotrophic green microalgae composed of single spherical cells
with diameters of ~2-10 µm (73).
They contain chlorophylls a and b in their chloroplasts, which help
them in photosynthesis. They require CO2, water,
sunlight and a small amount of minerals for rapid growth and can be
grown commercially in large circular tanks, photobioreactors and
ponds. Initially, Chlorella is grown indoors in small
culture flasks and then inoculated into outdoor tanks and ponds for
large-scale production. Chlorella is harvested through
centrifugation or auto-flocculation; the harvested biomass is then
dried, and the obtained powder is used for different purposes.
Chlorella contains high amounts of various biologically
active compounds and is also used as a source of food, feed and
medicine. The biochemical composition of Chlorella is 11-58%
protein, 12-28% carbohydrates and 2-46% lipids. Chlorella
also contains various vitamins, such as provitamin A, β-carotene,
vitamin E, thiamine B1, riboflavin B2, niacin B3, vitamin B6,
inositol, vitamin B12, biotin, folic acid and pantothenic acid
(74-77).
Chlorella has been found to have favourable environmental
remediation effects and bioenergy generation ability (51,78-80).
Microalgae are a reliable source of feedstock
because of their broad accessibility and can be a favourable source
of oil. A study investigated the calorific values of
Chlorella strains, including four freshwater strains
(Chlorella sorokiniana, Chlorella protothecoides,
Chlorella emerson and Chlorella vulgaris) and one
marine strain (C. minutissima) and suggested that
Chlorella strains might be suitable for use as a diesel
replacement (81). The utilisation
of C. protothecoides as a biodiesel source (82,83).
The role of iron in microalgae growth has been
reported in numerous studies. Through fluorescence methods and
estimations assumed over 12 years, it was determined that iron has
a prominent role in managing and directing phytoplankton biomass
under high nitrate low-chlorophyll conditions and in oligotrophic
waters close to the equator and further south (84,85).
However, the response of some biochemical compounds, for example,
lipids, in microalgae when the deficiency of ‘bioavailable’ iron is
the primary factor restricting algal biomass production has rarely
been reported (86,87). Nitzschia communis (88), Dunaliella (89), Botryococcus braunii
(90) and the diatom
Chaetoceros muelleri (91)
were also found to be valuable biofuel sources. Previous
investigations have revealed that the lipid content of microalgae
might change under different conditions, such as fluctuating
salinity (92), silicon deficiency
(88), nitrogen deficiency
(89), phosphate limitation
(90) and excessive cadmium
(91), and alternately
co-immobilised over alginate globules by the bacterium
Azospirillum brasilense (90,93).
Dry biomass oil levels of 20-50% can be obtained and might reach
80%.
Chlorella strains have great potential to be
used as a tool for biodiesel development because of their
straightforward cultivation and fast growth. Under general growth
conditions, the lipid content of Chlorella reaches ~14-30%
by dry biomass weight (85,94),
which cannot satisfy the commercial criteria for biodiesel output.
As already stated, iron is the main factor in improving
phytoplankton abundance in ocean waters. In addition, some
cultivation conditions may increase lipid quantities. The effect of
iron on the production and lipid composition of the marine strain
C. vulgaris has been studied to determine whether iron may
facilitate biomass productivity or lipid aggregation under
laboratory conditions. Studies on microalgae and their associations
are demonstrated in Table II.
These studies demonstrated that the final cell density and lipid
content of the marine strain C. vulgaris increased by
introducing chelated iron into cultures in the late exponential
growth stage and C. vulgaris increased considerably when its
cells were reinoculated into new media with high iron
concentrations (95).
 | Table IIStudies on microalgae and
pharmaceutical significance. |
Table II
Studies on microalgae and
pharmaceutical significance.
| First author/s,
year | Disease | Microalgae type,
extract or active compound | Type of cell
lines | Significance | (Refs.) |
|---|
| Renju et al,
2014 | Prostate
cancer | Chlorella
marina carotenoids (lycopene), Dose 50 µM | PC-3 cell line | Significant | (101) |
| Saad et al,
2006 | Liver cancer | Chlorella vulgaris
Hot water extract, 1,600 µg/ml | HepG2 | Significant | (102) |
| Wu et al,
2005 | Liver fibrosis | Spirulina and
Chlorella water extract | HepG2 and HSCs
cells | Significant | (103) |
| Yusof 2010 | Hepatoma | Hot water extract
of Chlorella vulgaris 1.6 mg/ml | HepG2 and WRL68
normal liver cells | The anticancer
mechanism of C. Vulgaris in hepatoma cells (HepG2) is by inhibiting
DNA synthesis, triggering DNA damage and inducing apoptosis | (104) |
| Pugh et al,
2001 | Immunosti mulatory
activity | Polysaccharides
from Spirulina platensis (Immulina), Aphanizomenon
flos-aquae (Immunon) and Chlorella pyrenoidosa
(Immurella) | THP-1 human
monocytes | Microalgal
polysaccharides have the main role in immunotherapy in the
treatment of cancer and infectious diseases | (105) |
| Jayshree et
al, 2016 | Breast cancer | C. vulgaris
and C. reinhardtii methanol extract | MCF-7 | Significant | (106) |
| Cha et al,
2011 | Human colon
cancer | Carotenoids
extracted from Chlorella ellipsoidea and Chlorella
vulgaris | HCT116 | xanthophylls of
C. ellipsoidea significant and more effective than
Chlorella vulgaris extracts | (66) |
| Sedighi et
al, 2016 | Breast cancer | Chlorella
vulgaris peptides 50 mg/ml | Breast cancer
cells | Significant | (107) |
| Yasukawa et
al, 1996 | Tumor promotion
mouse skin | Sterols from
Chlorella vulgaris | In vivo
mice | Significant | (108) |
The Antimicrobial Stewardship Programme studied the
autotrophic growth of microalgae. Autotrophic growth provides
several benefits. For example, i) microalgae can convert and
produce energy from the sun at the cost of inexpensive natural
resources (for example, H2O and CO2)
(96), leading to global
CO2 reduction; and ii) microalgae may flourish in areas
wherein salty water, excessive solar exposure and lack of vital
nutrients prevent certain crops from developing (97,98).
The concept of the autotrophic growth of microalgae for biodiesel
development is fascinating, enticing and technically feasible.
Nevertheless, this culture style makes achieving high microalgal
biomass densities difficult because light absorption is inversely
proportional to cell density (85). Cell coverage can also induce
insufficiency, resulting in the low productivity of algal lipids
and biomass (99). Additionally,
the insufficient production of biomass often increases the cost of
biomass processing (100,101).
Microalgae can be grown in heterotrophic systems for
developing cost-effective algal oil production wherein organic
compounds, such as organic acids and sugars, act as carbon sources.
This culture style removes the need for light and thus offers the
possibility of drastically increasing cell density, efficiency and
productivity (102). Some
microalgae can proliferate heterotrophically (103). The heterotrophic algal growth
system has been documented to produce not only high algal biomass
productivity but also high cellular oil contents (104,105). The heterotrophic growth of C.
protothecoides on corn compound hydrolysate resulted in a
3.4-fold higher biomass yield than the autotrophic growth system
and increased lipid content by 4.2-fold.
Compared with that during autotrophic growth, the
production of biomass and lipids during heterotrophic growth is
higher; however, it remains not as cost-effective because it
requires a higher amount of organic carbon (acetate or glucose)
than that of all other nutrients. A cheap tool has to be sought to
offset high carbon expenses. Crude glycerol obtained during
biodiesel processing can offer such a tool. While the demand for
biodiesel continues to increase, crude glycerol is flooding the
market (106). The prices of
synthetic glycerol decreased from $0.25/lb in 2004 to
$0.025-0.05/lb in 2006(66). The
low demand and increased supply of crude glycerol have driven
biodiesel manufacturers to search for ways to dispose of this
by-product (107).
4. Tamoxifen
Tamoxifen is one of the well-recognised and most
recommended specific oestrogen receptor (ER) modulators (108). The FDA affirmed its use for the
treatment of women and men with early-stage breast cancer or
malignant growth after a medical procedure or to diminish the
danger of disease recurrence (109). Tamoxifen has been used to
decrease the chance of breast malignancies in women who have not
been diagnosed; however, they are at higher-than-normal risk for
these malignancies. In women with ER-positive early breast cancer,
the risk of recurrence reduced to 50% after 5 years of treatment
with tamoxifen [Early Breast Cancer Trialists' Collaborative Group
(2015)]. The long-term use of tamoxifen is related to extreme
reactions, for example, nausea, and can cause aneuploidy and
endometrial and hepatic diseases. The critical characteristics of
tamoxifen are revealed in Table
III.
 | Table IIITamoxifen characteristics |
Table III
Tamoxifen characteristics
| Name | Tamoxifen |
|---|
| Molar mass | 371.515 g/mol |
| Formula |
C26H29NO |
| Other names | TMX; ICI-46474 |
| Trade name | Nolvadex, Genox,
Tamifen, and others |
| Elimination
half-life | 5-7 days |
|
Bioavailability | ~100% |
Tamoxifen (ICI 46, 474) is a triphenylethylene
derivative and exists in trans-isomeric form. This
tetra-substituted olefin is a nonsteroidal antihormonal drug used
for the treatment of breast cancer. It is administered orally and
available in the form of citrate salt with the common marketed name
Nolvadex (110-114).
The citrate salt of tamoxifen exists in the form of a fine white
crystalline powder, which is readily soluble in organic solvents,
for example acetone, methanol and ethanol, and is partially soluble
in water. The stability of tamoxifen citrate depends on its
exposure to light and moisture. If properly stored, it can be
stable for 5 years. However, humid conditions are unsuitable for
this compound, and it becomes hygroscopic under conditions of high
humidity. It has been applied worldwide for >40 years and is the
most common antioestrogen drug used in chemotherapy. It is usually
prescribed for the treatment of oestrogen-positive breast cancer.
Tamoxifen binds competitively to the ER of cancer cells to form a
nuclear complex, which results in oestrogen inhibition and
decreases DNA synthesis. The pharmacological activity of this drug
requires metabolic activation. The 2D6 cytochrome P450 converts the
pharmacologically inactive tamoxifen into endoxifen (100). Tamoxifen has been found to
decrease the rate of breast cancer with its long-term use. It is
amongst the highly recommended breast cancer drugs due to its fewer
side effects than other treatments. It has been the primary choice
for the therapy of postmenopausal, node-positive,
oestrogen/progesterone receptor-positive and postmenopausal,
node-negative, oestrogen/progesterone receptor-positive women since
the ‘90s. It is one of the common drugs recommended after breast
cancer surgery and radiation therapy and for the treatment of women
who are at risk of developing invasive carcinoma (115).
Nausea, vaginal dryness, irregular periods, hot
flashes and decreased urine amount are amongst the common side
effects of tamoxifen (92,116). Another important side effect of
tamoxifen is its agonistic effect on all tissues, namely, bones,
ovaries, heart and liver, including breast tissues. It participates
in forming excess reactive oxygen species within the mitochondria,
leading to cell apoptosis (94).
In a few cases, the long-term use of tamoxifen also involves the
blistering, peeling and loosening of the skin, cataracts, anxiety,
chest pain, cough, confusion, pelvic pain, pain and swelling of
arms and legs and diarrhoea.
5. Immunological mechanisms of action
As aforementioned, tamoxifen has differential
effects on various tissues. Two types of ER exist, namely, ER-α and
ER-β (117). Tamoxifen exhibits
differential expression with both of these ligand receptors
(81). For decades, the
antiproliferative action of tamoxifen was considered to be due to
its agonistic effect on ER-α. However, in 1996, tamoxifen was
reported to have an antagonistic effect on ER-β (118,119). The expression of tamoxifen on
ER-α either leads to the inhibition of cell growth or death of
cancer cells. The anti-oestrogenic mechanism of tamoxifen on ER-α
can induce apoptosis in vivo and in vitro. However,
the in vitro mechanism of tamoxifen was found to be
concentration-dependent; at low concentrations (nM), tamoxifen can
only inhibit the growth of cells, whereas at high concentrations
(µM), it leads to cell death (120).
6. Metabolism of tamoxifen
N-desmethyl tamoxifen and
4-hydroxy-N-desmethyl tamoxifen are the primary metabolites
found after the oral administration of tamoxifen. The enzyme
responsible for the metabolism of tamoxifen into 4-hydroxy
tamoxifen is cytochrome P450 2D6, which undergoes further
metabolism to afford endoxifen. These active metabolites account
for tamoxifen's antiproliferative and ER binding ability, and their
decreased production will affect the efficacy of the drug because
they have the potential to induce apoptotic cell death in MCF-7
(ER-positive), MDA MB 231 (ER-negative) and BT-20 (ER-negative)
cell lines (121). Therefore,
induced apoptosis might be the primary mechanism of the antitumour
activity of tamoxifen. Nevertheless, several attempts have been
made to study the non-ER-mediated mechanism of tamoxifen at the
cellular and molecular levels due to its efficacy in ER-negative
cancers. It has also been used to treat other malignancies,
including hepatocellular, ovarian, renal and pancreatic cancers,
other than breast carcinoma (122). Therefore, the antitumour effect
of tamoxifen is due to a combination of ER-mediated (genomic) and
non-ER-mediated (non-genomic) mechanisms. Various signalling
proteins are involved in nongenomic pathways, including TGF-β,
calmodulin, MAP kinases and protein kinase C (123-125).
The statistics of tamoxifen are revealed in Table IV.
 | Table IVStatistics of Tamoxifen. |
Table IV
Statistics of Tamoxifen.
| First author/s,
year | Disease | Treatment | Type of cells | Significance | (Refs.) |
|---|
| Shen et al,
2010 | Lung cancer | (Tamoxifen +
Gefitinib) 106 mol/l for 72 h | A549 and H1650 | Significant | (130) |
| Torti et al,
1984 | Prostate
cancer | Tamoxifen Citrate,
Oral (10-50 mg/twice day) | In vivo |
Non-significant | (131) |
| Hoelting et
al, 1995 | Follicular and
papillary thyroid cancer | 1.5 µM | FTC133, FTC236, and
FTC238 | Significant | (132) |
| Chen and Thompson,
2003 | Breast cancer | 10 µM | MDA-MB-435 and
MDA-MB-231 | Significant | (133) |
| He et al,
2015 | Glioblastoma | 15 µM | A172, U251, BT325
and U87 | Significant | (134) |
7. Other related drugs
Other antioestrogen drugs are used as the second
line of breast cancer treatment. Several studies have been
conducted to evaluate the comparative effects of tamoxifen and
other antioestrogen drugs, for example megestrol acetate. In a
survey, Nie et al concluded that tamoxifen and megestrol
acetate could induce responses as initial treatments. They also
studied the combined effects of both drugs however they did not
obtain encouraging results (126). However, tamoxifen is still
preferable because it has fewer side effects than megestrol
acetate. Letrozole, an aromatase inhibitor, has been used to treat
metastatic breast cancer in postmenopausal women who are steroid
hormone receptor-positive. Compared with tamoxifen, letrozole
effectively reduced the risk of periodic disease development in
postmenopausal women with endocrine-responsive breast cancer
(127).
Tamoxifen is one of the most common drugs used as a
first-line breast cancer treatment around the globe. Although the
pharmacokinetics and pharmacodynamics parameters of tamoxifen have
been widely studied, its paragenetic factor still remains to be
explored. In vivo studies are performed to evaluate and
screen the effects of new drug molecules for the treatment of any
disease. Various studies have been conducted to assess the in
vivo effects of tamoxifen and reported different results. The
over-dosage of tamoxifen has not demonstrated any acute toxicity.
However, tamoxifen has been found to induce retinopathy in a
dose-dependent manner (128).
Data on toxicity in children are unavailable. Tamoxifen has
exhibited osteogenic agonist effects in some animal species at high
dosages (129). The effect of
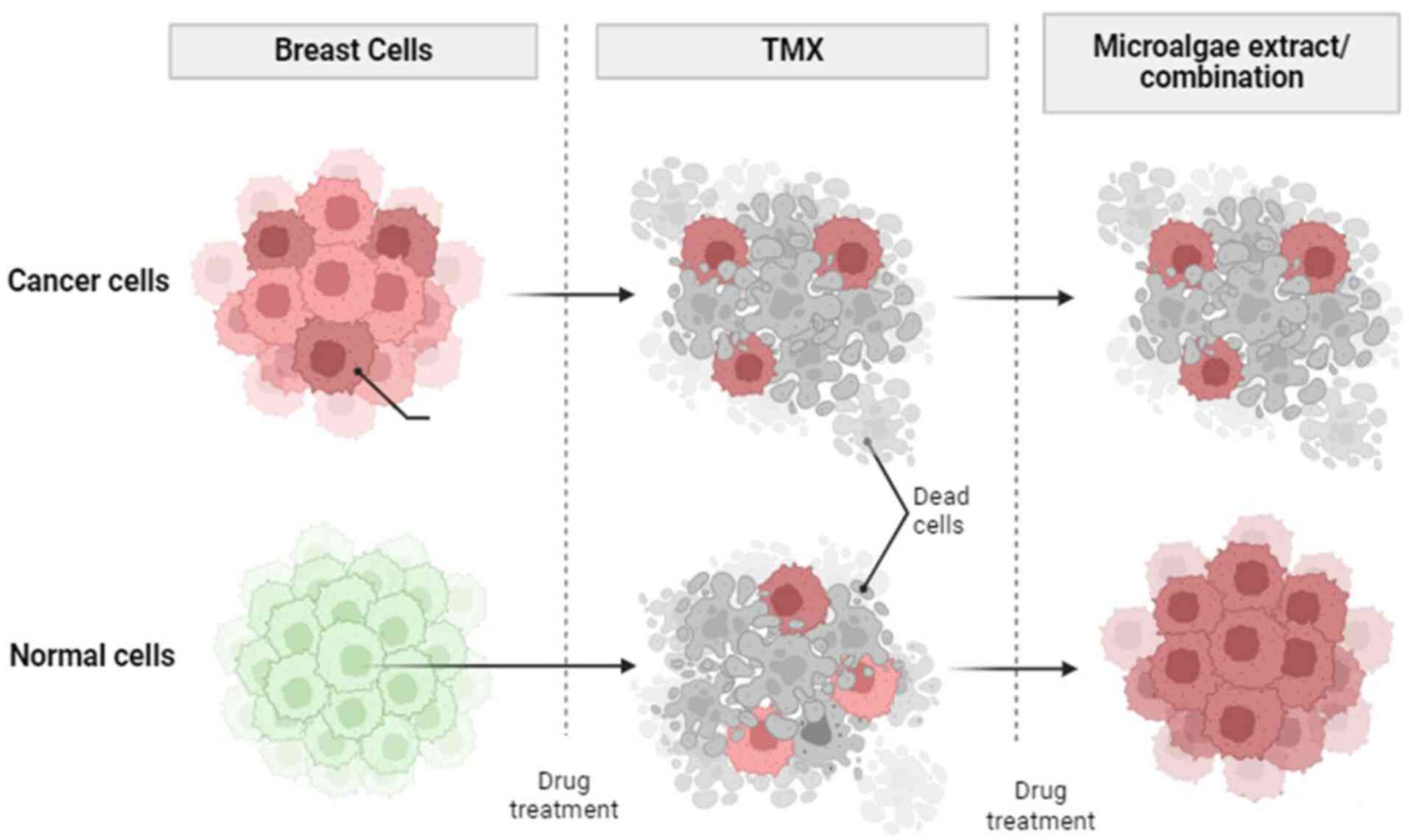
tamoxifen and microalgal extracts on normal and breast cancer cells
is depicted in Fig. 3. As
aforementioned, tamoxifen is used as a first-line breast cancer
treatment.
Nonetheless, an important side effect of tamoxifen
is damage to normal breast cells. Microalgal extracts may affect
both cell lines; however, not normal cells. The present review
highlights that the co-application of tamoxifen with microalgal
extracts may lead to the targeted apoptosis of breast cancer cells
and have minimal cytotoxic effects on normal cells.
8. Perspectives
The prospective product areas of algae include
nutrient-rich food, bioenergy, bioactive medicine, novel enzymes,
special chemicals, bio-fertilisers and bioremediation. They do not
depend on one factor and are instead multifactorial with multiple
target pathways. Therefore, further studies must be conducted to
enhance the positive effects of already available drugs. Given that
natural products have always been a great source of interest, the
co-application of active algal extracts with tamoxifen can enhance
activity against cancer cells or might be a helpful treatment for
minimising the side effects of tamoxifen in other ways. Microalgal
extracts may affect both cell lines; however, not normal cells. The
present review highlights that the co-application of tamoxifen with
microalgal extracts may lead to the targeted apoptosis of breast
cancer cells and have minimal cytotoxic effects on normal cells.
More research interest is necessary to clarify their ideal dosages,
mechanisms of action and potential adverse effects.
9. Future directions
Tamoxifen is one of the most common drugs used in
the first-line treatment of breast cancer worldwide. Although its
pharmacokinetics and pharmacodynamics parameters have been widely
studied, its paragenetic factor has yet to be explored. Previous
studies have determined that microalgal extracts, especially those
with astaxanthin, have an essential role in anticancerous effects.
However, to date, no studies have been conducted to investigate the
modulatory action of microalgal extracts targeting specific
mechanisms related to inflammation or immunological pathways.
Previous studies have demonstrated that the cell cycle and
treatment progression of cancer are unclear. They do not depend on
one factor and are instead multifactorial with multiple target
pathways. Therefore, further studies must be conducted to enhance
the positive effects of already available drugs. Given that natural
products have always been a great source of interest, the
co-application of active algal extracts with tamoxifen can enhance
activity against cancer cells or might be a helpful treatment for
minimising the side effects of tamoxifen in other ways.
10. Conclusion
The regulation of breast cancer immunotherapy will
benefit immensely from the use of microalgal extracts and
tamoxifen. Both substances have the potential to strengthen the
immune system's response to breast cancer cells, thereby improving
therapy outcomes in affected patients. Tamoxifen's well-established
involvement in the treatment of breast cancer and astaxanthin's
antioxidant qualities imply a synergistic relationship that could
enhance the efficacy of immunotherapy. However, before considering
the inclusion of these two essential compounds in clinical practice
for improving breast cancer immunotherapy, additional research is
necessary to clarify their ideal dosages, mechanisms of action and
potential adverse effects.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study may be found
in PubMed and Google Scholar at the following URL: https://scholar.google.com/, https://pubmed.ncbi.nlm.nih.gov/.
Authors' contributions
OMAS and TADAATD conceptualized the study. OMAS and
HAA wrote the original draft. OMA, HAA, IAMT and TADAATD reviewed
and edited the manuscript. Data authentication is not applicable.
All authors read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Favoriti P, Carbone G, Greco M, Pirozzi F,
Pirozzi REM and Corcione F: Worldwide burden of colorectal cancer:
A review. Updates Surg. 68:7–11. 2016.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Ferlay J, Parkin DM and Steliarova-Foucher
E: Estimates of cancer incidence and mortality in Europe in 2008.
Eur J Cancer. 46:765–781. 2010.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Mohammadian M, Pakzad R,
Mohammadian-Hafshejani A and Salehiniya H: A study on the incidence
and mortality of leukemia and their association with the human
development index (HDI) Worldwide In 2012. WCRJ.
5(e1080)2018.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Nigjeh SE, Yeap SK, Nordin N, Rahman H and
Rosli R: In Vivo anti-tumor effects of citral on 4T1 breast cancer
cells via induction of apoptosis and downregulation of aldehyde
dehydrogenase activity. Molecules. 24(3241)2019.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Oh H, Eliassen AH, Beck AH, Rosner B,
Schnitt SJ, Collins LC, Connolly JL, Montaser-Kouhsari L, Willett
WC and Tamimi RM: Breast cancer risk factors in relation to
estrogen receptor, progesterone receptor, insulin-like growth
factor-1 receptor, and Ki67 expression in normal breast tissue. NPJ
Breast Cancer. 3(39)2017.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Brind J, Condly SJ, Lanfranci A and Rooney
B: Induced abortion as an independent risk factor for breast
cancer: A systematic review and meta-analysis of studies on South
Asian women. Issues Law Med. 33:32–54. 2018.PubMed/NCBI
|
|
7
|
Iyengar NM, Chen IC, Zhou XK, Giri DD,
Falcone DJ, Winston LA, Wang H, Williams S, Lu YS, Hsueh TH, et al:
Adiposity, inflammation, and breast cancer pathogenesis in Asian
women. Cancer Prev Res (Phila). 11:227–236. 2018.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Saini P, Henshall C, Brett J, Watson E and
Smith L: Interventions to improve the uptake of breast, cervical
and bowel cancer screening in South Asian women living in high
income countries. Prospero International prospective register of
systematic reviews. LJMU Research Online (In Press).
|
|
9
|
Varadhachary GR, Tamm EP, Abbruzzese JL,
Xiong HQ, Crane CH, Wang H, Lee JE, Pisters PW, Evans DB and Wolff
RA: Borderline resectable pancreatic cancer: Definitions,
management, and role of preoperative therapy. Ann Surg Oncol.
13:1035–1046. 2006.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Poste G and Fidler IJ: The pathogenesis of
cancer metastasis. Nature. 283:139–146. 1980.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Gupta GP and Massagué J: Cancer
metastasis: Building a framework. Cell. 127:679–695.
2006.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Shuda M, Arora R, Kwun HJ, Feng H, Sarid
R, Fernández-Figueras MT, Tolstov Y, Gjoerup O, Mansukhani MM,
Swerdlow SH, et al: Human Merkel cell polyomavirus infection I. MCV
T antigen expression in Merkel cell carcinoma, lymphoid tissues and
lymphoid tumors. Int J Cancer. 125:1243–1249. 2009.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Johnston WW: The malignant pleural
effusion. A review of cytopathologic diagnoses of 584 specimens
from 472 consecutive patients. Cancer. 56:905–909. 1985.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Cancer Genome Atlas Research Network.
Electronic address: elizabeth.demicco@sinaihealthsystem.ca 1;
Cancer Genome Atlas Research Network. Comprehensive and integrated
genomic characterization of adult soft tissue sarcomas. Cell.
171:950–965.e28. 2017.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Rybka J, Gębura K, Wróbel T, Wysoczańska
B, Stefanko E, Kuliczkowski K and Bogunia-Kubik K: Variations in
genes involved in regulation of the nuclear factor-KappaB pathway
and the risk of acute myeloid leukaemia. Int J Immunogenet.
43:101–106. 2016.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Riihimäki M, Hemminki A, Sundquist J and
Hemminki K: Patterns of metastasis in colon and rectal cancer. Sci
Rep. 6(29765)2016.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Curado MP: Breast cancer in the world:
Incidence and mortality. Salud Publica Mex. 53:372–384.
2011.PubMed/NCBI
|
|
18
|
Tan MM, Ho WK, Yoon SY, Mariapun S, Hasan
SN, Lee DS, Hassan T, Lee SY, Phuah SY, Sivanandan K, et al: A
case-control study of breast cancer risk factors in 7,663 women in
Malaysia. PLoS One. 13(e0203469)2018.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Ferlay J, Soerjomataram I, Dikshit R, Eser
S, Mathers C, Rebelo M, Parkin DM, Forman D and Bray F: Cancer
incidence and mortality worldwide: Sources, methods and major
patterns in GLOBOCAN 2012. Int J Cancer. 136:E359–E386.
2015.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Coccia M: What is the Cause of Breast
Cancer? A Socioeconomic Analysis. CocciaLab Working Paper 2017 –
No. 16. SSRN - Elsevier, Rochester, NY, 2017.
|
|
21
|
Murphy SL, Xu J, Kochanek KD and Arias E:
Mortality in the United States, 2017. NCHS Data Brief. (328):1–8.
2018.PubMed/NCBI
|
|
22
|
Miniño AM, Murphy SL, Xu J and Kochanek
KD: Deaths: Final data for 2008. Natl Vital Stat Rep. 59:1–126.
2011.PubMed/NCBI
|
|
23
|
Lengacher CA, Reich RR, Paterson CL,
Ramesar S, Park JY, Alinat C, Johnson-Mallard V, Moscoso M,
Budhrani-Shani P, Miladinovic B, et al: Examination of broad
symptom improvement resulting from mindfulness-based stress
reduction in breast cancer survivors: A randomized controlled
trial. J Clin Oncol. 34:2827–2834. 2016.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Flores-Pérez A, Marchat LA,
Rodríguez-Cuevas S, Bautista VP, Fuentes-Mera L, Romero-Zamora D,
Maciel-Dominguez A, de la Cruz OH, Fonseca-Sánchez M, Ruíz-García
E, et al: Suppression of cell migration is promoted by miR-944
through targeting of SIAH1 and PTP4A1 in breast cancer cells. BMC
Cancer. 16(379)2016.PubMed/NCBI View Article : Google Scholar
|
|
25
|
DeSantis CE, Ma J, Goding Sauer A, Newman
LA and Jemal A: Breast cancer statistics,. 2017, racial disparity
in mortality by state. CA Cancer J Clin. 67:439–448.
2017.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Leslie NS, Deiriggi P, Gross S, DuRant ME,
Smith C and Veshnesky JG: Knowledge, attitudes, and practices
surrounding breast cancer screening in educated Appalachian women.
Oncol Nurs Forum. 30:659–667. 2003.PubMed/NCBI View Article : Google Scholar
|
|
27
|
DeSantis CE, Ma J, Gaudet MM, Newman LA,
Miller KD, Goding Sauer A, Jemal A and Siegel RL: Breast cancer
statistics, 2019. CA Cancer J Clin. 69:438–451. 2019.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Hisham AN and Yip CH: Spectrum of breast
cancer in Malaysian women: Overview. World J Surg. 27:921–923.
2003.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Lim GC: Overview of cancer in Malaysia.
Jpn J Clin Oncol. 32 (suppl_1):S37–S42. 2002.PubMed/NCBI
|
|
30
|
Kan CS and Chan KM: A review of lung
cancer research in Malaysia. Med J Malaysia. 71 (Suppl 1):S70–S78.
2016.PubMed/NCBI
|
|
31
|
Naing C, Lai PK and Mak JW: Immediately
modifiable risk factors attributable to colorectal cancer in
Malaysia. BMC Public Health. 17(637)2017.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Rezakhani L, Rashidi Z, Mirzapur P and
Khazaei M: Antiproliferatory effects of crab shell extract on
breast cancer cell line (MCF7). J Breast Cancer. 17:219–225.
2014.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Singh S, Chitkara D, Mehrazin R, Behrman
SW, Wake RW and Mahato RI: Chemoresistance in prostate cancer cells
is regulated by miRNAs and Hedgehog pathway. PLoS One.
7(e40021)2012.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Fanciullino R, Ciccolini J and Milano G:
Challenges, expectations and limits for nanoparticles-based
therapeutics in cancer: A focus on nano-albumin-bound drugs. Crit
Rev Oncol Hematol. 88:504–513. 2013.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Farooqi AO, Cham M, Zhang L, Beasley MB,
Austin JH, Miller A, Zulueta JJ, Roberts H, Enser C, Kao SJ, et al:
Lung cancer associated with cystic airspaces. AJR Am J Roentgenol.
199:781–786. 2012.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Soriano ME and Scorrano L: Traveling Bax
and forth from mitochondria to control apoptosis. Cell. 145:15–17.
2011.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Foo CS, Su D, Chong CK, Chng HC, Tay KH,
Low SC and Tan SM: Breast cancer in young Asian women: Study on
survival. ANZ J Surg. 75:566–572. 2005.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Park HH: Structural features of
caspase-activating complexes. Int J Mol Sci. 13:4807–4818.
2012.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Takayama T, Miyanishi K, Hayashi T, Sato Y
and Niitsu Y: Colorectal cancer: Genetics of development and
metastasis. J Gastroenterol. 41:185–192. 2006.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Ma Y, Kepp O, Ghiringhelli F, Apetoh L,
Aymeric L, Locher C, Tesniere A, Martins I, Ly A, Haynes NM, et al:
Chemotherapy and radiotherapy: Cryptic anticancer vaccines. Semin
Immunol. 22:113–124. 2010.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Green DR: Apoptotic pathways: Ten minutes
to dead. Cell. 121:671–674. 2005.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Zhou X, Zhang J, Jia Q, Ren Y, Wang Y, Shi
L, Liu N, Wang G, Pu P, You Y and Kang C: Reduction of miR-21
induces glioma cell apoptosis via activating caspase 9 and 3. Oncol
Rep. 24:195–201. 2010.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Fischer M: Conservation and divergence of
the p53 gene regulatory network between mice and humans. Oncogene.
38:4095–4109. 2019.PubMed/NCBI View Article : Google Scholar
|
|
44
|
El Shamieh S, Saleh F, Assaad S and Farhat
F: Next-generation sequencing reveals mutations in RB1, CDK4 and
TP53 that may promote chemo-resistance to palbociclib in ovarian
cancer. Drug Metab Pers Ther
34:/j/dmdi.2019.34.issue-2/dmpt-2018-0027/dmpt-2018-0027.xml,
2019.
|
|
45
|
Miles J, Applebee CJ, Leboucher P, López
Fernández S, Lee DJ, Guarch R, Ward S, Parker PJ, López JI and
Larijani B: Time resolved amplified FRET identifies protein kinase B
activation state as a marker for poor prognosis in clear cell renal
cell carcinoma. BBA Clin. 8:97–102. 2017.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Abdullah MA, Bahamid AAA, Alshajrawi OMS,
Nazir MS and Tahir Z: Integrated biomaterials engineering of oil
palm fibres and microalgae for bioenergy, environmental
remediation, and conversion into value-added-products. OP Conf.
Ser: Earth Environ. Sci. 448(012091)2020.
|
|
47
|
Zhou Y, Yu F, Luo B, Luo H and Liu C:
Cytrarabine (Ara-c) promotes cell apoptosis by inhibiting the
phosphorylation of Protein Kinase B (AKT/PKB). Process
Biochemistry. Vol 82. Elsevier, Amsterdam, pp144-152, 2019.
|
|
48
|
Rahman KM: Microalgae biotechnology for
food, health and high value products. Springer, Singapore; 2020.
479 p. https://link.springer.com/chapter/10.1007/978-981-15-0169-2_1.
|
|
49
|
Antoprete G and Berni P: Algae: An
opportunity to obtain products with high value, to contribute to
the reduction of environmental pollution and to achieve
environmental sustainability. Journal of commodity science,
technology and quality. 50:101–117. 2011.https://www.sci.unich.it/ricerca/jcs/content/2011/2011-01-06.pdf.
|
|
50
|
Abdullah MA, Ahmad A, Shah SMU, Shanab
SMM, Ali HEA, Abo-State MAM and Othman MF: Integrated algal
engineering for bioenergy generation, effluent remediation, and
production of high-value bioactive compounds. Biotechnol Bioproc E.
21:236–249. 2016.
|
|
51
|
Abdullah MA, Shah SMU, Shanab SMM and Ali
HEA: Integrated Algal Bioprocess Engineering for Enhanced
Productivity of Lipid, Carbohydrate and High-Value Bioactive
Compounds. J Microbiol Biotechnol. 6:61–92. 2017.
|
|
52
|
Cioffi N, Torsi L, Ditaranto N, Tantillo
G, Ghibelli L, Sabbatini L, Bleve-Zacheo T, D'Alessio M, Zambonin
PG and Traversa E: Copper nanoparticle/polymer composites with
antifungal and bacteriostatic properties. Chem. Mater.
17:5255–5262. 2005.
|
|
53
|
Prakash JW, Marimuthu J and Jeeva S:
Antimicrobial activity of certain fresh water microalgae from
Thamirabarani River, Tamil Nadu, South India. Asian Pac J Trop
Biomed. 1:S170–S173. 2011.
|
|
54
|
Schade S and Meier T: A comparative
analysis of the environmental impacts of cultivating microalgae in
different production systems and climatic zones: A systematic
review and meta-analysis. Algal Res. 40(101485)2019.
|
|
55
|
Smit AJ: Medicinal and pharmaceutical uses
of seaweed natural products : A review. J Appl Phycol. 16:245–262.
2004.
|
|
56
|
Doušková I, Kaštánek F, Maléterová Y,
Kaštánek P, Doucha J and Zachleder V: Utilization of distillery
stillage for energy generation and concurrent production of
valuable microalgal biomass in the sequence : In: Energy Conversion
and Management. Vol 51. Elsevier, Amsterdam, p606-11, 2010.
|
|
57
|
Alam A and Wang Z: Microalgae
biotechnology for development of biofuel and wastewater treatment.
Springer, Singapore, p646, 2019.
|
|
58
|
Mata TM, Martins AA and Caetano NS:
Microalgae for biodiesel production and other applications : A
review. In: Renewable and sustainable energy reviews. Vol 14.
Elsevier, Amsterdam, pp217-232, 2010.
|
|
59
|
Hochman G and Zilberman D: Algae farming
and its bio-products. In: In Plants and BioEnergy. Springer, New
York, NY, pp49-64, 2014.
|
|
60
|
Guedes AC, Amaro HM and Malcata FX:
Microalgae as sources of carotenoids. Mar Drugs. 9:625–644.
2011.PubMed/NCBI View Article : Google Scholar
|
|
61
|
Apt KE and Behrens PW: Commercial
Developments In Microalgal Biotechnology. J Phycol. 35:215–226.
1999.
|
|
62
|
Martinez Hernandez A, Urbanke H, Gillman
AL, Lee J, Ryazanov S, Agbemenyah HY, Benito E, Jain G, Kaurani L,
Grigorian G, et al: The diphenylpyrazole compound anle 138b blocks
Aβ channels and rescues disease phenotypes in a mouse model for
amyloid pathology. EMBO Mol Med. 10:32–47. 2018.PubMed/NCBI View Article : Google Scholar
|
|
63
|
Enzing C, Ploeg M, Barbosa M and Sijtsma
L: Microalgae-based products for the food and feed sector: An
outlook for Europe. 2014. (PDF) Microalgae-based products for food
and feed sector: an outlook for Europe.
|
|
64
|
Boroumand Moghaddam A, Moniri M, Azizi S,
Abdul Rahim R, Bin Ariff A, Navaderi M and Mohamad R: Eco-friendly
formulated zinc oxide nanoparticles: Induction of cell cycle arrest
and apoptosis in the MCF-7 cancer cell line. Genes (Basel).
8(281)2017.PubMed/NCBI View Article : Google Scholar
|
|
65
|
Cole JJ: Interactions between bacteria and
algae in aquatic ecosystems. Annu Rev Ecol Syst. 13:291–314.
1982.
|
|
66
|
Cha KH, Koo SY and Lee DU:
Antiproliferative effects of carotenoids extracted from Chlorella
ellipsoidea and Chlorella vulgaris on human colon cancer cells. J
Agric Food Chem. 56:10521–10526. 2008.PubMed/NCBI View Article : Google Scholar
|
|
67
|
Kusaikin MI, Ermakova SP, Shevchenko NM,
Isakov VV, Gorshkov AG, Vereshchagin AL, Grachev MA and
Zvyagintseva TN: Structural characteristics and antitumor activity
of a new chrysolaminaran from the diatom alga Synedra acus. Chem
Nat Compd. 46:1–4. 2010.
|
|
68
|
Pasquet V, Morisset P, Ihammouine S,
Chepied A, Aumailley L, Berard JB, Serive B, Kaas R, Lanneluc I,
Thiery V, et al: Antiproliferative activity of violaxanthin
isolated from bioguided fractionation of Dunaliella tertiolecta
extracts. Mar Drugs. 9:819–831. 2011.PubMed/NCBI View Article : Google Scholar
|
|
69
|
Erfani N, Nazemosadat Z and Moein M:
Cytotoxic activity of ten algae from the Persian Gulf and Oman Sea
on human breast cancer cell lines; MDA-MB-231, MCF-7, and T-47D.
Pharmacognosy Res. 7:133–137. 2015.PubMed/NCBI View Article : Google Scholar
|
|
70
|
Srivastava A, Laidler P, Davies RP, Horgan
K and Hughes LE: The prognostic significance of tumor vascularity
in intermediate-thickness (0.76-4.0 mm thick) skin melanoma. A
quantitative histologic study. Am J Pathol. 133:419–423.
1988.PubMed/NCBI
|
|
71
|
Kim YS, Li XF, Kang KH, Ryu BM and Kim SK:
Stigmasterol isolated from marine microalgae Navicula incerta
induces apoptosis in human hepatoma HepG2 cells. BMB Rep.
47:433–438. 2014.PubMed/NCBI View Article : Google Scholar
|
|
72
|
Ciferri O: Spirulina, the Edible
Microorganism. Microbiol Rev. 47:551–578. 1983.PubMed/NCBI View Article : Google Scholar
|
|
73
|
Athasivam R, Radhakrishnan R, Hashem A and
Abd Allah EF: Microalgae metabolites: A rich source for food and
medicine. Saudi J Biol Sci. 26:709–722. 2019.PubMed/NCBI View Article : Google Scholar
|
|
74
|
Ang KK, Harris J, Wheeler R, Weber R,
Rosenthal DI, Nguyen-Tân PF, Westra WH, Chung CH, Jordan RC, Lu C,
et al: Human papillomavirus and survival of patients with
oropharyngeal cancer. N Engl J Med. 363:24–35. 2010.PubMed/NCBI View Article : Google Scholar
|
|
75
|
Ambati RR, Phang SM, Ravi S and
Aswathanarayana RG: Astaxanthin: Sources, extraction, stability,
biological activities and its commercial applications-a review. Mar
Drugs. 12:128–152. 2014.PubMed/NCBI View Article : Google Scholar
|
|
76
|
Cardozo KHM, Guaratini T, Barros MP,
Falcão VR, Tonon AP, Lopes NP, Campos S, Torres MA, Souza AO,
Colepicolo P and Pinto E: Metabolites from algae with economical
impact. Comp Biochem Physiol C Toxicol Pharmacol. 146:60–78.
2007.PubMed/NCBI View Article : Google Scholar
|
|
77
|
Grune T, Lietz G, Palou A, Ross AC, Stahl
W, Tang G, Thurnham D, Yin SA and Biesalski HK: Beta-Carotene is an
important vitamin A source for humans. J Nutr. 140:2268S–2285S.
2010.PubMed/NCBI View Article : Google Scholar
|
|
78
|
Rajendran S, Rathish RJ, Prabha SS and
Anandan A: Green electrochemistry-a versatile tool in green
synthesis: An overview. Port Electrochim Acta. 34:321–342.
2016.
|
|
79
|
Thomford NE, Dzobo K, Chopera D, Wonkam A,
Skelton M, Blackhurst D, Chirikure S and Dandara C:
Pharmacogenomics implications of using herbal medicinal plants on
African populations in health transition. Pharmaceuticals (Basel).
8:637–663. 2015.PubMed/NCBI View Article : Google Scholar
|
|
80
|
Balandrin MF, Klocke JA, Wurtele ES and
Bollinger WH: Natural plant chemicals: Sources of industrial and
medicinal materials. Science. 228:1154–1160. 1985.PubMed/NCBI View Article : Google Scholar
|
|
81
|
Johnson DT and Taconi KA: The glycerin
glut: Options for the value-added conversion of crude glycerol
resulting from biodiesel production. Environ Prog. 26:338–348.
2007.
|
|
82
|
Widjaja A, Chien CC and Ju YH: Study of
increasing lipid production from fresh water microalgae Chlorella
vulgaris. J Taiwan Inst Chem Eng. 40:13–20. 2009.
|
|
83
|
Nick GL: Addressing human exposure to
environmental toxins with Chlorella pyrenoidosa. (Medicinal
Properties in Whole Foods). Townsend Letter for Doctors and
Patients. (237):28–33. 2003.
|
|
84
|
Gerardo ML, Van Den Hende S, Vervaeren H,
Coward T and Skill SC: Harvesting of microalgae within a
biorefinery approach: A review of the developments and case studies
from pilot-plants. Algal Res. 11:248–262. 2015.
|
|
85
|
Illman AM, Scragg AH and Shales SW:
Increase in Chlorella strains calorific values when grown in low
nitrogen medium. Enzyme Microb Technol. 27:631–635. 2000.PubMed/NCBI View Article : Google Scholar
|
|
86
|
Fathima HM and Geetha RV: Phytochemical
screening and antioxidant activity of Thymus vulgaris. Drug
Invention Today. 11:1803–1806. 2019.
|
|
87
|
ABDULLAH MA, Shah SMU, Ahmad A and
El-Sayed H: ALGAL biotechnology for bioenergy, environmental
remediation and high-value biochemicals. biotechnology and
bioinformatics: advances and Applications for Bioenergy,
Bioremediation and Biopharmaceutical Research 301, 2014.
|
|
88
|
Dempster TA and Sommerfeld MR: Effects of
environmental conditions on growth and lipid accumulation in
Nitzschia communis (Bacillariophyceae). J Phycol. 34:712–721.
1998.
|
|
89
|
Takagi M and Karseno and Yoshida T: Effect
of salt concentration on intracellular accumulation of lipids and
triacylglyceride in marine microalgae Dunaliella cells. J Biosci
Bioeng. 101:223–226. 2006.PubMed/NCBI View Article : Google Scholar
|
|
90
|
Rao AR, Dayananda C, Sarada R, Shamala TR
and Ravishankar GA: Effect of salinity on growth of green alga
Botryococcus braunii and its constituents. Bioresour Technol.
98:560–564. 2007.PubMed/NCBI View Article : Google Scholar
|
|
91
|
McGinnis KM, Dempster TA and Sommerfeld
MR: Characterization of the growth and lipid content of the diatom
Chaetoceros muelleri. J Appl Phycol. 9:19–24. 1997.
|
|
92
|
Xu H, Miao X and Wu Q: High quality
biodiesel production from a microalga Chlorella protothecoides by
heterotrophic growth in fermenters. J Biotechnol. 126:499–507.
2006.PubMed/NCBI View Article : Google Scholar
|
|
93
|
Behrenfeld MJ and Milligan AJ:
Photophysiological expressions of iron stress in phytoplankton. Ann
Rev Mar Sci. 5:217–246. 2013.PubMed/NCBI View Article : Google Scholar
|
|
94
|
Lynn SG, Kilham SS, Kreeger DA and
Interlandi SJ: Effect of nutrient availability on the biochemical
and elemental stoichiometry in the freshwater diatom Stephanodiscus
minutulus (Bacillariophyceae). J Phycol. 36:510–522.
2000.PubMed/NCBI View Article : Google Scholar
|
|
95
|
Reitan KI, Rainuzzo JR and Olsen Y: Effect
of nutrient limitation on fatty acid and lipid content of marine
microalgae 1. J Phycol. 30:972–979. 1994.
|
|
96
|
Guschina IA and Harwood JL: Lipids and
lipid metabolism in eukaryotic algae. Prog Lipid Res. 45:160–186.
2006.PubMed/NCBI View Article : Google Scholar
|
|
97
|
Lebsky VK, Gonzalez-Bashan LE and Bashan
Y: Ultrastructure of interaction in alginate beads between the
microalga Chlorella vulgaris with its natural associative bacterium
Phyllobacterium myrsinacearum and with the plant growth-promoting
bacterium Azospirillum brasilense. Can J Microbiol. 47:1–8.
2001.PubMed/NCBI
|
|
98
|
Bashan Y and De-Bashan LE: Protection of
tomato seedlings against infection by Pseudomonas syringae pv.
tomato by using the plant growth-promoting bacterium Azospirillum
brasilense. Appl Environ Microbiol. 68:2637–2643. 2002.PubMed/NCBI View Article : Google Scholar
|
|
99
|
Spolaore P, Joannis-Cassan C, Duran E and
Isambert A: Commercial applications of microalgae. J Biosci Bioeng.
101:87–96. 2006.PubMed/NCBI View Article : Google Scholar
|
|
100
|
Liu ZY, Wang GC and Zhou BC: Effect of
iron on growth and lipid accumulation in Chlorella vulgaris.
Bioresour Technol. 99:4717–4722. 2008.PubMed/NCBI View Article : Google Scholar
|
|
101
|
Renju GL, Muraleedhara Kurup G and
Bandugula VR: Effect of lycopene isolated from Chlorella marina on
proliferation and apoptosis in human prostate cancer cell line
PC-3. Tumour Biol. 35:10747–10758. 2014.PubMed/NCBI View Article : Google Scholar
|
|
102
|
Saad SM, Yusof YAM and Ngah WZW:
Comparison between locally produced Chlorella vulgaris and
Chlorella vulgaris from Japan on proliferation and apoptosis of
liver cancer cell line, HepG2. Malays J Biochem Mol Biol, pp32-36,
2006.
|
|
103
|
Wu LC, Ho JA, Shieh MC and Lu IW:
Antioxidant and antiproliferative activities of Spirulina and
Chlorella water extracts. J Agric Food Chem. 53:4207–4212.
2005.PubMed/NCBI View Article : Google Scholar
|
|
104
|
Yusof YA, Saad SM, Makpol S, Shamaan NA
and Ngah WZ: Hot water extract of Chlorella vulgaris induced DNA
damage and apoptosis. Clinics (Sao Paulo). 65:1371–1377.
2010.PubMed/NCBI View Article : Google Scholar
|
|
105
|
Pugh N, Ross SA, ElSohly HN, ElSohly MA
and Pasco DS: Isolation of three high molecular weight
polysaccharide preparations with potent immunostimulatory activity
from Spirulina platensis, Aphanizomenon flos-aquae and Chlorella
pyrenoidosa. Planta Med. 67:737–742. 2001.PubMed/NCBI View Article : Google Scholar
|
|
106
|
Jayshree A, Jayashree S and Thangaraju N:
Chlorella vulgaris and Chlamydomonas reinhardtii: Effective
antioxidant, antibacterial and anticancer mediators. Indian J Pharm
Sci. 78:575–581. 2016.
|
|
107
|
Sedighi M, Jalili H, Ranaei-Siadat SO and
Amrane A: Potential health effects of enzymatic protein
hydrolysates from Chlorella vulgaris. Appl Food Biotechnol.
3:160–169. 2016.
|
|
108
|
Yasukawa K, Akihisa T, Kanno H, Kaminaga
T, Izumida M, Sakoh T, Tamura T and Takido M: Inhibitory effects of
sterols isolated from Chlorella vulgaris on
12-O-tetradecanoylphorbol-13-acetate-induced inflammation and tumor
promotion in mouse skin. Biol Pharm Bull. 19:573–576.
1996.PubMed/NCBI View Article : Google Scholar
|
|
109
|
Carvalho WF and Granéli E: Acidotropic
probes and flow cytometry: A powerful combination for detecting
phagotrophy in mixotrophic and heterotrophic protists. Aquat Microb
Ecol. 44:85–96. 2006.
|
|
110
|
Chisti Y: Biodiesel from microalgae.
Biotechnol Adv. 25:294–306. 2007.PubMed/NCBI View Article : Google Scholar
|
|
111
|
Chisti Y: Biodiesel from microalgae beats
bioethanol. Trends Biotechnol. 26:126–131. 2008.PubMed/NCBI View Article : Google Scholar
|
|
112
|
Roselet F, Vandamme D, Roselet M, Muylaert
K and Abreu PC: Effects of pH, salinity, biomass concentration, and
algal organic matter on flocculant efficiency of synthetic versus
natural polymers for harvesting microalgae biomass. Bioenergy Res.
10:427–437. 2017.
|
|
113
|
Martínez F and Orús MI: Interactions
between glucose and inorganic carbon metabolism in Chlorella
vulgaris strain UAM 101. Plant Physiol. 95:1150–1155.
1991.PubMed/NCBI View Article : Google Scholar
|
|
114
|
Benemann JR and Oswald WJ: Systems and
economic analysis of microalgae ponds for conversion of CO {sub 2}
to biomass. Final report. California Univ., Berkeley CA (United
States). Dept. of Civil Engineering; 1996. Systems and economic
analysis of microalgae ponds for conversion of CO{sub 2} to
biomass. Final report (Technical Report) | OSTI.GOV.
|
|
115
|
Lv JM, Cheng LH, Xu XH, Zhang L and Chen
HL: Enhanced lipid production of Chlorella vulgaris by adjustment
of cultivation conditions. Bioresour Technol. 101:6797–6804.
2010.PubMed/NCBI View Article : Google Scholar
|
|
116
|
Hu J, Nagarajan D, Zhang Q, Chang JS and
Lee DJ: Heterotrophic cultivation of microalgae for pigment
production: A review. Biotechnol Adv. 36:54–67. 2018.PubMed/NCBI View Article : Google Scholar
|
|
117
|
Thompson JC and He BB: Characterization of
crude glycerol from biodiesel production from multiple feedstocks.
Appl Eng Agric. 22:261–265. 2006.
|
|
118
|
Liang Y, Sarkany N and Cui Y: Biomass and
lipid productivities of Chlorella vulgaris under autotrophic,
heterotrophic and mixotrophic growth conditions. Biotechnol Lett.
31:1043–1049. 2009.PubMed/NCBI View Article : Google Scholar
|
|
119
|
Boele FW, Schilder CM, de Roode ML, Deijen
JB and Schagen SB: Cognitive functioning during long-term tamoxifen
treatment in postmenopausal women with breast cancer. Menopause.
22:17–25. 2015.PubMed/NCBI View Article : Google Scholar
|
|
120
|
Abdel-Qadir H, Amir E, Fischer HD, Fu L,
Austin PC, Harvey PJ, Rochon PA, Lee DS and Anderson GM: The risk
of myocardial infarction with aromatase inhibitors relative to
tamoxifen in post-menopausal women with early stage breast cancer.
Eur J Cancer. 68:11–21. 2016.PubMed/NCBI View Article : Google Scholar
|
|
121
|
Tamoxifen KS: Pharmacokinetics and
Pharmacodynamics. Open Access Journal of Pharmaceutical Research.
2017;1: 1-8, 2017. https://opus.lib.uts.edu.au/handle/10453/122001.
|
|
122
|
Buckley MM and Goa KL: Tamoxifen: A
reappraisal of its pharmacodynamic and pharmacokinetic properties,
and therapeutic use. Drugs. 37:451–490. 1989.PubMed/NCBI View Article : Google Scholar
|
|
123
|
Fukuno N, Matsui H, Kanda Y, Suzuki O,
Matsumoto K, Sasaki K and Tamura S: TGF-β-activated kinase 1
mediates mechanical stress-induced IL-6 expression in osteoblasts.
Biochem Biophys Res Commun. 408:202–207. 2011.PubMed/NCBI View Article : Google Scholar
|
|
124
|
Elnaggar YS, El-Massik MA and Abdallah OY:
Self-nanoemulsifying drug delivery systems of tamoxifen citrate:
Design and optimization. Int J Pharm. 380:133–141. 2009.PubMed/NCBI View Article : Google Scholar
|
|
125
|
Kamato D, Burch ML, Piva TJ, Rezaei HB,
Rostam MA, Xu S, Zheng W, Little PJ and Osman N: Transforming
growth factor-beta signalling: Role and consequences of Smad linker
region phosphorylation. Cell Signal. 25:2017–2024. 2013.PubMed/NCBI View Article : Google Scholar
|
|
126
|
Moon AS and Dorigo O: Long-term efficacy
of megestrol acetate and tamoxifen in a recurrent adult granulosa
cell tumor of the ovary. Gynecol Oncol Rep 36 100770, 2021.
|
|
127
|
Cuzick J, Forbes JF, Sestak I, Cawthorn S,
Hamed H, Holli K and Howell A: International Breast Cancer
Intervention Study I Investigators. Long-term results of tamoxifen
prophylaxis for breast cancer-96-month follow-up of the randomized
IBIS-I trial. J Natl Cancer Inst. 99:272–282. 2007.PubMed/NCBI View Article : Google Scholar
|
|
128
|
Cella D and Fallowfield LJ: Recognition
and management of treatment-related side effects for breast cancer
patients receiving adjuvant endocrine therapy. Breast Cancer Res
Treat. 107:167–180. 2008.PubMed/NCBI View Article : Google Scholar
|
|
129
|
Lash TL, Fox MP, Westrup JL, Fink AK and
Silliman RA: Adherence to tamoxifen over the five-year course.
Breast Cancer Res Treat. 99:215–220. 2006.PubMed/NCBI View Article : Google Scholar
|
|
130
|
Shen H, Yuan Y, Sun J, Gao W and Shu YQ:
Combined tamoxifen and gefitinib in non-small cell lung cancer
shows antiproliferative effects. Biomed Pharmacother. 64:88–92.
2010.PubMed/NCBI View Article : Google Scholar
|
|
131
|
Torti FM, Lum BL, Lo R, Freiha F and
Shortliffe L: Tamoxifen in advanced prostatic carcinoma. A dose
escalation study. Cancer. 54:739–743. 1984.PubMed/NCBI View Article : Google Scholar
|
|
132
|
Hoelting T, Siperstein AE, Duh QY and
Clark OH: Tamoxifen inhibits growth, migration, and invasion of
human follicular and papillary thyroid cancer cells in vitro and in
vivo. J Clin Endocrinol Metab. 80:308–313. 1995.PubMed/NCBI View Article : Google Scholar
|
|
133
|
Chen J and Thompson LU: Lignans and
tamoxifen, alone or in combination, reduce human breast cancer cell
adhesion, invasion and migration in vitro. Breast Cancer Res Treat.
80:163–170. 2003.PubMed/NCBI View Article : Google Scholar
|
|
134
|
He W, Liu R, Yang SH and Yuan F:
Chemotherapeutic effect of tamoxifen on temozolomide-resistant
gliomas. Anticancer Drugs. 26:293–300. 2015.PubMed/NCBI View Article : Google Scholar
|