Introduction
Despite recent improvements in treatment modalities,
ovarian carcinoma is the seventh most common cancer in women and
the eighth most common cause of carcinoma-related deaths worldwide
(1). The best management strategy
is aggressive treatment including maximal cytoreductive surgery and
subsequent adjuvant chemotherapy (2). Despite aggressive treatment, ovarian
carcinoma has a poor prognosis.
Management of complications associated with ovarian
cancer is essential for optimal treatment of patients with ovarian
carcinoma. Cancer-associated thromboembolism (CAT) is a prevalent
complication of ovarian carcinoma and includes venous
thromboembolic events (VTEs) and arterial thromboembolic events
(ATEs). The incidence of VTEs in all histological subtypes ranges
from 5.2 to 13.3%, and the incidence of ATEs is 1.1 to 3.2%
(3-5).
Previous reports have indicated that CAT developed more frequently
in ovarian clear cell carcinoma (OCCC) among the different
histologic subtypes of ovarian carcinoma (5). Therefore, the management of CAT,
particularly in patients with OCCC, is crucial in clinical
settings.
OCCC is a histological subtype of epithelial ovarian
carcinoma that comprises clear, proliferating, solid, tubular, or
papillary cells with hobnail features (6). The incidence of OCCC is higher in
Asia, particularly Japan (26.9%), which is higher than that in the
U.S. (7). Compared with other
histological subtypes, OCCC develops at a younger age, is
discovered at an earlier stage, is complicated by endometriosis,
and has a lower response to chemotherapy and a shorter response
period (8,9). However, few studies have examined the
association between CAT and OCCC (5,10,11).
The pathological mechanisms of CAT are complicated
and multifactorial, and include the tumor, tumor microenvironment,
and hemostatic system (12).
Tissue factor (TF) initiate the extrinsic coagulation pathway and
produce thrombin (13). Janus
kinases (JAK) are a family of intracellular non-receptor tyrosine
kinases that mediate signaling through the pathway of signal
transducer and activator of transcription (STAT) proteins (14). Some studies have shown that TF and
IL-6 are risk factors for OCCC (3,15),
and another in vitro study showed that JAK-STAT signaling causes
hypercoagulation through platelet activation (14). However, studies on the relationship
between CAT and the JAK/STAT pathway in patients with ovarian
carcinoma are scarce.
This study aimed to investigate the risk factors,
prognosis, and proteins associated with CAT in patients with OCCC
using previous data with extended follow-up and target periods.
Materials and methods
Patients and tissue samples
Patients with OCCC who underwent surgery at the
National Defense Medical College Hospital (Tokorozawa, Japan)
between January 2000 and December 2019 were included in this study.
The data of patients treated between January 2000 and December 2017
were identified in our previous reports (5). The observational period of these
patients was extended to approximately 2 years, and an analysis
using these data was performed. Patient data from January 2018 to
December 2019 were obtained and included in the final analysis.
Clinical data were obtained from the medical and surgical records.
Patients who did not receive primary treatment, including surgery;
refused chemotherapy; or had no clinical records were excluded.
To identify risk factors for CAT in OCCC, the
following variables were evaluated: Age at diagnosis, body mass
index, comorbid conditions (hypertension, diabetes, heart disease,
hyperlipidemia, stroke, and allergic immune disorders), performance
status score, International Federation of Gynecology and Obstetrics
(FIGO), residual tumor, response rates, ascites, recurrence, and
pattern of recurrence. Performance status was measured using the
World Health Organization Performance Status Scale. The diseases
were staged according to the 2014 FIGO staging system (16). Residual tumors were defined as the
presence or absence of residual tumors after the primary debulking
surgery. The response rates were evaluated according to the
Response Evaluation Criteria in Solid Tumors (RECIST) version
1.1(17). Evaluation was performed
only in patients with residual tumors. Platinum-sensitive
recurrence was defined as a disease that recurred more than six
months after the final cycle of first-line chemotherapy, whereas
platinum-resistant recurrence was defined as a disease that
recurred or progressed within less than six months from the final
cycles of first-line chemotherapy. This study was approved by the
Institutional Review Board of the National Defense Medical College
(approval no. 4346; Tokorozawa, Japan).
CAT evaluation protocol
Peripheral blood samples were obtained from all
patients at the initial visit and before several rounds of
treatment, including primary surgery and several courses of
chemotherapy; D-dimer levels were also measured. We used D-dimer
tests to detect CAT because D-dimer is useful for screening
thromboembolism in ovarian carcinoma according to several previous
reports (18-21).
Inherited predisposition for thromboembolism was additionally
examined if the patient had a family history of thrombotic
predisposition, such as hemophilia or protein C&S deficiency.
After the CAT incidents, if the symptoms or blood tests were
suspicious, we examined acquired predisposition for thromboembolism
such as anti-phospholipid syndrome and disseminated intravascular
coagulopathy (DIC). In addition, all patients underwent computed
tomography and magnetic resonance imaging before the primary
surgery. When symptoms of suspected CAT appeared, including chest
pain, dyspnea, pain, and swelling in one leg or elevated D-dimer
levels exceeding the normal limit (1.0 µg/l), we additionally
performed ultrasonography, computed tomography, magnetic resonance
imaging, and angiography. Furthermore, if the D-dimer levels
suddenly increased or symptoms were present during the observation
or treatment period, CAT screening was performed.
The timing of CAT development was classified as
before or after primary treatment, such as surgery or chemotherapy.
CAT was used to evaluate VTEs, including pulmonary embolism (PE)
and deep vein thrombosis (DVT), and ATEs, including acute
myocardial infarction (AMI) and cerebral infarction (CI), as
described in our previous report (5).
Immunohistochemistry (IHC)
IHC was performed on 111 formalin-fixed,
paraffin-embedded tissues in accordance with our previous study
(22). Tissue microarrays (TMA)
were constructed using a manual tissue array (KIN-2; AZUMAYA,
Tokyo, Japan). TMA slides were deparaffinized and rehydrated using
a stepwise ethanol series. Antigens were removed using citrate (pH
6.0) and Tris-EDTA (pH 9.0) buffers. TMA slides were autoclaved in
citrate buffer at 121˚C for 5 min or boiled in Tris/EDTA buffer at
98˚C for 40 min. The primary antibodies are listed in Table I. All TMA slides were incubated
with primary antibodies for 1 day at room temperature. After
incubation, the slides were incubated with the DAKO EnVision+
System-HRP Labeled Polymer (DAKO Denmark A/S, Glostrup, Denmark,
Code: K4000) as a secondary antibody for 30 min at room
temperature. Finally, we visualized specific antigen-antibody
reactions using 0.2% diaminobenzidine tetrahydrochloride (MUTO PURE
CHEMICALS CO. LTD, Tokyo, Japan, Code: 40651) and hydrogen peroxide
(FUJIFILM Wako Pure Chemical CO, Osaka Japan, Code: 08-0421), and
counterstained with Mayer's hematoxylin (MUTO PURE CHEMICALS CO.
LTD, Tokyo, Japan, Code: 30002). The proportion score was
determined as the proportion of cells in the carcinoma tissue as
follows: 0, no tumor cells stained; 1+, between 1 and 10% of cells
stained throughout the carcinoma tissue; 2+, between 10 and 50%;
3+, 50% or more. The staining intensity score was determined as
follows: 0, no tumor cells stained throughout the carcinoma tissue;
1+, incomplete staining and slight or mostly imperceptible
staining; and 2+, total staining and/or more than moderate
staining. The immunohistochemical interpretation is shown in
Table I.
 | Table IPrimary antibodies. |
Table I
Primary antibodies.
| Molecule | Type | Manufacturer | Antibody cat.
no. | Dilution | Localization | Control tissue | Antigen
retrieval | Interpretation |
|---|
| TF | Monoclonal
(Mouse) | Santa Crus | sc-374441 | 1:50 | Membrane | Kidney | Citrate | Proportion score 3
and staining intensity score 2 to 3 were defined as positive |
| IL-6 | Polyclonal
(Rabbit) | Abcam | ab6672 | 1:400 | Cytoplasm | Lung | EDTA | Proportion score 3
and staining intensity score 2 to 3 were defined as positive |
|
Phosphorylated-JAK2 | Monoclonal
(Rabbit) | Abcam | ab32101 | 1:100 | Nucleus | SCC | Citrate | Proportion score 3
and staining intensity score 2 to 3 were defined as positive |
|
Phosphorylated-STAT3 | Monoclonal
(Rabbit) | Cell Signaling | 9145 | 1:50 | Nucleus | Heart | EDTA | Proportion score 1 to
3 and staining intensity score 1 to 3 were defined as positive |
Statistical analysis
Using JMP 11.0 software (SAS Institute Inc., Tokyo,
Japan), statistical analyses were performed using the χ2
test and Fisher's exact test to compare the differences in
characteristics between the two groups. Progression-free survival
(PFS) was defined as the period from the date of primary treatment
to the date of disease progression or death. Overall survival (OS)
was defined as the period from the date of primary treatment to
death. Survival curves for PFS and OS were generated using the
Kaplan-Meier method. A log-rank test was conducted to compare the
survival distributions. Univariate and multivariate analyses of PFS
and OS were performed using Cox proportional hazards regression.
The variables in the multivariate analysis were those with
statistical significance as identified by univariate analysis.
P<0.05 was considered to indicate a statistically significant
difference.
Results
A total of 111 patients were enrolled during the
observation period. The median follow-up duration was 61 months
(range, 1-195 months). Among these 111 patients, 20 (18.0%) with
OCCC developed CAT complications. The prevalence of CAT is shown in
Table II. None of these patients
had acquired and inherited predisposition for thromboembolism
including DIC or a history of thromboembolism. Twelve patients
(10.8%) were diagnosed with CAT before primary treatment and eight
patients (7.2%) were diagnosed with CAT after primary
treatment.
 | Table IIThe prevalence of cancer-associated
thromboembolism in ovarian clear cell carcinoma. |
Table II
The prevalence of cancer-associated
thromboembolism in ovarian clear cell carcinoma.
| Variable | VTEs, n (%) | ATEs, n (%) | VTEs + ATEs, n
(%) |
|---|
| Total | 14 (70.0) | 3 (15.0) | 3 (15.0) |
| DVT | 8 (40.0) | - | - |
| PE | 3 (15.0) | - | - |
| DVT + PE | 3 (15.0) | - | - |
| CI | - | 3 (15.0) | - |
| DVT + CI | - | - | 1 (5.0) |
| PE + AMI | - | - | 1 (5.0) |
| DVT + PE + CI | - | - | 1 (5.0) |
Table III shows
the incidence of relapse in 13 patients (26.5%) with CAT and 36
patients (73.5%) without CAT, which was significantly higher than
that in patients with CAT (P=0.048). In addition,
platinum-resistant recurrence was significantly more frequent in
patients with CAT than in those without CAT (P=0.025). The
differences in the other characteristics between the two groups
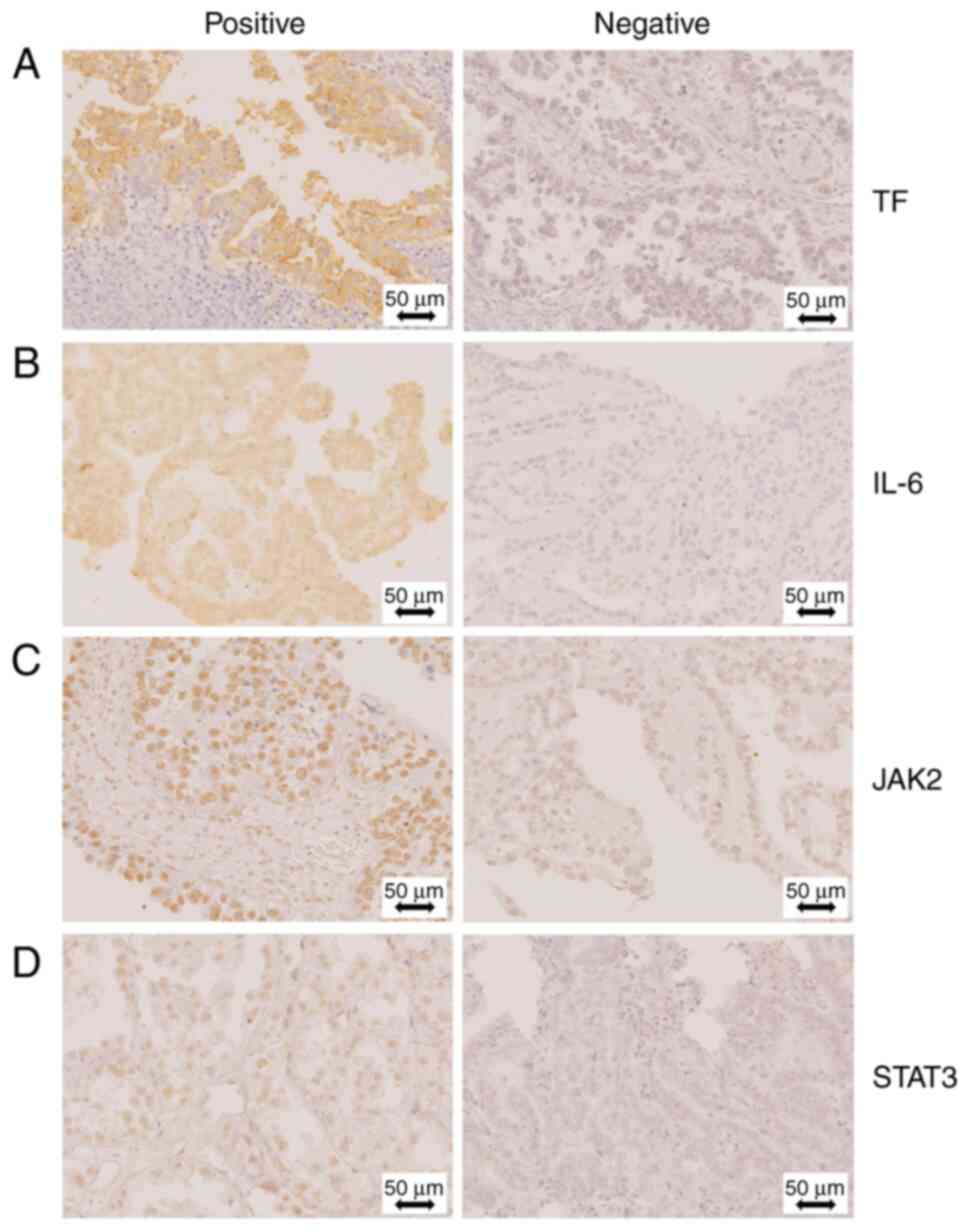
were not statistically significant. Representative images of IHC
staining are shown in Fig. 1.
Table III shows the results of
the immunohistochemical staining for OCCC. Although the IL-6 and
phosphorylated-STAT3 levels were not significantly different
between the two groups, cases of OCCC with CAT were more positive
for TF (P=0.030) and phosphorylated-JAK2 (P=0.034) than those with
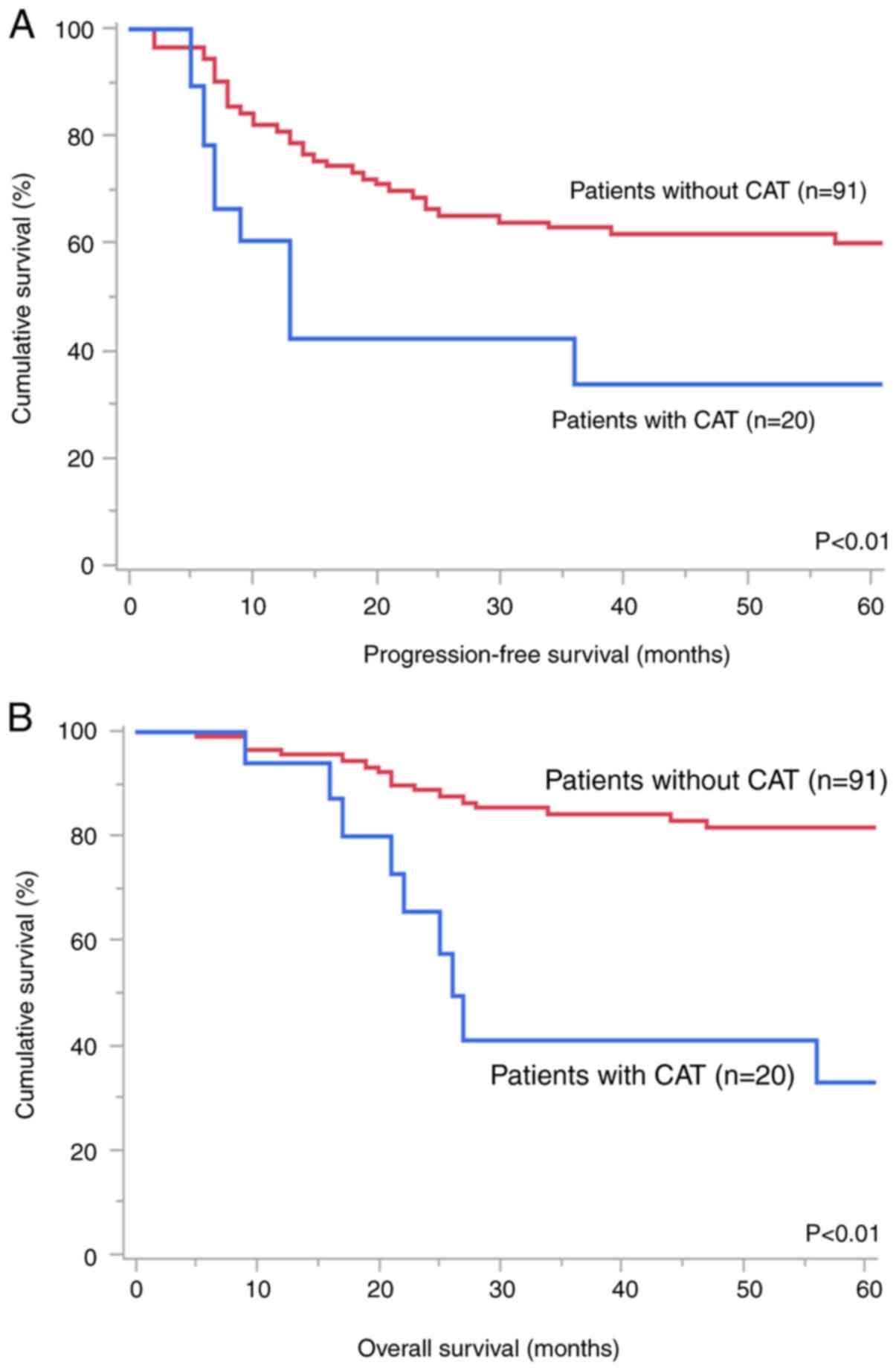
OCCC without CAT. Patients with CAT had worse PFS (Fig. 2A, P<0.01) and OS (Fig. 2B, P<0.01) than those without
CAT. Multivariate analysis (Table
IV) showed that CAT [hazard ratio (HR), 2.10; P=0.039] and
advanced stage (HR=3.46; P<0.01) were independent predictors of
worse PFS. In addition, CAT (HR=4.26; P<0.01) and residual tumor
(HR=3.53; P=0.018) were identified as significant worse prognostic
factors for OS.
 | Table IIICharacteristics and results of
immunohistochemistry staining of all ovarian clear cell carcinoma
patients with or without cancer-associated thromboembolism. |
Table III
Characteristics and results of
immunohistochemistry staining of all ovarian clear cell carcinoma
patients with or without cancer-associated thromboembolism.
| Variable | Patients with CAT,
n (%) | Patients without
CAT, n (%) | P-value |
|---|
| Total | 20 (18.6) | 91 (81.4) | |
| Age at
diagnosis | | | 0.560 |
|
≥65
years | 6 (30.0) | 20 (22.0) | |
|
<65
years | 14 (70.0) | 71 (78.0) | |
| Body mass
index | | | 0.760 |
|
≥25
kg/m2 | 3 (15.0) | 18 (19.8) | |
|
<25
kg/m2 | 17 (85.0) | 73 (80.2) | |
| Comorbid
conditions | | | 0.206 |
|
Yes | 6 (30.0) | 15 (16.5) | |
|
No | 14 (70.0) | 76 (83.5) | |
| Performance status
score | | | 0.150 |
|
0 | 18 (90.0) | 89 (97.8) | |
|
1 | 2 (10.0) | 2 (2.2) | |
| FIGO stage | | | 0.455 |
|
I | 12 (60.0) | 64 (70.3) | |
|
II | 4 (20.0) | 8 (8.8) | |
|
III | 4 (20.0) | 17 (18.7) | |
|
IV | 0 (0.0) | 2 (2.2) | |
| Residual tumor | | | 0.715 |
|
Yes | 3 (15.0) | 11 (12.1) | |
|
No | 17 (85.0) | 80 (87.9) | |
| Best response | | | 0.923 |
|
CR/PR | 1 (33.3) | 4 (36.3) | |
|
SD/PD | 2 (66.7) | 7 (63.7) | |
| Ascites | | | 0.228 |
|
Yes | 13 (65.0) | 45 (49.5) | |
|
No | 7 (35.0) | 46 (50.5) | |
| Recurrence | | | 0.048 |
|
Yes | 13 (65.0) | 36 (39.6) | |
|
No | 7 (35.0) | 55 (60.4) | |
| Pattern of
recurrence | | | 0.025 |
|
Platinum-sensitive
recurrence | 2 (15.4) | 19 (52.8) | |
|
Platinum-resistant
recurrence | 11 (84.6) | 17 (47.2) | |
|
Immunohistochemistry staining | | | |
|
TF (%) | | | 0.030 |
|
Positive | 18 (90.0) | 51 (63.0) | |
|
Negative | 2 (10.0) | 30 (37.0) | |
|
IL-6
(%) | | | 0.625 |
|
Positive | 11 (55.0) | 39 (48.2) | |
|
Negative | 9 (45.0) | 42 (51.8) | |
|
JAK2
(%) | | | 0.034 |
|
Positive | 11 (55.0) | 23 (28.4) | |
|
Negative | 9 (45.0) | 58 (71.6) | |
|
STAT3
(%) | | | 0.203 |
|
Positive | 11 (55.0) | 30 (37.0) | |
|
Negative | 9 (45.0) | 51 (63.0) | |
 | Table IVUnivariate and multivariate analysis
for progression-free survival and overall survival in all
patients. |
Table IV
Univariate and multivariate analysis
for progression-free survival and overall survival in all
patients.
| | Progression-free
survival | Overall
survival |
|---|
| | Univariate
analysis | Multivariate
analysis | Univariate
analysis | Multivariate
analysis |
|---|
| Variable | HR | 95% confidence
interval | P-value | HR | 95% confidence
interval | P-value | HR | 95% confidence
interval | P-value | HR | 95% confidence
interval | P-value |
|---|
| Age at
diagnosis | | | | | | | | | | | | |
|
≥65 years
vs. <65 years | 1.43 | 0.74-2.60 | 0.277 | | | | 1.46 | 0.61-3.19 | 0.374 | | | |
| Body mass
index | | | | | | | | | | | | |
|
≥25 vs.
<25 kg/m2 | 1.17 | 0.55-2.26 | 0.648 | | | | 1.22 | 0.48-2.71 | 0.656 | | | |
| Complications | | | | | | | | | | | | |
|
Yes vs.
no | 1.21 | 0.56-2.38 | 0.599 | | | | 1.12 | 0.41-2.62 | 0.801 | | | |
| Performance status
score | | | | | | | | | | | | |
|
≥1 vs.
0 | 2.43 | 0.59-6.73 | 0.166 | | | | 3.55 | 0.57-12.1 | 0.147 | | | |
| FIGO stage | | | | | | | | | | | | |
|
II-IV vs.
I | 4.31 | 2.42-7.73 | <0.01 | 3.46 | 1.77-6.70 | <0.01 | 3.10 | 1.49-6.48 | <0.01 | 1.87 | 0.76-4.38 | 0.170 |
| Residual tumor | | | | | | | | | | | | |
|
Yes vs.
no | 3.07 | 1.44-6.00 | <0.01 | 1.35 | 0.58-2.99 | 0.471 | 4.18 | 1.72-9.22 | <0.01 | 3.53 | 1.25-9.77 | 0.018 |
| Ascites | | | | | | | | | | | | |
|
Yes vs.
no | 1.94 | 1.09-3.56 | 0.022 | 1.13 | 0.59-2.22 | 0.699 | 1.71 | 0.83-3.67 | 0.146 | | | |
| CAT | | | | | | | | | | | | |
|
Yes vs.
no | 2.52 | 1.25-4.74 | 0.011 | 2.10 | 1.03-3.97 | 0.039 | 3.68 | 1.59-7.87 | <0.01 | 4.26 | 1.77-9.57 | <0.01 |
Discussion
In our study, the rate of CAT-related complications
in patients with OCCC was 18.0%. Patients with CAT were more likely
to experience relapse (P=0.048) and platinum-resistant recurrence
(P=0.02). CAT is a poor prognostic factor in patients with OCCC. In
addition, we showed for the first time that JAK2 signaling is
associated with CAT in OCCC.
Our results indicated that the incidence of OCCC
patients with CAT (18.0%) was within the range of previous studies
(14.5-27.3%) (10,23). Similarly, the incidence of patients
with OCCC complicated with CAT before primary treatment in our
study (10.8%) was within the range of previous studies (5.3-14.9%)
(11,23). Conversely, the incidence of CAT
after primary treatment (7.2%) was lower than that of previous
studies (9.3-19.7%) (11,23). Thus, the incidence at several time
points in our study did not differ significantly from that in
previous reports.
Our previous report demonstrated that CAT was
associated with worse prognosis and OCCC (5). We did not perform a sub-analysis to
determine the significance of CAT in patients with OCCC. Our
findings are consistent with those of previous studies that found
that patients with OCCC and CAT had a poor prognosis (10,11,23).
In this study, comorbid conditions were not risk factors to develop
CAT, and did not influence PFS or OS between patients with and
without CAT. A potential confounding factor that CAT might affect
patient outcomes is the delay or discontinuation of OCCC treatment
because CAT could induce a poor general condition. This study
showed that the development of severe CAT, such as massive PE, AMI,
and CI, could reduce performance status and require immediate
treatment, resulting in the delay and discontinuation of OCCC
treatment. From another perspective, bevacizumab, a humanized
anti-vascular endothelial growth factor monoclonal antibody, may
improve OCCC prognosis (24).
However, this is associated with severe thromboembolism as a side
effect. Thus, treatments with promising drugs are limited.
Our results also demonstrated that OCCC with CAT
recurred more frequently and was platinum resistant. CAT in OCCC
may indicate not only the formation of a tumor microenvironment,
such as hypoxia, but also immunosuppression due to TF and
IL-6/JAK2/STAT3. TF activates various signaling pathways that
promote cancer cell proliferation, metastasis, angiogenesis, and
cancer stem cell-like cell maintenance (25). In addition, TF is overexpressed
during inflammation, leading to the activation of JAK2, which
promotes platelet activation and epithelial-mesenchymal transition
(EMT) via STAT3 (14.26.27). Furthermore, JAK2/STAT3 signaling
suppresses tumor-infiltrating lymphocytes induced by hypoxia
(28). Therefore, the inhibition
of TF and JAK2 prevents EMT in cancer cells and improves the tumor
microenvironment. Thus, TF and JAK2 are potential therapeutic
candidates for OCCC.
From this perspective, several potential therapeutic
targets for OCCC with CAT are candidates for new treatments that
target the TF and JAK pathways compared with other histologic
subtypes. The Phase 1-2 trial demonstrated that the objective
response rate of tisotumab vedotin (TV), an antibody-drug conjugate
against TF expressed on the cell surface of tumor cells, was five
out of 36 (13.9%) (29). However,
the expression varied among the histological subtypes and was most
frequently found in OCCC. However, it was unclear whether OCCC was
included in this clinical trial. Therefore, TV treatment was
feasible. A clinical trial of TV for platinum-resistant ovarian
cancer is ongoing (NCT03657043) (30). In IL-6/JAK2/STAT3 pathway, two
studies have demonstrated that siltuximab, a humanized anti-IL-6
antibody, and tocilizumab, a humanized anti-human IL-6 receptor
antibody, inhibit the proliferation of ovarian cancer cells
(31,32). Recently, clinical trials have
explored the role of ruxolitinib, a JAK/STAT inhibitor, in solid
tumors (33). A phase I/II
randomized clinical trial (NCT02713386) investigated the
combination chemotherapy of ruxolitinib with paclitaxel and
carboplatin for epithelial ovarian, fallopian tube, and primary
peritoneal cancers. The study demonstrated that ruxolitinib was
well tolerated and prolonged the PFS (34). As another potential therapy for
OCCC, aspirin use might reduce the mortality of ovarian cancer, and
aspirin and other nonsteroidal anti-inflammatory drugs might
improve the prognosis owing to their anti-platelet activity
(35,36).
The limitations of this study are its retrospective
design, single-center analysis, and small sample size. In this
study, only immunohistochemistry was used to validate the activity
of the signaling pathways. And, we could not suggest any further
analysis of the molecular mechanisms which CAT may affect outcomes
in OCCC. Further studies using other experimental techniques and
methods are required for a comprehensive validation. Moreover,
studies with larger sample sizes are warranted to identify the
clinical significance of the association between OCCC and CAT, and
to evaluate other coagulation factors in OCCC.
In conclusion, our study found that the development
of CAT was a poor prognostic factor related to platinum resistance
and that TF and JAK2/STAT3 were associated with the occurrence of
CAT in OCCC. Further studies are required to prevent and treat CAT
in patients with OCCC.
Acknowledgements
The authors would like to thank Ms. Ayako Suzuki for
collecting the samples at the National Defense Medical College
Hospital.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
TI, MM, TH, SK and MT contributed to the study
conception and design. Material preparation, data collection and
analysis were carried out by TI, NK, JS and KK. All authors
contributed to the data interpretation. MM and TH confirm the
authenticity of all the raw data. The first draft of the manuscript
was written by TI, and all authors read and approved the final
version of the manuscript.
Ethics approval and consent to
participate
This study was approved by the Institutional Review
Board of the National Defense Medical College (Tokorozawa, Japan)
on January 20, 2021, approval no. 4346. Records and information of
all patients in this study were fully anonymized before the
analysis to prevent the disclosure of their identities. Before the
treatment, written informed consent for participation was obtained
from all patients.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Webb PM and Jordan SJ: Epidemiology of
epithelial ovarian cancer. Best Pract Res Clin Obstet Gynaecol.
41:3–14. 2017.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Chandra A, Pius C, Nabeel M, Nair M,
Vishwanatha JK, Ahmad S and Basha R: Ovarian cancer: Current status
and strategies for improving therapeutic outcomes. Cancer Med.
8:7018–7031. 2019.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Sakurai M, Matsumoto K, Gosho M, Sakata A,
Hosokawa Y, Tenjimbayashi Y, Katoh T, Shikama A, Komiya H,
Michikami H, et al: Expression of tissue factor in epithelial
ovarian carcinoma is involved in the development of venous
thromboembolism. Int J Gynecol Cancer. 27:37–43. 2017.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Yuan Z, Cao D, Yu M, Zhou H, Zhang Y, Yang
J and Shen K: Importance of standard treatment in prognosis of
patients with ovarian cancer and associated cerebral infarction.
Clin Interv Aging. 15:151–157. 2020.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Takasaki K, Miyamoto M, Takano M, Soyama
H, Aoyama T, Matsuura H, Iwahashi H, Ishibashi H, Sakamoto T and
Furuya K: Thrombotic events induce the worse prognosis in ovarian
carcinomas and frequently develop in ovarian clear cell carcinoma.
Int J Clin Oncol. 24:1273–1283. 2019.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Machida H, Matsuo K, Yamagami W, Ebina Y,
Kobayashi Y, Tabata T, Kanauchi M, Nagase S, Enomoto T and Mikami
M: Trends and characteristics of epithelial ovarian cancer in Japan
between 2002 and 2015: A JSGO-JSOG joint study. Gynecol Oncol.
153:589–596. 2019.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Takano M, Kikuchi Y, Yaegashi N, Kuzuya K,
Ueki M, Tsuda H, Suzuki M, Kigawa J, Takeuchi S, Tsuda H, et al:
Clear cell carcinoma of the ovary: A retrospective multicentre
experience of 254 patients with complete surgical staging. Br J
Cancer. 94:1369–1374. 2006.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Miyamoto M, Takano M, Goto T, Kato M,
Sasaki N, Tsuda H and Furuya K: Clear cell histology as a poor
prognostic factor for advanced epithelial ovarian cancer: A single
institutional case series through central pathologic review. J
Gynecol Oncol. 24:37–43. 2013.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Takano M, Goto T, Kato M, Sasaki N,
Miyamoto M and Furuya K: Short response duration even in responders
to chemotherapy using conventional cytotoxic agents in recurrent or
refractory clear cell carcinomas of the ovary. Int J Clin Oncol.
18:556–557. 2013.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Diaz ES, Walts AE, Karlan BY and Walsh CS:
Venous thromboembolism during primary treatment of ovarian clear
cell carcinoma is associated with decreased survival. Gynecol
Oncol. 131:541–545. 2013.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Ye S, Yang J, Cao D, Bai H, Huang H, Wu M,
Chen J, You Y, Lang J and Shen K: Characteristic and prognostic
implication of venous thromboembolism in ovarian clear cell
carcinoma: A 12-year retrospective study. PLoS One.
10(e0121818)2015.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Dunbar A, Bolton KL, Devlin SM,
Sanchez-Vega F, Gao J, Mones JV, Wills J, Kelly D, Farina M,
Cordner KB, et al: Genomic profiling identifies somatic mutations
predicting thromboembolic risk in patients with solid tumors.
Blood. 137:2103–2113. 2021.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Unruh D and Horbinski C: Beyond
thrombosis: The impact of tissue factor signaling in cancer. J
Hematol Oncol. 13(93)2020.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Lu WJ, Lin KC, Huang SY, Thomas PA, Wu YH,
Wu HC, Lin KH and Sheu JR: Role of a Janus kinase 2-dependent
signaling pathway in platelet activation. Thromb Res.
133:1088–1096. 2014.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Matsuo K, Hasegawa K, Yoshino K, Murakami
R, Hisamatsu T, Stone RL, Previs RA, Hansen JM, Ikeda Y, Miyara A,
et al: Venous thromboembolism, interleukin-6 and survival outcomes
in patients with advanced ovarian clear cell carcinoma. Eur J
Cancer. 51:1978–1988. 2015.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Prat J: FIGO Committee on Gynecologic
Oncology. FIGO's staging classification for cancer of the ovary,
fallopian tube, and peritoneum: Abridged republication. J Gynecol
Oncol. 26:87–89. 2015.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Eisenhauer EA, Therasse P, Bogaerts J,
Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S,
Mooney M, et al: New response evaluation criteria in solid tumours:
Revised RECIST guideline (version 1.1). Eur J Cancer. 45:228–247.
2009.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Zhou Q, Zhu C, Shen Z, Zhang T, Li M, Zhu
J, Qin J, Xie Y, Zhang W, Chen R, et al: Incidence and potential
predictors of thromboembolic events in epithelial ovarian carcinoma
patients during perioperative period. Eur J Surg Oncol. 46:855–861.
2020.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Kawaguchi R, Furukawa N and Kobayashi H:
Cut-off value of D-dimer for prediction of deep venous thrombosis
before treatment in ovarian cancer. J Gynecol Oncol. 23:98–102.
2012.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Tasaka N, Minaguchi T, Hosokawa Y, Takao
W, Itagaki H, Nishida K, Akiyama A, Shikama A, Ochi H and Satoh T:
Prevalence of venous thromboembolism at pretreatment screening and
associated risk factors in 2086 patients with gynecological cancer.
J Obstet Gynaecol Res. 46:765–773. 2020.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Habu Y, Mitsuhashi A, Hanawa S, Usui H,
Horikoshi T, Uno T and Shozu M: High prevalence of pulmonary
embolism prior to cancer therapies in patients with ovarian and
endometrial cancers detected by contrast-enhanced CT using D-dimer
as an index. J Surg Oncol. 124:106–114. 2021.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Hada T, Miyamoto M, Ishibashi H, Matsuura
H, Sakamoto T, Kakimoto S, Iwahashi H, Tsuda H and Takano M:
Survival and biomarker analysis for ovarian mucinous carcinoma
according to invasive patterns: Retrospective analysis and review
literature. J Ovarian Res. 14(33)2021.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Matsuura Y, Robertson G, Marsden DE, Kim
SN, Gebski V and Hacker NF: Thromboembolic complications in
patients with clear cell carcinoma of the ovary. Gynecol Oncol.
104:406–410. 2007.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Gallego A, Ramon-Patino J, Brenes J,
Mendiola M, Berjon A, Casado G, Castelo B, Espinosa E, Hernandez A,
Hardisson D, et al: Bevacizumab in recurrent ovarian cancer: Could
it be particularly effective in patients with clear cell carcinoma?
Clin Transl Oncol. 23:536–542. 2021.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Hisada Y and Mackman N: Tissue factor and
cancer: Regulation, tumor growth, and metastasis. Semin Thromb
Hemost. 45:385–395. 2019.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Shinohara A, Kutsukake M, Takahashi M, Kyo
S, Tachikawa E and Tamura K: Protease-activated receptor-stimulated
interleukin-6 expression in endometriosis-like lesions in an
experimental mouse model of endometriosis. J Pharmacol Sci.
119:40–51. 2012.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Huang B, Lang X and Li X: The role of
IL-6/JAK2/STAT3 signaling pathway in cancers. Front Oncol.
12(1023177)2022.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Hu R, Han Q and Zhang J: STAT3: A key
signaling molecule for converting cold to hot tumors. Cancer Lett.
489:29–40. 2020.PubMed/NCBI View Article : Google Scholar
|
|
29
|
de Bono JS, Concin N, Hong DS,
Thistlethwaite FC, Machiels JP, Arkenau HT, Plummer R, Jones RH,
Nielsen D, Windfeld K, et al: Tisotumab vedotin in patients with
advanced or metastatic solid tumours (InnovaTV 201): A
first-in-human, multicentre, phase 1-2 trial. Lancet Oncol.
20:383–393. 2019.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Blank S, Mahdi H, Tehrani O, Ghamande S,
Jain S, Nicacio L, Soumaoro I, O'Malley DM and Innova TV: 882TiP
InnovaTV 208: New weekly dosing cohort in the phase II study of
tisotumab vedotin in platinum-resistant ovarian cancer. Ann Oncol.
31(S646)2020.
|
|
31
|
Guo Y, Nemeth J, O'Brien C, Susa M, Liu X,
Zhang Z, Choy E, Mankin H, Hornicek F and Duan Z: Effects of
siltuximab on the IL-6-induced signaling pathway in ovarian cancer.
Clin Cancer Res. 16:5759–5769. 2010.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Yousefi H, Momeny M, Ghaffari SH,
Parsanejad N, Poursheikhani A, Javadikooshesh S, Zarrinrad G,
Esmaeili F, Alishahi Z, Sabourinejad Z, et al: IL-6/IL-6R pathway
is a therapeutic target in chemoresistant ovarian cancer. Tumori.
105:84–91. 2019.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Yunianto I, Currie M, Chitcholtan K and
Sykes P: Potential drug repurposing of ruxolitinib to inhibit the
JAK/STAT pathway for the treatment of patients with epithelial
ovarian cancer. J Obstet Gynaecol Res. 49:2563–2574.
2023.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Landen CN, Buckanovich RJ, Sill M, Mannel
RS, Walker JL, Disilvestro P, Mathews CA, Mutch G, Hernandez M,
Martin LP, et al: A phase I/II study of ruxolitinib with frontline
neoadjuvant and post-surgical therapy in patients with advanced
epithelial ovarian, fallopian tube, or primary peritoneal cancer. J
Clin Oncol. 40(5501)2022.
|
|
35
|
Wield AM, Walsh CS, Rimel BJ, Cass I,
Karlan BY and Li AJ: Aspirin use correlates with survival in women
with clear cell ovarian cancer. Gynecol Oncol Rep. 25:78–81.
2016.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Verdoodt FC, Dehlendorff C, Friis S and
Kjaer SK: Non-aspirin NSAID use and ovarian cancer mortality.
Gynecol Oncol. 150:331–337. 2018.PubMed/NCBI View Article : Google Scholar
|