Introduction
Gastric cancer (GC) ranks the fifth most prevalent cancer and the fifth leading cause of cancer-related deaths worldwide. Notably, >40% of the global GC mortality burden is noted in China, which is significantly higher than that observed in Europe, America, Japan and South Korea. Furthermore, the majority of Chinese patients with GC present at an advanced stage at diagnosis, resulting in an overall 5-year survival rate of 35.9% (1,2). In the past 10 years, only the targeted drug trastuzumab combined with chemotherapy for HER2 positive patients has achieved overall survival benefits in the first-line treatment of advanced gastric cancer, while other HER2 targeted drugs have not been successful. With the exception of a small subset of patients with human epidermal growth factor receptor-2 (HER2)-positive cancer eligible for anti-HER2 therapy, the vast majority of HER2-negative cases still depend on chemotherapy as their primary treatment. However, recent advancements in immunotherapy have introduced promising alternatives. Specifically, programmed cell death-1 (PD-1) inhibitors have demonstrated remarkable efficacy.
Attraction-2 study in 2017 is a phase III clinical trial conducted in the Asian region, which includes patients with advanced gastric and gastroesophageal junction adenocarcinomas who have been resistant or intolerant to at least two chemotherapy regimens. The ATTRACION-2 indicated that nivolumab showed significant OS benefits in patients with gastric cancer who had failed at least second-line treatment, rendering it the world's first PD-1 inhibitor to receive indications for advanced gastric cancer treatment. International guidelines unanimously recommend nivolumab as a new standard for the treatment of GC at or after the third line (3). In September 2020, the preliminary results of Checkmate 649 study were presented at the annual meeting of the European Society for Medical Oncology, bringing the first PD-1 antibody nivolumab that may prolong survival to the first-line treatment of HER2 negative advanced gastric cancer. The research results showed that nivolumab combined with chemotherapy has significant short-term efficacy and long-term survival benefits compared with chemotherapy alone. Based on the impressive OS outcomes reported in the study, the combination therapy of nivolumab and chemotherapy has become the first-line standard treatment for HER2 negative advanced gastric cancer and GEJ adenocarcinoma. In addition, the ORIENT-16 study, led by Chinese researchers, further supported the use of sintilimab plus chemotherapy as a viable first-line option, providing a robust foundation for the standard of care in treating patients with advanced GC in China (4,5).
However, second-line treatment for advanced GC remains challenging, despite the rapid advancements in single-agent immunotherapy under stringent limitations and chemotherapy alone. Consequently, significant potential has been revealed for the combination of PD-1 inhibitors with paclitaxel in the second-line treatment of GC, an area that currently lacks substantial exploration.
The present study focused on patients with HER2-negative GC who experienced recurrence or metastasis following treatment with the SOX or XELOX regimens. These patients were subsequently transitioned to second-line therapy with PD-1 antibodies, such as nivolumab, pembrolizumab, or camrelizumab in combination with taxanes (docetaxel or nab-paclitaxel). The primary objectives were to evaluate progression-free survival (PFS), OS, objective response rate (ORR), disease control rate (DCR) and adverse reactions in this cohort.
Materials and methods
Patients
The present study was a retrospective, non-interventional, single-center cohort study of patients with advanced GC who were initiated on second-line therapy between March 2021 and October 2023 (index date). For each patient, all available data between the index date and the end of clinical activity, end of follow-up (May 2024) or death (whichever occurred first) were retrospectively collected. The data set for the statistical analysis was prepared between May and June 2024, and the final study report was released in July 2024.
A total of 21 patients were selected with HER2-negative advanced GC who had progressed on standard regimens of SOX (130 mg/m2 of oxaliplatin a day, intravenous drip, 21 days for 1 cycle; 40 mg/(m2·day) of S-1 capsules, orally, days 1-14, 21 days for 1 cycle) or XELOX (130 mg/m2 of oxaliplatin per day, intravenous drip, 21 days for 1 cycle; 1,000 mg/m2 of capecitabine twice a day, days 1-14, 21 days for 1 cycle).
The eligibility criteria for the present study were as follows: i) Histologically proven HER2 (-) adenocarcinoma of the stomach or gastroesophageal junction, ii) administration of platinum and fluorouracil-based first-line therapy, iii) absence of concomitant advanced malignant disease, iv) refractory or intolerant to fuoropyrimidine and v) administration of taxane-based second-line chemotherapy following disease progression during first-line chemotherapy or recurrence within 6 months following the last adjuvant chemotherapy dose. Patients with a history of taxane treatment and/or those with serious complications were excluded, such as active infection, renal failure (serum creatinine level ≥3.0 mg/dl) and hepatic failure or obstructive jaundice (serum total bilirubin level ≥2.0 mg/dl).
Methods
The second-line regimen included taxanes (docetaxel, nab-paclitaxel) in combination with immunotherapy (nivolumab, pembrolizumab, camrelizumab); the patients were evaluated using RECIST 1.1 criteria (https://recist.eortc.org/) until disease progression or intolerable side effects.
Outcomes
The responses were evaluated by investigators per RECIST v1.1 using imaging at baseline and every 8 weeks until disease progression. A complete response (CR) or a partial response (PR) was confirmed with one sequential tumor assessment at least 4 weeks later.
Safety was evaluated according to the NCI Common Terminology Criteria for Adverse Events, version 5.0. Clinical examination, adverse events (AEs) reported by patients and blood count tests were conducted and carefully evaluated on a weekly basis during each cycle. Other general safety assessment examinations, including biochemistry tests, electrocardiograms and echocardiography and safety assessments were performed on day 1 of every cycle. The causality of AE classification was performed by the investigators.
The primary endpoint was ORR (confirmed CR or PR), as determined by the investigators. Secondary endpoints were investigator-assessed PFS (defined as the time from the initiation of second-line therapy to the first occurrence of progression or death, whichever occurred first), OS (initiation of second-line therapy until death from any cause), duration of response (DOR; first occurrence of response to disease progression or death from any cause, whichever occurred first), DCR; proportion of patients with an optimal Odds ratio (OR) of CR or PR or stable disease ≥8 weeks), safety and tolerability.
Statistical analysis
Statistical analysis was performed using the SPSS statistical software (version 26.0; IBM Corp.) and Prism software (version 9.5; GraphPad Software, Inc.). The Kaplan-Meier method was used to estimate survival rates. The log-rank test was used to evaluate the survival differences between subgroups. The Cox proportional hazards model for multivariate survival analysis was used to assess predictors related to survival, compute hazard ratios (HR) and 95% confidence intervals (CIs). Two-sided P<0.05 were considered to indicate a statistically significant difference.
Data availability
All data required to evaluate the conclusions in the study is included in the figures and/or tables of this article or can be requested via the leading corresponding author.
Ethics approval and consent to participate
The present study was approved by the Ethics Committee of Shanghai Changhai Hospital, Naval Medical University (approval no. KTSB20240716031; Shanghai, China). Patient informed consent was waived due to the retrospective nature of the study.
Results
Study design and participants
A total of 21 patients with HER2-negative advanced GC who progressed following platinum and fluorouracil-based therapy were enrolled. As of May 1, 2024, the median follow-up time was 14.4 months (range, 10.5-18.4), and six (28.6%) patients remained under treatment (Fig. 1). The baseline characteristics are summarized in Table I. Briefly, the median age was 55 years (range, 36-68); two patients (9.5%) exhibited three metastatic sites, one (4.8%) presented with lung metastasis, three (14.3%) presented with liver metastasis, four (19.0%) exhibited bone metastasis and five (23.8%) presented with peritoneal metastasis. All 21 patients (100.0%) had previously received fluorouracil combined with oxaliplatin.
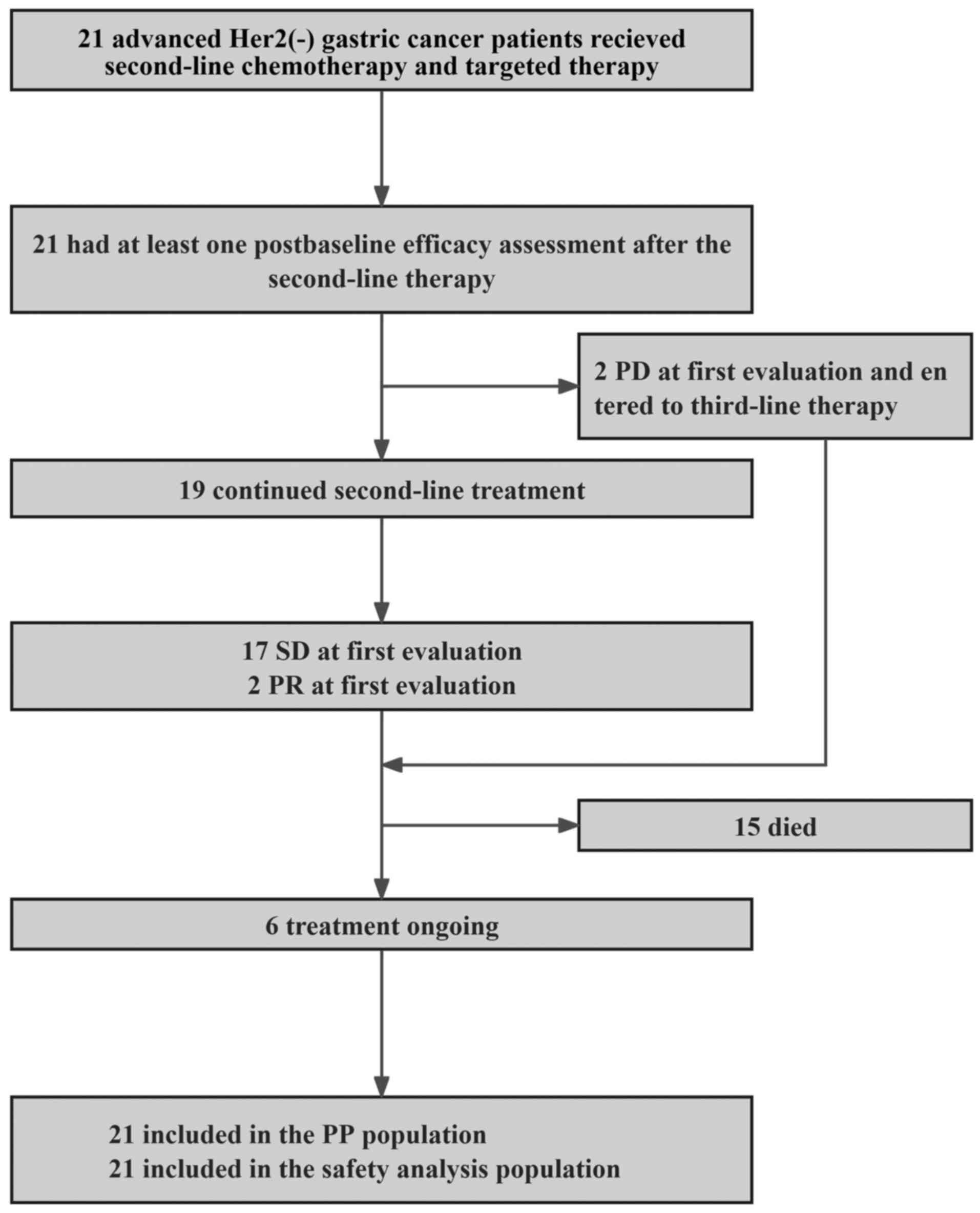 |
Figure 1
Research flowchart. HER2, growth factor receptor-2; SD, stable disease; PR, partial response; PD, progressive disease; PP, per protocol.
|
 |
Table I
Predictive variables for OS and PFS of patients with advanced stomach adenocarcinoma by log rank test.
|
Table I
Predictive variables for OS and PFS of patients with advanced stomach adenocarcinoma by log rank test.
| |
PFS (months) |
OS (months) |
| Variables |
Case |
Median |
P-value |
Case |
Median |
Pa |
| Age, years |
|
|
|
|
|
|
| ≤55 |
10 |
193 |
0.907 |
10 |
340 |
0.475 |
| >55 |
11 |
213 |
|
11 |
493 |
|
| Sex |
|
|
|
|
|
|
| Male |
16 |
212 |
0.961 |
16 |
340 |
0.948 |
| Female |
5 |
224 |
|
5 |
493 |
|
| Eastern cooperative oncology group |
|
|
|
|
|
|
| 0 |
2 |
n.a. |
|
2 |
n.a. |
0.336 |
| 1 |
19 |
212 |
|
19 |
340 |
|
| WHO Grade |
|
|
|
|
|
|
| G1 |
1 |
273 |
0.921 |
1 |
517 |
0.802 |
| G2 |
7 |
213 |
|
7 |
340 |
|
| G3 |
13 |
212 |
|
13 |
315 |
|
| T status |
|
|
|
|
|
|
| T1 |
0 |
n.a. |
0.674 |
0 |
n.a. |
0.943 |
| T2 |
0 |
n.a. |
|
0 |
n.a. |
|
| T3 |
14 |
213 |
|
14 |
493 |
|
| T4 |
7 |
212 |
|
7 |
265 |
|
| N status |
|
|
|
|
|
|
| N0 |
0 |
n.a. |
0.221 |
0 |
n.a. |
0.303 |
| N1 |
2 |
218 |
|
2 |
228 |
|
| N2 |
8 |
213 |
|
8 |
494 |
|
| N3 |
11 |
193 |
|
11 |
315 |
|
| M status |
|
|
|
|
|
|
| M0 |
3 |
n.a. |
0.156 |
3 |
340 |
0.644 |
| M1 |
18 |
212 |
|
18 |
315 |
|
| Metastatic site |
|
|
|
|
|
|
| Lung |
|
|
|
|
|
|
| Negative |
20 |
212 |
0.348 |
20 |
340 |
0.691 |
| Positive |
1 |
n.a. |
|
1 |
n.a. |
|
| Liver |
|
|
|
|
|
|
| Negative |
18 |
198 |
0.973 |
18 |
493 |
0.721 |
| Positive |
3 |
243 |
|
3 |
303 |
|
| Bone metastasis |
|
|
|
|
|
|
| Negative |
17 |
212 |
0.108 |
17 |
315 |
0.393 |
| Positive |
4 |
286 |
|
4 |
493 |
|
| Only lymph node or soft tissue |
|
|
|
|
|
|
| Negative |
8 |
224 |
0.451 |
8 |
517 |
0.367 |
| Positive |
13 |
193 |
|
13 |
315 |
|
| Ascitic fluid |
|
|
|
|
|
|
| Negative |
20 |
212 |
0.729 |
20 |
493 |
0.454 |
| Positive |
1 |
213 |
|
1 |
315 |
|
| Peritoneal metastasis |
|
|
|
|
|
|
| Negative |
16 |
213 |
0.800 |
16 |
493 |
0.946 |
| Positive |
5 |
193 |
|
5 |
340 |
|
| Number of distant metastases |
|
|
|
|
|
|
| 0 |
5 |
224 |
0.829 |
5 |
517 |
0.549 |
| 1 |
8 |
159 |
|
8 |
231 |
|
| 2 |
6 |
213 |
|
6 |
315 |
|
| 3 |
2 |
130 |
|
2 |
216 |
|
| 4 |
0 |
n.a. |
|
0 |
n.a. |
|
| 5 |
0 |
n.a. |
|
0 |
n.a. |
|
| Programmed cell death 1 ligand 1 |
|
|
|
|
|
|
| Negative |
17 |
212 |
0.680 |
17 |
340 |
0.397 |
| Positive |
4 |
218 |
|
4 |
228 |
|
| Ki67 |
|
|
|
|
|
|
| Low |
10 |
273 |
0.002 |
10 |
517 |
0.013 |
| High |
11 |
159 |
|
11 |
265 |
|
| Tumor marker-1 |
|
|
|
|
|
|
| Negative |
8 |
224 |
0.408 |
8 |
517 |
0.018 |
| Positive |
13 |
212 |
|
13 |
265 |
|
According to patient records, an anti-PD-1 inhibitor was administered in combination with taxane chemotherapy in the second line. The patients received 200 mg pembrolizumab, nivolumab at 3 mg/kg and 200 mg camrelizumab intravenously once every 3 weeks following chemotherapeutic agents.
Efficacy
In the population, 17 patients (81.0%) were evaluated as stable disease (SD), 0 (0.0%) achieved CR, 2 (9.5%) achieved PR and 6 (28.6%) patients remained under treatment. In addition, 2 events (9.5%) of disease progression and 15 (71.4%) deaths occurred (Figs. 1 and 2, Table II). The mPFS was 7.1 months (95% CI, 6.1-8.1 months) and the median OS was 11.3 months (95% CI, 4.5-18.2 months; Fig. 3A and B). The ORR was 9.5% (2/21) and the DCR was 90.5% (19/21) (Table II). Univariate and multivariate analyses indicated that Ki67 <70% (grouped by median 70%) and tumor marker-positive [one or two increases among carcinoembryogenic antigen (CEA), cancer antigen (CA)199 and CA125] were independent prognostic factors for PFS and OS in second-line treatment (Tables III and IV).
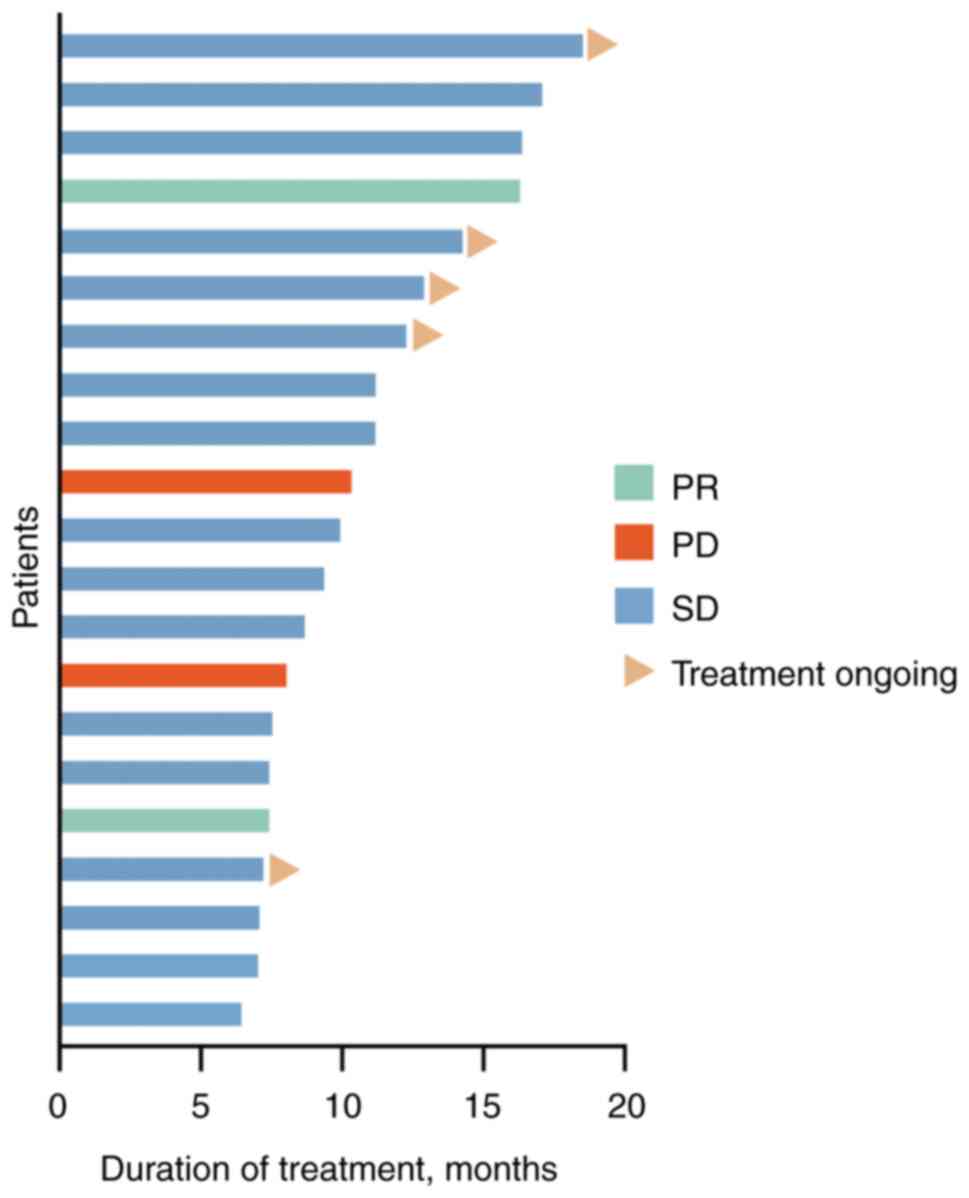 |
Figure 2
Tumor response of each patient. PR, partial response; PD, progressive disease; SD, stable disease.
|
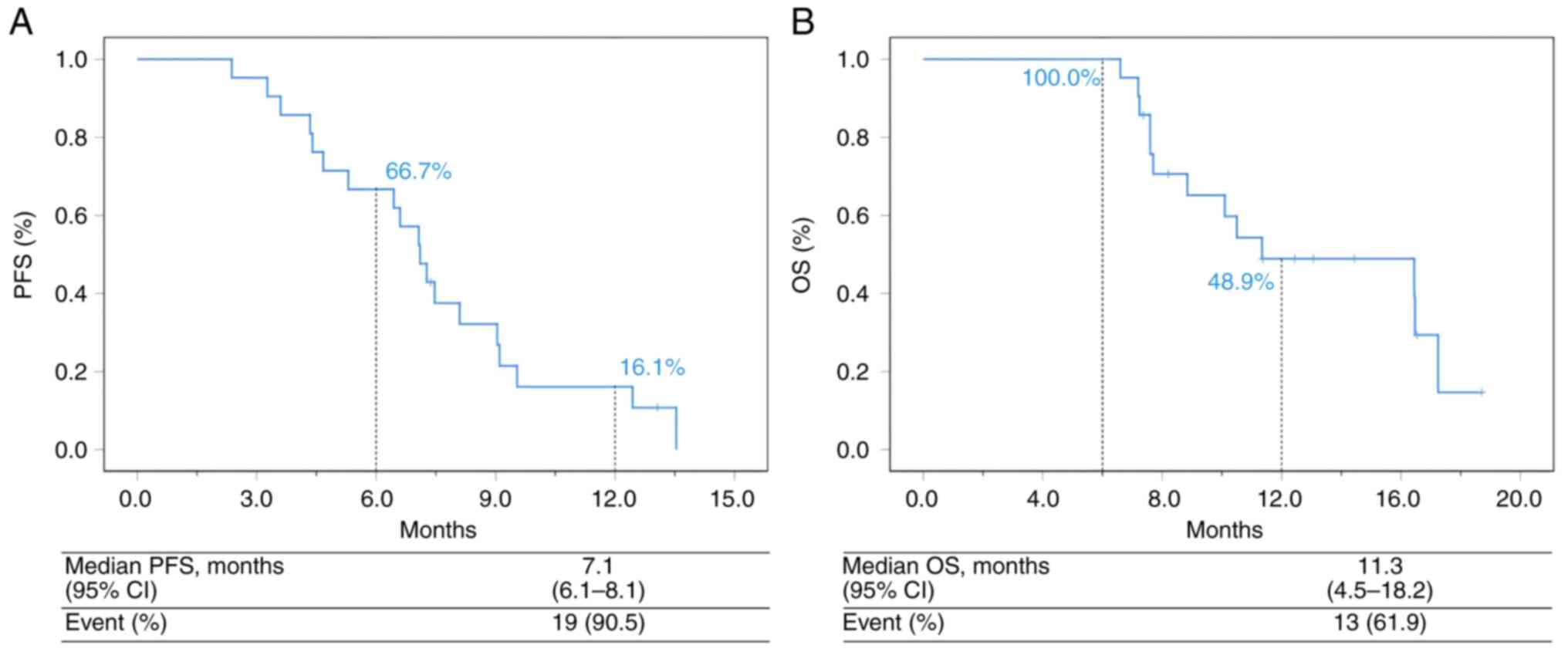 |
Figure 3
Analysis of PFS and OS in the overall population. (A) PFS of the total population. (B) OS of the total population. PFS, progression free survival; OS, overall survival; CI, confidence interval.
|
 |
Table II
Tumor response.
|
Table II
Tumor response.
| Best overall response |
N (%) |
| Complete response |
0 (0.0) |
| Partial response |
2 (9.5) |
| Stable disease |
17 (81.0) |
| Progressive disease |
2 (9.5) |
| Not evaluated |
0 (0.0) |
| Objective response rate |
2 (9.5) |
| Disease control rate |
19 (90.5) |
 |
Table III
Results of univariate Cox proportional-hazards analysis for overall survival for patients with advanced gastric cancer.
|
Table III
Results of univariate Cox proportional-hazards analysis for overall survival for patients with advanced gastric cancer.
| Variables |
Pa |
Hazard ratio |
95% Confidence interval |
| Age |
0.480 |
1.521 |
0.475 |
4.874 |
| Sex |
0.948 |
1.044 |
0.279 |
3.909 |
| World Health Organization Grade |
0.526 |
1.349 |
0.535 |
3.398 |
| T status |
0.943 |
0.957 |
0.291 |
3.150 |
| N status |
0.268 |
1.787 |
0.640 |
4.993 |
| M status |
0.648 |
1.619 |
0.204 |
12.820 |
| Ascitic fluid |
0.469 |
2.173 |
0.266 |
17.746 |
| Liver |
0.724 |
1.325 |
0.278 |
6.311 |
| Only lymph node or soft tissue |
0.375 |
1.712 |
0.522 |
5.611 |
| Peritoneal metastasis |
0.946 |
1.042 |
0.315 |
3.445 |
| Bone metastasis |
0.402 |
0.519 |
0.112 |
2.408 |
| Number of distant metastases |
0.863 |
1.050 |
0.603 |
1.830 |
| Programmed cell death 1 ligand 1 |
0.406 |
1.758 |
0.465 |
6.645 |
| Ki67 |
0.023 |
4.737 |
1.239 |
18.105 |
| Tumor marker |
0.033 |
5.596 |
1.151 |
27.212 |
 |
Table IV
Cox multivariate analyses of prognostic factors on overall survival.
|
Table IV
Cox multivariate analyses of prognostic factors on overall survival.
| Variables |
Pa |
Hazard ratio |
95% Confidence interval |
| Ki67 |
0.011 |
6.884 |
1.545 |
30.677 |
| Tumor marker |
0.017 |
8.642 |
1.482 |
50.406 |
Ki67 ≥70% (Ki67-high) and Ki67 <70% (Ki67-low) were used as stratification factors and significant statistical differences were observed in PFS (mPFS=5.3 months, 95% CI 3.1-7.5 months vs. mPFS=9.1 months, 95% CI 6.2-12.0 months, P=0.005) and OS (mOS=8.8 months, 95% CI 7.0-10.7 months vs. mOS=17.2 months, 95% CI 16.0-18.5 months, P=0.023) between the two groups. The OS rate was 100.0% at 6 months and 78.8% at 12 months in the Ki67-low group (Fig. 4A and B).
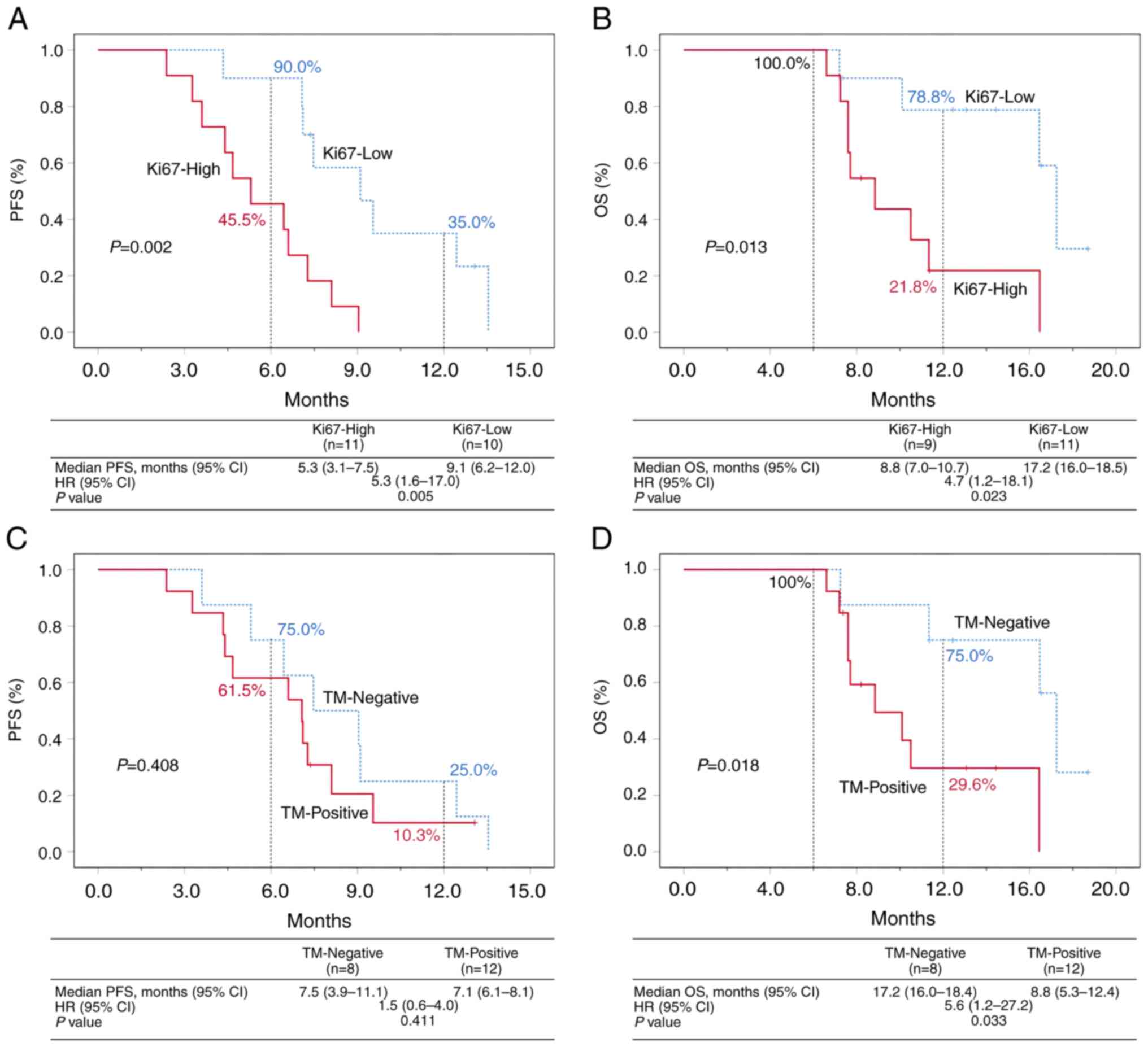 |
Figure 4
Analysis based on Ki67 and tumor marker expression. (A) and (B) PFS and OS of the Ki67-high and Ki67-low groups. (C) and (D) PFS and OS of the TM-negative and TM-positive groups. TM-Negative, CEA, CA199 and CA125 are all in the normal range; TM-Positive, one or two increases among CEA, CA199 and CA125. PFS, progression free survival; OS, overall survival; TM, tumor marker; CEA, carcinoembryogenic antigen; CA, cancer antigen; HR, hazard ratio.
|
The tumor marker-negative (CEA, CA199 and CA125 were all in the normal range) and tumor marker-positive (one or two increases among CEA, CA199 and CA125) were used as stratification factors; a significant difference was noted in OS between the two groups (mOS=17.2 months, 95% CI 16.0-18.4 months vs. mOS=8.8 months, 95% CI 5.3-12.4 months, P=0.018); however, no significant differences were noted in PFS between the two groups (mPFS=7.5 months, 95% CI 3.9-11.1 months vs. mPFS=7.1 months, 95% CI 6.1-8.1 months, P=0.408). The OS rate was 100.0% at 6 months and 75.0% at 12 months in the tumor marker-negative group (Fig. 4C and D).
Safety
All patients (100%; n=21) experienced at least one treatment-related AE (TRAE; Table V). A total of five patients (23.8%) experienced grade 3 or 4 TRAEs, namely neutropenia (14.3%; n=3), thrombocytopenia (9.5%; n=2) and pancreatitis (4.8%; n=1). A serious TRAE (grade 3 immune-related pancreatitis) was observed in one patient (4.8%); pancreatitis resolved following hormonal therapy, whereas immunotherapy was permanently discontinued. No treatment-related deaths occurred.
 |
Table V
AEs.
|
Table V
AEs.
| |
No. (%), n=21 |
| AEs |
All grade |
Grade 1-2 |
Grade 3 |
Grade 4 |
| Hematologic toxicity |
|
|
|
|
| Neutropenia |
10 (47.6) |
7 (33.3) |
2 (14.3) |
1 (4.8) |
| Anemia |
10 (47.6) |
10 (47.6) |
0 (0.0) |
0 (0.0) |
| Thrombocytopenia |
3 (14.3) |
1 (4.8) |
1 (4.8) |
1 (4.8) |
| Non-hematologic toxicity |
|
|
|
|
| Fatigue |
19 (90.5) |
19 (90.5) |
0 (0.0) |
0 (0.0) |
| Anorexia |
13 (61.9) |
13 (61.9) |
0 (0.0) |
0 (0.0) |
| Nausea |
19 (90.5) |
19 (90.5) |
0 (0.0) |
0 (0.0) |
| Vomiting |
9 (42.9) |
9 (42.9) |
0 (0.0) |
0 (0.0) |
| Hypertension |
6 (28.6) |
6 (28.6) |
0 (0.0) |
0 (0.0) |
| TSH increased |
9 (42.9) |
9 (42.9) |
0 (0.0) |
0 (0.0) |
| Hypothyroidism |
4 (19.0) |
4 (19.0) |
0 (0.0) |
0 (0.0) |
| Alanine transaminase/aspartate aminotransferase increased |
4 (19.0) |
4 (19.0) |
0 (0.0) |
0 (0.0) |
| Palmar-plantar erythrodysesthesia |
9 (42.9) |
9 (42.9) |
0 (0.0) |
0 (0.0) |
| Proteinuria |
2 (9.5) |
2 (9.5) |
0 (0.0) |
0 (0.0) |
| Pancreatitis |
1 (4.8) |
0 (0.0) |
1 (4.8) |
0 (0.0) |
| Erythra |
1 (4.8) |
1 (4.8) |
0 (0.0) |
0 (0.0) |
| Hepatitis |
1 (4.8) |
1 (4.8) |
0 (0.0) |
0 (0.0) |
| Potential immune-related AEs |
|
|
|
|
| Pancreatitis |
1 (4.8) |
0 (0.0) |
1 (4.8) |
0 (0.0) |
| Erythra |
1 (4.8) |
1 (4.8) |
0 (0.0) |
0 (0.0) |
| Thyroid stimulating hormone increased |
9 (42.9) |
9 (42.9) |
0 (0.0) |
0 (0.0) |
| Hypothyroidism |
3 (14.3) |
3 (14.3) |
0 (0.0) |
0 (0.0) |
| Hepatitis |
1 (4.8) |
1 (4.8) |
0 (0.0) |
0 (0.0) |
Potential immune-related AEs included increased thyroid-stimulating hormone (42.9%; n=9), as well as hypothyroidism (14.3%; n=3), hepatitis (4.8%; n=1) and erythra (4.8%; n=1).
Discussion
Currently, the second-line regimens recommended by guidelines for patients with HER2-negative GC are considered ineffective, and the use of PD-1 inhibitors is subject to stringent criteria. Previous studies exploring second-line treatments for advanced GC, such as KEYNOTE-061, have investigated the efficacy of pembrolizumab. In the present study, when PD-L1 Combined Positive Score (CPS) was ≥1, pembrolizumab did not demonstrate statistically significant improvements in PFS, OS, or ORR compared with paclitaxel. Statistically significant improvements in OS were observed only in tumors with PD-L1 CPS ≥10 or microsatellite instability-high (MSI-H) (6).
The study Checkmate-032 evaluated the effectiveness of nivolumab, either alone or in combination with ipilimumab, as second-line and subsequent therapy for advanced GC. However, the present study did not yield ideal outcomes with regard to OS.
While previous clinical trials have not revealed significant benefits of immune monotherapy across the entire population of patients with HER2-negative GC, there is potential for exploration in combining immunotherapy with chemotherapy or targeted therapies (7).
To further explore the use of chemotherapeutic drugs that can be effectively co-administered with immunotherapy, previous research studies have highlighted the widespread consideration among clinical trials that platinum-based chemotherapies and PD-1 inhibitors exhibit a synergistic effect. As a result, tumor treatment guidelines for GC, esophageal cancer, lung cancer and other malignancies recommend the combination of immunotherapy with platinum-based agents (8-11).
In addition, the combination of paclitaxel and immunotherapy has demonstrated promising outcomes. Notably, recent Chinese clinical trials, such as the ESCORT-1st study, have demonstrated that the use of the PD-1 inhibitor camrelizumab in conjunction with paclitaxel and cisplatin significantly improves PFS and OS rates in patients with advanced esophageal squamous cell carcinoma compared with standard first-line chemotherapy. This combination achieved an impressive response rate of 72.1% (12).
The present study selected a chemotherapy regimen (paclitaxel combined with cisplatin) that exhibited a superior synergistic effect with immunotherapy, rather than the more commonly used 5-fluorouracil and cisplatin combination. These findings have important implications for guiding clinical practice in the treatment of esophageal cancer in Chinese patients.
For HER2-negative patients with GC, if the first-line treatment with platinum and fluorouracil has progressed, the second-line treatment options are still limited to taxanes, such as paclitaxel, albumin paclitaxel, and docetaxel, or irinotecan. The mPFS data for single-agent taxanes were reported to be slightly improved compared with those for irinotecan (3.6 vs. 2.3 months) (13,14). The only double-agent treatment used was ramucirumab combined with paclitaxel; however, its mPFS only reached 4.14 months, which was slightly higher than the 3.15 months noted for paclitaxel (15). According to the NCCN guidelines, the use of immune checkpoint inhibitors as monotherapy is only recommended for patients with MSI-H/deficient mismatch repair. For the second-line treatment of HER2-negative GC, no mature data have been reported for the combination of chemotherapy and immunotherapy to date. In a phase II clinical trial (NCT04982276) reported at the 2024 ASCO conference, the combination of cadonilimab with pulocimab and paclitaxel as a second-line treatment for patients with advanced gastric or gastroesophageal junction cancer indicated encouraging efficacy and controllable safety. The mPFS values for the two groups (cadonilimab + pulocimab + paclitaxel vs. pulocimab + paclitaxel) were 6.8 months (95% CI, 4.1-11.2) and 4.9 months (95% CI, 3.2-7.1), respectively (16). Irinotecan monotherapy is not as effective as taxanes and there is no clear report on its ability to enhance efficacy in tumor treatment when combined with immunotherapy. The application of platinum-based drugs on second-line patients with HER2 negative GC who have already used them in the first line results in the selection of the combination of taxanes and immune checkpoint inhibitors as a second-line treatment for GC.
Therefore, the present study conducted a retrospective analysis of patients with HER2-negative GC who used taxanes combined with immunotherapy in the second line therapy; their mPFS reached 7.1 months, which was approximately twice as long as that of taxanes. The most common adverse reactions of the combination of chemotherapy and immunotherapy were safe and controllable and there was only one case of serious adverse reactions leading to discontinuation of medication. The present study suggested that a longer OS may be related to normal levels of tumor markers (the levels of CEA, CA199 and CA125 were all in the normal range) and PFS did not reach a statistically significant difference, which may be related to the limited sample size. This is consistent with certain previous studies, which reported that patients with locally advanced GC and normal CEA/CA19-9 ratio may be associated with longer survival (17). In addition, for patients with advanced cancer stage who only underwent palliative resection of metastatic foci, as well as patients with GC who underwent neoadjuvant chemotherapy and surgery or direct radical gastrectomy, the normalization of the levels of CEA or CA19-9 in the early postoperative period was a strong prognostic factor for GC, notably in patients with high levels of tumor markers prior to surgery (17-19). Moreover, an increase in Ki67 levels also indicated that it was an independent prognostic factor for PFS and OS in the second-line treatment of HER2-negative GC. Following grouping, it was suggested that a significant statistical difference was noted in PFS and OS between the two groups. A lower number of studies have explored the association of Ki67 in GC, and it has been reported that a decrease in Ki67 expression is an independent predictor of recurrence-free survival in patients with GC and peritoneal metastasis following induction of chemotherapy (20). With regard to the immunotherapy, a previous study suggested that CPS ≥1/CPS ≥5/CPS ≥10 was significantly and independently related to the Ki67 index, which was explored as a potential biomarker for GC anti-PD-1 treatment (21). The results also revealed that the Ki67 index exhibited a certain predictive significance for the prognosis of chemotherapy combined with immunotherapy.
In the present study, the PD-1 inhibitors including nivolumab, pembrolizumab and camrelizumab have not yet obtained the second-line indication for HER2-negative GC (22-24) and their application is limited. The current retrospective analysis provides another possibility for the effectiveness of the scheme.
The swift advancement of immunotherapy has enabled a significant leap in the quality of cancer treatment outcomes. Existing and ongoing clinical studies have demonstrated that by leveraging additional molecular markers to identify suitable patients, the combination of immunotherapy with other therapeutic approaches holds great promise. This integrated strategy is expected to yield superior benefits and improve clinical outcomes for a broader range of patients with cancer.
However, the small sample size of the present study necessitates further supplementation with additional clinical cases to fully validate the conclusions drawn. Early patient data lacked CPS and other genetic testing results, introducing limitations in data grouping. These limitations will be addressed in subsequent data collection efforts. The combination of immunotherapy and taxanes has shown significant improvements in mPFS (increase to 7.1 months) and mOS (increase to 11.3 months) for second-line treatment in patients with advanced HER2-negative GC. Notably, these findings are yet to be incorporated into clinical guidelines. In addition, the expression levels of Ki67 and the status of tumor markers, such as CEA, CA19-9, and CA125 have emerged as important prognostic factors for these patients.
Acknowledgements
The authors wish to thank Dr Xiaobo Peng (Institute for Department of Oncology, Shanghai Changhai Hospital, Naval Medical University, Shanghai, China) for advice on experimental design.
Funding
Funding: The present study was supported by the National Natural Science Foundation of China (grant no. 82072707), the basic research project of Naval Medical University (grant no. 2023MS023), the basic research project of Naval Medical University (grant no. 2022MS011), the Changhai Hospital CHANGHONG Project and Changhai Hospital GUHAI Project (grant no. 2022009592) and the Teaching Achievement Cultivation Project of Naval Medical University (grant no. CHPY2021B06). 2020 Shanghai Municipal Health Commission Health Industry Clinical Research Special Project, General Project. Project Number: 202040412.
Availability of data and materials
The data generated in the present study are not publicly available due to data access and ownership compliance regulations but may be requested from the corresponding author.
Authors' contributions
MW, YZ, XZ and MY contributed to the study conception and design. TC, JX and MX acquired materials, and collected and analyzed data. All authors substantially contributed to critically reviewing the manuscript for important intellectual content. XZ and MW confirm the authenticity of all the raw data. TC prepared the first draft of the manuscript and all authors commented on previous versions of the manuscript. All authors read and approved the final version of the manuscript.
Ethics approval and consent to participate
The present study was approved (approval no. KTSB20240716031) by the Ethics Committee of Shanghai Changhai Hospital, Naval Medical University (Shanghai, China). Patient informed consent was was waived due to the retrospective nature of the study.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
|
1
|
Bray F, Laversanne M, Sung H, Ferlay J, Siegel RL, Soerjomataram I and Jemal A: Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 74:229–263. 2024.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Allemani C, Matsuda T, Di Carlo V, Harewood R, Matz M, Nikšić M, Bonaventure A, Valkov M, Johnson CJ, Estève J, et al: Global surveillance of trends in cancer survival 2000-14 (CONCORD-3): Analysis of individual records for 37 513 025 patients diagnosed with one of 18 cancers from 322 population-based registries in 71 countries. Lancet. 391:1023–1075. 2018.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Kang YK, Boku N, Satoh T, Ryu MH, Chao Y, Kato K, Chung HC, Chen JS, Muro K, Kang WK, et al: Nivolumab in patients with advanced gastric or gastro-oesophageal junction cancer refractory to, or intolerant of, at least two previous chemotherapy regimens (ONO-4538-12, ATTRACTION-2): A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 390:2461–2471. 2017.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Janjigian YY, Shitara K, Moehler M, Garrido M, Salman P, Shen L, Wyrwicz L, Yamaguchi K, Skoczylas T, Bragagnoli AC, et al: First-line nivolumab plus chemotherapy versus chemotherapy alone for advanced gastric, gastro-oesophageal junction, and oesophageal adenocarcinoma (CheckMate 649): A randomised, open-label, phase 3 trial. Lancet. 398:27–40. 2021.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Xu J, Jiang H, Pan Y, Gu K, Cang S, Han L, Shu Y, Li J, Zhao J, Pan H, et al: Sintilimab plus chemotherapy for unresectable gastric or gastroesophageal junction cancer: The ORIENT-16 randomized clinical trial. JAMA. 330:2064–2074. 2023.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Liang F: The KEYNOTE-061 trial. Lancet. 393(1098)2019.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Janjigian YY, Bendell J, Calvo E, Kim JW, Ascierto PA, Sharma P, Ott PA, Peltola K, Jaeger D, Evans J, et al: CheckMate-032 study: Efficacy and safety of nivolumab and nivolumab plus ipilimumab in patients with metastatic esophagogastric cancer. J Clin Oncol. 36:2836–2844. 2018.PubMed/NCBI View Article : Google Scholar
|
|
8
|
An M, Mehta A, Min BH, Heo YJ, Wright SJ, Parikh M, Bi L, Lee H, Kim TJ, Lee SY, et al: Early immune remodeling steers clinical response to first-line chemoimmunotherapy in advanced gastric cancer. Cancer Discov. 14:766–785. 2024.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Janjigian YY, Kawazoe A, Bai Y, Xu J, Lonardi S, Metges JP, Yanez P, Wyrwicz LS, Shen L, Ostapenko Y, et al: Pembrolizumab plus trastuzumab and chemotherapy for HER2-positive gastric or gastro-oesophageal junction adenocarcinoma: Interim analyses from the phase 3 KEYNOTE-811 randomised placebo-controlled trial. Lancet. 402:2197–2208. 2023.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Xu J, Kato K, Raymond E, Hubner RA, Shu Y, Pan Y, Park SR, Ping L, Jiang Y, Zhang J, et al: Tislelizumab plus chemotherapy versus placebo plus chemotherapy as first-line treatment for advanced or metastatic oesophageal squamous cell carcinoma (RATIONALE-306): A global, randomised, placebo-controlled, phase 3 study. Lancet Oncol. 24:483–495. 2023.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Reck M, Rodríguez-Abreu D, Robinson AG, Hui R, Csőszi T, Fülöp A, Gottfried M, Peled N, Tafreshi A, Cuffe S, et al: Pembrolizumab versus chemotherapy for PD-L1-positive non-small-cell lung cancer. N Engl J Med. 375:1823–1833. 2016.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Luo H, Lu J, Bai Y, Mao T, Wang J, Fan Q, Zhang Y, Zhao K, Chen Z, Gao S, et al: Effect of camrelizumab vs placebo added to chemotherapy on survival and progression-free survival in patients with advanced or metastatic esophageal squamous cell carcinoma: The ESCORT-1st randomized clinical trial. JAMA. 326:916–925. 2021.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Hironaka S, Ueda S, Yasui H, Nishina T, Tsuda M, Tsumura T, Sugimoto N, Shimodaira H, Tokunaga S, Moriwaki T, et al: Randomized, open-label, phase III study comparing irinotecan with paclitaxel in patients with advanced gastric cancer without severe peritoneal metastasis after failure of prior combination chemotherapy using fluoropyrimidine plus platinum: WJOG 4007 trial. J Clin Oncol. 31:4438–4444. 2013.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Hawkes E, Okines AF, Papamichael D, Rao S, Ashley S, Charalambous H, Koukouma A, Chau I and Cunningham D: Docetaxel and irinotecan as second-line therapy for advanced oesophagogastric cancer. Eur J Cancer. 47:1146–1151. 2011.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Xu RH, Zhang Y, Pan H, Feng J, Zhang T, Liu T, Qin Y, Qin S, Yin X, Liu B, et al: Efficacy and safety of weekly paclitaxel with or without ramucirumab as second-line therapy for the treatment of advanced gastric or gastroesophageal junction adenocarcinoma (RAINBOW-Asia): A randomised, multicentre, double-blind, phase 3 trial. Lancet Gastroenterol Hepatol. 6:1015–1024. 2021.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Zhang X, Wang Y, Xiang X, Pan H, Zhang J, Chen X, Ba Y, Jieer Y, He Y, Yin X, et al: Efficacy and safety of cadonilimab in combination with pulocimab and paclitaxel as second-line therapy in patients with advanced gastric or gastroesophageal junction (G/GEJ) cancer who failed immunochemotherapy: A multicenter, double-blind, randomized trial. Journal of Clinical Oncology, 2024ASCO (abstract 4012), 2024. https://ascopubs.org/doi/10.1200/JCO.2024.42.16_suppl.4012.
|
|
17
|
Tang XH, Wu XL, Gan XJ, Wang YD, Jia FZ, Wang YX, Zhang Y, Gao XY and Li ZY: Using normalized carcinoembryonic antigen and carbohydrate antigen 19 to predict and monitor the efficacy of neoadjuvant chemotherapy in locally advanced gastric cancer. Int J Mol Sci. 24(12192)2023.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Kawahara K, Makino H, Kametaka H, Hoshino I, Fukada T, Seike K, Kawasaki Y and Otsuka M: Outcomes of surgical resection for gastric cancer liver metastases: A retrospective analysis. World J Surg Oncol. 18(41)2020.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Nam DH, Lee YK, Park JC, Lee H, Shin SK, Lee SK, Lee YC, Cheong JH, Hyung WJ, Noh SH and Kim CB: Prognostic value of early postoperative tumor marker response in gastric cancer. Ann Surg Oncol. 20:3905–3911. 2013.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Torun BC, Sobutay E, Akbulut OE, Saglam S, Yilmaz S, Yonemura Y and Canbay E: Important predictive factors for the prognosis of patients with peritoneal metastasis of gastric cancer. Ann Surg Oncol. 31:5975–5983. 2024.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Chen X, Zhang H, Wang M, Liu H, Hu Y, Lin T, Chen H, Zhao M, Chen T, Li G, et al: relationship between programmed death ligand 1 expression and other clinicopathological features in a large cohort of gastric cancer patients. Front Immunol. 13(783695)2022.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Maio M, Ascierto PA, Manzyuk L, Motola-Kuba D, Penel N, Cassier PA, Bariani GM, De Jesus Acosta A, Doi T, Longo F, et al: Pembrolizumab in microsatellite instability high or mismatch repair deficient cancers: Updated analysis from the phase II KEYNOTE-158 study. Ann Oncol. 33:929–938. 2022.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Xu J, Zhang Y, Jia R, Yue C, Chang L, Liu R, Zhang G, Zhao C, Zhang Y, Chen C, et al: Anti-PD-1 antibody SHR-1210 combined with apatinib for advanced hepatocellular carcinoma, gastric, or esophagogastric junction cancer: An open-label, dose escalation and expansion study. Clin Cancer Res. 25:515–523. 2019.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Smyth EC, Nilsson M, Grabsch HI, van Grieken NC and Lordick F: Gastric cancer. Lancet. 396:635–648. 2020.PubMed/NCBI View Article : Google Scholar
|


















