Introduction
Recently, single nucleotide polymorphisms (SNPs)
have begun to be considered as potential biomarkers to select the
appropriate opioid for use in patients with cancer pain. A
CYP2D6 SNP (rs1065852) has been shown to affect the
metabolism of several opioids (e.g., codeine and tramadol) and been
proposed as a predictive biomarker for selection of the most
suitable opioid for treating cancer pain (1-3).
The µ-opioid receptor gene (OPRM1) has a SNP that could
allow individualization of pain treatment based on the predicted
response. For G-allele carriers of this SNP, tapentadol and
methadone may be more suitable than hydromorphone, oxycodone or
fentanyl (4). In Japan, morphine
and oxycodone are the most frequently used opioids, although there
is still a lack of consensus on which of the two would be the
better choice in individual patients (5). Since the sensitivity to and side
effects of opioids vary widely among patients, we also attempted to
identify SNPs of genes that could potentially predict differences
in the responses of patients to morphine and oxycodone. To identify
the most appropriate biomarker SNP(s) for predicting the efficacy
of each opioid, we conducted a randomized controlled trial, the
RELIEF study (Trial registration number: UMIN000015579; date of
registration: November 4, 2014, patients recruited: November 2014
to February 2020 in Kindai University Hospital, Osaka, Japan.), in
which we randomized a total of 138 patients (1:1) to receive either
morphine (Group M) or oxycodone (Group O), based on the COMT
rs4680 SNP as a biomarker; we identified several candidate SNPs in
this trial from among the SNPs that have previously been suggested
as possibly being linked to pain sensitivity and/or opioid efficacy
(6-8).
Based on further screening, we identified a SNP, CCL11
rs17809012, as having the potential to predict the response to
morphine (6). We assessed the
analgesic response in each patient on a numerical rating scale
(NRS) for pain. The ∆NRS, defined as the difference between the NRS
score recorded before the start of opioid treatment and that
recorded after opioid dose titration, was smaller (namely, the
degree of pain relief was smaller) in patients with the
CCL11 rs17809012 AA genotype treated with morphine [least
square mean (LSM) for ∆NRS, 2.33] as compared with that in the
AA+oxycodone (LSM 3.48), AG/GG+morphine (LSM 3.58), and
AG/GG+oxycodone (LSM 3.16) groups (6). These results suggest that the
CCL11 rs17809012 SNP could be a predictive biomarker for the
effect of morphine.
In regard to the key mechanisms underlying chronic
pain, it has come to be increasingly accepted that chemokines (such
as CCL11) and cytokines serve as major mediators that activate
glial cell interactions with neurons (9,10).
Therefore, we sought to explore additional biomarkers, besides the
CCL11 rs17809012 SNP, from among the 39 chemokines/cytokines
included as analytes in the Bio-Plex Pro Human Chemokine assay kit
used by us. In this study, we measured the plasma concentrations of
these 39 chemokines/cytokines in pre-treatment plasma samples
collected from a total of 138 patients enrolled in the RELIEF
study, and found that one cytokine, IL-16, showed a bias in plasma
concentrations between patients who responded well and responded
poorly to oxycodone. Moreover, genetic analysis also showed that
rs4778889 SNP genotype residing in the IL-16 gene may allow
discrimination between patients who responded well and responded
poorly to oxycodone. Based on these findings of our current and
previous studies, we propose that the dual-biomarker combination of
CCL11 rs17809012 and IL-16 rs4778889 SNPs could be
useful to accurately guide selection of the more appropriate opioid
between morphine and oxycodone in individual patients with cancer
pain.
Patients and methods
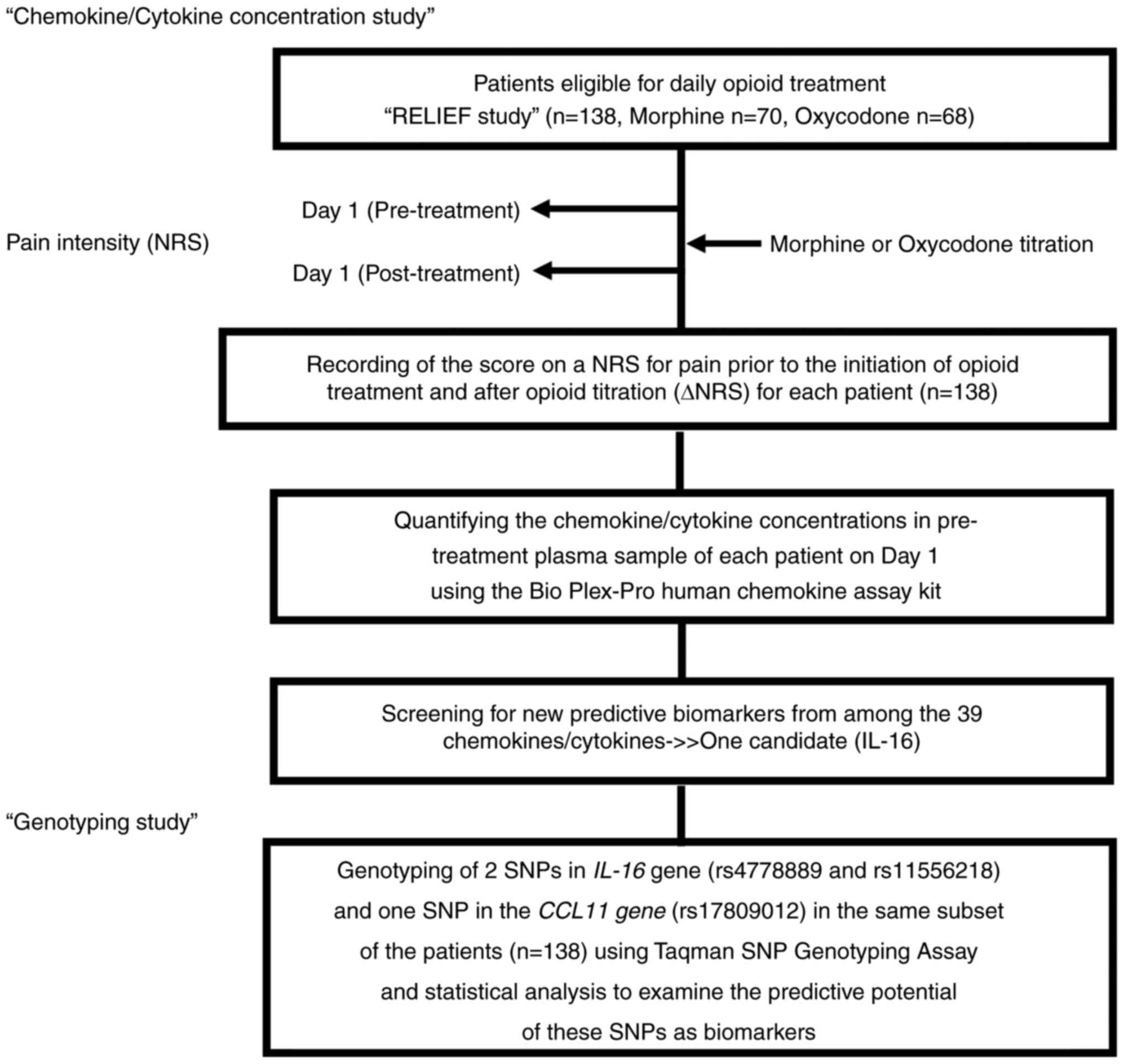
Patients and samples
We enrolled a total of 138 patients with advanced
malignancies based on our eligibility criteria in the RELIEF study,
a randomized controlled trial recently conducted by us (Trial
registration number: UMIN000015579) (8). Our cohort did not include any
subjects whose families could influence the genotype independency.
In the present study, we measured the plasma concentrations of
chemokines/cytokines in pre-treatment plasma samples of the study
subjects and performed genotyping of the IL-16 SNPs in their
DNA samples. The 138 patients who fulfilled the eligibility
criteria of suffering from cancer pain that necessitated daily
treatment with opioids were randomized to either morphine (Group M;
n=70) or oxycodone (Group O; n=68) (Fig. 1).
Calculation of the optimal study sample size and the
inclusion and exclusion criteria have been described in our
previous trial report (8). The
baseline characteristics of the 138 patients are presented in
Table I.
 | Table IBaseline characteristics of the
patients. |
Table I
Baseline characteristics of the
patients.
| | Morphine group
(n=70) | Oxycodone group
(n=68) |
|---|
| Required dose | Number of
patients | Low (n=41) | High (n=29) | P-value | Low (n=53) | High (n=15) | P-value |
|---|
| Age (years), n
(%)a | | | | 0.052 | | | 1 |
|
<70 | 62 | 23 (56.1) | 9 (31.0) | | 23 (43.4) | 7 (46.7) | |
|
≥70 | 76 | 18 (43.9) | 20 (69.0) | | 30 (56.6) | 8 (53.3) | |
| Sex, n
(%)a | | | | 0.225 | | | 0.382 |
|
Males | 79 | 22 (53.7) | 20 (69.0) | | 27 (50.9) | 10 (66.7) | |
|
Females | 59 | 19 (46.3) | 9 (31.0) | | 26 (49.1) | 5 (33.3) | |
| Performance status,
n (%)a,c | | | | 1 | | | 1 |
|
0 | 10 | 3 (7.3) | 0 (0) | | 3 (5.7) | 4 (26.7) | |
|
1 | 80 | 23 (56.1) | 18 (62.1) | | 31 (58.5) | 6 (40.0) | |
|
2 | 35 | 11 (26.8) | 7 (24.1) | | 15 (28.3) | 3 (20.0) | |
|
3 | 10 | 3 (7.3) | 3 (10.3) | | 2 (3.8) | 2 (13.3) | |
|
4 | 4 | 1 (2.4) | 1 (3.5) | | 2 (3.8) | 0 (0) | |
| Pre-NRS score, mean
(SD)b | | 4.9 (2.0) | 5.9 (2.1) | 0.031 | 5.0 (1.9) | 7.0 (1.6) | 0.0006 |
| HADS score, mean
(SD)b | | 15.3 (7.5) | 16.6 (7.9) | 0.5 | 14.5 (7.3) | 15.5 (6.9) | 0.641 |
| SF-MPQ-2 score,
mean (SD)b | | 38.9 (28.5) | 44.2 (31.2) | 0.459 | 52.1 (40.2) | 75.1 (36.4) | 0.055 |
Opioid administration and dose
titration
The opioid-naïve patients were started on treatment
with an intermediate-release (IR) opioid, according to the
guidelines for opioid use and titration (NCCN Guidelines™, Adult
Cancer Pain) (11,12), by specialized palliative care
physicians. Opioid titration on day 1 following onset of cancer
pain has been described in detail in a previous report (6). In brief, the minimum standard
starting dose of an IR opioid, that is, 5 mg for morphine and 2.5
mg for oxycodone, is administered repeatedly to the patients until
a decrease of the score on an NRS for pain (0=no pain to 10=maximal
pain) by ≥33% or by ≤3 is recorded post-titration (day 1) as
compared with the score recorded prior to the start of opioid
treatment (6). Classification of
the patients according to the required opioid dose (high-dose
group/low-dose group) for each opioid was as defined previously
(6,8); namely, patients requiring ≥10 mg of
IR morphine, or ≥7.5 mg of IR oxycodone were classified into the
high-dose group, while those requiring 5 mg of IR morphine or 5 mg
or less of IR oxycodone were classified into the low-dose
group.
Measurement of the plasma
chemokine/cytokine concentrations
Plasma samples of the patients were collected on day
1 prior to the start of treatment (pre-treatment samples) using a
Venoject II vacuum blood-collecting tube (Terumo). The blood
samples were centrifuged for 10 min at 1,200 g, and the
supernatants (pre-treatment plasma samples) were frozen immediately
and stored at -80˚C until use. The concentrations of the 39
chemokine/cytokines listed in Table
II were measured in the pre-treatment plasma samples of the
patients using a BioPlex 200 System (Bio-Rad Laboratories), in
accordance with the manufacturer's protocols. Levels of one of the
cytokines (GM-CSF) included as an analyte in the kit were omitted
from the analysis, because only 29 out of the 138 patients had
detectable amounts of this cytokine in the plasma.
 | Table IIChemokines/cytokines measured for
plasma concentrations. |
Table II
Chemokines/cytokines measured for
plasma concentrations.
| Items | Non-abbreviated
form | Median
concentrationa |
|---|
| CCL21 | C-C Motif Chemokine
Ligand 21 | 3426.4 |
| CXCL13 | C-X-C Motif
Chemokine Ligand 13 | 38.2 |
| CCL27 | C-C Motif Chemokine
Ligand 27 | 950.2 |
| CXCL5 | C-X-C Motif
Chemokine Ligand 5 | 1048.1 |
| CCL11 | C-C Motif Chemokine
Ligand 11 | 95.1 |
| CCL24 | C-C Motif Chemokine
Ligand 24 | 68.6 |
| CCL26 | C-C Motif Chemokine
Ligand 26 | 15.0 |
| CX3CL1 | C-X3-C Motif
Chemokine Ligand 1 | 154.6 |
| CXCL6 | C-X-C Motif
Chemokine Ligand 6 | 55.8 |
| CXCL1 | C-X-C Motif
Chemokine Ligand 1 | 186.7 |
| CXCL2 | C-X-C Motif
Chemokine Ligand 2 | 291.4 |
| CCL1 | C-C Motif Chemokine
Ligand 1 | 58.3 |
| IFN-γ |
Interferon-gamma | 2.4 |
| IL-1β | Interleukin-1
beta | 16.3 |
| IL-2 | Interleukin-2 | 10.1 |
| IL-4 | Interleukin-4 | 15.9 |
| IL-6 | Interleukin-6 | 21.8 |
| IL-8 | Interleukin-8 | 29.7 |
| IL-10 | Interleukin-10 | 25.7 |
| IL-16 | Interleukin-16 | 323.2 |
| CXCL10 | C-X-C Motif
Chemokine Ligand 10 | 72.0 |
| CXCL11 | C-X-C Motif
Chemokine Ligand 11 | 343.9 |
| CCL2 | C-C Motif Chemokine
Ligand 2 | 24.3 |
| CCL8 | C-C Motif Chemokine
Ligand 8 | 22.1 |
| CCL7 | C-C Motif Chemokine
Ligand 7 | 71.9 |
| CCL13 | C-C Motif Chemokine
Ligand 13 | 27.0 |
| CCL22 | C-C Motif Chemokine
Ligand 22 | 441.0 |
| MIF | Macrophage
Migration Inhibitory Factor | 689.9 |
| CXCL9 | C-X-C Motif
Chemokine Ligand 9 | 410.0 |
| CCL3 | C-C Motif Chemokine
Ligand 3 | 7.3 |
| CCL15 | C-C Motif Chemokine
Ligand 15 | 3458.4 |
| CCL20 | C-C Motif Chemokine
Ligand 20 | 17.4 |
| CCL19 | C-C Motif Chemokine
Ligand 19 | 109.8 |
| CCL23 | C-C Motif Chemokine
Ligand 23 | 285.4 |
| CXCL16 | C-X-C Motif
Chemokine Ligand 16 | 424.2 |
| CXCL12 | C-X-C Motif
Chemokine Ligand 12 | 607.7 |
| CCL17 | C-C Motif Chemokine
Ligand 17 | 88.7 |
| CCL25 | C-C Motif Chemokine
Ligand 25 | 435.1 |
| TNF-α | Tumor Necrosis
Factor-alpha | 23.2 |
Genotyping
Genomic DNA was isolated from the blood samples, as
described previously (13).
Genotyping was performed for 2 SNPs (rs4778889 and rs11556218) of
the IL-16 gene (Interleukin 16, Gene ID: 3603) and a SNP
(rs17809012) of the CCL11 gene (C-C Motif Chemokine 11, Gene
ID: 6356) using a PCR-based Taqman SNP Genotyping Assay, in
accordance with the manufacturer's instructions (Thermo Fisher
Scientific, Inc.).
Statistical analysis
The differences in the required dose (high or low)
were estimated for each opioid using Fisher's exact test for
categorical variables or Mann-Whitney U test for ordinal data
(Table I). To screen for
chemokines/cytokines relevant to the effects of the opioids,
patients were divided into high- and low-concentration groups for
each analyte according to its plasma concentration using the cutoff
value that had been defined as the median concentrations for all
patients (Table II). The ∆NRS,
defined as the difference in the score on an NRS for pain
(hereinafter, NRS score) before the start of opioid treatment on
day 1 and after opioid titration (day 1), was used as the dependent
variable for comparing between the high- and low-concentration
groups for each analyte using simple regression analyses. In
addition to the analytes, the independent variables considered were
the age (<70/≥70 years), sex, performance status score (1/≥2),
pre-NRS score (1-10),
total score on the HADS (Hospital Anxiety and Depression Scale)
(14), total score on the SF-MPQ-2
(Short-Form McGill Pain Questionnaire-2) (7), and the required drug dose (high or
low), among which the pre-NRS, HADS, and SF-MPQ-2 scores were
ordinal variables.
For the analysis in the genotypic study of the SNPs,
we characterized the SNPs of CCL11 rs17809012, IL-16
rs4778889 and IL-16 rs11556218 by performing simple
regression analyses separately for Group M and Group O. As the
CCL11 rs17809012 SNPs had already been characterized for 135
patients in our previous study (6), we additionally analyzed this SNP for
the 3 remaining patients in this study. ∆NRS was set as the
dependent variable.
We also analyzed the three SNPs (CCL11
rs17809012, IL-16 rs4778889 and IL-16 rs11556218) in
the entire subject population (n=138), adding ‘treatment (morphine
or oxycodone)’ as the independent variable in place of ‘dose’,
which was omitted due to the incompatible dosage forms between the
two opioids (8). We analyzed data
from the entire subject population by a simple regression analysis
and a multiple regression analysis with adjustments for confounding
variables. The variance inflation factor (VIF) was used to diagnose
problems of multicollinearity. P<0.05 was set as denoting
statistical significance. The analyses were performed using the JMP
statistical software (v14.2; SAS Institute).
Results
Screening for chemokines/cytokines
with predictive potential for opioid effect
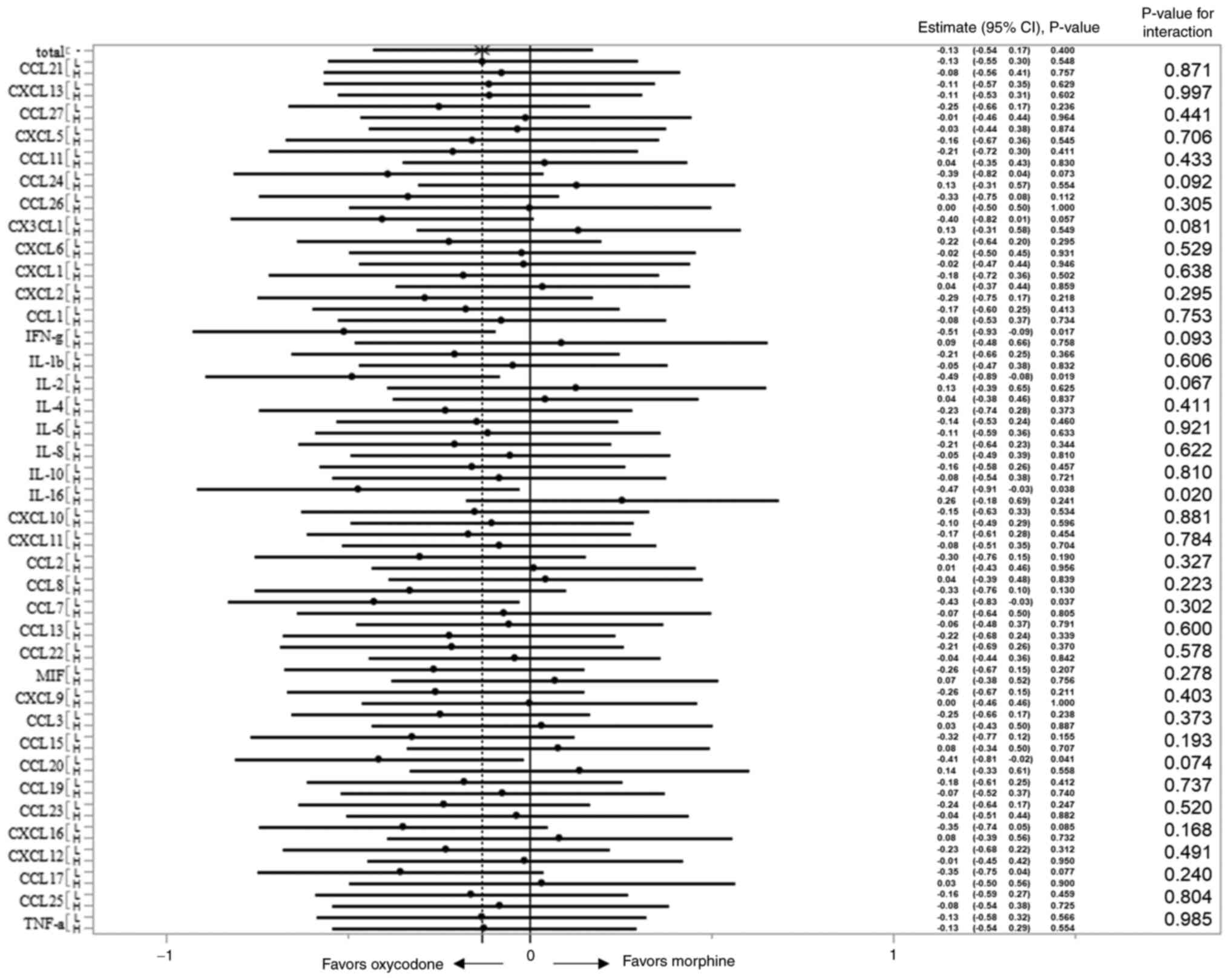
Out of the 39 cytokines/chemokines, our simple
regression analyses identified one candidate predictive factor,
IL-16, as a cytokine whose plasma concentrations were significantly
correlated with the effect of oxycodone (Table SI).
We also examined the concentration-treatment
interactions for the ∆NRS. A forest plot was constructed based on
the estimate (relative risk) with its 95% CI in the 2 concentration
groups (low and high) for each analyte (Fig. 2). Better efficacy with oxycodone
was observed in patients with plasma IL-16 concentrations in the
lower half of the concentration range, while morphine was more
effective in patients with IL-16 concentrations in the upper half
of the concentration range (P value for interaction=0.02).
Genotyping study
Next, we focused on the SNPs residing in the
IL-16 gene. We selected the rs4778889 and rs11556218 SNPs,
which have been identified previously as being functional (15-18).
As suggested before (19), these
two SNPs were found to be closely related. Patients with the major
genotype of rs4777889 (TT) exclusively showed the major genotype of
rs11556218 (TT). Meanwhile, patients with the minor allele (C) of
rs4777889 had either the major allele (T) or minor allele (G) of
rs11556218 (Table SII). These
results suggest that the minor allele in rs11556218 emerged in an
IL-16 gene with the minor nucleotide (C) in rs4777889 that
is more ancestral, and linkage disequilibrium was evident between
the two SNPs loci (~9.3 kbp) in our cohort (d'=0.999).
We first confirmed if these SNPs were linked to the
analgesic effect of oxycodone, as the analgesic effect of oxycodone
differed between patients with higher and lower plasma
concentrations of IL-16 (Table
SI), and the expression levels of the IL-16 gene could
be modulated by these IL-16 SNPs. A simple regression
analysis for Group O showed that the IL-16 rs4777889 and
IL-16 rs11556218 genotypes were correlated with the ∆NRS.
The ∆NRS values in the patients who were homozygous for the major
allele (IL-16 rs4777889 TT and IL-16 rs11556218 TT)
were significantly lower by 0.45 and 0.44, respectively, on
average, as compared with the ∆NRS values in patients who were
carrying the minor alleles (IL-16 rs4777889 TC/CC and
IL-16 rs11556218 TG/GG; P=0.027 and 0.034, respectively)
(Table III). In contrast, for
patients of Group M, the ∆NRS value was lower by 0.56 in the
patients who were homozygous for the major allele of CCL11
(rs17809012 AA) as compared with that in patients who were carrying
the minor allele [rs17809012 (AG/GG)] (P=0.019) (Table III) (6). These results confirmed that the
rs4777889 (or rs11556218) and rs17809012 SNPs could be specific
biomarkers to predict the analgesic effects of oxycodone and
morphine, respectively.
 | Table IIISimple regression analyses to
identify the SNP determinants of the ΔNRS on day 1 in the morphine
and oxycodone groups. |
Table III
Simple regression analyses to
identify the SNP determinants of the ΔNRS on day 1 in the morphine
and oxycodone groups.
| | Morphine
(n=70) | Oxycodone
(n=68) |
|---|
| Variable | β | t-value | Partial regression
coefficient (95% CI) | P-value | β | t-value | Partial regression
coefficient (95% CI) | P-value |
|---|
| Age | -0.02 | -0.19 | 0.85 (-0.53 to
0.43) | 0.851 | 0.04 | 0.30 | 0.76 (-0.33 to
0.44) | 0.764 |
| Sex | -0.03 | -0.22 | -0.05 (-0.54 to
0.44) | 0.828 | -0.12 | -0.95 | -0.18 (-0.57 to
0.21) | 0.345 |
| Performance
status | -0.07 | -0.60 | -0.15 (5-0.64 to
0.3) | 0.549 | 0.22 | 1.84 | 0.36 (-0.03 to
0.75) | 0.070 |
| Pre-NRS score | 0.58 | 5.81 | 0.55 (0.36 to
0.74) | <0.0001 | 0.60 | 6.11 | 0.47 (0.32 to
0.63) | <0.0001 |
| HADS score | 0.07 | 0.59 | 0.02 (-0.04 to
0.08) | 0.555 | 0.15 | 1.19 | 0.03 (-0.02 to
0.09) | 0.237 |
| SF-MPQ-2 Total
score | 0.26 | 2.26 | 0.02 (-0.00 to
0.03) | 0.027 | 0.29 | 2.42 | 0.01 (0.002 to
0.02) | 0.018 |
| Dose | -0.16 | -1.38 | -0.33 (-0.81 to
0.15) | 0.174 | -0.15 | -1.22 | -0.28 (-0.74 to
0.18) | 0.228 |
| Genotype | | | | | | | | |
|
IL-16
(rs4778889) | -0.01 | -0.10 | -0.03 (-0.52 to
0.47) | 0.918 | -0.27 | -2.27 | -0.45 (-0.84 to
-0.05) | 0.027 |
|
IL-16
(rs11556218) | -0.17 | -1.42 | -0.42 (-1.01 to
0.17) | 0.161 | -0.26 | -2.17 | -0.44 (-0.84 to
-0.03) | 0.034 |
|
CCL11
(rs17809012) | -0.28 | -2.41 | -0.56 (-1.01 to
-0.10) | 0.019 | 0.11 | 0.86 | 0.16 (-0.22 to
0.55) | 0.394 |
Regardless of the opioids that were used in the
overall subject population, these SNPs appeared to affect the ∆NRS,
although analysis using a simple regression model revealed that the
differences between the IL-16 rs4777889 genotype groups were
statistically insignificant (Table
SIII). We also performed a multiple linear regression analysis
with adjustments for the age, sex, ps, pre-NRS score, treatment
used, genotype, and total scores on the HADS and SF-MPQ-2, which
still revealed significant differences of the ∆NRS between the
CCL11 rs17809012 genotype groups (difference in ∆NRS between
the genotype groups=0.25 with P=0.049), but not between the
IL-16 rs4777889 genotype groups (difference in ∆NRS between
the genotype groups=0.14, with P=0.286) (Table SIV, multiple regression model 1).
We did not select IL-16 rs4777889 and IL-16
rs11556218 SNPs as covariables at the same time, because these SNPs
with linkage disequilibrium highly confounded each other (with VIFs
of 2.85 and 2.98, respectively; data not shown). However, the
analysis using CCL11 rs17809012 and IL-16 rs11556218
SNPs as covariables showed significant differences of the ∆NRS
between both the CCL11 rs17809012 genotype groups and
IL-16 rs11556218 genotype groups (differences in ∆NRS
between these genotype groups=0.28 and 0.31, with P=0.028 and
0.040, respectively; Table SIV,
multiple regression model 2), and there seemed to be no strong
confounding variables, with uniformly low VIF values for all SNPs
(<1.5) for both multiple regression models 1 and 2 shown in
Table SIV.
Predictive factors for opioid
selection
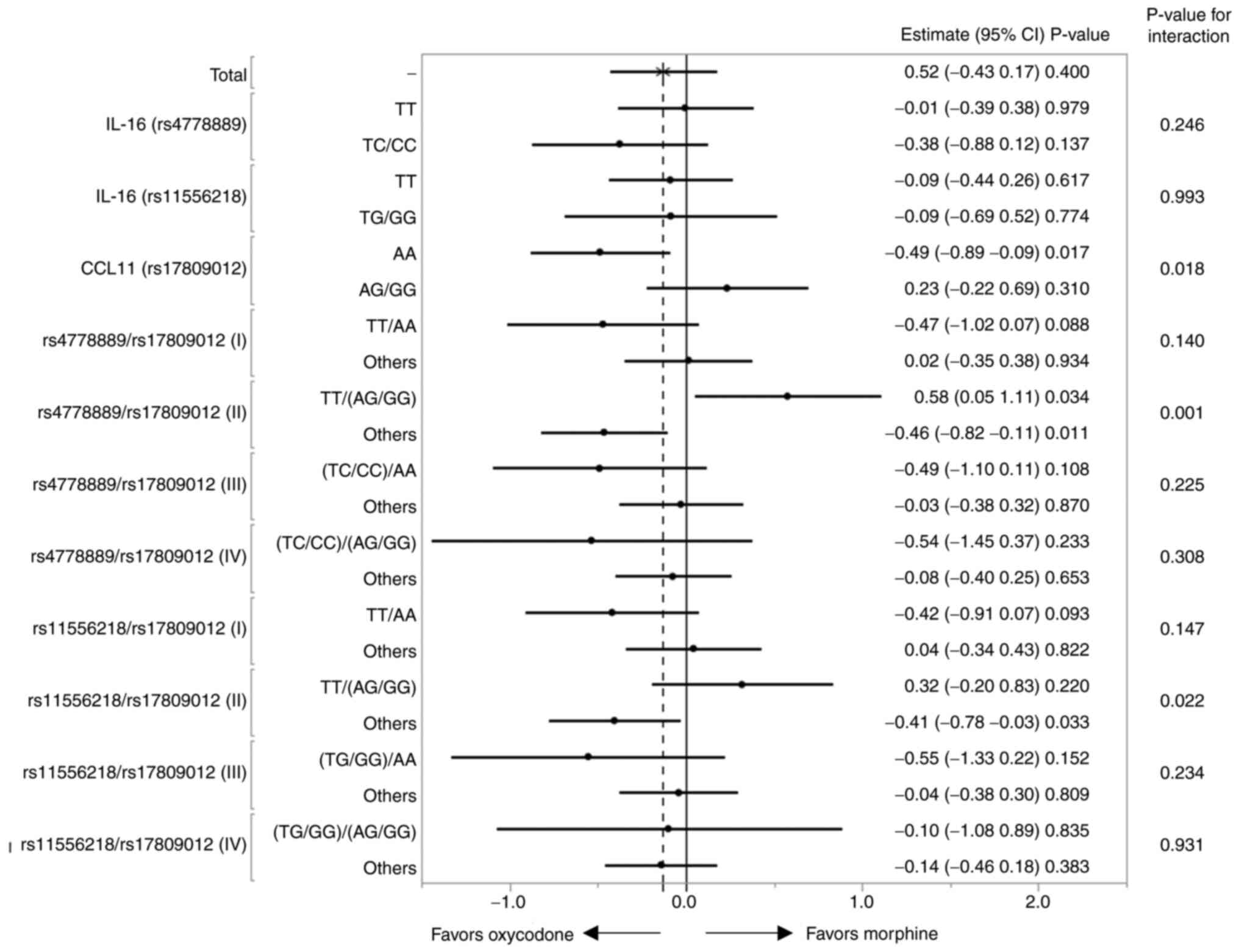
Next, we examined the genotype-treatment
interactions influencing the ∆NRS. The LSM for ∆NRS for each
genotype-treatment interaction was calculated based on the results
of the multiple regression analysis (Table SV). A significant interaction
(P=0.018) was observed between the CCL11 genotype and
treatment (Table SV and Fig. 3) (6), while no such interaction was observed
between the IL-16 rs4777889 or IL-16 rs11556218
genotype and treatment (Table SV
and Fig. 3).
Four combinations of genotypes can be generated from
the two SNPs, IL-16_rs4777889/CCL11_rs17809012, i.e.
i) TT/AA, ii) TT/(AG+GG), iii) (TC+CC)/AA, iv) (TC+CC)/(AG+GG). We
also analyzed the interactions influencing the ∆NRS between each of
the 4 genotype combinations and treatment (Table SV and Fig. 3). Significant interaction (P=0.001)
was detected when comparison was conducted between patients with
‘TT/AG+GG’ (n=45) and others (n=93) (rs4778889/rs17809012,
combination II; Table SV and
Fig. 3). As shown in Table IV, the LSM of the ∆NRS was 4.00
for the Group M patients with the TT/AG+GG genotype, which was
higher by 1.4 than the LSM in the Group M patients with the
remaining genotypes. In contrast, the LSM of the ∆NRS was 2.85 for
the Group O patients with the TT/AG+GG genotype, which was lower by
0.68 than the LSM for the Group O patients with the remaining
genotypes. No significant genotype-treatment interactions were
observed for any of the other genotype combinations (Fig. 3 and Table IV). A similar analysis was
performed for another IL-16 SNP (rs11556218), and we
detected a weaker significance level of the genotype-treatment
interaction for the ∆NRS (P=0.022; Fig. 3).
 | Table IVLSMs of ∆NRS for patients (n=138) in
terms of interaction between their genotype (combination) and
treatment. |
Table IV
LSMs of ∆NRS for patients (n=138) in
terms of interaction between their genotype (combination) and
treatment.
|
Variablea | Groupb | n | LSM | Standard error | 95% CI |
|---|
| IL-16
(rs4778889)*treatment | TT + morphine | 43 | 3.02 | 0.27 | 2.48-3.56 |
| | TC/CC +
morphine | 27 | 3.07 | 0.35 | 2.39-3.76 |
| | TT + oxycodone | 45 | 3.03 | 0.27 | 2.50-3.56 |
| | TC/CC +
oxycodone | 23 | 3.83 | 0.37 | 3.09-4.57 |
| CCL11
(rs17809012)*treatment | AA + morphine | 34 | 2.47 | 0.30 | 1.87-3.07 |
| | AG/GG +
morphine | 36 | 3.58 | 0.29 | 3.00-4.17 |
| | AA + oxycodone | 38 | 3.45 | 0.29 | 2.88-4.01 |
| | AG/GG +
oxycodone | 30 | 3.12 | 0.32 | 2.48-3.75 |
|
(rs4778889/rs17809012)*treatment | (TT/AA) +
morphine | 21 | 2.29 | 0.39 | 1.52-3.05 |
| | (Others) +
morphine | 49 | 3.37 | 0.25 | 2.87-3.87 |
| | (TT/AA) +
oxycodone | 22 | 3.23 | 0.38 | 2.48-3.98 |
| | (Others) +
oxycodone | 46 | 3.34 | 0.26 | 2.82-3.86 |
|
(rs4778889/rs17809012)*treatment | [TT/(AG/GG)] +
morphine | 22 | 4.00 | 0.37 | 3.27-4.73 |
| | (Others) +
morphine | 48 | 2.60 | 0.25 | 2.11-3.10 |
| | [TT/(AG/GG)] +
oxycodone | 23 | 2.85 | 0.36 | 2.13-3.56 |
| | (Others) +
oxycodone | 45 | 3.53 | 0.26 | 3.02-4.05 |
|
(rs4778889/rs17809012)*treatment | [(TC/CC)/AA] +
morphine | 13 | 2.77 | 0.50 | 1.78-3.76 |
| | (Others) +
morphine | 57 | 3.11 | 0.24 | 2.63-3.58 |
| | [(TC/CC)/AA] +
oxycodone | 16 | 3.75 | 0.45 | 2.86-4.64 |
| | (Others) +
oxycodone | 52 | 3.16 | 0.25 | 2.67-3.66 |
|
(rs4778889/rs17809012)*treatment | [(TC/CC)/(AG/GG)] +
morphine | 14 | 2.93 | 0.48 | 1.97-3.88 |
| | (Others) +
morphine | 56 | 3.07 | 0.24 | 2.59-3.55 |
| | [(TC/CC)/(AG/GG)] +
oxycodone | 7 | 4.00 | 0.68 | 2.65-5.35 |
| | (Others) +
oxycodone | 61 | 3.22 | 0.23 | 2.76-3.68 |
Discussion
In the previous study, we confirmed three SNPs
(TRPV1 rs222749, CCL11 rs17809012, HNMT
rs1050891) as being involved in the analgesic effect of morphine.
Out of the three, we found that the CCL11 rs17809012 SNPs
could be a potential biomarker to guide selection of the more
suitable opioid between morphine and oxycodone for treating cancer
pain (6). Patients of Group M with
the major CCL11 rs17809012 genotype (AA) showed a
significantly reduced ∆NRS (P=0.006), suggesting that oxycodone
should be preferred for patients with this genotype of CCL11
to obtain better pain relief. However, for the patients with the
minor allele of the rs17809012 (AG/GG), morphine appeared to be a
better choice, but this interaction was not statistically
significant (P=0.358) (6).
In the current study, we used pre-treatment plasma
samples of patients to screen for chemokines/cytokines with
potential value as biomarker(s) to guide opioid selection. From
among the 39 chemokines/cytokines measured, we identified only the
plasma concentrations of IL-16 as possibly having the potential to
predict the analgesic effect of oxycodone. Analysis of the
interaction between the plasma concentration of IL-16 and treatment
for the ∆NRS showed that patients with plasma IL-16 concentrations
in the lower half of the range of concentrations responded
significantly better to oxycodone treatment.
We next focused on several SNPs residing in the
IL-16 gene. We selected two (rs4778889 and rs11556218 SNPs),
which have been identified previously as being functional (15-18).
For both SNPs, in the Group O patients, homozygosity for the major
allele (TT for both SNPs) was associated with a reduced ∆NRS,
implying a lower analgesic effect, as compared with the genotypes
including at least one minor allele (C for rs4778889 or G for
rs11556218). These minor alleles may be linked to low plasma
concentrations (expression levels) of IL-16, because in the current
study, we showed that the effect of oxycodone in the Group O
patients was significantly better in those with lower
concentrations of IL-16. This result, however, appeared to be
inconsistent with the finding of Burkart et al (20), who reported a several-fold
increased expression of IL-16 associated with the minor
allele (C) as compared with the major allele (T) of rs4778889,
which is putatively located in the promoter region of the human
IL-16 gene (21). They used
a luciferase reporter assay in an in vitro experiment, which
may not have accurately reproduced the status in vivo
(22,23). Indeed, a positive association has
been reported between the rs4778889 TT genotype and IL-16
expression levels in Crohn's disease (24) and Grave's diseases (25), which are consistent with our
results. Further analyses may be required to clarify this
issue.
The rs11556218 SNP, another IL-16 SNP located
on exon 6 of the gene, can result in an asparagine to lysine
substitution in the IL-16 protein. This substitution may alter the
protein structure and function (26), but whether it affects the pain
perception or sensitivity remains largely unknown, although it has
been reported to be associated with an elevated risk of development
of gastric cancer, colorectal cancer and osteosarcoma (16,17).
We found that this SNP was in linkage disequilibrium with
rs4778889. Thus, a trend towards a reduced ∆NRS value associated
with homozygosity for the major allele (TT for both SNPs) as
compared with heterozygosity or homozygosity for the minor allele
was observed for both the SNPs in the Group O patients (Table III). When the interaction between
treatment and the genotype for the ∆NRS was analyzed, the trends
associated with the IL-16 rs4778889 genotypes were found to
be stronger because the combination of the rs4778889 TT genotype
with the CCL11 rs17809012 AG/GG genotype was associated with
a higher effect of morphine (P=0.034), while no such association
was observed for combination of the rs11556218 TT genotype with the
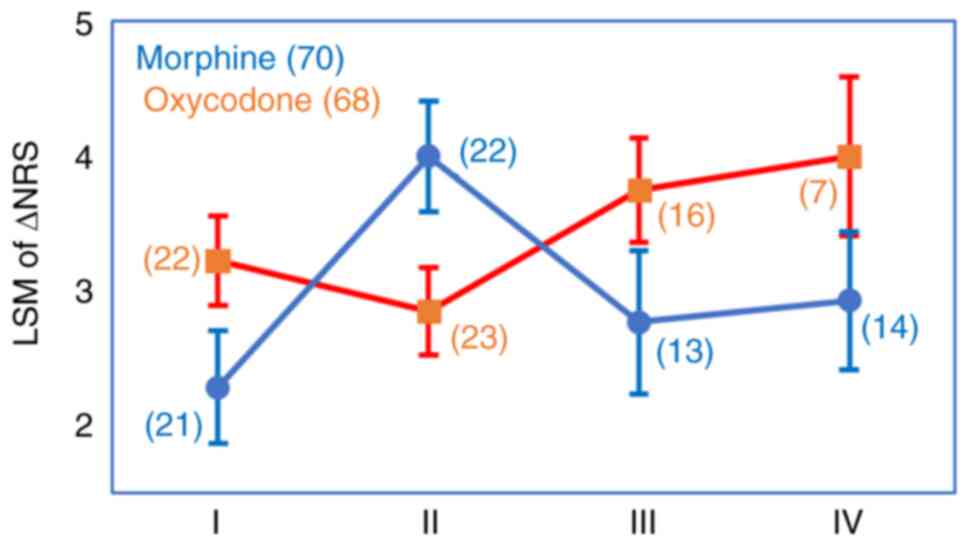
CCL11 rs17809012 AG/GG genotype (P=0.22) (Fig. 3). Thus, the Group M patients with
the rs4778889 TT genotype and rs17809012 AG/GG genotype showed an
LSM for the ∆NRS of 4.00, which was more than 1.00 higher than LSMs
in the Group M patients with the other rs4778889/rs17809012
genotype combinations and the Group O patients with the same
genotype combination (Fig. 4). In
contrast, the Group O patients with the genotype combinations other
than rs4778889 TT/rs17809012 (AG/GG) showed an LSM for the ∆NRS
that was about 1.00 higher than LSMs in the Group M patients with
the respective genotype combinations (Fig. 4).
SNPs of drug transporters, metabolizing enzymes, and
opioid receptors known to modulate the pharmacokinetic and
pharmacodynamic effects of opioids have been suggested as
potentially useful biomarkers for aiding in opioid selection for
patients with cancer pain (1-3).
To the best of our knowledge, this study, as an extension of our
previous study (6), is the first
to show that cytokine or chemokine SNPs could also be used to
choose between opioids (morphine or oxycodone). Our previous study
suggested that the CCL11 rs17809012 SNP could be a biomarker that
could predict the effect of morphine (6). However, our current study
demonstrated that two SNPs (i.e. CCL11 rs17809012 and IL-16
rs4778889) in combination could significantly predict the effect of
both opioids and, therefore, enhance the validity of the choice
(morphine or oxycodone) further than the use of CCL11 rs17809012
alone.
IL-16 is considered as being a proinflammatory
cytokine, and by binding to its receptor (CD4), it promotes the
secretion of inflammatory cytokines, such as TNF-α, IL-1β and
IL-6(11). IL-6 is also known as a
pronociceptive cytokine, like CCL11(27). These cytokines/chemokines form
networks that induce nociceptive and neuropathic pains. How these
networks contribute to the pathogenesis of cancer pain,
characterized by a mixed-mechanism pain state, is still unknown.
Our findings regarding the interactions between the cytokines (or
chemokines) and opioids may be expected to pave the way towards
elucidation of the mechanisms of cancer pain and its treatment.
Our study had some limitations. Cancer pain is
widely known to be an inflammatory response mediated by complex
interactions among many cytokines and chemokines. These humoral
factors are induced in different ways depending on the cancer type,
grade and stage. We enrolled subjects with a variety of cancer
types in the study (Table SVI),
however, classification of patients into these categories could not
be performed due to our small sample size and lack of data, which
could have introduced some biases. Second, we detected a positive
relationship between the genotype and plasma levels for IL-16 in
this study, but unexpectedly, this was not the case for CCL11.
While we measured the plasma concentrations of CCL11, we observed
no relationship of the plasma concentrations of CCL11 with the
treatment effect in this study, unlike the case for the
CCL11 genotype, which showed a significant correlation with
the treatment effect (6). This
divergence could weaken the reliability of our findings; however, a
positive relationship between the plasma concentrations and the
genotype may not necessarily be observed if the genotype is not
linked to regulation of the gene expression but to other biological
function(s) of the encoded protein.
In conclusion, we found two biomarker SNPs that can
be used in combination to guide treatment selection between
morphine and oxycodone for the treatment of cancer pain. Patients
with the IL-16 rs4778889 TT genotype and CCL11
rs17809012 AG/GG genotype may be expected to benefit from treatment
with morphine, while patients with the remaining genotype
combinations could be expected to benefit from treatment with
oxycodone, both of which are significant. Nucleotide sequencing of
these two SNP regions can be readily performed in patients with
cancer pain, so that physicians can have the option of selecting
the more effective opioid for individual patients with cancer pain,
a new therapeutic concept that warrants further clinical
evaluation.
Supplementary Material
Simple regression analyses to identify
determinants of the ∆NRS on day 1 in the Morphine and Oxycodone
groups.
Lists of genotypes and haplotypes of
the 2 SNPs of the IL-16 gene.
Independent determinants of the ∆NRS
on day 1 in the overall subject population (N = 138) (simple
regression model)
Independent determinants of the ∆NRS
on day 1 in the overall subject population (N = 138) (Multiple
regression model).
Multiple regression analysis to test
the interaction with treatment for each variable.
Cancer types of the patients.
Acknowledgements
The authors would like to thank Mrs. Mami Kitano,
Mrs. Haruka Sakamoto, Mrs. Yume Shinkai (all from the Department of
Medical Oncology, Kindai University Faculty of Medicine, Osaka,
Japan) and Dr Masato Terashima (Department of Genome Biology,
Kindai University Faculty of Medicine, Osaka, Japan) for genomic
DNA isolation.
Funding
Funding: This study was financially supported by the Health
Labor Sciences Research Grant (Grant for Innovative Clinical Cancer
Research: H26-Innovative Cancer-General-056; grant no.
16ck0106059h0003) and the Japan Agency for Medical Research and
Development (Innovative Clinical Cancer Research; grant no.
17ck0106328h0001).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
YF, HM, YC, JTs, AK, KNi and KNa designed the study.
YF performed the experiments and collected the data. HM, JTs, TY,
KS, MN, RS, CM, YO, KT, HH, TT and JTa collected the clinical data.
YF, HM, YC, JTs and TY analyzed and interpreted the data. YF and HM
drafted the manuscript. YF, HM, YC, JTs, AK, KNi and KNa revised
the manuscript critically. YF, HM and JTs confirm the authenticity
of all the raw data. All the authors have read and approved the
final manuscript.
Ethics approval and consent for
participation
The study was conducted according to the guidelines
of the Declaration of Helsinki and the Japanese ethical guidelines
for clinical research with the approval of the Ethical Committee of
Kindai University Faculty of Medicine (approval no. 26-130).
Written informed consent was obtained from all participants
involved in the study.
Patient consent for publication
The publication of data was approved by all
patients.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Owusu Obeng A, Hamadeh I and Smith M:
Review of opioid pharmacogenetics and considerations for pain
management. Pharmacotherapy. 37:1105–1121. 2017.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Crews KR, Monte AA, Huddart R, Caudle KE,
Kharasch ED, Gaedigk A, Dunnenberger HM, Leeder JS, Callaghan JT,
Samer CF, et al: Clinical pharmacogenetics implementation
consortium guideline for CYP2D6, OPRM1, and COMT genotypes and
select opioid therapy. Clin Pharmacol Ther. 110:888–896.
2021.PubMed/NCBI View
Article : Google Scholar
|
|
3
|
Vieira CMP, Fragoso RM, Pereira D and
Medeiros R: Pain polymorphisms and opioids: An evidence-based
review. Mol Med Rep. 19:1423–1434. 2019.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Takemura M, Niki K, Okamoto Y, Kawamura T,
Kohno M, Matsuda Y and Ikeda K: Comparison of the effects of OPRM1
A118G polymorphism using different opioids: A prospective study. J
Pain Symptom Manage. 67:39–49.e5. 2024.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Caraceni A, Hanks G, Kaasa S, Bennett MI,
Brunelli C, Cherny N, Dale O, De Conno F, Fallon M, Hanna M, et al:
Use of opioid analgesics in the treatment of cancer pain:
Evidence-based recommendations from the EAPC. Lancet Oncol.
13:e58–e68. 2012.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Fujita Y, Matsuoka H, Chiba Y, Tsurutani
J, Yoshida T, Sakai K, Nakura M, Sakamoto R, Makimura C, Ohtake Y,
et al: Novel single nucleotide polymorphism biomarkers to predict
opioid effects for cancer pain. Oncol Lett. 26(355)2023.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Matsuoka H, Tsurutani J, Chiba Y, Fujita
Y, Terashima M, Yoshida T, Sakai K, Otake Y, Koyama A, Nishio K and
Nakagawa K: Selection of opioids for cancer-related pain using a
biomarker: A randomized, multi-institutional, open-label trial
(RELIEF study). BMC Cancer. 17(674)2017.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Matsuoka H, Tsurutani J, Chiba Y, Fujita
Y, Sakai K, Yoshida T, Nakura M, Sakamoto R, Makimura C, Ohtake Y,
et al: Morphine versus oxycodone for cancer pain using a
catechol-O-methyltransferase genotype biomarker: A multicenter,
randomized, open-label, phase III clinical trial (RELIEF study).
Oncologist. 28:278–e166. 2023.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Ji RR, Berta T and Nedergaard M: Glia and
pain: Is chronic pain a gliopathy? Pain. 154 (Suppl 1):S10–S28.
2013.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Ramesh G, MacLean AG and Philipp MT:
Cytokines and chemokines at the crossroads of neuroinflammation,
neurodegeneration, and neuropathic pain. Mediators Inflamm.
2013(480739)2013.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Swarm R, Abernethy AP, Anghelescu DL,
Benedetti C, Blinderman CD, Boston B, Cleeland C, Coyle N,
Deleon-Casasola OA, Eilers JG, et al: Adult cancer pain. J Natl
Compr Canc Netw. 8:1046–1086. 2010.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Swarm RA and Dans M: NCCN frameworks for
resource stratification of NCCN guidelines: Adult cancer pain and
palliative care. J Natl Compr Canc Netw. 16:628–631.
2018.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Matsuoka H, Makimura C, Koyama A, Fujita
Y, Tsurutani J, Sakai K, Sakamoto R, Nishio K and Nakagawa K:
Prospective replication study implicates the
catechol-O-methyltransferase Val158Met polymorphism as a
biomarker for the response to morphine in patients with cancer.
Biomed Rep. 7:380–384. 2017.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Matsuoka H, Yoshiuchi K, Koyama A,
Makimura C, Fujita Y, Tsurutani J, Sakai K, Sakamoto R, Nishio K
and Nakagawa K: Expectation of a decrease in pain affects the
prognosis of pain in cancer patients: A prospective cohort study of
response to morphine. Int J Behav Med. 24:535–541. 2017.PubMed/NCBI View Article : Google Scholar
|
|
15
|
De Souza VH, De Alencar JB, Tiyo BT, Alves
HV, Vendramini ECL, Sell AM and Visentainer JEL: Association of
functional IL16 polymorphisms with cancer and cardiovascular
disease: A meta-analysis. Oncotarget. 11:3405–3417. 2020.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Gao LB, Rao L, Wang YY, Liang WB, Li C,
Xue H, Zhou B, Sun H, Li Y, Lv ML, et al: The association of
interleukin-16 polymorphisms with IL-16 serum levels and risk of
colorectal and gastric cancer. Carcinogenesis. 30:295–299.
2009.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Tang YJ, Wang JL, Xie KG and Lan CG:
Association of interleukin 16 gene polymorphisms and plasma IL16
level with osteosarcoma risk. Sci Rep. 6(34607)2016.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Zhu J, Qin C, Yan F, Wang M, Ding Q, Zhang
Z and Yin C: IL-16 polymorphism and risk of renal carcinoma:
Association in a Chinese population. Int J Urol. 17:700–707.
2010.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Liu Z, Ma L, Qiu S and Jia T: Genetic
polymorphisms of interleukin-16 are associated with susceptibility
to primary knee osteoarthritis. Int J Clin Exp Med. 8:1401–1405.
2015.PubMed/NCBI
|
|
20
|
Burkart KM, Barton SJ, Holloway JW, Yang
IA, Cakebread JA, Cruikshank W, Little F, Jin X, Farrer LA, Clough
JB, et al: Association of asthma with a functional promoter
polymorphism in the IL16 gene. J Allergy Clin Immunol. 117:86–91.
2006.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Nakayama EE, Wasi C, Ajisawa A, Iwamoto A
and Shioda T: A new polymorphism in the promoter region of the
human interleukin-16 (IL-16) gene. Genes Immun. 1:293–294.
2000.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Elvert G, Kappel A, Heidenreich R,
Englmeier U, Lanz S, Acke T, Rauter M, Plate K, Sieweke M, Breier G
and Flamme I: Cooperative interaction of hypoxia-inducible
factor-2alpha (HIF-2alpha) and Ets-1 in the transcriptional
activation of vascular endothelial growth factor receptor-2
(Flk-1). J Biol Chem. 278:7520–7530. 2003.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Kappel A, Schlaeger TM, Flamme I, Orkin
SH, Risau W and Breier G: Role of SCL/Tal-1, GATA, and ets
transcription factor binding sites for the regulation of flk-1
expression during murine vascular development. Blood. 96:3078–3085.
2000.PubMed/NCBI
|
|
24
|
Glas J, Török HP, Unterhuber H, Radlmayr M
and Folwaczny C: The-295T-to-C promoter polymorphism of the IL-16
gene is associated with Crohn's disease. Clin Immunol. 106:197–200.
2003.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Gu XJ, Cui B, Zhao ZF, Chen HY, Li XY,
Wang S, Ning G and Zhao YJ: Association of the interleukin (IL)-16
gene polymorphisms with Graves' disease. Clin Immunol. 127:298–302.
2008.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Matalliotakis M, Zervou MI, Eliopoulos E,
Matalliotaki C, Rahmioglu N, Kalogiannidis I, Zondervan K,
Spandidos DA, Matalliotakis I and Goulielmos GN: The role of IL-16
gene polymorphisms in endometriosis. Int J Mol Med. 41:1469–1476.
2018.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Pawlik K, Ciechanowska A, Ciapała K,
Rojewska E, Makuch W and Mika J: Blockade of CC chemokine receptor
type 3 diminishes pain and enhances opioid analgesic potency in a
model of neuropathic pain. Front Immunol. 12(781310)2021.PubMed/NCBI View Article : Google Scholar
|