Introduction
Pancreatic ductal adenocarcinoma (PDAC) has a poor
prognosis with an overall 5-year survival rate of 8% (1). Radical resection is currently the
only treatment that can increase the 5-year rate of survival to
10-25% (2-5).
However, only around 20% of patients suffering from PDAC are
suitable candidates for radical resection at the time of diagnosis
due to the lack of early symptoms or to its aggressive nature
(4,6).
Because of the poor prognosis even in the patients
with resectable PDAC (rPDAC), adjuvant chemotherapy has commonly
been performed. Its introduction has improved prognosis and is one
of the factors mainly associated with long-term survival. Despite
the use of the most effective regimens, such as modified
FOLFIRINOX, the recurrence rate of the disease still remains high,
with a disease-free survival rate at 5 years of only 26.1%
(7). This poor outcome can be
caused by early recurrence and the significantly higher rate of an
incomplete resection compared to resection for other
gastrointestinal cancers. Therefore, effective neoadjuvant
chemotherapy (NAC) is being sought for patients with rPDAC. The
results of various randomized controlled trials of NAC for rPDAC
such as NEONAX (8), SWOG
S1505(9), and the NORPACT-1 study
(10) have been reported. In
Japan, the Prep-02/JSAP05 trial was the first to show a survival
benefit of NAC with gemcitabine plus S-1 (GS) in patients with
resectable PDAC, and NAC-GS is now the standard regimen for rPDAC
in Japan (11,12). Nevertheless, as it remains
controversial whether NAC is actually required for rPDAC (13), the importance of NAC and whether
NAC-GS is appropriate therapy for rPDAC require examination.
Staging laparoscopy (SL) has been shown to identify
small peritoneal or liver metastases not observed in preoperative
imaging (14,15). For patients with distant occult
metastases, SL is more beneficial than exploratory laparotomy due
not only to its lower invasiveness but also rapid patient recovery,
which consequently leads to early induction of chemotherapy
(15,16). Therefore, it is meaningful to
identify the patients at possible risk of distant metastasis from
PDAC.
Therefore, the aim of this study was to investigate
the benefits of NAC for patients with rPDAC.
Patients and methods
Study population
In total, 170 patients who were diagnosed as having
rPDAC preoperatively at the Oita University Faculty of Medicine
from May 2005 to August 2023 were enrolled in this study. Patient
characteristics were retrospectively collected from the patients'
charts. This study was approved by the Ethics Committee of Oita
University Faculty of Medicine (approval number: 1502). The need
for written informed consent was waived owing to the retrospective
nature of the study.
Preoperative evaluation of PDAC with
computed tomography (CT)
According to general rules for the study of
pancreatic cancer edited by the Japan Pancreas Society (17), rPDAC was defined on CT imaging as
no contact with the major arteries (celiac axis, superior
mesenteric artery, or common hepatic artery), and no contact with
the superior mesenteric vein or portal vein or ≤ 180˚ contact
without vein contour irregularity. When a spicula-like protrusion
was observed toward the surrounding fat tissue of the pancreas
beyond the anterior or posterior pancreatic capsule, it was
classified as either invasion of the serosal side of the anterior
pancreatic tissue or invasion of retroperitoneal tissue.
Extrapancreatic invasion of the nerve plexus was diagnosed when a
continuous lesion surrounding the celiac artery or superior
mesenteric artery was observed. These invasions, except for the
major vascular invasion mentioned above, are diagnosed as rPDAC
according to these general rules.
Neoadjuvant chemotherapy
Until 2019, all patients who were diagnosed as
having rPDAC preoperatively underwent upfront surgery (UpS). NAC
was started from 2020 for all patients diagnosed as having rPDAC
preoperatively, and all patients were treated with GS (intravenous
gemcitabine at a dose of 1000 mg/m2 on days 1 and 8 plus
S-1 orally at a dose based on body surface area (BSA) (<1.25
m2, 40 mg; BSA 1.25-1.5 m2, 50 mg; BSA
>1.50 m2, 60 mg) twice daily on days 1-14 of a 21-day
cycle). The patients were divided into two groups: the UpS group
and the NAC group, and their selection was determined by the period
in which the treatment was performed. Tumor response was assessed
preoperatively according to RECIST version 1.1. by CT. For
pathological assessment, grading of the extent of residual
carcinoma in specimens was performed according to the grading
scheme reported by Evans et al (18), which is based on the percentage of
residual tumor cells present.
Staging laparoscopy
SL was performed for all patients at the beginning
of the surgery intended for resection. When occult distant
metastases were detected, the metastatic lesions were examined for
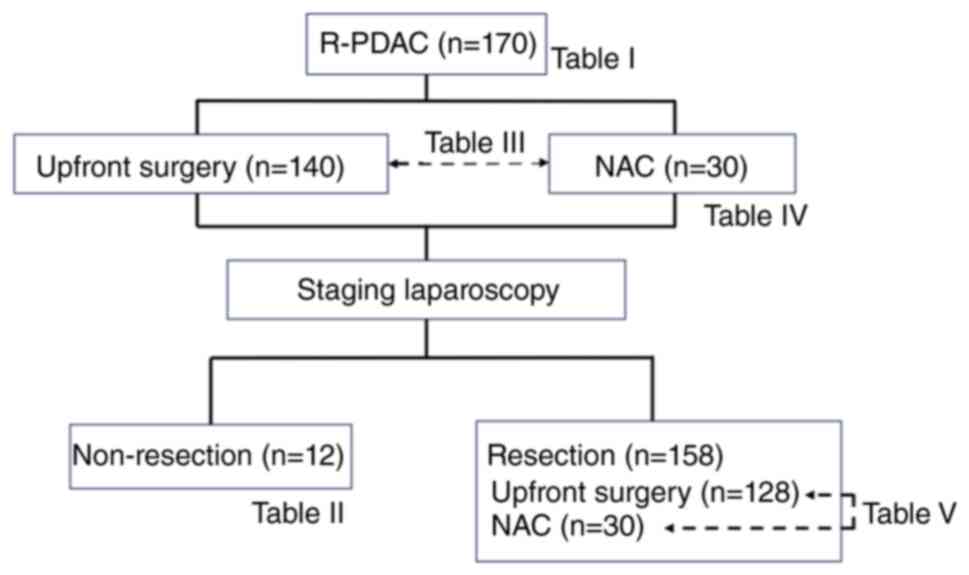
a diagnosis by a pathologist (19). Fig.
1 shows the treatment strategy and examinations of this
study.
Statistical analyses
All variables are expressed as the mean±standard
deviation for continuous data. Prior to analysis, continuous data
were divided into two groups using averages or abnormal values.
Univariate analyses were performed with the Student t-test
for continuous variables and the chi-squared test for categorical
variables. Statistical significance was defined as P<0.05. All
statistical analyses were performed using JMP 17 (SAS Institute
Inc., Cary, NC, USA). There were no missing data for the factors
examined in the 170 patients in this study, and all were eligible
for inclusion.
Results
Patient characteristics
The mean age of the 66 women and 104 men was
70.7±8.6 years (Table I). Tumors
were located at the pancreatic head in 89 patients (52%) and in the
pancreatic body and tail in 81 (48%), and the mean tumor size was
24.6±10.4 mm. Among the 170 patients receiving SL, occult distant
metastases were found in 12 patients (7%), with peritoneal
dissemination recognized in 7 patients, liver metastases in 4, and
both in 1 (Table II). The
remaining 158 patients (93%) were diagnosed as having rPDAC, and
these patients were assigned to undergo resection.
 | Table IPatient characteristics (n=170). |
Table I
Patient characteristics (n=170).
| Characteristics | Value |
|---|
| Preoperative
factors | |
|
Age,
years | 70.7±8.6 |
|
Sex
(female/male) | 66 (39%)/104
(61%) |
|
Body mass
index, kg/m2 | 22.6±4.0 |
|
Serum
albumin, g/dl | 4.0±0.5 |
|
HbA1C,
% | 6.8±1.4 |
|
CEA,
ng/ml | 4.7±5.8 |
|
CA19-9,
U/ml | 691±1811 |
|
Period from
first visit to treatment, days | 22.9±12.5 |
| Radiological
findings | |
|
Tumor
location (Ph/Pbt) | 89 (52%)/81
(48%) |
|
Tumor size,
mm | 24.6±10.4 |
|
Serosal side
of the anterior pancreatic tissue invasion, (-/+) | 42 (25%)/128
(75%) |
|
Retroperitoneal
tissue invasion, (-/+) | 58 (34%)/112
(66%) |
|
Extrapancreatic
nerve plexus invasion, (-/+) | 132 (78%)/38
(22%) |
|
Contact with
portal vein, (-/+) | 157 (92%)/13
(8%) |
|
Lymph node
metastasis, (-/+) | 130 (76%)/40
(24%) |
| Treatment | |
|
Neoadjuvant
chemotherapy, (-/+) | 140 (82%)/30
(18%) |
|
Operation
(resection/non-resection) | 158 (93%)/12
(7%) |
 | Table IICharacteristics of patients with
unresectable PDAC diagnosed by staging laparoscopy (n=12). |
Table II
Characteristics of patients with
unresectable PDAC diagnosed by staging laparoscopy (n=12).
| Characteristics | Value |
|---|
| Metastatic site | |
|
Peritoneal
dissemination | 7 |
|
Liver
metastasis | 4 |
|
Peritoneal
dissemination and liver metastasis | 1 |
| Operation | |
|
SL | 8 |
|
SL and
gastrojejunostomy | 1 |
|
SL and
colostomy | 2 |
| Operation time,
min | 96±35 |
| Blood loss, ml | 0 |
| Postoperative
complications | |
|
Gastritis | 1 |
|
Pancreatitis | 1 |
| Postoperative
hospital stay, days | 12.9±6.8 |
| Duration between SL
and induction of chemotherapy, days | 11.8±4.4 |
Comparison of pre-treatment clinical
and imaging factors between the UpS and NAC groups for all
patients
Preoperative and imaging factors of the UpS group
and NAC group are shown in Table
III. There were no significant differences in patient
characteristics and blood test data between the two groups. The
period from the first visit to treatment in the NAC group was
shorter than that in the UpS group (P<0.001). There were no
significant differences between the imaging factors. All patients
with occult distant metastases diagnosed by SL were in the UpS
group, and all patients in the NAC group underwent resection
(P=0.028).
 | Table IIIClinical and imaging factors before
treatment with UpS and NAC in all cases. |
Table III
Clinical and imaging factors before
treatment with UpS and NAC in all cases.
| Factor | UpS (n=140) | NAC (n=30) | P-value |
|---|
| Preoperative
factors | | | |
|
Age,
years | 70.8±9.0 | 70.5±6.5 | 0.895 |
|
Sex
(female/male) | 55/85 | 11/19 | 0.789 |
|
Body mass
index, kg/m2 | 22.5±4.1 | 22.8±3.7 | 0.775 |
|
Serum
albumin, g/dl | 4.0±0.5 | 4.0±0.5 | 0.345 |
|
HbA1C,
% | 6.8±6.7 | 6.7±1.1 | 0.686 |
|
CEA,
ng/ml | 4.9±6.2 | 4.2±2.9 | 0.565 |
|
CA19-9,
U/ml | 684±1888 | 696±1390 | 0.976 |
|
Period from
first visit to treatment, days | 25.2±11.6 | 12.7±11.0 | <0.001 |
| Imaging
findings | | | |
|
Tumor
location (Ph/Pbt) | 78/62 | 11/19 | 0.057 |
|
Tumor size,
mm | 24.2±10.7 | 26.4±8.7 | 0.325 |
|
Serosal side
of the anterior pancreatic tissue invasion, (-/+) | 37/103 | 5/25 | 0.244 |
|
Retroperitoneal
tissue invasion, (-/+) | 52/88 | 6/24 | 0.062 |
|
Extrapancreatic
nerve plexus invasion, (-/+) | 105/35 | 27/3 | 0.055 |
|
Contact with
portal vein, (-/+) | 132/8 | 25/5 | 0.063 |
|
Lymph node
metastasis, (-/+) | 105/35 | 25/5 | 0.314 |
| Treatment | | | |
|
Operation
(resection/non-resection) | 128/12 | 30/0 | 0.028 |
Comparison of tumor-related factors
before and after NAC
All patients received GS therapy, and one patient
received gemcitabine plus nab-paclitaxel (GnP) after GS (Table IV). Tumor size and CA19-9 level
decreased after NAC, but the differences were not significant. In
the preoperative imaging evaluation, 4 patients had a partial
response (PR) and 26 had stable disease (SD). All patients
underwent surgery, and the numbers of patients with pathological
responses of 1a, 1b, 2, and 3 were 7, 18, 3, and 2,
respectively.
 | Table IVTumor-related factors before and
after NAC. |
Table IV
Tumor-related factors before and
after NAC.
| Factor | Number | Before NAC | After NAC | P-value |
|---|
| Regimen
(GS/GS→GnP) | 29/1 | | | |
| Tumor size, mm | | 27.7±9.6 | 25.7±9.1 | 0.406 |
| Serum albumin,
g/dl | | 4.0±0.5 | 3.8±0.5 | 0.138 |
| CEA, ng/ml | | 4.2±2.9 | 4.3±3.2 | 0.855 |
| CA19-9, U/ml | | 696±1389 | 288±787 | 0.167 |
| Imaging evaluation
(CR/PR/SD/PD) | 0/4/26/0 | | | |
| Pathological
evaluation (1a/1b/2/3) | 7/18/3/2 | | | |
Comparison of perioperative factors
between the UpS and NAC groups in the 158 patients undergoing
resection
There were no significant differences in patient
characteristics and blood test data between the two groups
(Table V). The period from the
first visit to treatment in the NAC group was shorter than that in
the UpS group (P<0.001). Operative outcomes in the NAC group
were not inferior to those in the UpS group. Pathological
examination showed the pancreatic cut end margin and dissected
peripancreatic tissue margin to be negative in all NAC cases,
indicating that all cases were R0 resections in the NAC group. The
rates of early recurrence within 6 months after surgery were 10%
(3/30) in the NAC group and 29% (37/128) in the UpS group
(P=0.021).
 | Table VUpS vs. NAC in the resected
cases. |
Table V
UpS vs. NAC in the resected
cases.
| Parameter | UpS (n=128) | NAC (n=30) | P-value |
|---|
| Patient
characteristics | | | |
|
Age,
years | 70.9±8.9 | 70.5±6.5 | 0.822 |
|
Sex
(female/male) | 76/52 | 19/11 | 0.689 |
|
Body mass
index, kg/m2 | 22.5±4.2 | 22.8±3.7 | 0.771 |
|
Serum
albumin, g/dl | 4.0±0.5 | 3.7±0.5 | 0.011 |
|
HbA1C,
% | 6.8±1.5 | 6.7±1.1 | 0.679 |
|
CEA,
ng/ml | 4.9±6.4 | 4.2±2.9 | 0.538 |
|
CA19-9,
U/ml | 695±1936 | 696±1390 | 0.999 |
| Period from first
visit to treatment, days | 26±12 | 13±11 | <0.001 |
| Operation | | | |
|
Open/laparoscopy | 113/15 | 17/13 | 0.000 |
|
PD/DP/TP | 75/50/3 | 11/18/1 | 0.145 |
|
Operation
time, min | 444±124 | 395±126 | 0.056 |
|
Blood loss,
ml | 830±707 | 440±399 | 0.004 |
| Postoperative
course | | | |
|
POPF (≥grade
B) | 16 (13%) | 2 (7%) | 0.338 |
|
Postoperative
hospital stay, days | 29±18 | 20±20 | 0.031 |
| Pathological
findings | | | |
|
Tumor size,
mm | 30.3±12.9 | 26.1±9.7 | 0.094 |
|
UICC T stage
(1/2/3) | 27/78/23 | 8/20/2 | 0.241 |
|
LN
metastasis (-/+) | 58/70 | 16/14 | 0.429 |
|
PCM
(-/+) | 122/6 | 30/0 | 0.108 |
|
DPM
(-/+) | 113/15 | 30/0 | 0.010 |
| Early recurrence
(≤6 months) after surgery | 37 (29%) | 3 (10%) | 0.021 |
Discussion
The survival benefits of UpS and adjuvant therapy
for rPDAC remain limited, but NAC may result in an improved
outcome. NAC offers the following advantages over UpS: elimination
of micro-metastases before surgery and a high tolerance rate among
patients. These advantages can contribute to improvement in the
rate of negative margin resection, reduced nodal involvement, and
better survival. However, there are two concerns regarding this
approach: risk of tumor progression and toxicities during NAC.
Furthermore, NAC-related adverse events could worsen the patient's
condition, thereby delaying surgery and potentially depriving them
of a chance for a cure.
In this patient series, NAC-GS was started from 2020
for all patients diagnosed as having rPDAC preoperatively, and all
patients were treated with GS. We compared UpS and NAC and found
that the NAC group showed early delivery of the therapy and no
unresectable conditions after NAC. All 12 patients in whom SL
revealed occult distant metastases were treated with UpS.
Furthermore, NAC did not appear to reduce the complexity of the
surgery and showed a high rate of R0 resection. Compared with UpS,
NAC was not inferior in terms of perioperative factors, and
importantly, the rate of recurrence-free survival at 6 months was
significantly lower with NAC. These results suggest that NAC-GS may
be a suitable regimen for rPDAC, although the response rate of
NAC-GS was relatively low.
Some randomized controlled trials for NAC have been
reported. The NEONAX study (8)
showed an R0 resection rate of 88% and median OS of 25.5 months in
the patients who were treated with GnP, whereas NAC could not be
shown to prolong RFS, which was the primary endpoint. SWOG
S1505(9) reported the short-term
outcome of patients who were treated with modified FOLFIRINOX or
GnP. This report showed a high tolerance to these systemic
therapies by the patients, successful surgical resection without
prohibitive perioperative complications, and a major pathologic
response rate of 33%. Immunotherapy using pembrolizumab in NAC has
also been reported, and no additive effects were shown (20). Randomized ongoing trials are
eagerly awaited with more active combined regimens including
modified FOLFIRINOX (21).
Our study showed that occult distant metastases were
still found in 7% of the patients with PDAC when performing SL. It
is difficult to predict unresectable cases preoperatively, and we
reconfirmed the benefit of SL for the patients potentially having
rPDAC. SL has a precise effect in identifying patients with
unsuspected unresectable lesions and, therefore, in decreasing the
number of unnecessary laparotomies. Stefanidis et al
reported that SL could identify unsuspected metastases in a
significant proportion of patients (15-51%) (22). Karabicak et al also reported
that occult distant metastases could be detected by SL in 20% of
patients with PDAC although the diagnostic accuracy of CT has
improved (23). Therefore, SL is a
valuable option for PDAC staging. Nevertheless, some reports noted
that SL was not recommended in all cases for two reasons: the
number of occult cancers detected by SL has decreased due to the
increased sensitivity of CT, and the cost-effectiveness of SL
(24,25). However, some papers supported the
cost-effectiveness of SL (26,27).
In their recent guidelines, the Japan Pancreas Society also
recommended performing SL when distant metastasis cannot be ruled
out in patients with resectable or locally advanced pancreatic
cancer (28). De Rosa et al
reviewed 24 studies to try to identify indications for SL (29) and concluded that patients with
rPDAC identified by CT and who have a CA19-9 level ≥150 U/ml or
tumor size >3 cm should be considered for SL.
The limitations of this study include its
retrospective study design and small number of patients. Thus, it
will be necessary to examine long-term outcomes to further clarify
the significance of NAC-GS administration.
In conclusion, surgery after NAC can be performed
safely, and NAC for rPDAC is useful in terms of rapid induction of
the treatment, fewer occult distant metastases, and lower rate of
early recurrence.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
TH, KT, YN, HO, MK, AF, HT, YK, TM, YE and MI
substantially contributed to the conception and design of the study
and performed the acquisition of data for the study. TH and KT
performed analysis and interpretation of the data and drafted the
manuscript. MI contributed to the review and/or critical revision
of the article. Each author has participated sufficiently in the
work to be considered an author and agrees to be accountable for
all aspects of the work by ensuring that questions related to the
accuracy or integrity of any part of the work are appropriately
investigated and resolved. TH and KT confirm the authenticity of
all the raw data. All authors have read and approved the final
version of the manuscript.
Ethics approval and consent to
participate
The present study was conducted in accordance with
the Declaration of Helsinki, and was approved by the Ethics
Committee of Oita University Faculty of Medicine (approval no.
1502).
Patient consent for publication
The need for written informed consent was waived
owing to the retrospective nature of the study.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2018. CA Cancer J Clin. 68:7–30. 2018.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Schnelldorfer T, Ware AL, Sarr MG, Smyrk
TC, Zhang L, Qin R, Gullerud RE, Donohue JH, Nagorney DM and
Farnell MB: Long-term survival after pancreatoduodenectomy for
pancreatic adenocarcinoma: Is cure possible? Ann Surg. 247:456–462.
2008.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Al-Hawary MM, Kaza RK, Wasnik AP and
Francis IR: Staging of pancreatic cancer: Role of imaging. Semin
Roentgenol. 48:245–252. 2013.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Neoptolemos JP, Stocken DD, Friess H,
Bassi C, Dunn JA, Hickey H, Beger H, Fernandez-Cruz L, Dervenis C,
Lacaine F, et al: A randomized trial of chemoradiotherapy and
chemotherapy after resection of pancreatic cancer. N Engl J Med.
350:1200–1210. 2004.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Hsu CP, Hsu JT, Liao CH, Kang SC, Lin BC,
Hsu YP, Yeh CN, Yeh TS and Hwang TL: Three-year and five-year
outcomes of surgical resection for pancreatic ductal
adenocarcinoma: Long-term experiences in one medical center. Asian
J Surg. 41:115–123. 2018.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Andrén-Sandberg A and Neoptolemos JP:
Resection for pancreatic cancer in the new millennium.
Pancreatology. 2:431–439. 2002.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Conroy T, Castan F, Lopez A, Turpin A, Ben
Abdelghani M, Wei AC, Mitry E, Biagi JJ, Evesque L, Artru P, et al:
Five-year outcomes of FOLFIRINOX vs gemcitabine as adjuvant therapy
for pancreatic cancer: A randomized clinical trial. JAMA Oncol.
8:1571–1578. 2022.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Seufferlein T, Uhl W, Kornmann M, Algül H,
Friess H, König A, Ghadimi M, Gallmeier E, Bartsch DK, Lutz MP, et
al: Perioperative or only adjuvant gemcitabine plus nab-paclitaxel
for resectable pancreatic cancer (NEONAX)-a randomized phase II
trial of the AIO pancreatic cancer group. Ann Oncol. 34:91–100.
2023.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Ahmad SA, Duong M, Sohal DPS, Gandhi NS,
Beg MS, Wang-Gillam A, Wade JL III, Chiorean EG, Guthrie KA, Lowy
AM, et al: Surgical outcome results from SWOG S1505: A randomized
clinical trial of mFOLFIRINOX versus gemcitabine/nab-paclitaxel for
perioperative treatment of resectable pancreatic ductal
adenocarcinoma. Ann Surg. 272:481–486. 2020.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Labori KJ, Bratlie SO, Biörserud C,
Bjornsson B, Bringeland E, Elander N, Grønbech JK, Haux J,
Hemmingsson O, Nymo L, et al: Short-course neoadjuvant FOLFIRINOX
versus upfront surgery for resectable pancreatic head cancer: A
multicenter randomized phase-II trial (NORPACT-1). J Clin Oncol.
41(LBA4005)2023.
|
|
11
|
Motoi F, Kosuge T, Ueno H, Yamaue H, Satoi
S, Sho M, Honda G, Matsumoto I, Wada K, Furuse J, et al: Randomized
phase II/III trial of neoadjuvant chemotherapy with gemcitabine and
S-1 versus upfront surgery for resectable pancreatic cancer
(Prep-02/JSAP05). Jpn J Clin Oncol. 49:190–194. 2019.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Kitano Y, Inoue Y, Takeda T, Oba A, Ono Y,
Sato T, Ito H, Ozaka M, Sasaki T, Sasahira N, et al: Clinical
efficacy of neoadjuvant chemotherapy with gemcitabine plus S-1 for
resectable pancreatic ductal adenocarcinoma compared with upfront
surgery. Ann Surg Oncol. 30:5093–5102. 2023.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Liu S, Li H, Xue Y and Yang L: Prognostic
value of neoadjuvant therapy for resectable and borderline
resectable pancreatic cancer: A meta-analysis of randomized
controlled trials. PLoS One. 18(e0290888)2023.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Allen VB, Gurusamy KS, Takwoingi Y, Kalia
A and Davidson BR: Diagnostic accuracy of laparoscopy following
computed tomography (CT) scanning for assessing the resectability
with curative intent in pancreatic and periampullary cancer.
Cochrane Database Syst Rev. 7(CD009323)2016.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Hashimoto D, Chikamoto A, Sakata K,
Nakagawa S, Hayashi H, Ohmuraya M, Hirota M, Yoshida N, Beppu T and
Baba H: Staging laparoscopy leads to rapid induction of
chemotherapy for unresectable pancreatobiliary cancers. Asian J
Endosc Surg. 8:59–62. 2015.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Sell NM, Fong ZV, Del Castillo CF, Qadan
M, Warshaw AL, Chang D, Lillemoe KD and Ferrone CR: Staging
laparoscopy not only saves patients an incision, but may also help
them live longer. Ann Surg Oncol. 25:1009–1016. 2018.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Japan Pancreatic Society, General rules
for the pancreatic cancer, the 8th edition Kanehara & Co.,
Ltd., Tokyo, 2023.
|
|
18
|
Evans DB, Rich TA, Byrd DR, Cleary KR,
Connelly JH, Levin B, Charnsangavej C, Fenoglio CJ and Ames FC:
Preoperative chemoradiation and pancreaticoduodenectomy for
adenocarcinoma of the pancreas. Arch Surg. 127:1335–1339.
1992.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Ushimaru Y, Fujiwara Y, Shishido Y, Omori
T, Yanagimoto Y, Sugimura K, Moon JH, Miyata H and Yano M:
Condition mimicking peritoneal metastasis associated with
preoperative staging laparoscopy in advanced gastric cancer. Asian
J Endosc Surg. 12:457–460. 2019.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Katz MHG, Petroni GR, Bauer T, Reilley MJ,
Wolpin BM, Stucky CC, Bekaii-Saab TS, Elias R, Merchant N, Dias
Costa A, et al: Multicenter randomized controlled trial of
neoadjuvant chemoradiotherapy alone or in combination with
pembrolizumab in patients with resectable or borderline resectable
pancreatic adenocarcinoma. J Immunother Cancer.
11(e007586)2023.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Van Dam JL, Verkolf EMM, Dekker EN,
Bonsing BA, Bratlie SO, Brosens LAA, Busch OR, van Driel LMJW, van
Eijck CHJ, Feshtali S, et al: Perioperative or adjuvant mFOLFIRINOX
for resectable pancreatic cancer (PREOPANC-3): Study protocol for a
multicenter randomized controlled trial. BMC Cancer.
23(728)2023.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Stefanidis D, Grove KD, Schwesinger WH and
Thomas CR Jr: The current role of staging laparoscopy for
adenocarcinoma of the pancreas: A review. Ann Oncol. 17:189–199.
2006.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Karabicak I, Satoi S, Yanagimoto H,
Yamamoto T, Hirooka S, Yamaki S, Kosaka H, Inoue K, Matsui Y and
Kon M: Risk factors for latent distant organ metastasis detected by
staging laparoscopy in patients with radiologically defined locally
advanced pancreatic ductal adenocarcinoma. J Hepatobiliary Pancreat
Sci. 23:750–755. 2016.PubMed/NCBI View
Article : Google Scholar
|
|
24
|
Pisters PW, Lee JE, Vauthey JN,
Charnsangavej C and Evans DB: Laparoscopy in the staging of
pancreatic cancer. Br J Surg. 88:325–337. 2023.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Camacho D, Reichenbach D, Duerr GD, Venema
TL, Sweeney JF and Fisher WE: Value of laparoscopy in the staging
of pancreatic cancer. JOP. 6:552–561. 2005.PubMed/NCBI
|
|
26
|
Peng JS, Mino J, Monteiro R, Morris-Stiff
G, Ali NS, Wey J, El-Hayek KM, Walsh RM and Chalikonda S:
Diagnostic laparoscopy prior to neoadjuvant therapy in pancreatic
cancer is high yield: An analysis of outcomes and costs. J
Gastrointest Surg. 21:1420–1427. 2017.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Morris S, Gurusamy KS, Sheringham J and
Davidson BR: Cost-effectiveness of diagnostic laparoscopy for
assessing resectability in pancreatic and periampullary cancer. BMC
Gastroenterol. 15(44)2015.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Yamaguchi K, Okusaka T, Shimizu K, Furuse
J, Ito Y, Hanada K, Shimosegawa T and Okazaki K: Committee for
Revision of Clinical Guidelines for Pancreatic Cancer of the Japan
Pancreas Society. clinical practice guidelines for pancreatic
cancer 2016 from the Japan pancreas society: A synopsis. Pancreas.
46:595–604. 2017.PubMed/NCBI View Article : Google Scholar
|
|
29
|
De Rosa A, Cameron IC and Gomez D:
Indications for staging laparoscopy in pancreatic cancer. HPB
(Oxford). 18:13–20. 2016.PubMed/NCBI View Article : Google Scholar
|