Introduction
Squamous cell lung carcinoma (SqCLC) constitutes a
significant subset of non-small cell lung cancer (NSCLC) that is
characterized by its distinctive histological features and clinical
behavior (1). Despite advancements
in the management of NSCLC, therapeutic options for advanced SqCLC
remain limited, particularly in the elderly population. This is
mainly due to the presence of multiple comorbidities and decreased
tolerance to aggressive treatment methods (2). As the global population ages, the
incidence of SqCLC among the elderly is expected to rise,
necessitating the exploration of novel effective and tolerable
second-line therapies for this demographic population (3). Elderly patients with SqCLC pose
unique challenges, due to age-related physiological changes,
comorbid conditions and increased susceptibility to
treatment-related toxicities (4).
Standard first-line therapies, which typically involve
platinum-based chemotherapy, have demonstrated limited efficacy
towards SqCLC and are frequently associated with severe adverse
effects, which can be particularly debilitating for elderly
patients (5,6). These factors underscore the need for
alternative therapeutic approaches that offer a favorable balance
between efficacy and safety (7,8).
Anlotinib is an oral multi-targeted tyrosine kinase
inhibitor that has demonstrated promising antitumor activity in
various malignancies, including NSCLC (9). It exerts its effects by inhibiting
multiple pathways involved in tumor angiogenesis and proliferation,
including vascular endothelial growth factor receptors, fibroblast
growth factor receptors and platelet-derived growth factor
receptors. Previous clinical studies have demonstrated its efficacy
in prolonging progression-free survival (PFS) and overall survival
(OS) in patients with NSCLC, leading to its approval for use for
advanced stages of this disease (10,11).
Vinorelbine (NVB) is a semi-synthetic vinca alkaloid
that has been found to disrupt microtubule formation during cell
division, which exerts cytotoxic effects on rapidly proliferating
cancer cells (12). It has been
extensively used for the treatment of NSCLC, both as a monotherapy
(13,14) and in combination with other
chemotherapeutic agents (15). In
addition, the relatively mild toxicity profile of NVB compared with
other chemotherapeutics renders it a viable option for elderly
patients who may not tolerate more aggressive regimens (16,17).
Combining targeted therapies such as anlotinib with
traditional chemotherapeutic agents, such as NVB, represents a
strategic approach to enhance therapeutic efficacy whilst
potentially mitigating the dose-limiting toxicities associated with
single-agent therapy (18-20).
The rationale for this combination stems from their complementary
mechanisms of action, where anlotinib can inhibit key pathways in
tumor growth and angiogenesis, whilst NVB directly targets the cell
cycle. This dual approach may theoretically result in improved
tumor control by attacking the cancer through different biological
pathways (21,22). A previous study highlighted the
benefits of anlotinib in NSCLC, demonstrating significant
improvements in PFS and OS (9).
However, to the best of the authors' knowledge, there is a paucity
of data specifically addressing the combination of anlotinib and
NVB in the context of second-line treatment for elderly patients
with advanced SqCLC. Given the distinct biological behavior of
SqCLC and the specific considerations required for treating elderly
patients, there is a critical need to evaluate the efficacy and
safety of this combination therapy. Therefore, the present study
aimed to fill the existing knowledge gap by investigating the
clinical outcomes and safety profile of anlotinib combined with NVB
as a second-line treatment in elderly patients with advanced
SqCLC.
Patients and methods
Study design
The present study was a retrospective, multicenter
study conducted on elderly patients (aged ≥65 years; male-to-female
sex ratio is ~3:2) with advanced SqCLC who have previously failed
first-line treatment. The study protocol was approved (approval no.
HBCHEC2021108) by the Institutional Review Board Hubei Cancer
Hospital, affiliated with Tongji Medical College (Wuhan, China),
Since this is a retrospective study, the informed consent was
waived. Patient selection criteria was as follows: i) Age, ≥65
years; ii) histologically confirmed advanced SqCLC; iii) previous
failure of first-line treatment; iv) Eastern Cooperative Oncology
Group performance status of 0-2; v) adequate organ function; and
vi) absence of other active malignancies or medical conditions that
may affect treatment outcomes. Key exclusion criteria included
previous treatment included antitumor angiogenesis therapy and
chemotherapy with vincristine-containing regimens, symptomatic
brain metastasis, cachexia and the expectancy life of <3
months.
Treatment administration
Elderly patients with advanced SqCLC who progressed
after first-line chemotherapy received treatment with anlotinib
combined with oral NVB. Patients received anlotinib orally at a
standard dose of 12 mg once daily on days 1-14 of a 21-day cycle.
If adverse reactions are severe, the dose can be sequentially
reduced to 10 or 8 mg. If 8 mg cannot be tolerated, permanent
discontinuation of the drug would be considered. NVB is available
in capsule form for oral administration at a dose of 60 mg/m² once
a week. If adverse reactions are intolerable, the dose can be
sequentially reduced to 40 mg/m² and then to 20 mg/m². If the 20
mg/m² dose remains intolerable, permanent discontinuation of the
drug would be recommended. Each cycle of administration lasted 3
weeks, with treatment continuing until disease progression or the
occurrence of intolerable toxic side effects.
Assessment of efficacy and safety
The primary endpoints for efficacy evaluation are
OS, PFS, objective response rate (ORR) and disease control rate
(DCR). OS was the primary endpoint in the present study, which was
measured from the date of treatment initiation until the patient
succumbed to disease from any cause or last follow-up. PFS was
defined from treatment initiation to disease progression, based on
RECIST criteria or mortality. ORR was defined as the proportion of
patients achieving complete response (CR) or partial response (PR)
according to RECIST criteria. DCR was defined as the proportion of
patients achieving CR, PR or stable disease for ≥12 weeks according
to RECIST criteria. Adverse events (AEs) were assessed according to
the Common Terminology Criteria for AEs version 5.0. Laboratory
assessments, vital signs monitoring and physical examinations were
performed regularly, before they were used to classify the severity
of adverse reactions for adequate management during treatment
through dose reduction and symptomatic supportive care.
Statistical analysis
Descriptive statistics were used to summarize
patient demographics, baseline characteristics and treatment
compliance. Kaplan-Meier curves were applied to estimate OS and
PFS. Subgroup comparisons were conducted using the log-rank test.
The ORR and DCR were reported with corresponding confidence
intervals. Safety profiles were presented as frequency and
percentage of AEs. Data were statistically analyzed using SPSS 13.0
software, and graphs were generated using GraphPad Prism 5.0
Software (Dotmatics). A P-value of less than 0.05 was considered
statistically significant. The database used in the present study
can be accessed at the following URL: https://kmplot.com/analysis/index.php?p=service&cancer=lung.
Results
Patient demographics and baseline
characteristics
The present study enrolled 48 elderly patients (aged
≥65 years) with advanced SqCLC who had previously failed first-line
treatment. The median age of the patients was 72 years (range,
65-80 years), with a male predominance (58.33%). The patient
performance status scores ranged from 0 to 2, with 75% of patients
scoring 0 or 1 and 25% scoring 2. Baseline comorbidities (Table I) included hypertension (21%),
diabetes mellitus (15%) and chronic obstructive pulmonary disease
(18%). The proportions of patients with multiple metastases and
oligo-metastases were 81.25 and 18.75%, respectively. In addition,
the majority of patients had unknown PD-L1 expression status
(70.83%), with only a small proportion (<30%) undergoing PD-L1
testing on re-biopsy specimens. Among these patients, the
proportions of those with PD-L1 ≥1% and PD-L1 <1% were 8.34 and
20.83%, respectively. After disease progression, <30% of
patients received third-line treatment, with the treatment regimen
determined by clinicians based on the individual patient's
condition.
 | Table IBaseline clinical characteristics of
the study cohort. |
Table I
Baseline clinical characteristics of
the study cohort.
|
Characteristics | No. of patients
(%) |
|---|
| Age | |
|
Years | 72 |
|
Range | 65-80 |
| Sex | |
|
Male | 28 (58.33%) |
|
Female | 20 (41.67%) |
| Smoking
history | |
|
Never
smoker | 15 (31.25%) |
|
Former
smoker | 33 (68.75%) |
| ECOG score | |
|
0-1 | 36 (75.00%) |
|
≥2 | 12 (25.00%) |
| Previous
radiotherapy | |
|
Yes | 12 (25.00%) |
|
No | 36 (75.00%) |
| Brain
metastasis | |
|
Measurable | 11 (22.92%) |
|
Unmeasurable | 37 (77.08%) |
| Bone
metastasis | |
|
Yes | 34 (70.83%) |
|
No | 14 (29.17%) |
| Liver
metastasis | |
|
Yes | 8 (16.67%) |
|
No | 40 (83.33%) |
| Stage | |
|
IVA | 7 (14.58%) |
|
IVB | 19 (39.58%) |
|
IVC | 22 (45.83%) |
| Metastasis
type | |
|
Multi-site
metastases | 39 (81.25%) |
|
Oligo-metastases | 9 (18.75%) |
| PD-L1
expression | |
|
PD-L1(+) | 4 (8.34%) |
|
PD-L1(-) | 10 (20.83%) |
|
Unknown | 34 (70.83%) |
| Comorbidities | |
|
Yes | 15 (31.25%) |
|
No | 33 (68.75%) |
Efficacy outcomes
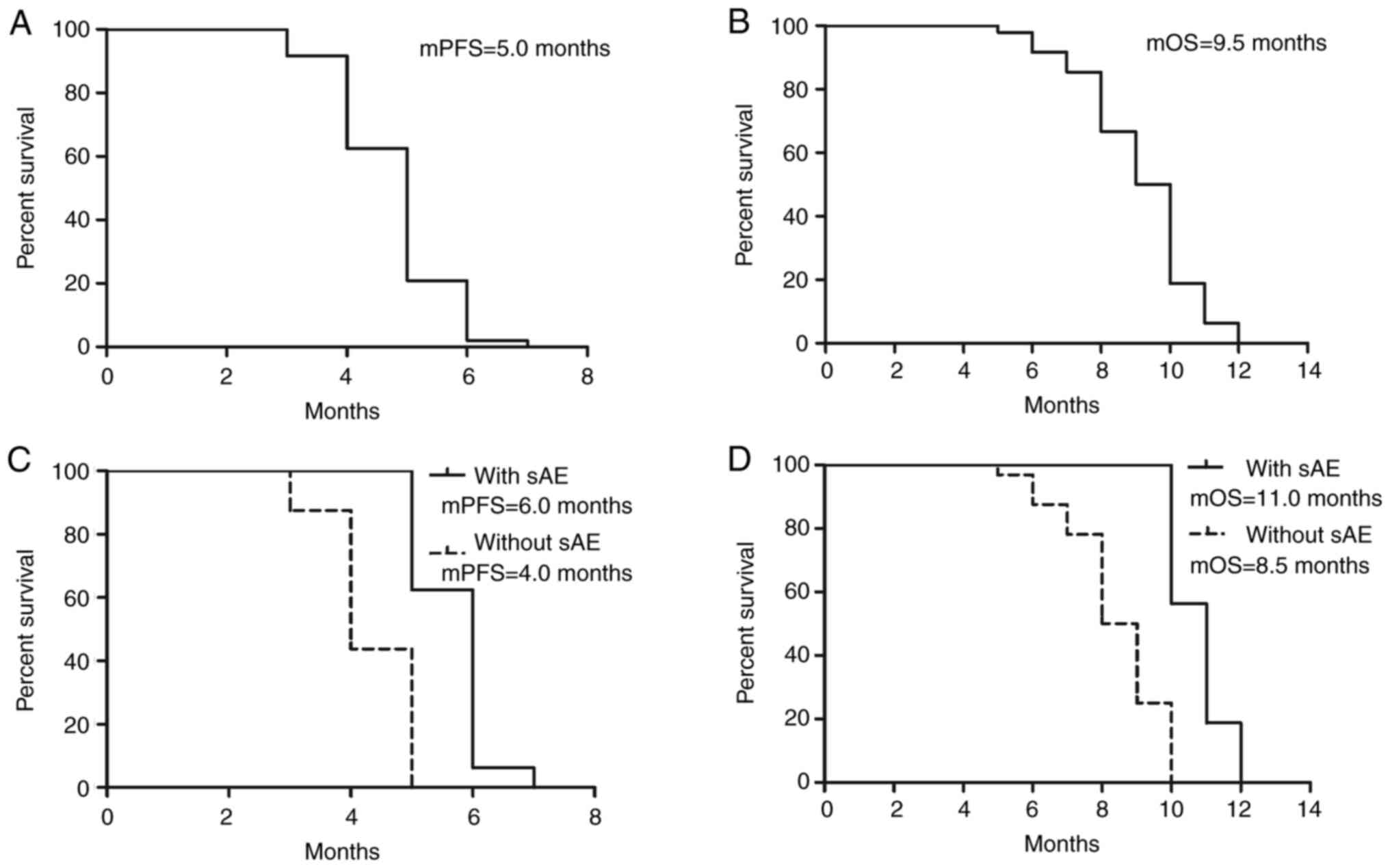
Preliminary results indicated that the median PFS
and OS for the drug combination of anlotinib and NVB in the second
line treatment of advanced SqCLC was 5.0 and 9.5 months,
respectively. In addition, the ORR was 29.17%, suggesting an
improvement compared with historical controls receiving
single-agent or best supportive care. Additionally, DCR was 70.83%
(Table II). This high rate of
disease control indicates that the combination regimen was
effective in managing tumor growth in the majority of patients
(Fig. 1; Table I).
 | Table IIClinical Activity of Anlotinib plus
NVB in advanced older cell lung carcinoma. |
Table II
Clinical Activity of Anlotinib plus
NVB in advanced older cell lung carcinoma.
| | Patient no. | Ratio |
|---|
| Complete
response | 0 | 0 |
| Partial
response | 14 | 29.17% (14/48) |
| Stable
response | 20 | 41.66% (20/48) |
| Progressive
disease | 14 | 29.17% (14/48) |
| Objective
response | | 29.17% |
| Disease control
rate | | 70.83% |
| Median
progression-free survival | | 5.0 months |
| Median overall
survival | | 9.5 months |
Safety and tolerability
The safety profile of the anlotinib-NVB combination
was consistent with known toxicities of the individual agents, but
no unexpected AEs were observed. Common hematological toxicities of
the regimen include leukopenia, neutropenia, anemia and
thrombocytopenia. Common non-hematological toxicities include
fatigue, decreased appetite, hypertension, proteinuria and
hand-foot syndrome, primarily of grade 1-2 severity. The incidence
of severe toxicities (grade 3 or higher) did not exceed 30%
(Table III). Dose modifications
were required in 24% patients due to AEs, primarily involving NVB.
Additionally, the proportion of patients who discontinued treatment
due to severe adverse reactions was 11%. Despite AEs, the overall
tolerability was deemed acceptable, particularly given the advanced
age and comorbidity burden of the patient population. Supportive
care measures, including dose modifications and symptomatic
treatments, successfully managed the majority of side effects.
 | Table IIIAdverse events of anlotinib plus NVB
in advanced older cell lung carcinoma. |
Table III
Adverse events of anlotinib plus NVB
in advanced older cell lung carcinoma.
| | Anlotinib plus NVB
(n (%)) |
|---|
| Adverse event | Any grade | Grade 3 or 4 |
|---|
| Hematological | | |
|
Leukopenia | 9 (18.75%) | 2 (4.17%) |
|
Neutropenia | 8 (16.67%) | 2 (4.17%) |
|
Anemia | 7 (14.58%) | 0% |
|
Thrombocytopenia | 7 (14.58%) | 0% |
|
Non-hematological | | |
|
Peripheral
neuropathy | 10 (20.83%) | 1 (2.08%) |
|
Hypertension | 12 (25.00%) | 3 (6.25%) |
|
Hand-foot
syndrome | 13 (27.08%) | 2 (4.17%) |
|
Proteinuria | 11 (22.92%) | 2 (4.17%) |
|
Elevated
transaminase | 6 (12.50%) | 1 (2.08%) |
|
Hyperbilirubinemia | 2 (4.17%) | 0% |
|
Bleeding | 0% | 0% |
|
Fatigue | 14 (29.17%) | 0% |
|
ALP
increased | 2 (4.17%) | 0% |
|
Elevated
gamma-glutamyl transpeptidase | 3 (6.25%) | 0% |
|
Abdominal
pain | 4 (8.33%) | 0% |
|
Decreased
appetite | 17 (35.42%) | 0% |
|
Hypoproteinemia | 3 (6.25%) | 0% |
|
Diarrhea | 4 (8.33%) | 0% |
|
Elevated
lactate dehydrogenase | 2 (4.17%) | 0% |
|
Oral
ulcer | 5 (10.42%) | 0% |
|
Stomatitis | 6 (12.50%) | 0% |
|
Dysphagia | 4 (8.33%) | 0% |
|
Dysphonia | 3 (6.25%) | 0% |
|
Rash | 2 (4.17%) | 0% |
Subgroup analysis
To further identify the beneficial patient
population for this treatment regimen, a subgroup analysis was
conducted. The results revealed that patients who experienced
specific AEs during treatment (mainly hypertension, proteinuria and
hand-foot syndrome) generally had superior efficacy compared with
those who did not experience these AEs [mPFS, 6.0 vs. 4.0 months;
hazard ratio (HR), 1.500; 95% confidence interval (CI),
0.9513-2.049; P<0.0001; mOS, 11.0 vs. 8.5 months; HR, 1.294; 95%
CI, 0.7454-1.843; P<0.0001; Fig.
1]. This suggests that recognizing these toxicities during
treatment may serve as potential markers for predicting treatment
efficacy (Fig. 1; Table I).
Discussion
The treatment of advanced SqCLC in elderly patients
presents substantial challenges due to the typically poor
performance status and comorbidities associated with this
population (2,3). Traditional chemotherapy regimens
frequently result in significant toxicity, which can outweigh the
benefits in frail patients (23).
Therefore, it is crucial to identify novel therapeutic strategies
that are both effective and tolerable for elderly individuals
(4).
Based on the current drug treatment models,
combination therapy is likely to become a trend in future treatment
development (24-26).
From the perspective of efficacy and tolerability, the combination
of anlotinib with metronomic chemotherapy using NVB has
demonstrated significant potential for application in elderly
patients with advanced SqCLC (19,27).
However, to the best of the authors' knowledge, there are currently
no similar research reports available (28). The present study primarily enrolled
elderly patients with advanced SqCLC. This decision was based on
existing research suggesting that age can serve as an independent
prognostic factor (29), a finding
also supported by data in the diagnosis and treatment of SqCLC
(30). However, there is still
some debate in the academic community regarding the definition of
‘elderly’ (31). Taking into
account the specific context of China (32,33)
and referring to relevant literature, in the present study
‘elderly’ was defined as individuals aged ≥65 (34-36).
Consistent with previous studies, the present results demonstrated
a notable improvement in ORR and PFS with the combination therapy
compared with historical controls receiving NVB monotherapy or best
supportive care (13,14,27).
Specifically, the ORR reached ~30%, which is encouraging given the
typically low response rates in this patient cohort. The median PFS
was extended to 5.0 months, suggesting a meaningful delay in
disease progression. These outcomes are particularly significant in
the context of treating elderly patients, who typically have
limited therapeutic options and lower tolerance for aggressive
treatments. Median OS was observed at 9.5 months, representing a
positive trend compared with existing second-line treatments for
this demographic population (7,16,37).
Furthermore, the present study included a portion of patients with
a performance status of 2 (accounting for 25% of the cohort), in
order to reflect the treatment needs of elderly patients with
advanced SqCLC (38,39). These patients have their own
characteristics, such as being often excluded from enrollment in
previous clinical studies, having poor tolerance to intravenous
chemotherapy, and suffering from multiple comorbidities such as
hypertension, diabetes and chronic obstructive pulmonary disease.
They also lack treatment options, and the efficacy is limited.
Therefore, from both the efficacy and safety perspectives, this
population is likely to represent a clinical application advantage
of this regimen (40,41). Additionally, the efficacy of this
regimen was found to be associated with the occurrence of specific
AEs (hypertension, proteinuria and hand-foot syndrome), suggesting
that the identification of these toxicities can serve as potential
markers for predicting treatment efficacy (42,43).
Safety and tolerability are crucial considerations
in the elderly population. The AEs observed in the present study
were consistent with the known profiles of anlotinib (44,45)
and NVB (46,47). Common AEs included fatigue,
hypertension, hand-foot syndrome and neutropenia. Importantly, the
majority of these AEs were manageable and reversible. Dose
reductions and supportive care measures were effective in
mitigating severe toxicities, allowing for the majority of patients
to continue treatment without interruption (46). Notably, no new or unexpected safety
signals emerged during the study period.
The combination of anlotinib and NVB appeared to
have provided a balance between efficacy and safety. The dual
approach targets both angiogenesis and cell division, potentially
overcoming resistance mechanisms that limit the effectiveness of
monotherapies (22,48-50).
In addition, the favorable safety profile supports the feasibility
of this regimen in elderly patients, who are frequently excluded
from clinical trials due to concerns about toxicity (2). Several factors may have contributed
to the observed benefits of this combination therapy. Anlotinib's
multi-targeted approach can effectively disrupt angiogenic
signaling, which is critical in SqCLC pathogenesis (51,52).
In addition, NVB's ability to induce mitotic arrest may have
complemented anlotinib's anti-angiogenic effects, enhancing overall
antitumor activity (22,53,54).
The oral administration of anlotinib and NVB can also provide
convenience and flexibility, improving adherence in the elderly
population (13,14,55).
However, certain limitations must be acknowledged. The present
study's sample size was relatively small and the single-arm design
limits direct comparisons with other treatment modalities.
Additionally, although subgroup analysis revealed a statistical
difference in efficacy between patients who did not experience
hypertension, proteinuria, hand-foot syndrome, and those who did,
the 95% CI was within 1 (with sAE vs. without sAE; mPFS 6.0 months
vs. 4.0 months; HR=1.500, 95% CI=0.9513-2.049; P<0.0001; mOS
11.0 months vs. 8.5 months; HR=1.294, 95% CI, 0.7454-1.843;
P<0.0001). This raises the question of whether this result is
due to an insufficient sample size in the present study, or whether
there is inherently little difference in efficacy between these two
groups. Alternatively, it may suggest that using treatment-related
AEs as a predictor of efficacy is not a reliable indicator, which
is clearly an issue worth further exploration. Future randomized
controlled trials with larger cohorts are necessary to validate
these findings and determine the optimal sequencing and dosing
strategies for anlotinib and NVB in this setting (25,48,56,57).
In conclusion, anlotinib combined with NVB
demonstrated promise as a second-line treatment for elderly
patients with advanced SqCLC, offering improved efficacy and a
manageable safety profile. This combination represents a viable
therapeutic option, addressing the unmet need for effective and
tolerable treatments in this vulnerable population. Ongoing
research and clinical trials should further elucidate the potential
of this regimen and refine its role in the management of advanced
SqCLC (1,58).
Acknowledgements
The authors would like to thank Mrs Xichen Wang
(MSDChina, Shanghai, China) for providing academic information
consulting support. The authors would also like to express their
gratitude to Professor Kaiyan Liu (Department of Urology, Zhejiang
Provincial People's Hospital, Hangzhou, China) for her editing and
proofreading of the manuscript.
Funding
Funding: The present study was supported by the Natural Science
Foundation of Hubei (grant no. 2019CFC929).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
YoL and WL were responsible for data analysis and
interpretation. YiL and YP collected the data. JT and XL
conceptualized and designed the study. All authors read and
approved the final version of the manuscript. JT and XL confirm the
authenticity of all the raw data.
Ethics approval and consent to
participate
The present study complied with the principles
outlined in the Declaration of Helsinki (revised in 2013). Ethical
approval (approval no. HBCHEC2021108) for this retrospective trial
was obtained from the Ethics Committee of Hubei Cancer Hospital,
affiliated with Tongji Medical College (Wuhan, China). Since this
is a retrospective study, the informed consent was waived.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Hsu EC, Wu KL, Tsai YM, Lee MH, Tsai MJ,
Kuo CY, Liu YC, Liang FW, Yang CJ and Hung JY: Real-world treatment
pattern and prognostic factors of stage IV lung squamous cell
carcinoma patients. Kaohsiung J Med Sci. 38:1001–1011.
2022.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Ganti AK, Klein AB, Cotarla I, Seal B and
Chou E: Update of incidence, prevalence, survival, and initial
treatment in patients with non-small cell lung cancer in the US.
JAMA Oncol. 7:1824–1832. 2021.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Santos ES and Rodriguez E: Treatment
considerations for patients with advanced squamous cell carcinoma
of the lung. Clin Lung Cancer. 23:457–466. 2022.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Basnet A, Alahmadi A and Gajra A: Older
patients with lung cancer: A summary of seminal contributions to
optimal patient care. Curr Oncol Rep. 24:1607–1618. 2022.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Novello S, Kowalski DM, Luft A, Gümüş M,
Vicente D, Mazières J, Rodríguez-Cid J, Tafreshi A, Cheng Y, Lee
KH, et al: Pembrolizumab plus chemotherapy in squamous
non-small-cell lung cancer: 5-year update of the phase III
KEYNOTE-407 Study. J Clin Oncol. 41:1999–2006. 2023.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Uprety D, Remon J and Peters S: First-line
dual immunotherapy, a treatment option in first-line metastatic
non-small-cell lung cancer: Are we ready to use it? J Clin Oncol.
42:378–382. 2024.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Lau SCM, Pan Y, Velcheti V and Wong KK:
Squamous cell lung cancer: Current landscape and future therapeutic
options. Cancer Cell. 40:1279–1293. 2022.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Filipits M: New developments in the
treatment of squamous cell lung cancer. Curr Opin Oncol.
26:152–158. 2014.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Han B, Li K, Wang Q, Zhang L, Shi J, Wang
Z, Cheng Y, He J, Shi Y, Zhao Y, et al: Effect of anlotinib as a
third-line or further treatment on overall survival of patients
with advanced non-small cell lung cancer: The ALTER 0303 phase 3
randomized clinical trial. JAMA Oncol. 4:1569–1575. 2018.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Zhang C, Kong FW, Wu WB, Zhang M, Yu GM,
Wang X and Liu YY: First-line pemetrexed and carboplatin plus
anlotinib for epidermal growth factor receptor wild-type and
anaplastic lymphoma kinase-negative lung adenocarcinoma with brain
metastasis: A case report and review of the literature. Medicine
(Baltimore). 99(e22128)2020.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Jiang S, Liang H, Liu Z, Zhao S, Liu J,
Xie Z, Wang W, Zhang Y, Han B, He J and Liang W: The impact of
anlotinib on brain metastases of non-small cell lung cancer: Post
Hoc Analysis of a Phase III Randomized Control Trial (ALTER0303).
Oncologist. 25:e870–e874. 2020.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Capasso A: Vinorelbine in cancer therapy.
Curr Drug Targets. 13:1065–1071. 2012.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Bilir C, Durak S, Kızılkaya B,
Hacıbekiroglu I, Nayır E and Engin H: Efficacy of metronomic
vinorelbine in elderly patients with advanced non-small-cell lung
cancer and poor performance status. Curr Oncol. 24:e199–e204.
2017.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Platania M, Pasini F, Porcu L, Boeri M,
Verderame F, Modena Y, Del Conte A, Nichetti F, Garassino MC,
Martinetti A, et al: Oral maintenance metronomic vinorelbine versus
best supportive care in advanced non-small-cell lung cancer after
platinum-based chemotherapy: The MA.NI.LA. multicenter, randomized,
controlled, phase II trial. Lung Cancer. 132:17–23. 2019.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Grossi F, Jaśkiewicz P, Ferreira M,
Czyżewicz G, Kowalski D, Ciuffreda L, Garcia-Gomez R, Caruso S,
Bosch-Barrera J, Gautier S, et al: Oral vinorelbine and cisplatin
as first-line therapy for advanced squamous NSCLC patients: A
prospective randomized international phase II study (NAVoTrial 03).
Ther Adv Med Oncol. 13(17588359211022905)2021.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Rossi D, Lippe P, Rocchi MBL, Sarti D,
Catalano V, Graziano F, Giordani P, Baldelli A, Fedeli SL,
Imperatori L, et al: Metronomic oral vinorelbine: an alternative
schedule in elderly and patients PS2 with local/advanced and
metastatic NSCLC Not Oncogene-addicted. In Vivo. 34:2687–2691.
2020.PubMed/NCBI View Article : Google Scholar
|
|
17
|
D'Ascanio M, Pezzuto A, Fiorentino C,
Sposato B, Bruno P, Grieco A, Mancini R and Ricci A: Metronomic
chemotherapy with vinorelbine produces clinical benefit and low
toxicity in frail elderly patients affected by advanced non-small
cell lung cancer. Biomed Res Int. 2018(6278403)2018.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Xiang M, Yang X, Ren S, Du H, Geng L, Yuan
L, Wen Y, Lin B, Li J, Zhang Y, et al: Anlotinib combined with S-1
in third- or later-line stage IV non-small cell lung cancer
treatment: A phase II clinical trial. Oncologist. 26:e2130–e2135.
2021.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Li DD, Tao ZH, Wang BY, Wang LP, Cao J, Hu
XC and Zhang J: Apatinib plus vinorelbine versus vinorelbine for
metastatic triple-negative breast cancer who failed
first/second-line treatment: the NAN trial. NPJ Breast Cancer.
8(110)2022.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Huang JY, Chen XL, Xie XF, Song L, Chen
LP, Lan XF, Bai X, Chen X and Du CW: The efficiency and safety of
low-dose apatinib combined with oral vinorelbine in pretreated
HER2-negative metastatic breast cancer. Cancer Med.
13(e7181)2024.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Xu H, Lv D, Meng Y, Wang M, Wang W, Zhou
C, Zhou S, Chen X and Yang H: Endostar improved efficacy of
concurrent chemoradiotherapy with vinorelbine plus carboplatin in
locally advanced lung squamous cell carcinoma patients with high
serum Lp(a) concentration. Ann Palliat Med. 9:298–307.
2020.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Ito K, Hamamichi S, Abe T, Akagi T,
Shirota H, Kawano S, Asano M, Asano O, Yokoi A, Matsui J, et al:
Antitumor effects of eribulin depend on modulation of the tumor
microenvironment by vascular remodeling in mouse models. Cancer
Sci. 108:2273–2280. 2017.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Liao BC, Shao YY, Chen HM, Shau WY, Lin
ZZ, Kuo RN, Lai CL, Chen KH, Cheng AL, Yang JC and Lai MS:
Comparative effectiveness of first-line platinum-based chemotherapy
regimens for advanced lung squamous cell carcinoma. Clin Lung
Cancer. 16:137–143. 2015.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Li X, Wu D, Tang J and Wu Y: The
efficiency and safety of triple-drug combination of albumin-bound
paclitaxel, anlotinib and PD-1/L1 Inhibitors in the 2(nd) or above
line of advanced NSCLC: A retrospective cohort study. Cancer Manag
Res. 16:1003–1012. 2024.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Tang J, Jiang H, Xiang Z, Zhu X, Xie R, Wu
D, Peng L and Li X: Apatinib plus docetaxel or pemetrexed shows
promising activities against non-small cell lung cancer with brain
metastasis: A retrospective analysis. J Thorac Dis. 16:615–622.
2024.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Yin C, Zou GR, He Y, Li J, Yan HW, Su Z,
Cao XL and Li XB: Efficiency and toxicity of nab-paclitaxel and
camrelizumab in the second or above line treatment of advanced
non-small cell lung cancer: A retrospective cohort study. J Thorac
Dis. 15:1838–1847. 2023.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Wang L, He Z, Yang S, Tang H, Wu Y, Li S,
Han B, Li K, Zhang L, Shi J, et al: The impact of previous therapy
strategy on the efficiency of anlotinib hydrochloride as a
third-line treatment on patients with advanced non-small cell lung
cancer (NSCLC): A subgroup analysis of ALTER0303 trial. Transl Lung
Cancer Res. 8:575–583. 2019.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Zhang X, Xiong Y, Xia Q, Wu F, Liu L, Zhou
Y, Zeng L, Zhou C, Xia C, Jiang W, et al: Efficacy and safety of
apatinib plus vinorelbine in patients with wild-type advanced
non-small cell lung cancer after second-line treatment failure: A
nonrandomized clinical trial. JAMA Netw Open.
3(e201226)2020.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Howard FM and Pearson AT: Prognosis and
treatment of non-small cell lung cancer in the age of deep
learning. JAMA Netw Open. 3(e206368)2020.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Kukulj S, Aukst Margetic B, Jakovljevic M
and Samarzija M: Temperament and character and quality of life in
lung cancer patients. Tumori. 99:708–714. 2013.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Okamoto J, Kubokura H and Usuda J: Factors
determining the choice of surgical procedure in elderly patients
with non-small cell lung cancer. Ann Thorac Cardiovasc Surg.
22:131–138. 2016.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Chen K, Yang D, Li F, Gao L, Tian Y, Xu B,
Xu X, Xu Q and Cao J: Changes in the symptom clusters of elderly
patients with lung cancer over the course of postoperative
rehabilitation and their correlation with frailty and quality of
life: A longitudinal study. Eur J Oncol Nurs.
67(102388)2023.PubMed/NCBI View Article : Google Scholar
|
|
33
|
She J, Yang P, Hong Q and Bai C: Lung
cancer in China: Challenges and interventions. Chest.
143:1117–1126. 2023.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Kwan SW, Mortell KE, Talenfeld AD and
Brunner MC: Thermal ablation matches sublobar resection outcomes in
older patients with early-stage non-small cell lung cancer. J Vasc
Interv Radiol. 25:1–9.e1. 2014.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Fang Z, He J, Fang W, Ruan L and Fang F:
Long-term outcomes of thoracoscopic anatomic resections and
systematic lymphadenectomy for elderly high-risk patients with
stage IB non-small-cell lung cancer. Heart Lung Circ. 25:392–397.
2016.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Ye X, Liu Y, Yang J, Wang Y, Cui X, Xie H,
Song L, Ding Z, Zhai R, Han Y, et al: Do older patients with stage
IB non-small-cell lung cancer obtain survival benefits from
surgery? A propensity score matching study using SEER data. Eur J
Surg Oncol. 48:1954–1963. 2022.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Lee SM, Schulz C, Prabhash K, Kowalski D,
Szczesna A, Han B, Rittmeyer A, Talbot T, Vicente D, Califano R, et
al: First-line atezolizumab monotherapy versus single-agent
chemotherapy in patients with non-small-cell lung cancer ineligible
for treatment with a platinum-containing regimen (IPSOS): A phase
3, global, multicentre, open-label, randomised controlled study.
Lancet. 402:451–463. 2023.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Sun L, Zhao Q, Wang Y, Wang Y, Zheng M,
Ding X and Miao L: Efficacy and safety of anlotinib-containing
regimens in advanced non-small cell lung cancer: A real-world
study. Int J Gen Med. 16:4165–4179. 2023.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Kokkotou E, Anagnostakis M, Evangelou G,
Syrigos NK and Gkiozos I: Real-world data and evidence in lung
cancer: A review of recent developments. Cancers (Basel).
16(1414)2024.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Nobili S, Lavacchi D, Perrone G, Vicini G,
Tassi R, Landini I, Grosso A, Roviello G, Mazzanti R, Santomaggio C
and Mini E: Vinorelbine in non-small cell lung cancer: Real-World
data from a single-institution experience. Oncol Res. 28:237–248.
2020.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Wang M, Mao M, Yang Y, Cai Z, Li Y, Chen
Y, Cai J and Ye Q: Safety and efficacy of anlotinib hydrochloride
capsules in advanced non-small-cell lung cancer: A multicenter,
real-world study. Future Oncol. 19:1729–1739. 2023.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Wei W, Ban X, Yang F, Li J, Cheng X, Zhang
R, Huang X, Huang Y, Li Q, Qiu Y, et al: Phase II trial of
efficacy, safety and biomarker analysis of sintilimab plus
anlotinib for patients with recurrent or advanced endometrial
cancer. J Immunother Cancer. 10(e004338)2022.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Jiang M, Zhang C, Hu Y, Li T, Yang G, Wang
G, Zhu J, Shao C, Hou H, Zhou N, et al: Anlotinib combined with
toripalimab as second-line therapy for advanced, relapsed gastric
or gastroesophageal junction carcinoma. Oncologist. 27:e856–e869.
2022.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Alshangiti A, Chandhoke G and Ellis PM:
Antiangiogenic therapies in non-small-cell lung cancer. Curr Oncol.
25 (Suppl 1):S45–S58. 2018.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Si X, Zhang L, Wang H, Zhang X, Wang M,
Han B, Li K, Wang Q, Shi J, Wang Z, et al: Management of
anlotinib-related adverse events in patients with advanced
non-small cell lung cancer: Experiences in ALTER-0303. Thorac
Cancer. 10:551–556. 2019.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Carlson K and Ocean AJ: Peripheral
neuropathy with microtubule-targeting agents: Occurrence and
management approach. Clin Breast Cancer. 11:73–81. 2011.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Noguchi E and Maeda Y:
Chemotherapy-induced peripheral neuropathy. Gan To Kagaku Ryoho.
38:1773–1776. 2011.PubMed/NCBI(In Japanese).
|
|
48
|
Xu B, Sun T, Wang S and Lin Y: Metronomic
therapy in advanced breast cancer and NSCLC: Vinorelbine as a
paradigm of recent progress. Expert Rev Anticancer Ther. 21:71–79.
2021.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Li X, Peng Y, Wu D, Tang J and Wu Y:
Efficacy and safety of anlotinib as maintenance therapy in patients
with advanced non-small cell lung cancer achieving SD post
first-line chemotherapy combined with immunotherapy. J Chemother:
Sep 1, 2024 (Epub ahead of print).
|
|
50
|
Glorieux C, Xia X, You X, Wang Z, Han Y,
Yang J, Noppe G, Meester C, Ling J, Robert A, et al: Cisplatin and
gemcitabine exert opposite effects on immunotherapy with PD-1
antibody in K-ras-driven cancer. J Adv Res. 40:109–124.
2022.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Blumberg N: Tumor angiogenesis factor.
Speculations on an approach to cancer chemotherapy. Yale J Biol
Med. 47:71–81. 1974.PubMed/NCBI
|
|
52
|
Cabebe E and Wakelee H: Role of
anti-angiogenesis agents in treating NSCLC: Focus on bevacizumab
and VEGFR tyrosine kinase inhibitors. Curr Treat Options Oncol.
8:15–27. 2007.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Zhang Y, Yang SH and Guo XL: New insights
into Vinca alkaloids resistance mechanism and circumvention in lung
cancer. Biomed Pharmacother. 96:659–666. 2017.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Laquente B, Viñals F and Germà JR:
Metronomic chemotherapy: An antiangiogenic scheduling. Clin Transl
Oncol. 9:93–98. 2007.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Wang J, Li X, Zhou J, Qiu D, Zhang M, Sun
L and Li SC: Long-term survival with anlotinib as a front-line
treatment in an elderly NSCLC patient: A case report. Front Oncol.
13(1043244)2023.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Qiang H, Chang Q, Xu J, Qian J, Zhang Y,
Lei Y, Han B and Chu T: New advances in antiangiogenic combination
therapeutic strategies for advanced non-small cell lung cancer. J
Cancer Res Clin Oncol. 146:631–645. 2020.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Li X, Wu D, Tang J and Wu Y: The
efficiency and safety of temozolomide and PD-1/L1 inhibitors in
pretreated NSCLC with brain metastasis: A retrospective cohort. J
Cancer Res Clin Oncol. 150(271)2024.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Niu Z, Jin R, Zhang Y and Li H: Signaling
pathways and targeted therapies in lung squamous cell carcinoma:
Mechanisms and clinical trials. Signal Transduct Target Ther.
7(353)2022.PubMed/NCBI View Article : Google Scholar
|