Introduction
Pleomorphic adenoma (PA) is the most common salivary
gland neoplasm (1). Fine-needle
aspiration (FNA) cytology is a well-established pre-operative
approach for salivary gland tumour evaluation (2-4).
The characteristic cytological features of PA, such as the presence
of a biphasic cell population composed of myoepithelial and ductal
cells and chondromyxoid material, are well recognised (5). Therefore, the cytological diagnosis
of PA is not difficult in most cases. However, the cytological and
histological features overlap with those of malignant neoplasms
(including predominant cellular components without the
characteristic chondromyxoid stroma and the presence of nuclear
atypia); thus, diagnosis is challenging in some cases (5,6).
Oncocytes are histopathologically characterised by
the presence of a rich eosinophilic granular cytoplasm owing to the
abundance of mitochondria, well-defined cell boundaries, and
hyperchromatic nuclei that accompany the nucleoli (7,8). In
individuals aged >50 years, oncocytic metaplasia is a common
finding in non-neoplastic ductal and acinar epithelial cells of the
salivary gland (7,8). Moreover, Warthin's tumour, the second
most common salivary gland tumour, as well as oncocytoma and
oncocytic carcinomas of the salivary gland, display characteristic
oncocytic morphology (9). PA
occasionally presents with various types of metaplastic changes,
including squamous metaplasia (5).
Oncocytic metaplasia is rare in PA but can pose diagnostic
challenges (7,10-14).
Moreover, only two cytological reports on oncocytic PA have been
published in the English literature (15,16),
and oncocytic neoplastic cells contain enlarged nuclei, leading to
overdiagnosis (16).
In 2018, the Milan System for Reporting Salivary
Gland Cytopathology (MSRSGC) was created as a standardised and
reproducible reporting system for salivary FNA cytology specimen
classification (17), with the
second edition published in 2023(18). MSRSGC risk stratification is based
on the assumed risk of malignancy (ROM) and recommendations of
therapeutic management for each tumour category (18). MSRSGC classifies tumours into seven
diagnostic cytomorphological-specific categories: I,
non-diagnostic; II, non-neoplastic; III, atypia of undetermined
significance (AUS); IVA, benign neoplasm; IVB, salivary gland
neoplasm of uncertain malignant potential (SUMP); V, suspicious for
malignancy; and VI, malignant (18). The MSRSGC system is useful for the
cytological diagnosis of salivary gland neoplasms (19-22).
Oncocytic neoplastic lesions with non-specific atypical
cytomorphological features are classified as SUMP (IVB) (18).
In this study, we have retrospectively analysed
patients with oncocytic PA of the salivary gland that underwent
preoperative FNA to describe the clinicopathological features of
salivary gland oncocytic PA.
Materials and methods
Patient selection
Patients diagnosed with oncocytic PA of the salivary
gland by postoperative pathological examination at Osaka Medical
and Pharmaceutical University Hospital (Osaka, Japan), who
underwent preoperative FNA from December 2020 to June 2023 were
included in the study.
This retrospective, single-institution study was
conducted in accordance with the tenets of the Declaration of
Helsinki. The study protocol was approved by the Institutional
Review Board of Osaka Medical and Pharmaceutical University
Hospital (approval #2023-073). All data were anonymised. Owing to
the retrospective study design, the Institutional Review Board
waived the requirement for informed consent, as medical records and
archived samples were used with no risk to the participants.
Moreover, the present study did not include children. Information
regarding this study, such as the inclusion criteria and
opportunity to opt-out, was provided using the institutional
website (https://www.ompu.ac.jp/u-deps/path/img/file19.pdf).
No statistical analysis was performed in this
article.
Cytological analysis
The FNA specimens were stained with Papanicolaou and
Giemsa stains. The cytological characteristics of the salivary
gland FNA specimens, such as background features (presence of
chondromyxoid material) and types of epithelial cells, were
evaluated.
MSRSGC (second edition) was used to classify FNA
specimens into the following seven categories: I, non-diagnostic;
II, non-neoplastic; III, AUS; IVA, benign neoplasm; IVB, SUMP; V,
suspicious for malignancy; and VI, malignant (18). At least two researchers
independently evaluated the cytological features of all
specimens.
Histopathological analysis
Surgically resected salivary gland specimens were
fixed in 10% buffered formalin, dehydrated, embedded in paraffin,
sectioned, and stained with haematoxylin and eosin. At least two
researchers independently evaluated the histopathological features
of all specimens. Histopathological features, such as the type of
epithelium and the presence of chondromyxoid material, were
evaluated and compared with the cytological features of the FNA
specimens. Oncocytic metaplasia was defined as the presence of
neoplastic cells containing rich eosinophilic granular cytoplasm,
well-defined cell boundaries, and hyperchromatic nuclei, with or
without nucleoli (7,8).
Immunohistochemical analysis
Immunohistochemical analysis was performed using an
autostainer (Discovery Ultra System; Roche Diagnostics) according
to the manufacture's instructions. 4-micrometer sections were
incubated with mouse monoclonal antibody against pleomorphic
adenoma gene 1 (PLAG1) (cat. no. 3B7; Abnova, dilution; 1:50) for
20 min at room temperature. Secondary antibodies were pre-diluted
and were used to incubate the sections for 8minutes at room
temperature [Optivew DAB Universal Kit (cat. no. 518-11427; Roche
Diagnostics)].
Results
Patient characteristics
Of the patients with PA of the salivary gland who
underwent pre-operative cytological and postoperative pathological
examinations at Osaka Medical and Pharmaceutical University
Hospital (n=142) between December 2020 and June 2023, only 2.1%
(n=3) of all patients had oncocytic PA. The clinicopathological
features of the study cohort are summarised in Table I. Ultimately, the study cohort
included three patients with oncocytic PA of the salivary gland
(Patients 1-3). This cohort included two males and one female. The
median age of the patients was 34 years (range: 22-51 years). The
study population comprised two males and one female. Patients 1-3
each had a lesion in the parotid gland (one and two patients on the
right and left sides, respectively). No molecular tests examining
PLAG1 fusions were performed in all three tumours.
 | Table IClinicocytological and pathological
features of oncocytic pleomorphic adenoma of the salivary gland in
the present series and previously reported patients. |
Table I
Clinicocytological and pathological
features of oncocytic pleomorphic adenoma of the salivary gland in
the present series and previously reported patients.
| | Cytological
features | Histopathological
features | |
|---|
| First author/s,
year | Patient | Age, years | Sex | Background | Epithelial
cells | Oncocytic
cells | Conventional
myoepithelial cells | Conventional ductal
cells | Initial MSRSGC | Oncocytic cells,
% | Chondromyxoid
material | (Refs.) |
|---|
| Present study | 1 | 22 | Male | Clean | Small clusters | 95% | 2% | 3% | IVB | 30 | + | - |
| Present study | 2 | 34 | Male | Myxoid
material | Small and large
clusters | 100% | None | None | V | 60 | + | - |
| Present study | 3 | 51 | Female | Myxoid
material | Small clusters | 95% | 5% | None | V | 60 | + | - |
|
Jiménez-Heffernanet al, 2001 | 4 | 61 | Male | Myxoid
material | NA | 100% | None | None | NA | >85 | + | (15) |
| Ito et al,
2020 | 5 | 62 | Female | Myxoid
material | NA | Present | Present | None | VI | 80 | + | (16) |
Cytological features
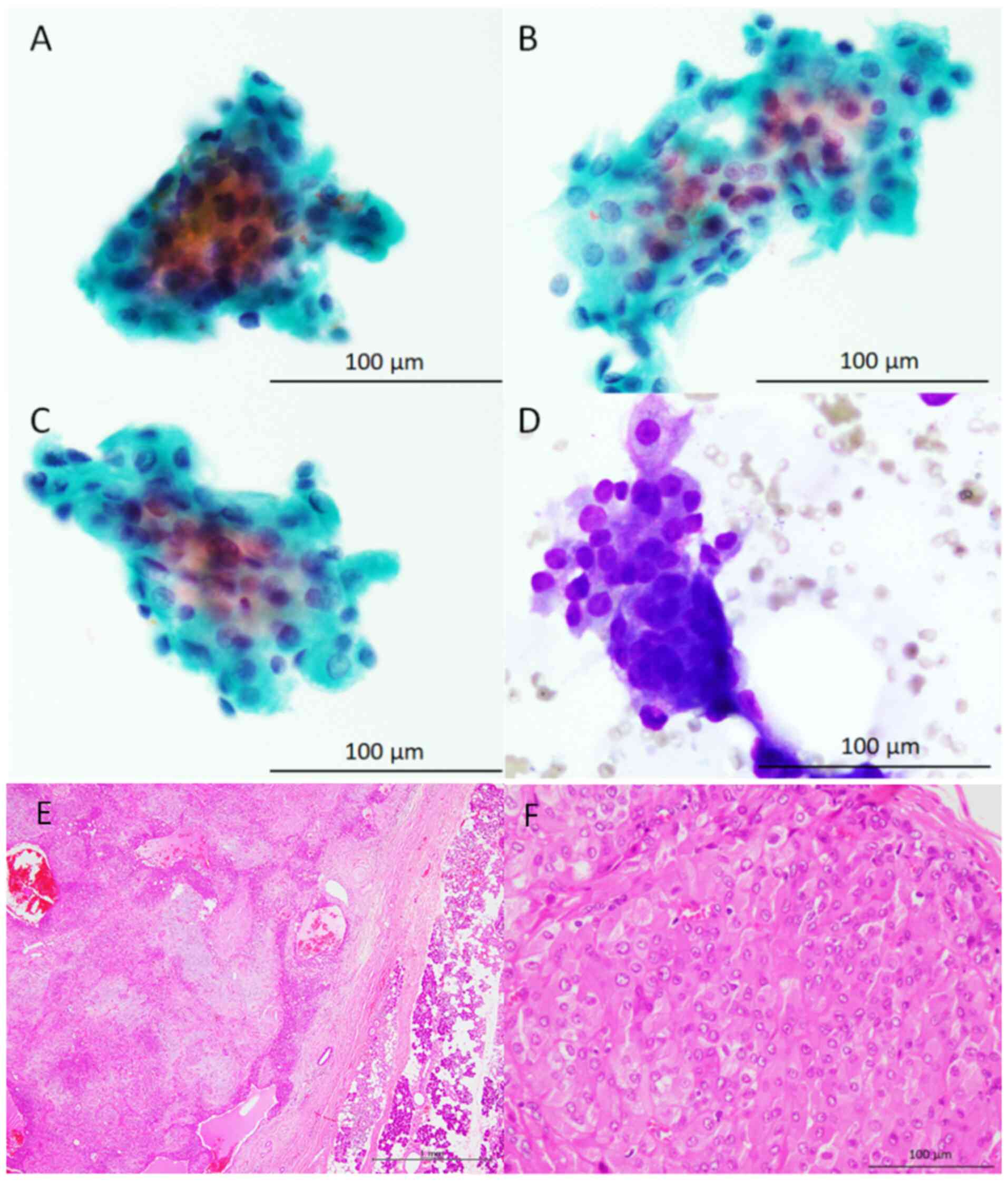
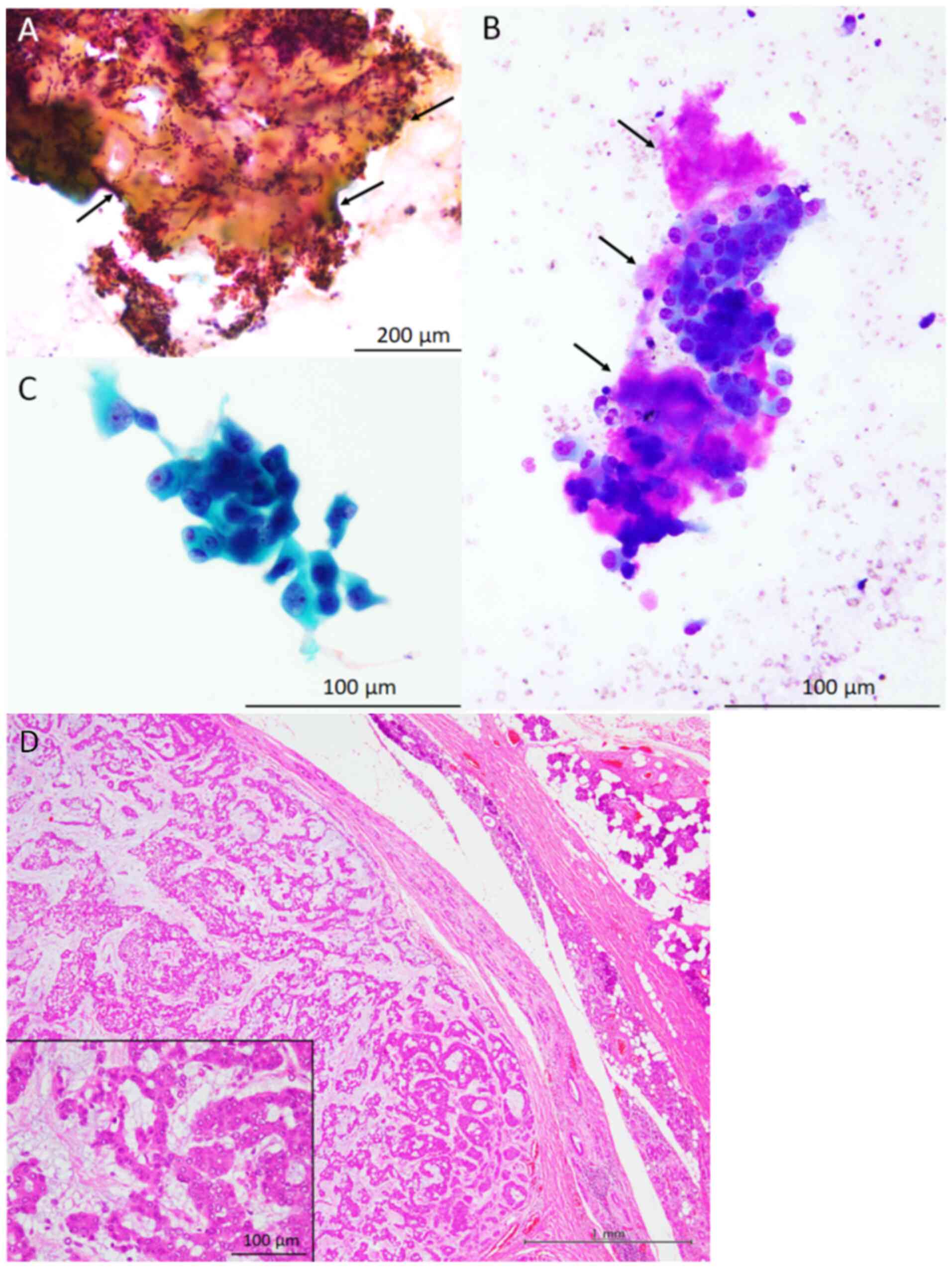
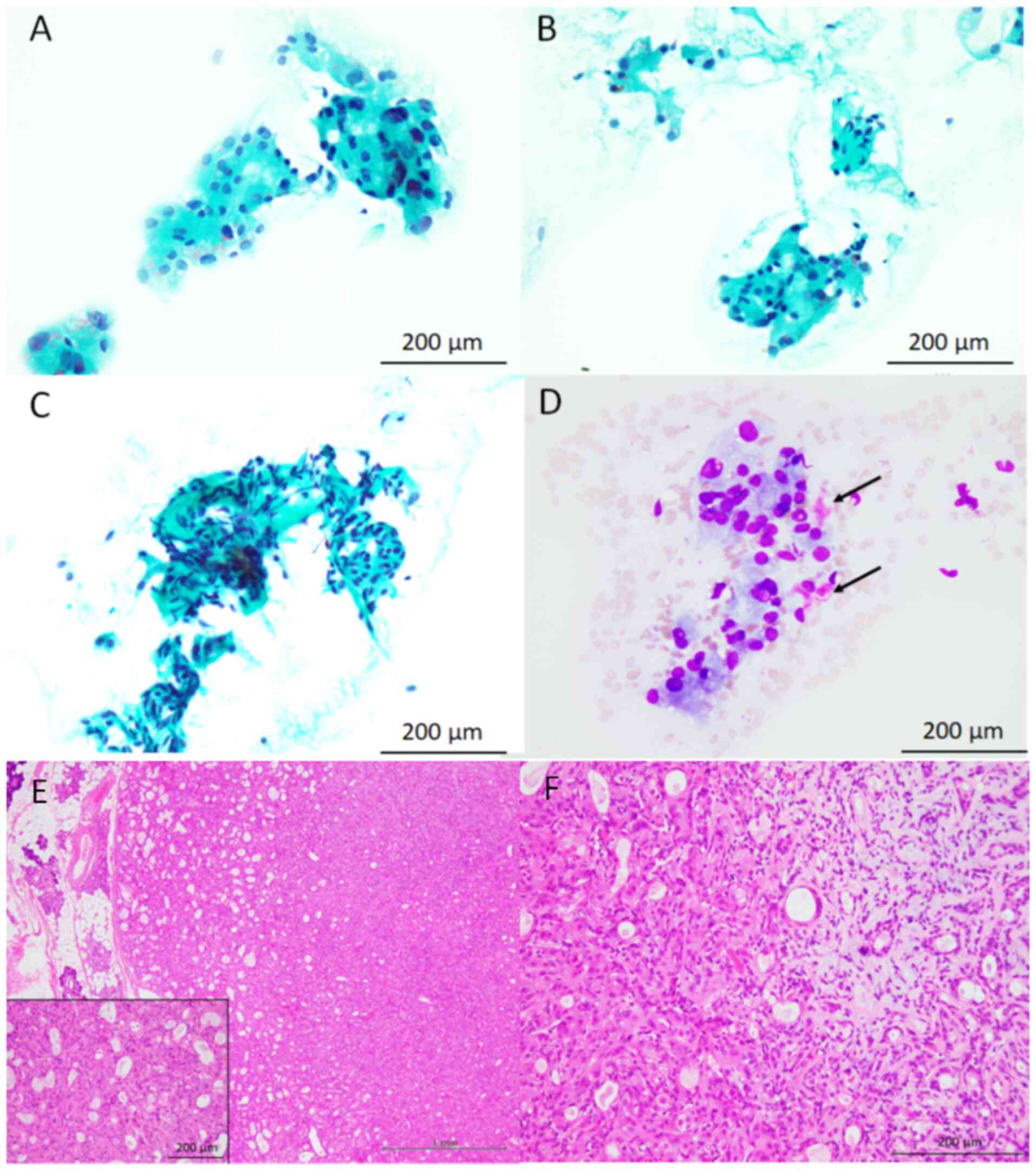
The cytological features of the study samples are
presented in Fig. 1, Fig. 2 and Fig. 3 (Table
I). Giemsa staining revealed myxoid material in two of the
three patient samples. In the remaining patient, a clear background
without myxoid material was observed. Necrotic material was not
observed in any of the specimens. Small and/or large clusters of
oncocytic cells, cytologically characterised by the presence of
rich granular cytoplasm and relatively large round nuclei
accompanying nucleoli, with a low nuclear/cytoplasmic ratio, were
observed in all three patients (95-100% of the epithelial cells
present in the cytological specimens were oncocytic cells).
Metachromatic material, revealed by Giemsa staining, was also
present around these oncocytic cells. A small number of
conventional bland myoepithelial and ductal cells were observed in
two of the three patient samples. No mitotic figures were observed
in any specimen.
The initial cytological diagnosis according to
MSRSGC (18) was SUMP (category
IVB) in patient 1 and suspicious for malignancy (category V)
(especially carcinoma ex pleomorphic adenoma (CXPA)) in patients 2
and 3.
Histopathological features
The histopathological features of the resected
parotid gland tumours are presented in Fig. 1, Fig.
2 and Fig. 3 (Table I). The resected specimens
demonstrated a relatively well-circumscribed tumour, and invasive
neoplastic growth into the surrounding salivary gland tissue was
not observed in any of the three tumour samples. The tumours were
primarily composed of neoplastic myoepithelial cells containing
small round-to-oval nuclei without rich eosinophilic granular
cytoplasm or occasional ductal formations. Although cellular
components without myxoid material were predominant in all tumours,
these neoplastic myoepithelial cells blurred into myxoid or
chondromyxoid material in at least some parts of the tumours.
Oncocytic cells containing rich granular eosinophilic cytoplasm and
relatively large round-to-oval nuclei with nucleoli were observed
in 30-60% of the tumours. Neither necrosis nor mitotic figures were
observed in the oncocytic cells of any of the tumours. Based on
these features, all patients were diagnosed with oncocytic PA.
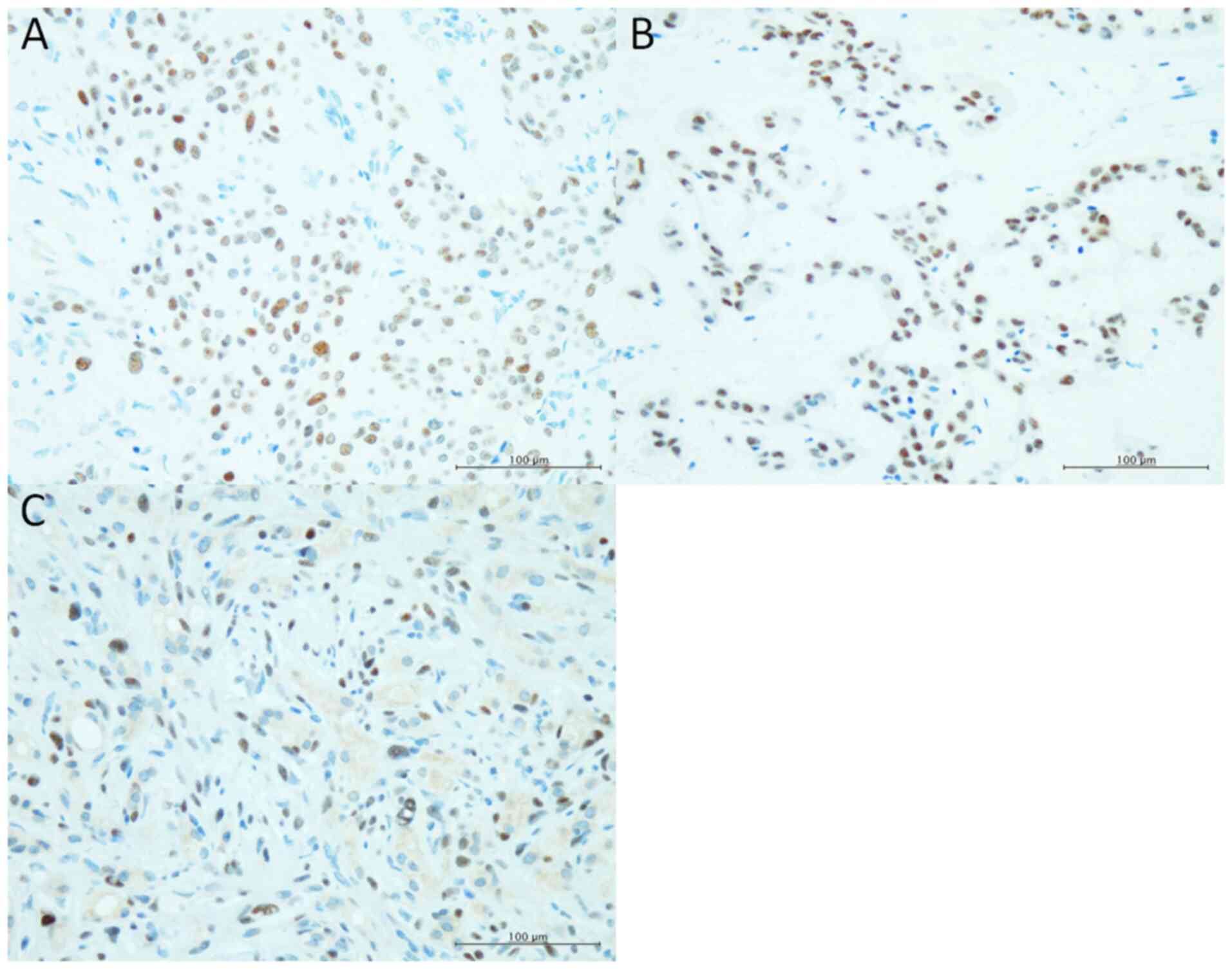
Immunohistochemical results
PLAG1 expression was noted in oncocytic cells of all
three tumours (Fig. 4).
Discussion
In this study, we describe the cytological features
of oncocytic PA. To the best of our knowledge, this is the first
cytological case series of this rare PA variant. Various types of
benign and malignant salivary gland tumours are known to have
oncocytic cells (9,23). Therefore, the presence of oncocytic
cells in cytological specimens from salivary gland FNA is a
well-known phenomenon, and oncocytic lesions represent a subset of
salivary gland tumours (18,23).
Oncocytic cells are observed in Warthin's tumours, acinic cell
carcinomas, mucoepidermoid carcinomas, secretory carcinomas, and
salivary duct carcinoma (SDC) (18,23).
Although rare, oncocytic cells have been observed in PA (7-14).
Skálová et al (7) reported
nine cases of oncocytic PA and 11 cases of oncocytic
myoepithelioma. The researchers described the histopathological
features of oncocytic PA and highlighted the oncocytic changes in
PA that could create challenges in the differential diagnosis of
salivary gland tumours. In particular, oncocytic cells in PA are
characterised by nuclear enlargement, hyperchromasia, and
pleomorphism, leading to confusion about the malignant nature of
the tumour (7). Although the
incidence of oncocytic PA in the major salivary glands remains
unknown (that of this cohort was 2.1%), a high frequency of
oncocytic metaplasia (47.6% 10 of 21 patients) in intraoral PA has
been reported (14). Di Palma
et al (11) reported a case
of oncocytic PA in which both conventional and oncocytic components
showed the same genetic amplification, indicating the same clonal
origin and subsequent acquisition of an oncocytic phenotype.
Advances in molecular genetics have meant that most PA cases are
now characterised by gene rearrangements resulting from gene
fusions containing PLAG1 or high mobility group 2
(24). Some oncocytic PA harbour
PLAG1 fusion, and in some cases, initially diagnosed
oncocytoma harbours PLAG1 gene rearrangements, indicating
that these tumours should be reclassified as pure-type oncocytic PA
(25). In addition, a recent study
demonstrated that pure oncocytic PA possesses a novel PLAG1
fusion, such as ZBTB47-AS1::PLAG1 (26). Accordingly, compared to
conventional PA, oncocytic PA may have distinct molecular
characteristics (25,26), and the molecular differences might
be present between pure type oncocytic PA and PA with focal
oncocytic cells (25,26). Disease concept and classification
between PA and oncocytoma may be changing. In addition, the lack of
the molecular tests examining PLAG1 fusions was a limitation
of the present report, although all of three oncocytic PA showed
positive immunoreactivity for PLAG1. Molecular tests may be useful
for diagnosis of salivary gland tumours showing oncocytic
feature.
Only two cytological reports exist on oncocytic PA
(15,16). The clinicopathological features of
the previously reported oncocytic PA cases, as well as those of the
present three patients, are summarised in Table I (15,16).
In this study, all the tumours were located in the parotid glands.
The median age of the patients was 51 years (range: 22-62 years).
Four of the five cytological specimens contained myxoid material
showing metachromasy with Giemsa staining in the background.
Although the proportion of oncocytic cells in the cytological
specimens was not available for one previously reported patient
(16), most of the neoplastic
cells present in the cytological specimens showed oncocytic
morphology (n=4) and conventional PA neoplastic cells were absent
in two of the patients. The histopathological features of the
resected tumour showed that oncocytic cells were predominant
(ranging from 30 to >85%), and cytological FNA specimens were
obtained from these regions. Avoiding malignant tumour
overdiagnosis remains crucial during oncocytic PA cytodiagnosis. As
described above, oncocytic cells have relatively large nuclei with
nucleoli; thus, malignancy is commonly overdiagnosed. The initial
cytological diagnoses for the present and previously reported
oncocytic PA cases were malignant (CXPA) (patient 5), suspicious
for malignancy (patients 2 and 3), undetermined (patient 1), and
oncocytoma (patient 4) (15,16).
Therefore, three of the five patients were suspected to have
malignancies. To avoid overdiagnosis, the presence of oncocytic
cells in PA specimens must be considered, and oncocytic PA must be
included in the differential diagnosis of oncocytic lesions of the
salivary gland (16,23).
MSRSGC has been widely used for the cytological
diagnosis of salivary gland tumours (19-22).
This system provides ROM and recommendations for therapeutic
management for each tumour category (18). In four patients with oncocytic PA
for whom information on MSRSGC was available, one, two, and one
patient were classified as SUMP (IVB), suspicious for malignancy
(V), and malignant (VI), respectively. This lesion should be
categorised as SUMP (oncocytic/oncocytoid neoplasm) (16).
Cytological differential diagnostic considerations
for oncocytic PA include various types of benign and malignant
salivary gland tumours with oncocytic features (23). The most important cytological
differential diagnosis is CXPA because myxoid material showing
metachromasy in the background suggests the presence of PA and the
presence of oncocytic neoplastic cells with large nuclei and
occasional nucleoli, which lead to the suspicion of carcinoma
cells. CXPA is defined as a carcinoma that develops from primary or
recurrent PA and accounts for 12% of all salivary gland
malignancies (27). Although
various histological subtypes of carcinoma occur as components in
CXPA, SDC, a common high-grade carcinoma of the salivary gland, is
the most frequent (27). The
characteristic cytological features of SDC are the presence of
small and large epithelial cell clusters in a necrotic background.
These neoplastic cells have large round to oval nuclei with
conspicuous nucleoli and a relatively rich eosinophilic cytoplasm
(28,29). In a review of the cytological
features of CXPA, both carcinoma and PA components were noted in
eight out of ten cytological specimens that can be cytodiagnosed as
CXPA (28). Thus, careful
observation enables the detection of carcinoma components, even in
specimens with small amounts of carcinoma components or when
carcinoma cells are intermingled within the PA component (28). These cytological features partially
resemble those of oncocytic PA in the present series. Two of three
tumours of the present series were cytodiagnosed as suspected for
malignancy (especially CXPA), because the most common lesion
containing rich eosinophilic cytoplasm and large nuclei in the
salivary gland tumour is SDC. The most important differential
diagnostic feature is the presence of necrotic material in CXPA and
the absence of necrosis in oncocytic PA (15,16,28).
Although oncocytic cells in PA have relatively large nuclei with
occasional nucleoli, typical SDC shows high-grade nuclear atypia
(28,29). Thus, the degree of nuclear atypia
and the necrosis status allows for differential diagnosis.
Immunohistochemical staining for PLAG1 may be useful
for diagnosing oncocytic PA (both pure oncocytic PA and PA with
focal oncocytic metaplasia) (25).
In the present series, all of three oncocytic PA showed positive
immunoreactivity for PLAG1. Moreover, the usefulness of
immunocytochemical staining for PLAG1 has been reported in
cytological specimens categorised as SUMP (30). Although SDC, a carcinoma component
of CXPA, also exhibits PLAG1 expression, other neoplastic lesions
showing oncocytic features, such as Warthin's tumour,
mucoepidermoid carcinoma, acinic cell carcinoma, and secretory
carcinoma, do not present with PLAG1 positivity (24). Thus, immunocytochemical analysis of
PLAG1 in oncocytic cells may be useful for detecting the origin of
PA.
In conclusion, we described the cytological features
of a series of cases of oncocytic PA in the salivary gland.
Although rare, oncocytic cells may be present in cytological
specimens of PA. These oncocytic cells have relatively large nuclei
with occasional nucleoli; thus, a carcinoma overdiagnosis,
particularly CXPA, is common. The absence of high-grade nuclear
atypia and necrotic material is an important diagnostic criterion
for the differential oncocytic PA diagnosis, and cytological
specimens with atypical oncocytic cells in PA should be categorised
as SUMP.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
NK and MI conceived the study. NK, MI, MO, HO, MT,
KA, IK, YN, MU, CD, SO, RT, TT, SIH and YH analysed the cytological
and/or clinicopathological data. NK and MI prepared the figures. NK
and MI wrote the original draft and edited the draft. NK and MI
confirm the authenticity of all the raw data. All authors have read
and approved the final version of the manuscript.
Ethics approval and consent to
participate
This study was conducted in accordance with the
tenets of the Declaration of Helsinki, and the study protocol was
approved by the Institutional Review Board of Osaka Medical and
Pharmaceutical University (protocol no. 2023-073; Takatsuki,
Japan). All data were anonymised. The Institutional Review Board
waived the requirement for informed consent due to the
retrospective study design with no risk of patient identity
exposure. In addition, the present study did not include
children.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Hernandez-Prera JC, Altemani AM, de Sousa
SOM, Faquin WC, Ihrler S, Katabi N, Wasserman JK and Weinreb I:
Pleomorphic adenoma. In: WHO Classification of Tumours. 5th
edition. IARC, Lyon, pp167-170, 2024.
|
|
2
|
Schmidt RL, Hall BJ, Wilson AR and
Layfield LJ: A systematic review and meta-analysis of the
diagnostic accuracy of fine-needle aspiration cytology for parotid
gland lesions. Am J Clin Pathol. 136:45–59. 2011.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Eytan DF, Yin LX, Maleki Z, Koch WM,
Tufano RP, Eisele DW, Boahene KDO, Fakhry C, Bishop JA, Westra WH
and Gourin CG: Utility of preoperative fine needle aspiration in
parotid lesions. Laryngoscope. 128:398–402. 2018.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Taniuchi M, Terada T and Kawata R:
Fine-needle aspiration cytology for parotid tumors. Life (Basel).
12(1897)2022.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Klijanienko J and Vielh P: Fine-needle
sampling of salivary gland lesions I. Cytology and histology
correlation of 412 cases of pleomorphic adenoma. Diagn Cytopathol.
14:195–200. 1996.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Hernandez-Prera JC, Skálová A, Franchi A,
Rinaldo A, Poorten VV, Zbären P, Ferlito A and Wenig BM:
Pleomorphic adenoma: The great mimicker of malignancy.
Histopathology. 79:279–290. 2021.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Skálová A, Michal M, Ryska A, Simpson RH,
Kinkor Z, Walter J and Leivo I: Oncocytic myoepithelioma and
pleomorphic adenoma of the salivary glands. Virchows Arch.
434:537–546. 1999.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Dardick I, Birek C, Lingen MW and Rowe PE:
Differentiation and the cytomorphology of salivary gland tumors
with specific reference to oncocytic metaplasia. Oral Surg Oral Med
Oral Pathol Oral Radiol Endod. 88:691–701. 1999.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Brandwein MS and Huvos AG: Oncocytic
tumors of major salivary glands. A study of 68 cases with follow-up
of 44 patients. Am J Surg Pathol. 15:514–528. 1991.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Pulitzer DR and Reitmeyer WJ: Oncocytic
pleomorphic adenoma of the parotid gland. J Surg Oncol. 34:198–201.
1987.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Di Palma S, Lambros MBK, Savage K, Jones
C, Mackay A, Dexter T, Iravani M, Fenwick K, Ashworth A and
Reis-Filho JS: Oncocytic change in pleomorphic adenoma: Molecular
evidence in support of an origin in neoplastic cells. J Clin
Pathol. 60:492–499. 2007.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Mariano FV, Vidaurre EC, Bologna-Molina
RE, Carlos-Bregni R and de Almeida OP: Histopathological and
immunohistochemical analysis of oncocytic pleomorphic adenoma.
Indian J Pathol Microbiol. 54:193–195. 2011.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Sarode GS, Sarode SC, Patil S and Anil S:
Oncocytic pleomorphic adenoma of palatal salivary gland with
macrophages and giant cells associated with cholesterol crystals.
Clin Pract. 6(884)2016.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Pérez-de-Oliveira ME, da Silva Leonel ACL,
de Castro JFL, Carvalho EJA, Vargas PA and da Cruz Perez DE:
Histopathological findings of intraoral pleomorphic adenomas: A
retrospective study of a case series. Int J Surg Pathol.
27:729–735. 2019.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Jiménez-Heffernan JA, Ortega L and Viguer
JM: Cytologic features of oncocytic pleomorphic adenoma. Diagn
Cytopathol. 24:147–148. 2001.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Ito H, Ishida M, Miyasaka C, Okano K,
Sandoh K, Fujisawa T, Iwai H and Tsuta K: Prominent oncocytic
metaplasia in pleomorphic adenoma: A potential diagnostic pitfall.
Diagn Cytopathol. 48:765–768. 2020.PubMed/NCBI View
Article : Google Scholar
|
|
17
|
Faquin WC, Rossi ES, Baloch Z, Barkan GA,
Foschini MP, Kurtycz DF, Pusztaszeri M and Vielh P: The Milan
system for reporting salivary gland cytopathology. Springer
International Publishing, AG Switzerland, 2018.
|
|
18
|
Faquin WC and Rossi ES: The Milan system
for reporting salivary gland cytopathology. Springer International
Publishing, AG. 2nd edition Switzerland, 2023.
|
|
19
|
Jalaly JB, Farahani SJ and Baloch ZW: The
Milan system for reporting salivary gland cytopathology: A
comprehensive review of the literature. Diagn Cytopathol.
48:880–889. 2020.PubMed/NCBI View
Article : Google Scholar
|
|
20
|
Higuchi K, Urano M, Akiba J, Nogami M,
Hirata Y, Zukeran Y, Moriyoshi K, Tada Y, Fukushima M, Obayashi M,
et al: A multi-institutional study of salivary gland cytopathology:
Application of the Milan system for reporting salivary gland
cytopathology in Japan. Cancer Cytopathol. 130:30–40.
2022.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Tochtermann G, Nowack M, Hagen C, Rupp NJ,
Ikenberg K, Broglie MA, Saro F, Lenggenhager D and Bode PK: The
Milan system for reporting salivary gland cytopathology-A
single-center study of 2156 cases. Cancer Med. 12:12198–12207.
2023.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Taniuchi M, Kawata R, Omura S, Haginomori
SI, Terada T, Higashino M, Kurisu Y and Hirose Y: A novel
clinically oriented classification of fine-needle aspiration
cytology for salivary gland tumors: A 20-year retrospective
analysis of 1175 patients. Int J Clin Oncol. 26:326–334.
2021.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Lubin D, Song S, Zafar HM and Baloch Z:
The key radiologic and cytomorphologic features of oncocytic and
oncocytoid lesions of the salivary gland. Diagn Cytopathol.
47:617–636. 2019.PubMed/NCBI View
Article : Google Scholar
|
|
24
|
Stenman G, Fehr A, Skálová A, Poorten VV,
Hellquist H, Mikkelsen LH, Saba NF, Guntinas-Lichius O,
Chiesa-Estomba CM, Andersson MK and Ferlito A: Chromosome
translocations, gene fusions, and their molecular consequences in
pleomorphic salivary gland adenomas. Biomedicines.
10(1970)2022.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Baněčková M, Uro-Coste E, Ptáková N,
Šteiner P, Stanowska O, Benincasa G, Colella G, Vondrák J Jr,
Michal M, Leivo I and Skálová A: What is hiding behind S100 protein
and SOX10 positive oncocytomas? Oncocytic pleomorphic adenoma and
myoepithelioma with novel gene fusions in a subset of cases. Hum
Pathol. 103:52–62. 2020.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Alsugair Z, Perrot J, Descotes F, Lopez J,
Champagnac A, Pissaloux D, Castain C, Onea M, Céruse P, Philouze P,
et al: Characterization of a molecularly distinct subset of
oncocytic pleomorphic adenomas/myoepitheliomas harboring recurrent
ZBTB47-AS1::PLAG1 gene fusion. Am J Surg Pathol. 48:551–561.
2024.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Griffith CC, Thompson LDR, Assaad A,
Purgina BM, Lai C, Bauman JE, Weinreb I, Seethala RR and Chiosea
SI: Salivary duct carcinoma and the concept of early carcinoma ex
pleomorphic adenoma. Histopathology. 65:854–860. 2014.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Okano K, Ishida M, Sandoh K, Fujisawa T,
Iwai H and Tsuta K: Cytological features of carcinoma ex
pleomorphic adenoma of the salivary glands: A diagnostic challenge.
Diagn Cytopathol. 48:149–153. 2020.PubMed/NCBI View
Article : Google Scholar
|
|
29
|
Klijanienko J, El-Naggar AK and Vielh P:
Fine-needle sampling findings in 26 carcinoma ex pleomorphic
adenomas: Diagnostic pitfalls and clinical considerations. Diagn
Cytopathol. 21:163–166. 1999.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Sanchez-Avila M, Tjendra Y, Zuo Y,
Ruiz-Cordero R, Garcia-Buitrago M, Jorda M, Gomez-Fernandez C and
Velez Torres JM: Don't SUMP it! Utility of PLAG1
immunocytochemistry in basaloid SUMP subcategory. Cancer
Cytopathol. 132:60–68. 2024.PubMed/NCBI View Article : Google Scholar
|