Introduction
Ovarian cancer can metastasize to the peritoneum,
omentum, lymph nodes, liver, lungs, and bones (1). However, reports on bone marrow
metastasis (BMM) in ovarian cancer are rare (2-5).
Most cancers that metastasize hematogenously are thought to have
the potential to metastasize to the bone marrow; however, the
frequency is low at approximately 1% (2,6,7).
When cancers metastasize to the bone marrow, they rapidly progress
to bone marrow carcinomatosis, a life-threatening condition
characterized by disseminated intravascular coagulation (DIC) and
microangiopathic hemolytic anemia. Although BMM generally has a
poor prognosis, one prior study (8) revealed that appropriate supportive
care and aggressive anti-tumor therapy tailored to the patient's
condition might improve the prognosis. As such, early diagnosis is
key in BMM. BMM is frequently detected late because clinicians have
a poor understanding of the condition owing to its rarity,
nonspecific symptoms, and blood test abnormalities, which can
easily be mistaken for bone marrow suppression caused by
chemotherapy. Herein, we present a two-center retrospective study
and literature review of BMM caused by ovarian cancer.
Materials and methods
Retrospective analysis
We retrospectively analyzed the existing medical
records of patients admitted to Oita University Hospital and
Saitama Medical University International Medical Center between
April 2014 and March 2023. We searched the medical records for
cases that met both the criteria of ‘ovarian cancer’ and ‘bone
marrow metastasis.’ One case was identified from each institution.
The Institutional Review Board of the Ethics Committee of each
institution approved this study (approval Code: 2586 and 16-257,
approval Date: August 4, 2023 and September 5, 2018 respectively).
Data were extracted from obstetric and gynecological records of
patients diagnosed with BMM of ovarian cancer. This study adhered
to the guidelines of the Declaration of Helsinki and was performed
in conjunction with the prevailing ethical regulations. All
patients provided written informed consent for publication. Patient
information, including the age at diagnosis, chief complaint,
history of pregnancy and delivery, past medical history, family
history, clinical courses, treatment methods, blood tests, clinical
imaging, pathological findings, and outcome, was collected.
Literature review
The PubMed® database was searched from
inception until June 2024. The keywords used were ‘ovarian cancer,’
‘ovarian carcinoma,’ ‘bone marrow metastasis,’ ‘bone marrow
carcinomatosis,’ and ‘disseminated carcinomatosis of bone marrow.’
Junya Nakajima and Mitsutake Yano screened titles, abstracts, and
full-text articles. Articles/case reports discussing BMM of ovarian
cancer were included. The article also needed to be available in
English, and either be open-access or available through the library
of Oita University or Saitama Medical University International
Medical Center. Articles with incomplete data, articles for which
original data could not be obtained, conference abstracts, or
animal/in vitro studies were excluded. Patient information,
including the number of cases, age at diagnosis, chief complaint,
clinical courses, treatment methods, blood tests, clinical imaging,
pathological findings, and outcome, was collected.
Results
Patient characteristics
We identified two and six cases of BMM of ovarian
cancer in our institutions and the literature review, respectively.
Table I summarizes these cases
(2-5).
Eight cases have been reported; however, detailed pieces of
information, such as blood tests, imaging tests, symptoms, and
clinical courses, were only described in the present two cases. The
patients' ages ranged from 37 to 71 years (median 51 years), while
tumor histology was described in five of the eight cases. Notably,
all three previous cases involved rare histological types (small
cell carcinoma, carcinosarcoma, and mucinous carcinoid) of ovarian
cancer, and only the present two cases involved the common
histological types (high-grade serous carcinoma and clear cell
carcinoma). Two of the three available cases had a history of
chemotherapy; however, only case 1 received platinum-based
chemotherapy and maintenance chemotherapy with poly (adenosine
diphosphate-ribose) polymerase (PARP) inhibitors, which are the
standard treatments for epithelial ovarian cancer. Detailed
information regarding two cases in our institutes is provided
below.
 | Table IReview of the present and previously
published cases involving bone marrow metastases of ovarian
cancer. |
Table I
Review of the present and previously
published cases involving bone marrow metastases of ovarian
cancer.
| First author/s,
year | Histological
type | Patients, n | Agea, years | Other
lesionsa | LDHa, IU/l | ALPa, U/l | Previous
chemotherapy | Maintenance
chemotherapy | Hematological
abnormalitiesa | (Refs.) |
|---|
| Present case 1 | High-grade serous
carcinoma | 1 | 71 | None | 2,712 | 81 | TC, 12 courses | Olaparib for 18
months | Pancytopenia,
DIC | - |
| Present case 2 | Clear cell
carcinoma | 1 | 51 | Only bones | 712 | 526 | No | No | Pancytopenia,
DIC | - |
| Xiao et al,
2009 | NA | 3 | NA | NA | NA | NA | NA | NA | NA | (2) |
| Saikia et al,
2005 | Small cell carcinoma,
hypercalcemic type | 1 | NA | NA | NA | NA | NA | NA | Hypercalcemia | (3) |
| Sreenan and Hart,
1995 | Carcinosarcoma | 1 | NA | NA | NA | NA | NA | NA | NA | (4) |
| Alenghat et
al, 1986 | Mucinous
carcinoid | 1 | 37 | NA | NA | NA | Cyclophosphamide,
doxorubicin and 5- fluorouracil | NA | NA | (5) |
Case 1
A 64-year-old woman visited Oita University Hospital
for evaluation of a pelvic mass. A summary of the patient's
clinical course is illustrated in Fig.
1. The patient experienced frequent urination and constipation.
Contrast-enhanced magnetic resonance imaging (MRI) and computed
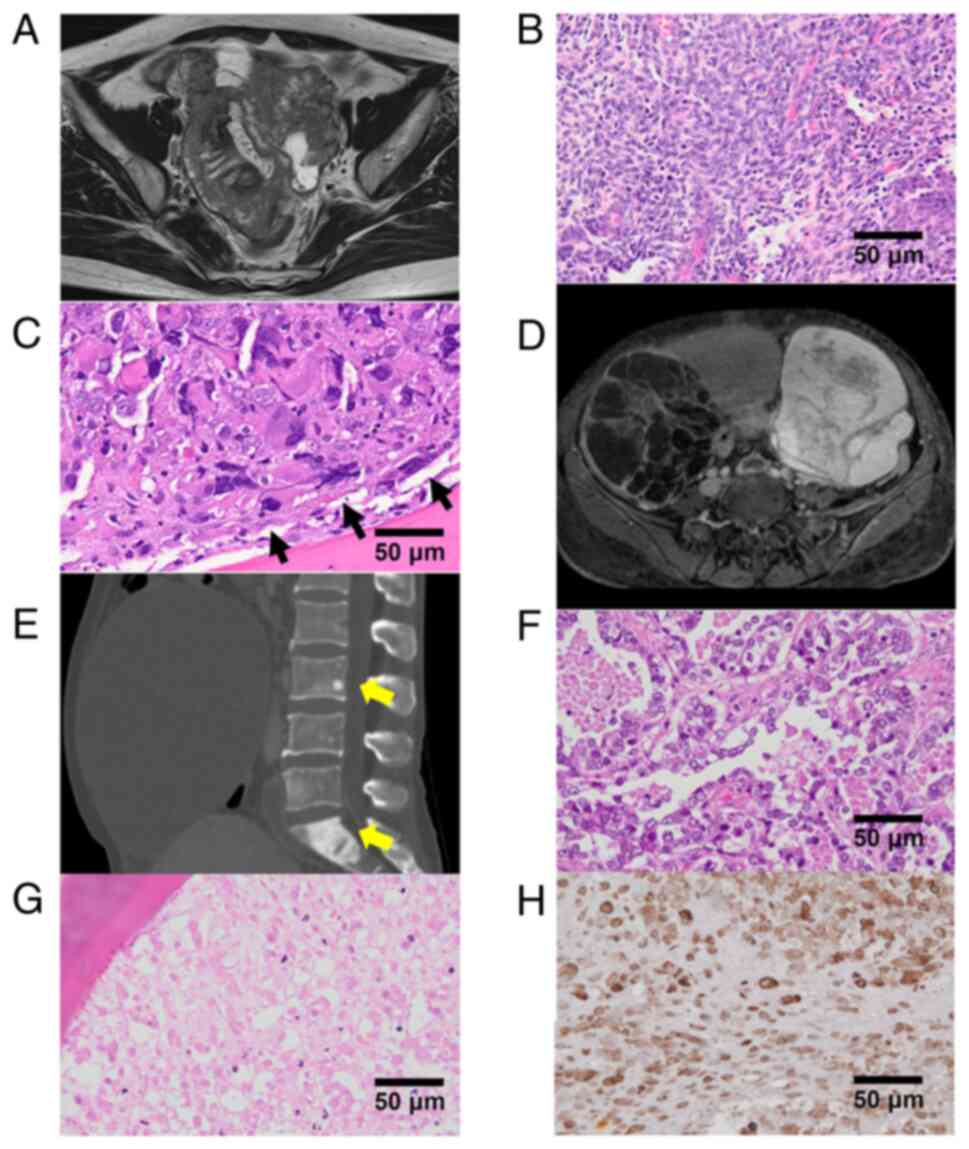
tomography (CT) revealed an 80 mm-sized pelvic tumor with
peritoneal dissemination, enlarged pelvic lymph nodes, and ascites
(Fig. 2A). The serum cancer
antigen 125 (CA125) level was elevated (7,198 U/ml). Tumor biopsy
revealed a high-grade serous carcinoma. The patient was diagnosed
with stage IIIC ovarian cancer (cT3cN1M0). Debulking surgery
(including hysterectomy and bilateral salpingo-oophorectomy), with
each of three courses of neoadjuvant and adjuvant chemotherapy
(paclitaxel: 80 mg/m2 [weekly]; carboplatin: area under
the blood concentration-time curve [AUC], 6 [tri-weekly]), was
performed. All tumors were resected. Postoperative histological
examination revealed stage IIIC ovarian cancer (ypT3cN1M0)
(Fig. 2B). An isolated splenic
metastasis was detected 43 months after the final round of
chemotherapy. The patient subsequently underwent abdominal
splenectomy and six courses of adjuvant chemotherapy (paclitaxel:
180 mg/m2; carboplatin: AUC 6, tri-weekly). A complete
response was obtained, and olaparib was initiated. Precisely 19
months after initiation, olaparib was withdrawn because of Common
Terminology Criteria for Adverse Events (CTCAE) grade 3 anemia.
After 1 month, CTCAE grade 3 anemia and thrombocytopenia were
diagnosed, and a blood transfusion was performed. This point was
retrospectively considered to be the onset of BMM based on
persistent cytopenia despite olaparib withdrawal and elevated CA125
levels. Exactly 1 month later, severe anemia and thrombocytopenia
continued, and she had a fever of 38.7˚C. Her blood tests revealed
the following levels: C-reactive protein (CRP), 11.11 mg/dl; white
blood cell (WBC), 3.72x103 cells/µl; hemoglobin, 6.1
g/dl; platelet, 1.7x103 cells/µl; aspartate
aminotransferase (AST), 74.6 U/l; lactate dehydrogenase (LDH),
2,712 U/l; and alkaline phosphatase (ALP), 81 U/l. The CA125 level
was elevated to 117 U/ml. Contrast-enhanced CT revealed no obvious
recurrence or focus of infection. Bone marrow biopsy revealed
BMM/carcinomatosis of ovarian cancer (Fig. 2C). Immunohistochemically, the BMM
tumor cells were positive for p53 and negative for AE1/AE3. The
patient died owing to BMM of ovarian cancer and DIC 4 weeks
following BMM onset and 1 week after admission/serum LDH
elevation.
 | Figure 1Summary of the clinical course of case
1, including the (A) course of treatments, (B) blood cell counts,
and (C) serum CA125 and LDH levels. CA125, cancer antigen 125; CR,
complete response; Hb, hemoglobin; LDH, lactate dehydrogenase; PD,
progressive disease; Plt, platelets; RECIST, Response Evaluation
Criteria in Solid Tumors; SDS, secondary debulking surgery; TC,
combination of paclitaxel and carboplatin; WBC, white blood
cell. |
Case 2
A 51-year-old woman was admitted to Saitama Medical
University International Medical Center owing to abdominal
bloating, severe anemia, and elevated CA125 levels.
Contrast-enhanced MRI and CT revealed bilateral ovarian tumors
(Fig. 2D) and multiple bone masses
(Fig. 2E). No peritoneal
dissemination or lymph node metastasis were observed. Her blood
tests revealed the following levels: CRP, 15.28 mg/dl; WBC,
12.11x103 cells/µl; hemoglobin, 3.3 g/dl; platelet,
426x103 cells/µl; AST, 22 U/l; LDH, 339 U/l; and ALP,
526 U/l. The CA125 level was elevated to 3,306 U/ml. The patient
underwent abdominal total hysterectomy, bilateral
salpingo-oophorectomy, and omentectomy. Intraoperative findings
revealed no peritoneal dissemination or ascites. Her peritoneal
cytology results were negative for malignancy. On postoperative day
5, the patient experienced hemorrhagic shock caused by bleeding
from the internal iliac artery, for which hemostasis was achieved
using interventional radiology. Pathologically, the right and left
ovarian tumors were clear cell carcinoma (Fig. 2F) and endometriotic cysts,
respectively. On postoperative day 21, bone marrow biopsy revealed
carcinomatous necrosis with immunohistochemical AE1/AE3 positivity
(Fig. 2G and H). Subsequent positron emission
tomography (PET)-CT confirmed the presence of multiple bone
metastases without other metastases. The patient was diagnosed with
stage IVB ovarian cancer (pT1aN0M1b, clear cell carcinoma) and
BMM/carcinomatosis. She did not receive systemic chemotherapy
because of her poor general health. She died of BMM and DIC 50 days
postoperatively.
Discussion
This is the first retrospective study of BMM of
ovarian cancer. To date, only eight cases of BMM of ovarian cancer
have been reported. However, we believe that the frequency of BMM
of ovarian cancer has been underestimated. BMM has been observed in
autopsies of 6-79% of patients with breast cancer; however, only
27% of cases were clinically diagnosed prior to autopsy (9). BMM is frequently accompanied by bone
metastases (10). In stage IV
ovarian cancer, bone metastasis accounts for 5% of cases and is the
fourth most common site for distant metastasis (1). Among BMM cases, ovarian cancer
accounts for 6% of cases and is the fifth most common cancer. As
BMM of ovarian cancer is less rare than previously thought,
clinicians should be aware of its characteristics and diagnostic
markers. Additionally, BMM can occur not only in special cases, but
also in common histological types treated with standard
chemotherapy. To date, BMM of ovarian cancer has only been reported
in cases involving rare histological subtypes, such as small cell
carcinoma (hypercalcemic type), carcinosarcoma, and mucinous
carcinoids, with frequencies of <1% (11), 2% (12), and <1% (13), respectively. The present study
provided detailed clinical information regarding BMM of high-grade
serous carcinoma (approximately 70%) (14), the most common histological type of
ovarian cancer, and clear cell carcinoma (10-27%) (14,15),
the second or third most common histological type, especially in
East Asia. Furthermore, Case 1 is the first reported case of BMM
occurring during standard chemotherapy for epithelial ovarian
cancer (platinum-based chemotherapy and maintenance PARP
inhibitor).
The difficulty in diagnosing BMM stems from a lack
of established diagnostic markers. ALP and LDH are both considered
useful diagnostic markers for BMM (8); however, these markers may not aid in
early diagnosis. Al-Ibraheem et al (16) previously reported a case of
laryngeal cancer with oligo-BMM without elevated ALP levels. In
cases of isolated, few, or early BMM, these markers are not
elevated, and may therefore not serve as diagnostic markers. In
contrast, Chan et al (17)
reported that serum carcinoembryonic antigen (CEA) is a potentially
useful diagnostic marker of BMM in colorectal cancer. Wang et
al (18) also showed that the
serum CEA levels were more frequently elevated than the ALP or LDH
levels in BMM of lung adenocarcinoma. In another study, the serum
prostate-specific antigen level was correlated with the presence of
micro-BMM in prostate cancer (19). In Case 1, elevated CA125 levels and
decreased blood counts were detected earlier than LDH and ALP
levels. These results indicate that a combination of serum tumor
markers and blood counts could be useful in the early detection of
BMM. PET-CT is useful for evaluating bone metastasis as a predictor
of BMM (8); however, bone marrow
biopsy should be considered because of isolated BMM without other
lesions, including bone lesions, as seen in Case 1 and the study by
Chan et al (17). Bone
marrow biopsy is also useful for ruling out secondary leukemias.
Additionally, poorly differentiated cancers have a higher
occurrence of BMM and poorer prognosis than well-differentiated
gastric (20,21) and breast cancers (22,23).
In the present case 1, BMM after long-term chemotherapy became
morphologically and immunohistochemically (AE1/AE3 became negative)
poorly differentiated. Notably, some preclinical studies have
demonstrated that long-term chemotherapy induces cancer stemness
and tumor-promoting cytokines/chemokines (24,25).
Clinicians should consider BMM during or after long-term
chemotherapy.
BMM rapidly leads to death owing to severe blood
cell depletion and DIC. Niu et al (26) and Hung et al (27) reported that vigorous anti-tumor
therapy, including systemic chemotherapy and hormone therapy, led
to longer survival in patients with BMM. The cornerstone of DIC
management is specific and vigorous treatment of the underlying
conditions (28). Systemic
therapy, such as chemotherapy, requires a stable general condition
and adequate bone marrow function. Early detection of BMM and
treatment of the primary cancer can prevent severe thrombocytopenia
and DIC (16). Blood transfusion,
granulocyte colony-stimulating factor, erythropoietin, and
thrombopoietin are also effective for BMM as supportive therapies
in addition to anti-tumor medications (8). A combination of vigorous anti-tumor
therapy and adequate supportive care is the key to the treatment of
BMM. However, this study was limited by its small sample size, and
further evidence is required before generalizing the management of
BMM of ovarian cancer.
In conclusion, the frequency of BMM due to ovarian
cancer may be underestimated. BMM progresses rapidly, leading to
DIC and resulting in death; as such, early diagnosis is crucial.
When decreasing blood counts are accompanied by elevated tumor
marker levels, BMM should be considered in the differential
diagnosis, regardless of the histological type.
Acknowledgements
Not applicable.
Funding
Funding: The present study was supported by a Grant-in-Aid for
Scientific Research (grant no. 22K15409) from the Ministry of
Education, Culture, Sports, Science and Technology.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
TY, JN, YO and MiY conceived the study. TY, JN, YO,
AY, YY, MasakY, MasanY, MiY and EK contributed to conception,
design and acquisition of data. TY, JN, YO, AY, YY, MasakY, MasanY
and MiY curated data. TY, JN, YO, MiY and EK analyzed and
interpreted data. MiY acquired funding. TY, JN, YO, AY, YY, MasakY,
MiY and EK contributed to the clinical management of the patients.
MiY and EK supervised the study. JN, AY, MasakY, MasanY and MiY
were involved in validation. TY, JN, YO, AY, YY, MasanY and MiY
were involved in visualization. TY, JN and MiY wrote the original
draft. MiY and EK reviewed and edited the manuscript. MiY and TY
confirm the authenticity of all the raw data. All authors have read
and approved the final version of the manuscript.
Ethics approval and consent to
participate
The present study was approved by the Institutional
Review Board of the Ethics Committee of the Oita University and
Saitama Medical University International Medical Centre (IRB nos.
2585 and 16-257; Hidaka, Japan), and was performed in accordance
with the guidelines of the Helsinki Declaration of 1975, as revised
in 1983. All study participants provided written informed consent
to participate (or a formal waiver of consent).
Patient consent for publication
All study participants provided informed consent to
publish medical records and images.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Deng K, Yang C, Tan Q, Song W, Lu M, Zhao
W, Lou G, Li Z, Li K and Hou Y: Sites of distant metastases and
overall survival in ovarian cancer: A study of 1481 patients.
Gynecol Oncol. 150:460–465. 2018.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Xiao L, Luxi S, Ying T, Yizhi L, Lingyun W
and Quan P: Diagnosis of unknown nonhematological tumors by bone
marrow biopsy: A retrospective analysis of 10,112 samples. J Cancer
Res Clin Oncol. 135:687–693. 2009.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Saikia UN, Malhotra P, Khandelwal N, Varma
S and Joshi K: Small cell carcinoma of the ovary presenting as bone
marrow metastasis: A rare presenting feature. Indian J Pathol
Microbiol. 48:402–404. 2005.PubMed/NCBI
|
|
4
|
Sreenan JJ and Hart WR: Carcinosarcomas of
the female genital tract. A pathologic study of 29 metastatic
tumors: Further evidence for the dominant role of the epithelial
component and the conversion theory of histogenesis. Am J Surg
Pathol. 19:666–674. 1995.PubMed/NCBI
|
|
5
|
Alenghat E, Okagaki T and Talerman A:
Primary mucinous carcinoid tumor of the ovary. Cancer. 58:777–783.
1986.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Ozkalemkas F, Ali R, Ozkocaman V, Ozcelik
T, Ozan U, Ozturk H, Kurt E, Evrensel T, Yerci O and Tunali A: The
bone marrow aspirate and biopsy in the diagnosis of unsuspected
nonhematologic malignancy: A clinical study of 19 cases. BMC
Cancer. 5(144)2005.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Kucukzeybek BB, Calli AO, Kucukzeybek Y,
Bener S, Dere Y, Dirican A, Payzin KB, Ozdemirkiran F and Tarhan
MO: The prognostic significance of bone marrow metastases:
Evaluation of 58 cases. Indian J Pathol Microbiol. 57:396–399.
2014.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Zhang L, Chen F, Xu L, Li N, Zhuo Q, Guo
Y, Wang X, Wen M, Zhao Z and Li M: Comprehensive review of solid
tumor bone marrow metastasis. Crit Rev Oncol Hematol.
194(104248)2024.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Shinden Y, Sugimachi K, Tanaka F,
Fujiyoshi K, Kijima Y, Natsugoe S and Mimori K: Clinicopathological
characteristics of disseminated carcinomatosis of the bone marrow
in breast cancer patients. Mol Clin Oncol. 8:93–98. 2018.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Kopp HG, Krauss K, Fehm T, Staebler A,
Zahm J, Vogel W, Kanz L and Mayer F: Symptomatic bone marrow
involvement in breast cancer-clinical presentation, treatment, and
prognosis: A single institution review of 22 cases. Anticancer Res.
31:4025–4030. 2011.PubMed/NCBI
|
|
11
|
Witkowski L, Goudie C, Ramos P, Boshari T,
Brunet JS, Karnezis AN, Longy M, Knost JA, Saloustros E, McCluggage
WG, et al: The influence of clinical and genetic factors on patient
outcome in small cell carcinoma of the ovary, hypercalcemic type.
Gynecol Oncol. 141:454–460. 2016.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Kunkel J, Peng Y, Tao Y, Krigman H and Cao
D: Presence of a sarcomatous component outside the ovary is an
adverse prognostic factor for primary ovarian malignant mixed
mesodermal/mullerian tumors: A clinicopathologic study of 47 cases.
Am J Surg Pathol. 36:831–837. 2012.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Baker PM, Oliva E, Young RH, Talerman A
and Scully RE: Ovarian mucinous carcinoids including some with a
carcinomatous component: A report of 17 cases. Am J Surg Pathol.
25:557–568. 2001.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Peres LC, Cushing-Haugen KL, Anglesio M,
Wicklund K, Bentley R, Berchuck A, Kelemen LE, Nazeran TM, Gilks
CB, Harris HR, et al: Histotype classification of ovarian
carcinoma: A comparison of approaches. Gynecol Oncol. 151:53–60.
2018.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Machida H, Matsuo K, Yamagami W, Ebina Y,
Kobayashi Y, Tabata T, Kanauchi M, Nagase S, Enomoto T and Mikami
M: Trends and characteristics of epithelial ovarian cancer in Japan
between 2002 and 2015: A JSGO-JSOG joint study. Gynecol Oncol.
153:589–596. 2019.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Al-Ibraheem A, Al-Adhami DA, Abdlkadir AS,
Mohamad I, Ghatasheh H and Qandeel M: FDG PET/CT reveals bone
marrow oligometastasis in laryngeal squamous carcinoma: A case
report with favorable outcome. BJR Case Rep.
9(20230065)2023.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Chan WK, Lin NS, Tay KV and Teo LT:
Systematic review of isolated disseminated carcinomatosis of bone
marrow from colorectal cancer. ANZ J Surg. 93:1169–1175.
2023.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Wang D, Luo Y, Shen D, Yang L, Liu HY and
Che YQ: Clinical features and treatment of patients with lung
adenocarcinoma with bone marrow metastasis. Tumori. 105:388–393.
2019.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Bretton PR, Melamed MR, Fair WR and Cote
RJ: Detection of occult micrometastases in the bone marrow of
patients with prostate carcinoma. Prostate. 25:108–114.
1994.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Kwon JY, Yun J, Kim HJ, Kim KH, Kim SH,
Lee SC, Kim HJ, Bae SB, Kim CK, Lee NS, et al: Clinical outcome of
gastric cancer patients with bone marrow metastases. Cancer Res
Treat. 43:244–249. 2011.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Suto H, Inui Y and Okamura A: Long-term
survival of a patient with gastric cancer with bone marrow
metastasis receiving S-1 plus oxaliplatin beyond three years: A
case report and literature review. Front Oncol.
14(1449212)2024.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Braun S, Vogl FD, Naume B, Janni W,
Osborne MP, Coombes RC, Schlimok G, Diel IJ, Gerber B, Gebauer G,
et al: A pooled analysis of bone marrow micrometastasis in breast
cancer. N Engl J Med. 353:793–802. 2005.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Hartkopf AD, Brucker SY, Taran FA, Harbeck
N, von Au A, Naume B, Pierga JY, Hoffmann O, Beckmann MW, Rydén L,
et al: Disseminated tumour cells from the bone marrow of early
breast cancer patients: Results from an international pooled
analysis. Eur J Cancer. 154:128–137. 2021.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Colucci M, Zumerle S, Bressan S, Gianfanti
F, Troiani M, Valdata A, D'Ambrosio M, Pasquini E, Varesi A, Cogo
F, et al: Retinoic acid receptor activation reprograms senescence
response and enhances anti-tumor activity of natural killer cells.
Cancer Cell. 42:646–661.e9. 2024.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Nacarelli T, Fukumoto T, Zundell JA,
Fatkhutdinov N, Jean S, Cadungog MG, Borowsky ME and Zhang R: NAMPT
inhibition suppresses cancer stem-like cells associated with
therapy-induced senescence in ovarian cancer. Cancer Res.
80:890–900. 2020.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Niu L, Lv H, Zhang M, Zeng H, Fu S, Cui S,
Liu Z and Yan M: Clinicopathological features and prognosis of
breast cancer combined with symptomatic bone marrow metastases: A
10-year, single-center, real-world study of 67 cases. Cancer Med.
12:10672–10683. 2023.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Hung YS, Chou WC, Chen TD, Chen TC, Wang
PN, Chang H, Hsu HC, Shen WC, Cheng WH and Chen JS: Prognostic
factors in adult patients with solid cancers and bone marrow
metastases. Asian Pac J Cancer Prev. 15:61–67. 2014.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Gando S, Levi M and Toh CH: Disseminated
intravascular coagulation. Nat Rev Dis Primers.
2(16037)2016.PubMed/NCBI View Article : Google Scholar
|