Introduction
Liposarcoma (LPS) represents the most prevalent type
of soft tissue sarcoma, comprising ~20% of all soft tissue sarcomas
(1). In 2020, the World Health
Organization classified these tumors as atypical lipomatous tumors
(ALT)/well-differentiated LPS (WDL), dedifferentiated LPS (DDLPS),
myxoid LPS, pleomorphic LPS and myxoid pleomorphic LPS (2). DDLPS, a rare subtype of LPS,
predominantly manifests in the interstitial limbs between the
posterior peritoneum and the pelvic soft tissues. It is exceedingly
uncommon in the head and neck region, where it accounts for only 1%
of all sarcomas in this area (3).
The histological features of DDLPS are characterized by the
transformation of mature adipocytes into cells exhibiting marked
atypia. This transformation typically results in the formation of
regions with two distinct components: WDL and dedifferentiated
non-adipose high-grade malignant components (4).
The present study reported a case of DDLPS with a
history spanning 30 years, characterized by multiple recurrent
episodes and culminating in the development of a substantial DDLPS
in the neck, exhibiting components of osteosarcoma and
chondrosarcoma. This case is intended to heighten awareness among
clinicians and radiologists, offering critical insights for
diagnosing and treating patients with DDLPS.
Case report
A 72-year-old male patient had initially presented
with a pebble-sized, painless lump in the left neck ~30 years
previously, which lacked ulceration. The lump gradually enlarged to
the size of an adult's fist over five years. Following surgical
excision at a local hospital, the patient experienced temporary
improvement. However, five years post-surgery, a mass of a similar
size re-emerged in the same location, necessitating further
surgical intervention at the same hospital. At two years subsequent
to the second surgery, the mass recurred and pathological analysis
confirmed it as a lipoma. In April 2023, the patient sought care at
The 940th Hospital of Joint Logistic Support Force of Chinese
People's Liberation Army (Gansu, China), reporting numbness and
heaviness in the left upper limb, significantly impacting the
patient's daily activities. Palpatory examination of the size,
texture and mobility of the mass revealed a 13x22 cm mass in the
left posterior region of the head and neck, characterized by a hard
texture and limited mobility.
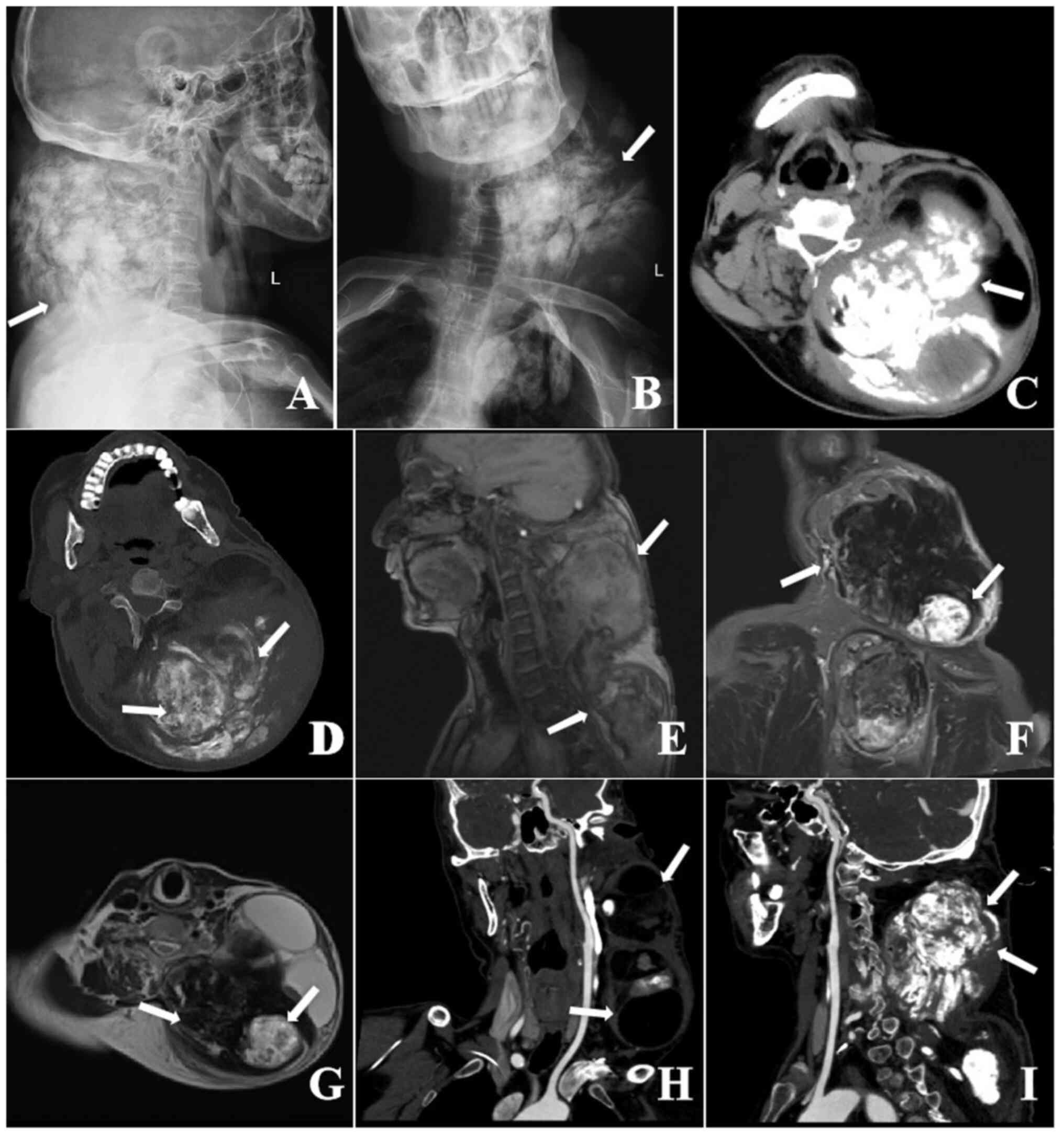
X-ray imaging revealed a large, dense mass in the
subcutaneous soft tissue of the left posterior neck. This mass
displayed inhomogeneous density, exerting pressure on adjacent
bronchial tubes and causing lumen narrowing (Fig. 1A and B). Computed tomography (CT) scans
demonstrated a substantial lobulated mass with mixed densities in
the left neck, shoulder and back, measuring ~13x11x22 cm. The
borders of this mass were poorly defined, containing multiple
flakes, extensive flaky calcifications and hypodense areas
resembling fat, with indistinct demarcation from surrounding
tissues (Fig. 1C and D). Magnetic resonance imaging (MRI)
showed a large soft tissue mass characterized by signal
inhomogeneity. The primary body of the lesion presented with low
signals on both T1-weighted MRI (T1WI) and T2WI, as well as in the
fat suppression sequences. In addition, multiple patchy high-signal
areas were observed on both T1WI and T2WI within the lesion, with
the posterior part displaying rounded high-signal shadows on T1WI,
low on T2WI, and high on fat suppression sequences; overall, the
lesion exhibited heterogeneous signal characteristics (Fig. 1E-G).
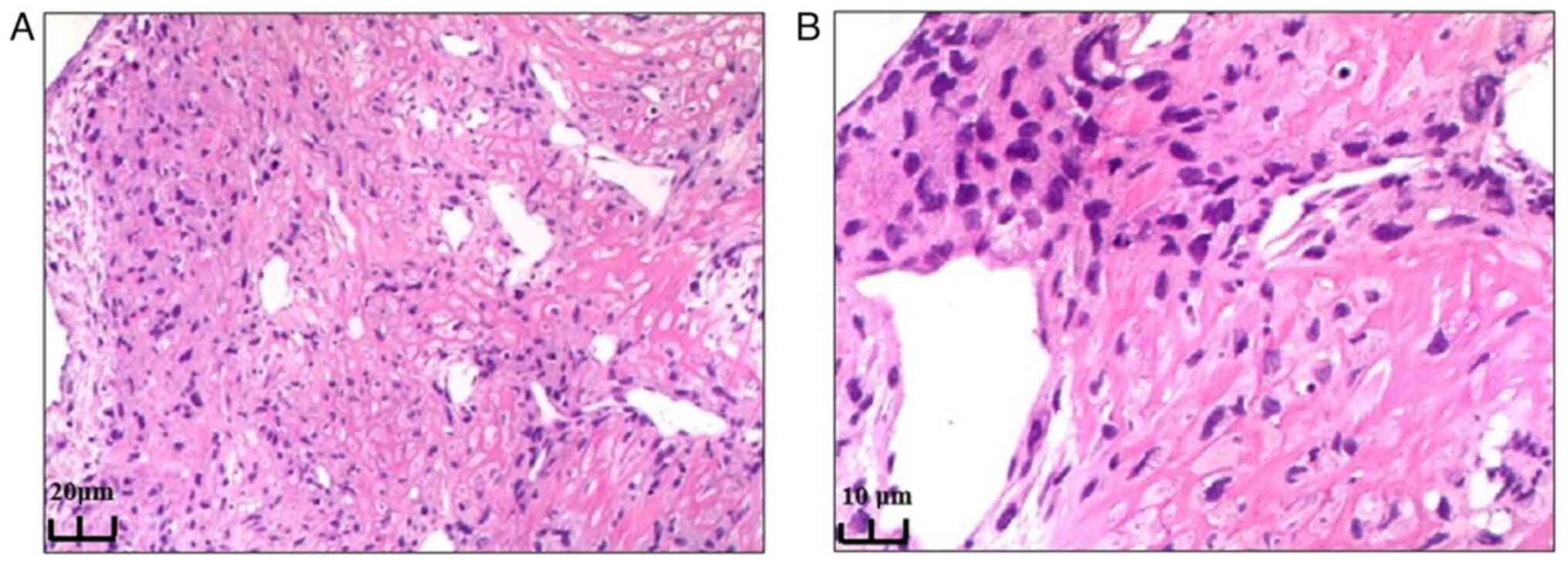
Preoperative ultrasound-guided aspiration biopsy of
the left neck mass was performed. Histological staining was
performed using hematoxylin and eosin (H&E), with a standard
protocol including the application of GM hematoxylin (Abcam) for 4
min and pure eosin (Abcam) for 2 min at room temperature. H&E
staining revealed a fat-rich mesenchymal tumor with ossification
and visible heterologous components, predominantly chondrosarcoma,
highly suggestive of DDLPS (Fig.
2). For further analysis, immunohistochemistry (IHC) was
carried out. The paraffin-embedded sections of the tumour were cut
to a thickness of 4 µm and placed onto slides. Sections were
immersed in citrate buffer (Gibco; Thermo Fisher Scientific, Inc.)
and heated in a water bath for 25 min at 95-100˚C for antigen
retrieval. Prior to staining of the sections, endogenous peroxidase
activity was blocked using 3% H2O2 with
incubation for 10 min at 37˚C. The sections were then incubated
with specific primary antibodies for 2 h at 37˚C, and subsequently,
anti-rabbit IgG (cat. no. RAB-0102; 1:500; Maixin Biotechnologies)
was applied to the sections and incubated for 10 min at 37˚C.
Visualization was performed using a diaminobenzidine chromogen
(Abcam) as a substrate (incubation for 3-5 min at room
temperature). Sections were counterstained with hematoxylin
(Abcam). A final drop of neutral resin was added for sealing with a
coverslip and slides were observed under a light microscope. The
IHC staining results using antibodies from Maixin Biotechnologies
were as follows: i) Murine double minute 2 [MDM2, (+); cat. no.
MAB-0744; pre-diluted 5 µg/ml]; ii) cyclin-dependent kinase 4
[CDK4, (+); cat. no. RMA-0741; pre-diluted 5 µg/ml]; iii) P16, (+);
cat. no. MAB-0673; pre-diluted 10 µg/ml; iv) P53, (wild-type); cat.
no. MAB-0674; pre-diluted 5 µg/ml; v) cytokeratin pan, (-); cat.
no. RAB-0050; pre-diluted 10 µg/ml; vi) vimentin, (+); cat. no.
Kit-0019; pre-diluted 10 µg/ml; vii) S-100, (-); cat. no. RAB-0150;
pre-diluted 30 µg/ml; viii) CD34, (-, microvessel +); cat. no.
Kit-0004; pre-diluted 10 µg/ml; ix) smooth muscle actin, (-); cat.
no. Kit-0006; pre-diluted 10 µg/ml; x) Ki-67, (index: 70%); cat.
no. MAB-0672; pre-diluted 20 µg/ml.
The patient exhibited a prolonged disease course,
characterized by a high propensity for recurrence. Prior to
admission, the patient had undergone three surgical interventions.
Considering both the clinical findings and auxiliary examination
results, the diagnosis was strongly suggestive of a DDLPS in the
soft tissue of the neck (2). At
the time of presentation at our hospital, the mass was substantial
in size and associated with numbness and heaviness in the upper
limbs. After departmental discussions, it was decided to administer
two cycles of chemotherapy drugs (isocyclophosphamide 12.5 mg +
epirubicin 120 mg) through a peripherally inserted central catheter
with the aim of reducing the tumor size. The multidisciplinary team
formulating the treatment plan comprised an attending physician,
the deputy chief of spine surgery, the chief of medical oncology
and the chief of pain medicine. Subsequent imaging with CT
post-chemotherapy revealed a decrease in tumor size relative to the
baseline measurements (Fig. 1H and
I). Before surgically removing the
tumor, a carotid angiography and thyroid artery embolization were
performed. The purpose was to reduce the tumor's blood supply and
surgical bleeding, clarify the surgical field, shorten the
operation time and improve the tumor resection rate. As the tumor
was too large, complete removal in a single piece was difficult.
Therefore, a bone knife was used to divide the tumor into several
large sections before removing the entire mass. Finally, the tumor
was completely removed and sent to the Department of Pathology.
The resected neck mass consisted of several pieces
of unshaped tissue, grayish-yellow to grayish-brown in color, with
a total size of 30x25x6 cm. The postoperative pathological results
were as follows: DDLPS, with differentiated components being
ALT/WDL, and dedifferentiated components being osteosarcoma and
chondrosarcoma (Fig. 3A-C). The
excised tissue's cut surface was grayish-yellow, solid and hard;
portions of the tissue exhibited bone that was visibly gray and
translucent, resembling cartilage. Adipose tissue adhered to
certain areas of the unplasticized tissue, which was enveloped by
periosteum; these sections were grayish-yellow, solid and soft.
Certain local sections appeared gray and solid with a medium
texture (Fig. 3D). The main reason
for the moderate texture of the mass in our patient was considered
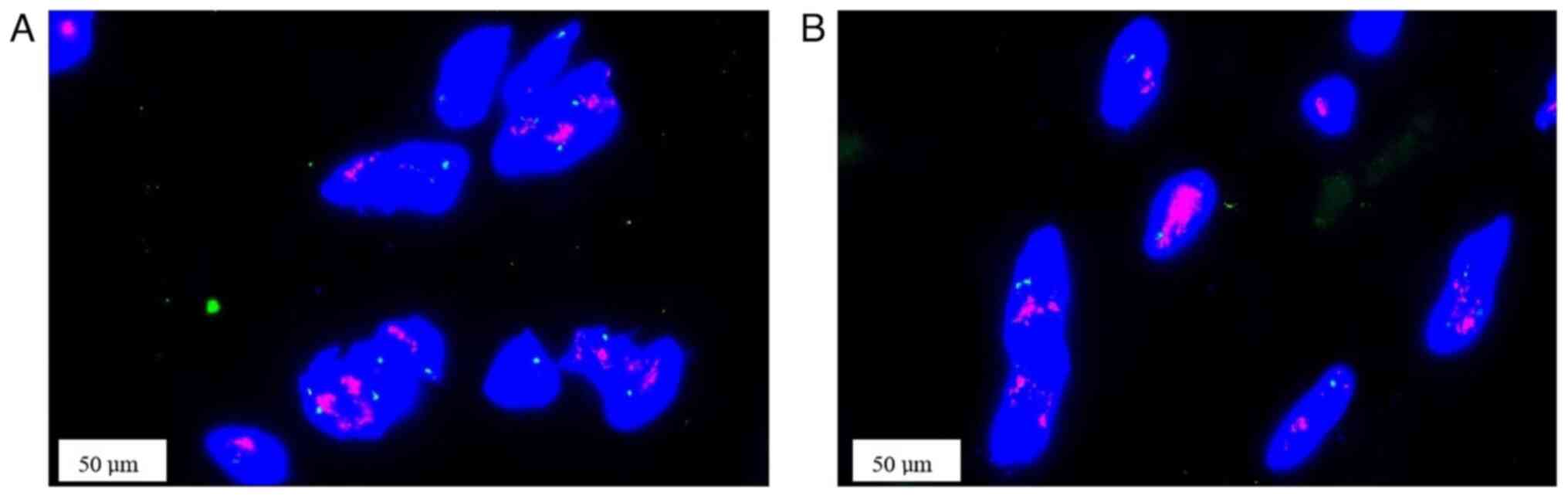
to be its nature in between hard and soft. Fluorescence in
situ hybridization (FISH) analysis showed amplification of the
MDM2 and CDK4 genes (Fig. 4). For
FISH, 2.5 µm paraffin-embedded sections were baked at 65˚C for 2 h.
Sections were deparaffinized with xylene and washed with 100%
ethanol. After pre-treatment in boiling water for 20 min, the
sections were incubated in a 0.5 mg/ml trypsin solution (Gibco;
Thermo Fisher Scientific, Inc.) at 37˚C for 7 min. The probe
mixture (probe/hybridization buffer/purified H2O=1:7:2;
Beijing GP Medical Technologies Co., Ltd.) was then added to the
slides. The slides were then incubated at 83˚C for 5 min to
denature them and then hybridized with the probe at 42˚C overnight.
After hybridization, the slides were counterstained with 10 µl of
DAPI reagent and mounted with coverslips. Finally, the fluorescent
signals were observed by fluorescence microscopy.
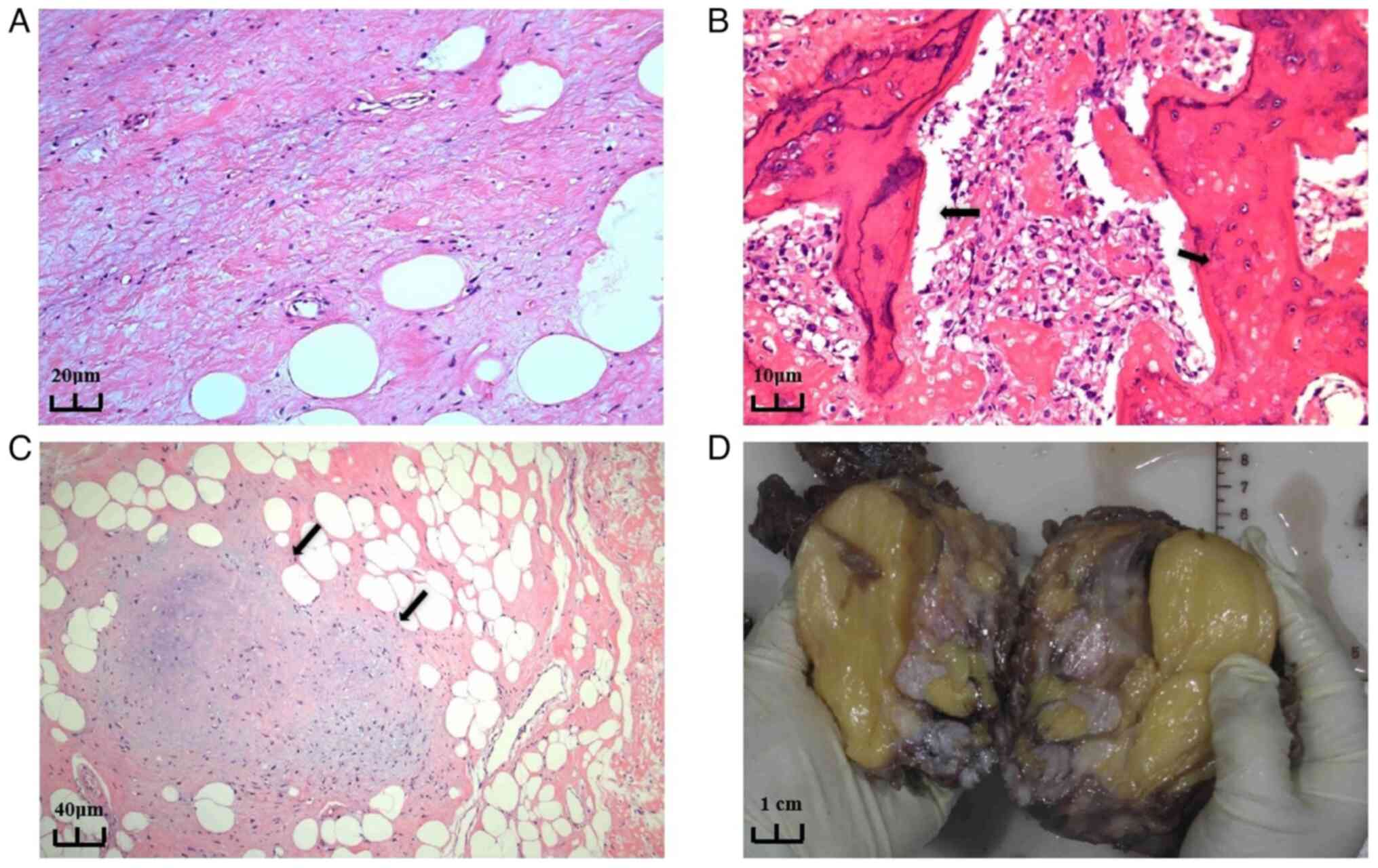 | Figure 3Pathologic findings. (A) Part of the
well-differentiated liposarcoma shows mature adipocytes and
abundant fibrous tissue. There are more collagen fibers in the
surrounding area and the diversity of cell nuclei is relatively
low, indicating a lower degree of heterogeneity (H&E staining;
magnification, x20). (B) A large number of spindle-shaped cells and
pleomorphic tumor cells are present, with a significantly increased
cell density. The stroma shows sclerosis accompanied by
infiltration of inflammatory cells (as indicated by the arrows).
Nuclear atypia of the tumor cells is evident, displaying typical
features of dedifferentiated liposarcoma. Cell proliferation is
active, exhibiting malignant characteristics, along with
osteosarcoma and tumor-related osteogenesis (H&E staining;
magnification, x40). (C) The combination of adipocytes and fibrous
tissue still exhibits highly differentiated characteristics in its
organizational structure. However, in certain areas, the
arrangement of cells shows abnormalities, suggesting the presence
of potential dedifferentiation. This indicates a transitional zone
between high differentiation and dedifferentiation, hinting at
possible malignant changes (H&E staining; magnification, x10).
(D) Gross appearance of cross-sectional view of the resected neck
mass. |
Pathology combined with clinical and molecular
findings led to the diagnosis of DDLPS with a differentiated
component of ALT/WDL and a dedifferentiated component of
osteosarcoma and chondrosarcoma. At 10 days after surgery, the
patient was discharged normally, and after discharge, celecoxib was
given orally for pain relief for two weeks. The patient was
followed for up to one year after the operation. During this
period, phone follow-ups indicated that the patient had recovered
well with no recurrence of the local mass. The patient was
repeatedly advised to visit either our hospital or a local hospital
for postoperative checkups. However, residing in a remote
mountainous area of China and facing financial and geographical
constraints, the patient did not undergo professional checkups.
Consequently, telephone inquiries were performed to monitor the
patient's condition. Follow-up assessments primarily focused on the
healing of surgical incisions, recurrence of local lumps, numbness
in the upper limbs, alleviation of severe symptoms, as well as the
patient's mental status, diet and bowel movements. The timing of
these assessments coincided with that of the post-operative review
noted in the patient's discharge certificate (1, 3, 6 months and 1
year after the operation).
Discussion
ALT/WDL exhibits few or no mitotic figures and
generally lacks prominent fibrous or sarcomatous features
macroscopically. Microscopically, fat vacuoles of varying sizes and
lipoblasts are typically observed and the stroma contains
irregular, hyperchromatic and pleomorphic atypical spindle cells
(5). ALT/WDL is considered a
different stage of the same tumor type, characterized by slow
growth, with numerous patients having had it for several years or
even decades by the time of diagnosis (6). DDLPS usually arises from ALT/WDL,
presenting as a sudden, gradual or mixed transition from ALT/WDL to
non-lipogenic sarcoma; in this zone, cellular atypia gradually
increases, fibrous septa become thicker and stromal cells
proliferate, ultimately developing into high-grade sarcomatous
components (7). This means that
within the same tumor, both well-differentiated lipogenic
components and dedifferentiated non-lipogenic malignant components
are present (8,9).
DDLPS is more commonly observed in middle-aged and
older individuals, with rare occurrences in children and
adolescents (10). DDLPS may
result from the transformation of these lower-grade tumors into
higher-grade sarcomas, typically accompanied by rapid symptom
deterioration and a sharp increase in tumor growth. A history of
illness lasting up to 30 years is often associated with the
presence of WDL. However, certain studies suggested that ALT/WDL
can transform into DDLPS, a relatively rare process, often
accompanied by changes in specific biomarkers (11). The amplification of the MDM2 and
CDK4 genes is commonly associated with DDLPS, while it is not
evident in most ALT/WDL cases (12).
In DDLPS, the dedifferentiated component and the ALT
component coexist within the same tumor and the boundary between
the two may be clear, ambiguous or even exhibit mixed growth
(13). In certain cases, there is
a distinct histological boundary between the ALT component and the
dedifferentiated high-grade sarcoma component, and under the
microscope, there is a clear transition from mature, less atypical
adipose tissue to highly undifferentiated sarcoma tissue (7).
‘Dedifferentiation’ refers to the progression of
low-grade sarcomas to high-grade sarcomas, including low-grade
osteosarcomas, chondrosarcomas and fibrosarcomas (14). In certain cases, the
dedifferentiated and transformed component may be a homologous
pleomorphic LPS (15).
Dedifferentiation can occur in any part of the body, with an
estimated frequency of ~10% (16).
In ~5-10% of cases, DDLPS may exhibit heterologous differentiation,
including rhabdomyosarcomas, leiomyosarcomas, osteosarcomas and
chondrosarcomas (17).
Dedifferentiation is time-dependent, with an increased likelihood
of occurrence as time progresses, with an average time to
dedifferentiation of 7-8 years (18). DDLPS is highly invasive, with a
strong tendency for distant lung metastasis and local recurrence
(4). The local recurrence rate is
41%, the metastasis rate is 17% and the disease-related mortality
rate is 28% (19). In the present
case, the dedifferentiated components of osteosarcoma and
chondrosarcoma were observed in the tumor, which was partially
replaced by fibrotic tissue, leading to erosive damage to the
surrounding bone and resulting in local metastases, as shown on
preoperative imaging.
In terms of symptoms, DDLPS typically presents as a
syndrome caused by the compression of surrounding tissues. However,
LPS in the retroperitoneal region of the pelvis may grow for
extended periods without causing any symptoms (20). In the present study, the tumor was
located in the patient's neck, resulting in numbness and a
sensation of heaviness in the distal left upper extremity, symptoms
that are not specific to DDLPS diagnosis. Therefore, other
diagnostic methods are necessary to identify the tumor. Ultrasound
findings of DDLPS are typically heterogeneous, solid and
hypoechoic, and LPS should be suspected when the tumor exhibits
structural heterogeneity and relatively low vascular echoes. In
addition to ultrasonography, CT and MRI offer greater precision in
differentiating between adipose and other soft tissue components
(21). MRI provides information on
the exact location of the tumor but does not allow for definitive
pathological staging. Imaging can determine the extent of the tumor
and its infiltration of surrounding organs, but a final diagnosis
requires pathological examination.
Preoperative diagnosis of DDLPS is challenging.
Davis et al (22) reported
that one-third of patients with head and neck LPS are misdiagnosed
upon initial pathology, and diagnostic errors can lead to delayed
or inadequate treatment. Immunohistochemistry, FISH, quantitative
PCR and comparative genomic hybridization are commonly used to
identify MDM2 amplification in lipomatous tumors, requiring a
highly experienced pathologist for accurate diagnosis (23). A characteristic feature of DDLPS is
the amplification of the chromosomal region 12q13-15, which encodes
potential oncogenes, including MDM2 and CDK4, with MDM2 being the
most prominent. This feature is highly informative for diagnosing
DDLPS (24). MDM2 is an oncogene
that promotes the degradation of the tumor suppressor protein p53,
which is considered the key gene in DDLPS tumorigenesis. MDM2 is
widely amplified and expressed in nearly all DDLPS cases (25). The amplification and overexpression
of the 12q13-15 region correspond to elevated levels of MDM2 and
CDK4 proteins, and MDM2, CDK4 and p16 are present in both the WDL
and DDLPS components. While MDM2 and p16 exhibit high sensitivity,
they lack specificity and can be found in various high-grade
sarcomas (25). Therefore, the
concurrent use of MDM2, CDK4 and p16 is often required to improve
diagnostic specificity in DDLPS. Immunohistochemical staining for
MDM2 and CDK4 is therefore highly useful in diagnosing DDLPS
(4). FISH is a molecular
cytogenetics technique. It utilizes fluorescently labeled specific
nucleic acid probes to hybridize with target DNA or RNA sequences
in cells or tissues, allowing for the detection of the presence,
location and quantity of the target sequences using fluorescence
microscopy (26). A study shown
that FISH is more sensitive in detecting MDM2 amplification and can
be incorporated into diagnostic protocols for refractory lipomas
(27). The present case is
consistent with positive expression of MDM2, CDK4 and p16.
The primary treatment for DDLPS involves extensive
surgical resection, radiation therapy and adjuvant chemotherapy.
Certain studies have indicated that systemic radiotherapy combined
with surgical treatment reduces the recurrence of DDLPS (3). However, even with multidisciplinary
approaches, DDLPS often recurs rapidly at the local site and can
metastasize to the lungs, bones or liver, leading to disease
progression that becomes difficult to manage (28). Furthermore, certain studies suggest
that potentially targeted molecular therapies, such as tyrosine
kinase inhibitors, MDM2 antagonists, CDK4 inhibitors, peroxisome
proliferator-activated receptor-γ agonists and nelfinavir, may have
therapeutic effects on DDLPS (11,29).
Early diagnosis of the present case was difficult. Although early
surgical intervention was performed, the disease kept recurring,
later leading to repeated exacerbations, which seriously affected
the patient's quality of life and physical and mental
well-being.
The current study presented a case of giant DDLPS of
the neck with osteosarcoma and chondrosarcoma components and
reported on its diagnosis, pathology, imaging manifestations and
course of treatment in the hope of gaining the attention of
clinicians and radiologists. In patients with DDLPS, early and
accurate diagnosis can significantly improve prognosis and quality
of life. In addition, the complete resection of the tumor during
surgery combined with the use of radiotherapy may be reasons for
the lack of recurrence after surgery in this patient. However, the
prognosis of patients with DDLPS is poor and new treatment
strategies are needed in the future to improve the prognosis and
treatment.
Acknowledgements
Not applicable.
Funding
Funding: This study was funded by the High-Level Talent
Cultivation Project at the 940th Hospital of Joint Logistics
Support Force (grant no. 2024-G3-5), the Lanzhou Science and
Technology Project (grant nos. 2023-2-11 and 2023-ZD-170) and the
Gansu University of Traditional Chinese Medicine Mentorship Project
(grant no. 2023YXKY015).
Authors' contributions
JZ obtained the data and wrote the manuscript. RG
and KY acquired the data and provided clinical advice. FT, PL, JL,
XW and JW evaluated the images. YQ and SZ made significant
contributions to the acquisition, analysis and interpretation of
the histopathology data. JZ and SZ confirm the authenticity of all
the raw data. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
The study was approved by the Ethics Committee of
the No. 940 Hospital of the People's Liberation Army Joint
Logistics Support Force (approval no.2023KYLL242).
Patient consent for publication
Written informed consent has been obtained from the
patient.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Blumberg JM, Jedrych J, Costa J and Judson
B: Cervical dedifferentiated liposarcoma with meningothelial-like
whorling. Head Neck Pathol. 6:476–480. 2012.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Anderson WJ and Doyle LA: Updates from the
2020 World Health Organization classification of soft tissue and
bone tumours. Histopathology. 78:644–657. 2021.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Gahvari Z and Parkes A: Dedifferentiated
liposarcoma: Systemic therapy options. Curr Treat Options Oncol.
21(15)2020.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Thway K: Well-differentiated liposarcoma
and dedifferentiated liposarcoma: An updated review. Semin Diagn
Pathol. 36:112–121. 2019.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Haddox CL, Hornick JL, Roland CL, Baldini
EH, Keedy VL and Riedel RF: Diagnosis and management of
dedifferentiated liposarcoma: A multidisciplinary position
statement. Cancer Treat Rev. 131(102846)2024.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Crago AM and Singer S: Clinical and
molecular approaches to well differentiated and dedifferentiated
liposarcoma. Curr Opin Oncol. 23:373–378. 2011.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Jebastin JAS, Perry KD, Chitale DA, Mott
MP, Sanchez J, Fritchie KJ, Palanisamy N and Williamson SR:
Atypical lipomatous tumor/well-differentiated liposarcoma with
features mimicking spindle cell lipoma. Int J Surg Pathol.
28:336–340. 2020.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Barisella M, Giannini L and Piazza C: From
head and neck lipoma to liposarcoma: a wide spectrum of
differential diagnoses and their therapeutic implications. Curr
Opin Otolaryngol Head Neck Surg. 28:136–143. 2020.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Zhan H, Cao S, Gao T, Zhang B, Yu X, Wang
L, Zeng J and Dai M: Giant atypical lipomatous
tumor/well-differentiated liposarcoma affects lower limb activity:
A case report. Medicine (Baltimore). 98(e17619)2019.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Kishimoto Y, Kishimoto AO, Yamada Y,
Kitano M, Kitada Y, Kitamura M, Tateya I, Sonobe M and Omori K:
Dedifferentiated liposarcoma of the thyroid gland: A case report.
Mol Clin Oncol. 11:219–224. 2019.PubMed/NCBI View Article : Google Scholar
|
|
11
|
M SA, K C, Bhargavan RV, Somanathan T and
Subhadradevi L: An overview on liposarcoma subtypes: Genetic
alterations and recent advances in therapeutic strategies. J Mol
Histol. 55:227–240. 2024.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Italiano A, Toulmonde M, Cioffi A, Penel
N, Isambert N, Bompas E, Duffaud F, Patrikidou A, Lortal B, Le
Cesne A, et al: Advanced well-differentiated/dedifferentiated
liposarcomas: Role of chemotherapy and survival. Ann Oncol.
23:1601–1607. 2012.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Weiss SW: Lipomatous tumors. Monogr
Pathol. 38:207–239. 1996.PubMed/NCBI
|
|
14
|
Mariño-Enríquez A, Fletcher CDM, Dal Cin P
and Hornick JL: Dedifferentiated liposarcoma with ‘homologous’
lipoblastic (pleomorphic liposarcoma-like) differentiation:
Clinicopathologic and molecular analysis of a series suggesting
revised diagnostic criteria. Am J Surg Pathol. 34:1122–1131.
2010.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Jo VY and Doyle LA: Refinements in sarcoma
classification in the current 2013 World Health Organization
classification of tumours of soft tissue and bone. Surg Oncol Clin
N Am. 25:621–643. 2016.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Weiss SW and Rao VK: Well-differentiated
liposarcoma (atypical lipoma) of deep soft tissue of the
extremities, retroperitoneum, and miscellaneous sites. A follow-up
study of 92 cases with analysis of the incidence of
‘dedifferentiation’. Am J Surg Pathol. 16:1051–1058.
1992.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Sbaraglia M, Bellan E and Dei Tos AP: The
2020 WHO classification of soft tissue tumours: News and
perspectives. Pathologica. 113:70–84. 2021.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Henricks WH, Chu YC, Goldblum JR and Weiss
SW: Dedifferentiated liposarcoma: A clinicopathological analysis of
155 cases with a proposal for an expanded definition of
dedifferentiation. Am J Surg Pathol. 21:271–281. 1997.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Zajicek AK, Bridge JA, Akers JW, McGarry
SV and Walker CW: Dedifferentiated liposarcoma of the lower
extremity with low-grade dedifferentiation and low-grade
osteosarcomatous component. Skeletal Radiol. 46:265–271.
2017.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Bourgeau M, Gandhi JS, Deeb KK and Bahrami
A: Superficial dedifferentiated liposarcoma: A clinicopathologic
study. Hum Pathol. 145:63–70. 2024.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Patel NG, Rajagopalan A and Shrotri NS:
Scrotal liposarcoma-a rare extratesticular tumour. JRSM Short Rep.
2(93)2011.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Davis EC, Ballo MT, Luna MA, Patel SR,
Roberts DB, Nong X and Sturgis EM: Liposarcoma of the head and
neck: The University of Texas M. D. Anderson cancer center
experience. Head Neck. 31:28–36. 2009.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Ray-Coquard I, Thiesse P, Ranchère-Vince
D, Chauvin F, Bobin JY, Sunyach MP, Carret JP, Mongodin B,
Marec-Bérard P, Philip T and Blay JY: Conformity to clinical
practice guidelines, multidisciplinary management and outcome of
treatment for soft tissue sarcomas. Ann Oncol. 15:307–315.
2004.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Weaver J, Downs-Kelly E, Goldblum JR,
Turner S, Kulkarni S, Tubbs RR, Rubin BP and Skacel M: Fluorescence
in situ hybridization for MDM2 gene amplification as a diagnostic
tool in lipomatous neoplasms. Mod Pathol. 21:943–949.
2008.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Yamashita K, Kohashi K, Yamada Y, Akatsuka
S, Ikuta K, Nishida Y, Toyokuni S and Oda Y: Prognostic
significance of the MDM2/HMGA2 ratio and histological tumor grade
in dedifferentiated liposarcoma. Genes Chromosomes Cancer.
60:26–37. 2021.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Bertram S and Schildhaus HU: Fluorescence
in situ hybridization for the diagnosis of soft-tissue and bone
tumors. Pathologe. 41:589–605. 2020.PubMed/NCBI View Article : Google Scholar : (In German).
|
|
27
|
Clay MR, Martinez AP, Weiss SW and Edgar
MA: MDM2 amplification in problematic lipomatous tumors: Analysis
of FISH testing criteria. Am J Surg Pathol. 39:1433–1439.
2015.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Murphey MD, Arcara LK and Fanburg-Smith J:
From the archives of the AFIP: Imaging of musculoskeletal
liposarcoma with radiologic-pathologic correlation. Radiographics.
25:1371–1395. 2005.PubMed/NCBI View Article : Google Scholar
|
|
29
|
De Vita A, Mercatali L, Recine F, Pieri F,
Riva N, Bongiovanni A, Liverani C, Spadazzi C, Miserocchi G,
Amadori D and Ibrahim T: Current classification, treatment options,
and new perspectives in the management of adipocytic sarcomas. Onco
Targets Ther. 9:6233–6246. 2016.PubMed/NCBI View Article : Google Scholar
|