Introduction
Triple-negative breast cancer (TNBC) refers to any
breast cancer that does not express the genes for the estrogen
receptor, progesterone receptor and HER2. TNBC accounts for 10-15%
of all breast cancers. These cancers tend to be more common in
women aged <40 years, who are African American, or who have a
BRCA1 mutation. This tumor differs from other breast cancer
subtypes because it grows and spreads faster, has limited treatment
options, and has a worse outcome (1,2). A
previous study by the authors found that the frequency of TNBC
cancers was high (29.3%), with large size, high-grade, and 70% with
lymph node metastasis (3).
TNBC is unresponsive to endocrine therapy or other
available targeted agents. Cytotoxic chemotherapy remains the
primary treatment for TNBC disease, along with surgery and/or
radiotherapy (4-6).
Novel drug developments in TNBC include antibody-drug conjugates,
immune checkpoint inhibitors, PARP inhibitors, and androgen
receptor-targeted agents (7).
TNBC is partly a basal-like subtype, with increased
expression of basal cytokeratins, such as cytokeratin (CK) 5/6, CK
17 and epidermal growth factor receptor (EGFR). Basal-like cancer
occurs mainly in young women, often relapsing rapidly, with
aggressive characteristics such as high-grade, high-proliferation
indexes, p53 mutation, EGFR overexpression, c-MYC amplification,
loss of phosphatase and tensin analog tumor suppressor gene, and
the loss of function of BRCA1 (8-10).
High recurrence and poor response to chemotherapy in TNBC are
probably due to the presence of basal-like cancer (11).
The identification of BRCA mutations in patients
with TNBC can have a significant effect on treatment. The BRCA
mutation status of patients with TNBC may predict the response to
treatment with inhibitors of poly (ADP ribose) polymerase (PARP)
(12,13). Identification by
immunohistochemistry (IHC) is a simple and reliable method to
access the expression of BRCA1 protein in tumor tissues. The
present study aimed to investigate the prognostic and predictive
value of BRCA1 expression by using the IHC method in TNBC.
Materials and methods
Data collection
A total of 57 samples of patients with TNBC received
from Sardjito General Hospital (Yogyakarta, Indonesia) from January
2015 to December 2020 were used in the present retrospective study.
Samples included patients who underwent breast surgery with
axillary dissection and had never received neoadjuvant therapy.
Clinicopathological data were collected from the medical records.
The present study was conducted after obtaining permission from the
ethics committee of Faculty of Medicine, Public Health and Nursing,
University Gadjah Mada/Sardjito General Hospital (approval no.
KE/FK/1291/E1; date, December 2021; Yogyakarta, Indonesia) and
patient consent for sample collection was waived by the ethics
committee.
IHC examination
Out of 57 samples, only 48 sample cases were found
eligible to be stained. Tissues were fixed in 10% Neutral Buffered
Formalin (NBF) for 24 h at room temperature. The paraffin-embedded
sections were cut into a 3-µm slice and subjected to
deparaffinization in xylene, rehydrated in series grade of ethanol,
and incubated with 3% H2O2 for 20 min. SNIPER
Reagent (BioCare Medical) were used as blocking agent for 20 min at
room temperature. The sections were incubated with the primary
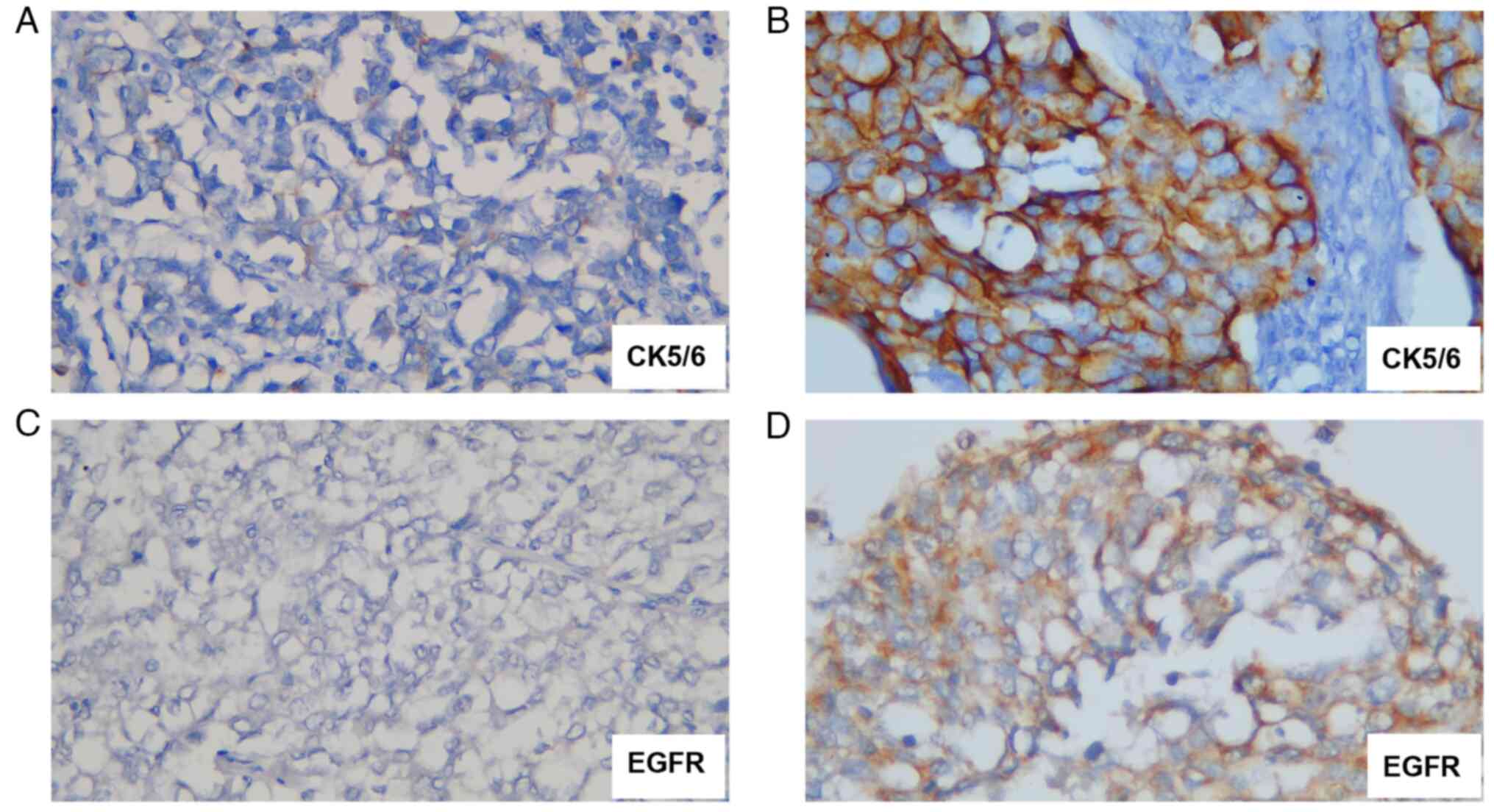
antibodies of EGFR and CK 5/6 and observed under light microscope
at x400 magnification (CX33; Olympus Corporation) to classify TNBC
into basal-like and non-basal-like. The expression of CK 5/6 and
EGFR was considered positive when stained in >10% of the tumor
cells and defined as negative when stained in <10% of the tumor
cells. Based on CK 5/6 and EGFR IHC results, TNBC was divided into
basal-like TNBC when deemed positive for either or both CK 5/6
(1:100; cat. no. 6057682) and EGFR (1:100; cat. no. 6067929; both
from Novocastra Laboratories Ltd.) and non-basal-like TNBC when
both CK 5/6 and EGFR were negative (14) (Fig.
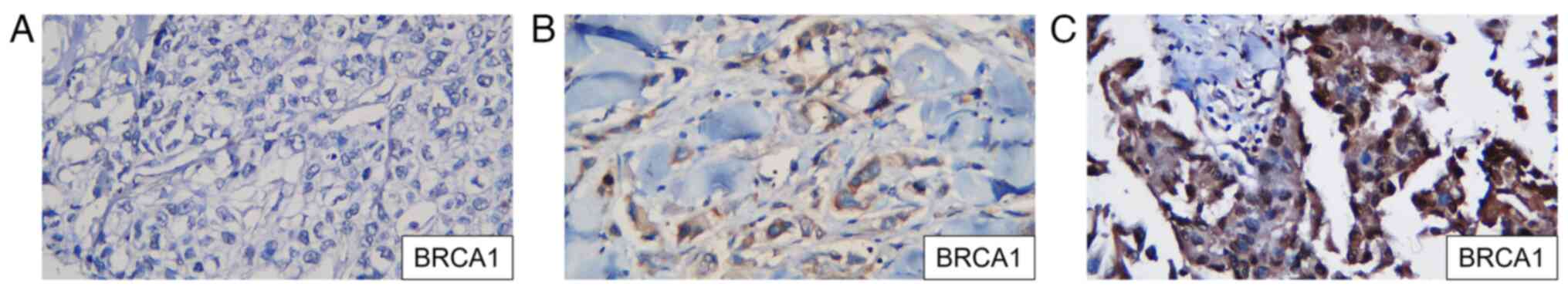
1). All samples were stained by IHC using a primary monoclonal
antibody against the BRCA1 mutation (clone MS110; CM; 1:100; cat.
no. 345A.C; BioCare Medical, LLC) to identify the expression of
BRCA1 in TNBC tissues. The primary antibody was incubated for 60
min at room temperature, followed by incubation with the secondary
antibody for 30 min at room temperature. Chromogen DAB was used to
visualize the BRCA1 protein expression. BRCA1 was positive if the
expression was in the tumor nuclei. A nuclear stain that appeared
brownish and accounted for <20% of the nucleus is considered
negative, while nuclear staining that accounted for >20% is
considered positive, according to the American Society of Clinical
Oncology/College of American Pathologists Clinical Practice
Guideline Update 2013(15)
(Fig. 2). The BRCA1 expression was
determined using ImageJ software version 1.54 (National Institutes
of Health) with a median cut-off of 30%.
Statistical analysis
Statistical analysis was performed with SPSS Version
26 (IBM Corp.) and presented in ± standard deviation. Pearson's
Chi-square test was used to analyze the correlation between several
variables, including age, grade, stage, histological type, type of
therapy and TNBC subtype. Fisher's exact test was used to analyze
stage. Follow-up survival was started in January 2019 and completed
in March 2023. Survival was analyzed using Kaplan-Meier (not
followed by the log-rank test) and P<0.05 was considered
statistically significant.
Results
There were 100 cases of TNBC reported between 2015
and 2020 with complete clinicopathological data available. However,
only 57 cases were accompanied by data on survival and therapy. Of
the 57 cases studied, the mean patient age was 55.18±10.014
(32-83). Patients aged ≥50 years were more frequent (70.2%)
compared with patients aged <50 years (29.8%). More patients
were high-grade, advanced staged, alive, received
non-platinum-based therapy, and non-specific type. The BRCA1
expression was detected in 44 cases (77.19%). The median value of
the BRCA1 expression was 30, and it was used as a cut-off to
categorize BRCA1 expression into negative and positive. The number
of negative BRCA1 expression cases was 52.6%. Of the 48 cases
studied, 72.9% were basal-like subtypes. The characteristics of the
samples are presented in Table
I.
 | Table ICharacteristics of the TNBC samples
(total n=57). |
Table I
Characteristics of the TNBC samples
(total n=57).
| Characteristics | Number, (%) |
|---|
| Age, years | |
|
<50 | 17 (29.8) |
|
≥50 | 40 (70.2) |
| Histological
grade | |
|
Poorly | 43 (75.4) |
|
Low | 14 (24.6) |
| Stage | |
|
Advanced | 47 (82.5) |
|
Early | 10 (17.5) |
| Status | |
|
Dead | 25 (43.9) |
|
Alive | 32 (56.1) |
| BRCA1 expression | |
|
Positive | 27 (47.4) |
|
Negative | 30 (52.6) |
| Therapy | |
|
Platinum-based | 20 (35.1) |
|
Non-platinum-based | 37 (64.9) |
| Histological
type | |
|
NST | 45 (78.9) |
|
Specific | 12 (21.1) |
| TNBC subtype | |
|
Basal-like | 35 (72.9) |
|
Non-basal-like | 13 (27.1) |
Fisher's exact test analysis revealed a correlation
between the expression of BRCA1 and the disease stage, as
demonstrated in Table II. A
negative expression of BRCA-1 was significantly associated with a
more advanced disease stage (P=0.035). However, there was no
correlation between the expression of BRCA-1 and other
clinicopathological characteristics, such as the type of
therapy.
 | Table IICorrelation between the BRCA1
expression and several characteristics of TNBC cases. |
Table II
Correlation between the BRCA1
expression and several characteristics of TNBC cases.
| | Expression level of
BRCA1 | |
|---|
| Characteristics | Positive (%) | Negative (%) | P-value |
|---|
| Age, years | | | 0.583
(0.444-4.291) |
|
<50 | 9 (15.7) | 8 (14.1) | |
|
≥50 | 18 (31.6) | 22 (38.6) | |
| Histological
grade | | | 0.125
(0.115-1.396) |
|
Poorly | 18 (31.7) | 25 (43.8) | |
|
Low | 9 (15.8) | 5 (8.7) | |
| Stage | | | 0.035 |
|
Advanced | 19 (33.3) | 28 (49.1) | |
|
Early | 8 (14.1) | 2 (3.5) | |
| Therapy | | | 0.396
(0.537-4.795) |
|
Platinum-based | 11 (19.3) | 9 (15.8) | |
|
Non-platinum-based | 16 (28.1) | 21 (36.8) | |
| Histological
type | | | 0.392
(0.157-2.052) |
|
NST | 20 (35.2) | 25 (43.8) | |
|
Specific | 7 (12.3) | 5 (8.7) | |
| Status | | | 0.325
(0.204-1.697) |
|
Dead | 10 (17.6) | 15 (26.3) | |
|
Alive | 17 (29.8) | 15 (26.3) | |
| TNBC subtype | | | 0.978
(0.274-3.542) |
|
Basal-like | 16 (33.3) | 6 (12.5) | |
|
Non-basal-like | 19 (39.6) | 7 (14.6) | |
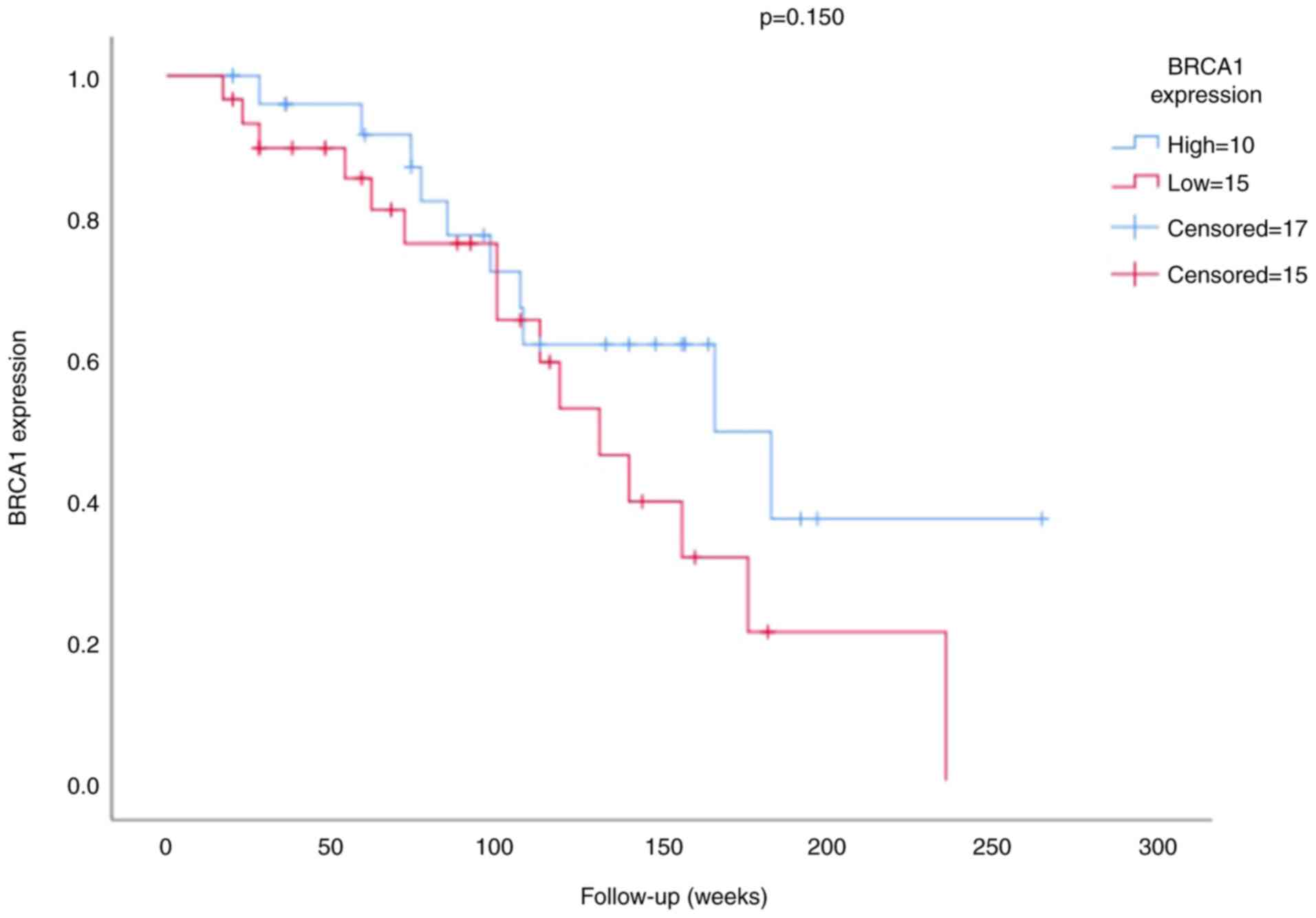
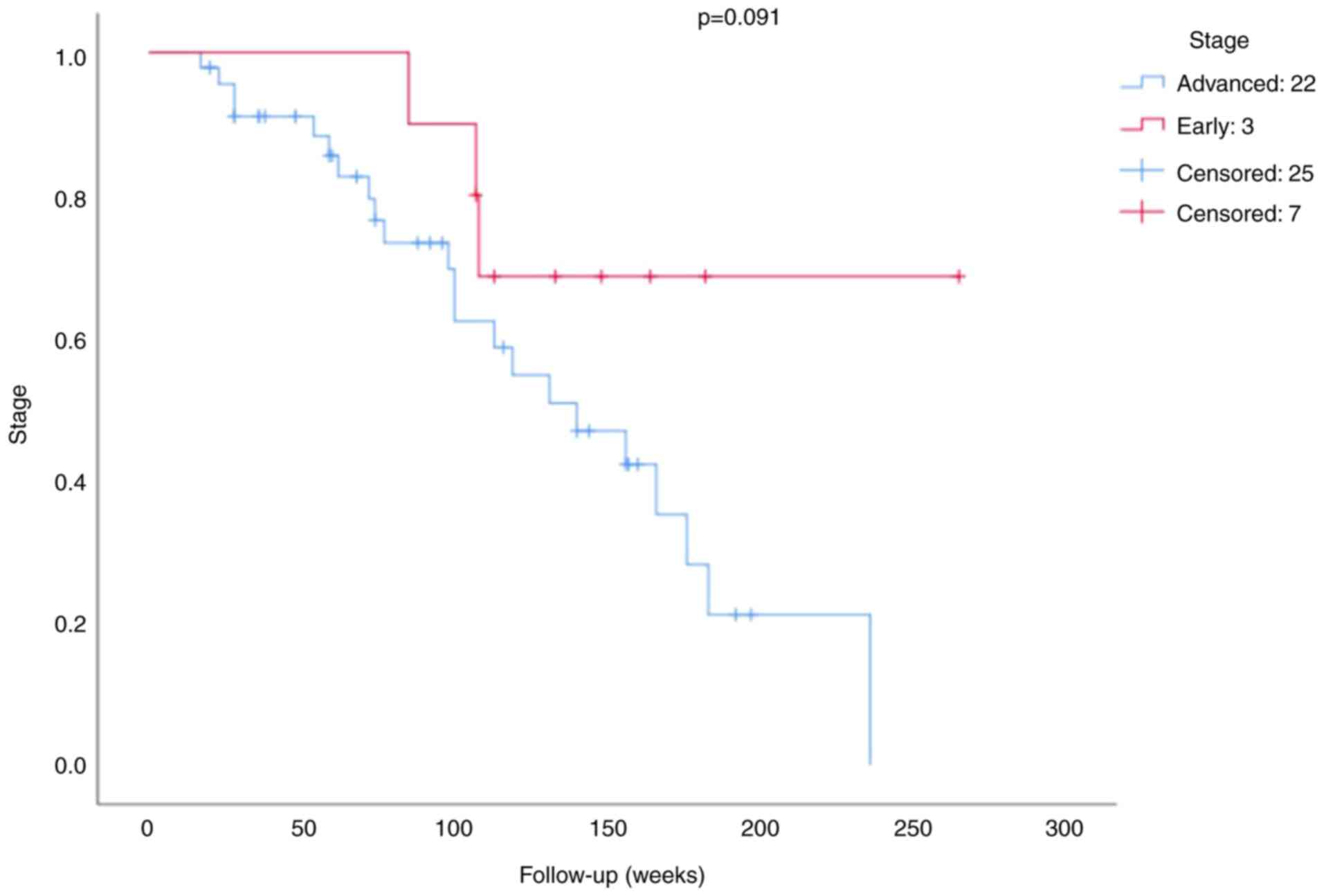
The survival analysis of the BRCA1 expression is
presented in Figs. 3 and 4. The mean survival time for patients was
100.79 weeks (minimum 17 weeks and maximum 265 weeks). The results
of the survival analysis demonstrated that neither BRCA1 expression
(P=0.150; Fig. 3) nor stage
(P=0.091; Fig. 4) were significant
prognostic factors in patients with TNBC.
Discussion
In the present study, the mean age of patients with
TNBC at diagnosis was 55.18±10.014 years. The number of patients
aged >50 years was high (70.2%). Previous studies concluded that
TNBC more frequently occurred at the age of ≤40 years, especially
among African-Americans (16-18),
and mostly has a poorer prognosis than other breast cancer subtypes
(1-3).
The present study found that 75.4% of TNBC cases were high-grade
and 82.5% were advanced stage. In total, 60-80% of TNBC cases are
basal-like subtypes with poor prognoses because they tended to
recur and were resistant to therapy (19-21).
Our cases were also dominated by basal-like subtypes (72.9%); of
these cases, 63% were high-grade, 74% were advanced-stage, and
52.6% succumbed.
The prevalence of BRCA mutations differs across
various ethnic groups. Previous studies on BRCA1/2 mutations in
TNBC predominantly focused on Caucasian populations. Studying
within the Asian population is crucial, as Asian patients with
breast cancer manifest the disease at a younger age compared with
their Caucasian counterparts. The frequency of BRCA1/2 mutations in
Korean patients with non-familial high-risk breast cancer and
familial breast cancer was 17.8 and 21.7%, respectively (22). The prevalence of BRCA1/2 mutations
in patients with familial breast cancer and early-onset breast
cancer in China ranged from 8-13.5% and from 8.7-11.4%,
respectively (23). A study of
Japanese patients with familial breast cancer indicated that
15-31.8% expressed mutations in the BRCA1/2 genes (24).
Several methods are available for detecting BRCA1
and BRCA2 dysfunction. Identification through IHC is a simple and
reliable method to assess the expression of the BRCA1 protein in
tumor tissues. Using the IHC method, cancer with positive BRCA1
expression in the present study was 47.4%. BRCA1-positive
expression reported by other studies was 17.5% (25) and 20.5%, (15) possibly due to differences in the
method and cut-off value of the BRCA1 expression. Previous research
used the cystoscope method, and BRCA1 was considered positive if
the score was ≥4(25). Meanwhile,
other groups used the cut-off value research of 20% and found that
a cut-off of 20% is improved for avoiding missing variants and has
a lower false positive rate compared with a cut-off of 10% (0.14
vs. 6.82%) (15,26).
In the present study, a negative BRCA1 expression
was correlated with advanced-stage cancer, but not with other
clinicopathological characteristics. Altered BRCA1 expression was
significantly associated with high-grade and advanced-stage breast
carcinoma; however, there was no correlation of the BRCA1
expression with clinicopathological parameters (15,25).
A previous study proved that reduced BRCA1 expression was
associated with high-grade tumors, negative hormone receptors and
HER2 status (27). Differences in
the number of samples, method and type of antibody used influence
these controversial results.
In the present study, basal-like and non-basal-like
subtype cancers tended to have a positive BRCA1 expression and were
not statistically significant. This result differed from those of a
previous study wherein positive BRCA1 expression correlated with
basal-like tumors (27). In
relation to mutation, basal-like breast cancer did not improve the
estimate of BRCA1 mutation risk (12).
Chemotherapy in TNBC can be platinum- or
non-platinum-based, including taxane, and anthracycline, among
others. The number of patients treated with non-platinum-based
treatment was higher (64.9%) than that of patients treated with
platinum-based treatment (35.1%). The therapy type did not
correlate with the BRCA1 expression. However, the mutation status
of BRCA1 in patients with TNBC was considered essential as it
influences the treatment choice. Patients with TNBC with BRCA1
mutation respond well to platinum-based therapy and PARP inhibitors
(12,13,28).
It was necessary to investigate the relation between the BRCA1
expression at the protein level and its mutation status considering
that protein detection is markedly simpler, cheaper and visible in
different laboratories in developing countries, such as
Indonesia.
The disease stage did not act as a prognostic factor
for patients with TNBC in the present study. It was recently
concluded that the advanced stage was related to significant
overall and disease-free survival reduction (29). Another study confirmed that young
patients with TNBC have a higher pathological stage and worse
long-term survival than young patients with other breast cancer
subtypes (30). The present study
has several limitations that need to be addressed in future
studies. Only 57 cases were included due to difficulty in obtaining
survival and therapy data and challenges in reaching the patients.
Transportation costs could constrain the distance from the
patient's house; therefore, check-ups were irregular. In addition,
limited therapy options for TNBC and national health insurance are
occasionally not easily accessible. Therefore, further study using
a more significant number of cases is needed to elaborate on the
prognostic significance of the disease stage in Indonesian patients
with TNBC.
The BRCA1 expression in the present study did not
act as a prognostic factor for TNBC cancer. A 20% cut-off was
employed for the BRCA1 expression, considering the lack of a
standard consensus on the cut-off point (15). The present study focuses on the
Indonesian population as it is currently an underexplored topic,
especially within the Indonesian demographic. Consequently, the
current study can significantly impact future research using
samples from the Southeast Asian region, specifically the
Indonesian population. Comprehensive research must therefore be
conducted to determine the standard value of BRCA1 expression,
especially when later BRCA1 protein expression can be applied to
determine the treatment choice in patients with TNBC.
In conclusion, the present study concluded that a
negative BRCA1 expression was correlated with the advanced stage of
Indonesian patients with TNBC, albeit it was not a prognostic
factor.
Acknowledgements
The authors would like to express their gratitude to
Mrs. Agustin from Department of Anatomical Pathology, Faculty of
Medicine, Public Health and Nursing, University Gadjah
Mada/Sardjito General Hospital (Yogyakarta, Indonesia) for valuable
contributions in facilitating the administrative and technical
tasks involved in the present study.
Funding
Funding: The present study was supported by the Sardjito General
Hospital (grant no. HK.02.03/XI.2/42771/2021).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
TCG, EKD and II conceived the research. EKD and II
wrote the manuscript, with significant contributions from SLA and
RGB. EKD and II confirm the authenticity of all the raw data. All
authors read and approved the final version of the manuscript.
Ethics approval and consent to
participate
The present study was approved (approval no.
KE/FK/1291/E1; date, December 2021) by the ethics committee. and
patient consent for sample collection was waived by the ethics
committee Faculty of Medicine, Public Health and Nursing,
University Gadjah Mada/Sardjito General Hospital (Yogyakarta,
Indonesia) and patient consent for sample collection was waived by
the ethics committee.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Boyle P: Triple-negative breast cancer:
Epidemiological considerations and recommendations. Ann Oncol. 23
(Suppl 6):vi7–vi12. 2012.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Yeh J, Chun J, Schwartz S, Wang A, Kern E,
Guth AA, Axelrod D, Shapiro R and Schnabel F: Clinical
characteristics in patients with triple negative breast cancer. Int
J Breast Cancer. 2017(1796145)2017.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Widodo I, Dwianingsih EK, Aryandono T and
Soeripto S: Clinicopathological characteristic and prognostic
significance of Indonesian triple-negative breast cancer. Indones
Biomed J. 11:286–292. 2019.
|
|
4
|
Jang MH, Kim HJ, Kim EJ, Chung YR and Park
SY: Expression of epithelial-mesenchymal transition-related markers
in triple-negative breast cancer: ZEB1 as a potential biomarker for
poor clinical outcome. Hum Pathol. 46:1267–1274. 2015.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Fedele M, Cerchia L and Chiappetta G: The
epithelial-to-mesenchymal transition in breast cancer: Focus on
basal-like carcinomas. Cancers (Basel). 9(134)2017.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Han J, Lim W, You D, Jeong Y, Kim S, Lee
JE, Shin TH, Lee G and Park S: Chemoresistance in the human
triple-negative breast cancer cell line MDA-MB-231 induced by
doxorubicin gradient is associated with epigenetic alterations in
histone deacetylase. J Oncol. 2019(1345026)2019.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Bou Zerdan M, Ghorayeb T, Saliba F, Allam
S, Bou Zerdan M, Yaghi M, Bilani N, Jaafar R and Nahleh Z: Triple
negative breast cancer: Updates on classification and treatment in
2021. Cancers (Basel). 14(1253)2022.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Yamamoto Y, Ibusuki M, Nakano M, Kawasoe
T, Hiki R and Iwase H: Clinical significance of basal-like subtype
in triple-negative breast cancer. Breast Cancer. 16:260–267.
2009.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Toft DJ and Cryns VL: Minireview:
Basal-like breast cancer: From molecular profiles to targeted
therapies. Mol Endocrinol. 25:199–211. 2011.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Badowska-Kozakiewicz AM and Budzik MP:
Immunohistochemical characteristics of basal-like breast cancer.
Contemp Oncol (Pozn). 20:436–443. 2016.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Bao B, Mitrea C, Wijesinghe P, Marchetti
L, Girsch E, Farr RL, Boerner JL, Mohammad R, Dyson G, Terlecky SR,
et al: Treating triple negative breast cancer cells with erlotinib
plus a select antioxidant overcomes drug resistance by targeting
cancer cell heterogeneity. Sci Rep. 7(44125)2017.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Jung J, Kang E, Gwak JM, Seo AN, Park SY,
Lee AS, Baek H, Chae S, Kim EK and Kim SW: Association between
basal-like phenotype and BRCA1/2 germline mutations in Korean
breast cancer patients. Curr Oncol. 23:298–303. 2016.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Maksimenko J, Irmejs A,
Nakazawa-Miklasevica M, Melbarde-Gorkusa I, Trofimovics G,
Gardovskis J and Miklasevics E: Prognostic role of BRCA1 mutation
in patients with triple-negative breast cancer. Oncol Lett.
7:278–284. 2014.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Botti G, Cantile M, Collina F, Cerrone M,
Sarno S, Anniciello A and Di Bonito M: Morphological and
pathological features of basal-like breast cancer. Transl Cancer
Res. 8 (Suppl 5):S503–S509. 2019.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Hussein IA, Ahmed ST, Hameedi AD, Naji RZ,
Alharbawi L, Alkhaytt M and Pity IS: Immunohistochemical expression
of BRCA1 protein, ER, PR and Her2/neu in breast cancer: A
clinicopathological study. Asian Pac J Cancer Prev. 21:1025–1029.
2020.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Abulkhair O, Moghraby JS, Badri M and
Alkushi A: Clinicopathologic features and prognosis of
triple-negative breast cancer in patients 40 years of age and
younger in Saudi Arabia. Hematol Oncol Stem Cell Ther. 5:101–106.
2012.PubMed/NCBI View Article : Google Scholar
|
|
17
|
McGuire A, Brown JA, Malone C, McLaughlin
R and Kerin MJ: Effects of age on the detection and management of
breast cancer. Cancers (Basel). 7:908–929. 2015.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Tzikas AK, Nemes S and Linderholm BK: A
comparison between young and old patients with triple-negative
breast cancer: Biology, survival and metastatic patterns. Breast
Cancer Res Treat. 182:643–654. 2020.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Badve S, Dabbs DJ, Schnitt SJ, Baehner FL,
Decker T, Eusebi V, Fox SB, Ichihara S, Jacquemier J, Lakhani SR,
et al: Basal-like and triple-negative breast cancers: A critical
review with an emphasis on the implications for pathologists and
oncologists. Mod Pathol. 24:157–167. 2011.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Leidy J, Khan A and Kandil D: Basal-like
breast cancer: Update on clinicopathologic, immunohistochemical,
and molecular features. Arch Pathol Lab Med. 138:37–43.
2014.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Guterson B and Eaves CJ: Basal-like breast
cancers: From pathology to biology and back again. Stem Cell
Reports. 10:1676–1686. 2018.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Kim H, Cho DY, Choi DH, Choi SY, Shin I,
Park W, Huh SJ, Han SH, Lee MH, Ahn SH, et al: Characteristics and
spectrum of BRCA1 and BRCA2 mutations in 3,922 Korean patients with
breast and ovarian cancer. Breast Cancer Res Treat. 134:1315–1326.
2012.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Kwong A, Wong CHN, Suen DTK, Co M, Kurian
AW, West DW and Ford JM: Accuracy of BRCA1/2 mutation prediction
models for different ethnicities and genders: Experience in a
southern Chinese cohort. World J Surg. 36:702–713. 2012.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Sugano K, Nakamura S, Ando J, Takayama S,
Kamata H, Sekiguchi I, Ubukata M, Kodama T, Arai M, Kasumi F, et
al: Cross-sectional analysis of germline BRCA1 and BRCA2 mutations
in Japanese patients suspected to have hereditary breast/ovarian
cancer. Cancer Sci. 99:1967–1976. 2008.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Hedau S, Batra M, Singh UR, Bharti AC, Ray
A and Das BC: Expression of BRCA1 and BRCA2 proteins and their
correlation with clinical staging in breast cancer. J Cancer Res
Ther. 11:158–163. 2015.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Shin S, Kim Y, Chul Oh S, Yu N, Lee ST,
Rak Choi J and Lee KA: Validation and optimization of the Ion
Torrent S5 XL sequencer and Oncomine workflow for BRCA1 and BRCA2
genetic testing. Oncotarget. 8:34858–34866. 2017.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Mahmoud AM, Macias V, Al-Alem U, Deaton
RJ, Kadjaksy-Balla A, Gann PH and Rauscher GH: BRCA1 protein
expression and subcellular localization in primary breast cancer:
Automated digital microscopy analysis of tissue microarrays. PLoS
One. 12(e0184385)2017.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Guney Eskiler G, Cecener G, Egeli U and
Tunca B: Triple negative breast cancer: New therapeutic approaches
and BRCA status. APMIS. 126:371–379. 2018.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Costa REARD, Oliveira FTR, Araújo ALN and
Vieira SC: Prognostic factors in triple-negative breast cancer: A
retrospective cohort. Rev Assoc Med Bras (1992). 67:950–957.
2021.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Chen B, Zhang X, Liu Y and Wang C:
Prognostic disparities in young patients based on breast cancer
subtype: A population-based study from the SEER database. Medicine
(Baltimore). 102(e33416)2023.PubMed/NCBI View Article : Google Scholar
|