1. Introduction
The metabolome, a final product of the
transcriptome, genome and proteome, contains small-molecule
metabolites correlated with specific metabolic phenotypes. It
provides insights into the pathophysiology and therapeutic targets
of numerous illnesses (1). The
metabolome has previously shown considerable advantages for
identifying biomarkers, diagnosing and treating illnesses, and
defining metabolic-control mechanisms. Comprehensive metabolic
fingerprints can identify treatment targets and infer potential
illness mechanisms. In the rapidly expanding science of
metabolomics, tiny molecules in biological processes known as
metabolites undergo comprehensive investigation. As a potential
method of identifying new biomarkers, pharmacological targets and
therapeutic agents, metabolomics in cancer research has attracted
considerable attention (2).
The results of protein translation, gene
transcription, or structural modifications to the proteome, genome,
or transcriptome are called metabolites. Metabolites have the
potential to play a significant role in the interaction between
genotype and environment and give a clearer picture of the final
phenotype. A publicly available human metabolome primarily includes
comprehensive data on 41,993 small-molecule metabolites (1-3).
In addition to acting as cofactors, energy producers' storage
units, signalling molecules, metabolites may also start regulatory
processes. Compared with other omic methods, metabolomics focuses
on metabolites and has several benefits. Although metabolomics may
directly identify the biochemical reaction to a stimulus, genomics
may not have considerable influence on how a protein's expression
induces its function (3). The
present review aims to cover various aspects of metabolomics in the
context of cancer research, including fundamentals, the role of
metabolites in cancer development, analysis methods, applications
in cancer detection and diagnostics, comparison of metabolomic
analysis instruments, and potential clinical uses in cancer and
breast cancer (BC) research (4).
Overall, a comprehensive overview of metabolomics in cancer
research is provided, highlighting its potential as a powerful tool
for understanding cancer biology and improving clinical outcomes
through early detection and personalised treatment strategies.
Finally, we discuss metabolomics' possible clinical uses in cancer
and BC research. It is aimed to identify and discuss the
possibility of metabolites' early detection through metabolomics
research.
2. Discussion
BC
Malignant tumours are divided into locally malignant
at the same organ or tissue without spreading and tumours spreading
to other organs or parts of the body, which is called metastasis.
Not all tumours arrive at the metastatic stage, especially if
diagnosed early. The tumour derives nutrients from other
surrounding healthy cells. As a result, the healthy cells die,
which allows the tumour cells to grow even faster. The process of
spreading the cancer cells to other body parts and growing
continuously in those locations is known as metastasis. BC is a
type of cancer affecting one in every eight women in high-income
nations by the age of 85, and it will continue to be the primary
source of disease burden for women (5). BC remains a severe health issue
despite significant advancements in the field of cancer research
and is now a high focus for biomedical research. The most frequent
disease amongst women worldwide is BC, and its incidence and
mortality rates are predicted to rise sharply in the coming years
(6). With over 1,700,000 new cases
each year, the frequency of this aggressive illness remains
disturbingly high, and these numbers point to decreased progress in
the preventative field (7). The
estimated number of deaths globally in 2020 according to Globocan
2020 (WHO) is 684,996 cases, which comprises 15.5% of the total
worldwide death percentage. Genes, the fundamental building blocks
of inheritance, can change in ways that lead to cancer (8). Genetic changes that cause cancer can
happen because of errors that occur as cells divide or DNA damage
inflicted by harmful environmental substances (such as the
chemicals in tobacco smoke and ultraviolet rays from the sun). Such
changes can also be inherited from parents (9). In elderly people, whose bodies become
less capable of eliminating damaged and old cells, the chance of
developing cancer later increases. The genetic mutations in every
individual cancer differ from one another. Further changes occur
when the cancer spreads. Several cells in the same tumour may have
distinct genetic changes (10).
Types of BC. BC is categorized into several
types based on the characteristics of the cancer cells and their
behaviour (11-14).
The major types of BC are as follows: i) Ductal carcinoma in
situ (DCIS); DCIS is a non-invasive cancer where abnormal cells
are found in the lining of a breast duct. While it is not
life-threatening, it can increase the risk of developing invasive
BC later. ii) invasive ductal carcinoma (IDC); IDC is the most
common type of BC, accounting for ~70-80% of cases. It begins in
the milk ducts and invades surrounding breast tissue. Symptoms may
include a lump or changes in breast shape. iii) invasive lobular
carcinoma (ILC); This type starts in the lobules (milk-producing
glands) and accounts for ~10-20% of invasive BCs. ILC may present
as a thickening or swelling rather than a distinct lump, making it
harder to detect via mammograms. iv) human epidermal growth factor
receptor 2 (HER2)-positive BC; HER2-positive BC tests positive for
excess HER2 proteins, which promote cell proliferation. This type
tends to be more aggressive but responds well to targeted therapies
that inhibit HER2. A total of ~15-20% of BCs are HER2-positive. v)
triple-negative BC (TNBC); TNBC lacks three common receptors:
Estrogen, progesterone and HER2. This type is more prevalent among
younger women and tends to be more aggressive with fewer treatment
options available compared with other types.
Metabolites
The metabolism or metabolic reaction can be defined
as the sum of all biochemical reactions carried out by an organism.
Metabolites have various roles, including those related to energy,
structure, signalling, catalysis, defence and interactions with
other organisms. Plants, humans and microbes, all produce
metabolites. Metabolites can be divided into two different types,
namely, primary metabolites and secondary metabolites. Metabolites
are the intermediates or final products of metabolic reactions,
which are typically limited to small molecules and are catalysed by
several enzymes that naturally exist within cells (15,16).
The cell produces primary metabolites and typically participates in
respiration and photosynthesis, the two main metabolic activities.
Primary metabolites can keep the body's physiological processes
running smoothly. Considering this function, it is often referred
to as the central metabolite. Amino acids, alcohols, polyols,
organic acids, vitamins (B2 and B12), inosine-5'-monophosphate, and
guanosine-5'-monophosphate are notable examples of primary
metabolites. Ethanol, citric acid, lactic acid and acetic acid are
primary metabolites necessary for healthy development, growth and
reproduction. Cells utilise primary metabolites, intermediate
by-products of anabolic metabolism, to create necessary
macromolecules (17).
Secondary metabolites are substances an organism
produces that are not necessary for primary metabolic activities
but may serve crucial ecological and other purposes. Secondary
metabolites are not involved in cell proliferation and development
and are synthesised at or near the end of the stationary growth
phase (18). Given that secondary
metabolites are produced by the same metabolic pathways that
primary metabolites use, secondary metabolites are known as the
final products of primary metabolites. Primary metabolites are
present in every living cell with the ability to divide. Secondary
metabolites are present merely incidentally and are not crucial to
an organism's survival (16).
However, secondary metabolites are produced from primary
metabolites, which do not constitute the organism's fundamental
molecular structure. Primary metabolites' absence does not
immediately shorten an organism's lifespan; instead, survival is
compromised to a greater extent. Within a phylogenetic group, its
existence and synthesis are found in ecologically disadvantageous
species (19). Drugs, flavours,
scents, dyes, pigments, insecticides and food additives are
examples of secondary metabolites used in pharmaceuticals,
industries and agriculture (20).
Numerous intermediates in primary metabolism overlap
with the intermediates of secondary metabolites, thus
distinguishing between primary and secondary metabolites is not
easy. Amino acids, considered primary metabolites, are also
unquestionably secondary metabolites (16), in contrast to the claim that
sterols are secondary metabolites essential to numerous cellular
structural frameworks. The mosaic structure of an intermediate
suggests that primary and secondary metabolism share the same
metabolic pathway. Adding extra nitrogen and carbon can be directed
into the secondary metabolites, which operate as a buffer zone to
produce an inactive primary metabolism. When needed, the metabolic
disintegration of secondary metabolites can convert the stored
carbon and nitrogen back into primary metabolites. The primary and
secondary metabolisms are dynamic and in a delicate balance with
the growth, tissue differentiation, and development of the cell or
organism, as well as external influences, all impacting. Secondary
metabolites, also known as natural products or heterogeneous groups
of natural metabolic products, are considered to play adaptive
roles in ecological interactions, symbiosis, metal transport,
competition and other processes even though they are not required
for the vegetative growth of the producing organisms (21). For instance, they may act as
defence compounds or signalling molecules.
According to Jones et al (23), a comprehensive analysis reveals
that a typical human body contains ~2,500 metabolites. Arachidonic
acid is a metabolite of prostaglandin, and the two compounds share
numerous of the same functional groups, physical characteristics
and formulae. Additionally, a specific sequence of enzyme-catalysed
reactions that follow a rational path of chemical change connects
both chemicals (24). Tyrosine is
an amino acid that produces catecholamines, whereas cholesterol
creates steroid hormones. By making only minor modifications to the
cholesterol ring's superstructure, steroid hormones that differ
biochemically from the cholesterol source molecule can be produced
(2). Tyrosine is the starting
point for an irreversible route that leads to catecholamines, such
as norepinephrine or dopamine. Moreover, all precursors of
catecholamine must pass through a tyrosine intermediate owing to
biochemical principles (3).
According to the free-energy exchange theory,
inosine-5'-monophosphate is a metabolite that develops from the
one-way condensation of two or more intermediates, specifically
glutamine and phosphoribosyl-pyrophosphate (4). Small molecules are complex to define
precisely because they quickly diverge from their parent structure.
A metabolite may also be a component of a larger structure or a
degraded product that needs to be disposed. A freely available
electronic database including comprehensive data on metabolites
discovered in the human body is known as the Human Metabolome
Database (3-5).
BC metabolomics
In attempts to discover potential biomarkers that
can be used to detect cancer cells in their earliest stages,
numerous studies have been performed on the biological samples of
patients with BC. Samples from patients such as tissues, blood and
urine have been collected and examined to obtain the best results
that can benefit individuals. Tumour DNA is the element that has
been most thoroughly evaluated, including DNA concentrations,
integrity, mutations and methylation status. The main aim is to
gauge its potential clinical relevance (24,25).
Cancer cells also have the exact needs and capacities for energy as
regular cells. It has been demonstrated that most cancer cells
produce energy through cytoplasm glycolysis. Energy generation is
typically utilised by several contemporary technologies to detect
malignancy. The rate of protein turnover and lipolysis, which is
the breakdown of fat stored in fat cells, increases in cancer cells
(26).
Cancer cells undergo significant metabolic changes
compared with normal cells. These changes are critical to cancer
cells' survival and proliferation, providing a unique opportunity
to differentiate cancer cells from normal cells. Metabolomics can
be used to identify these metabolic changes and thus help diagnose
and treat cancer. It can also help in the discovery of new
biomarkers and therapeutic targets. Some researchers have focused
on potential indicators found in urine samples of patients with BC.
The metabolomics approach is used for the test and research, which
involves running tests on technologies such as nuclear magnetic
resonance (NMR), high-performance liquid chromatography (HPLC), gas
chromatography (GC)-mass spectrometry (MS), or other suitable
analytical tools to obtain the most accurate results. In total, 44
pair-wise rates of RNA metabolites exist for BC urinary tests.
Numerous different indicators or biomarkers can be found in the
urine samples of patients with BC. Based on a study by Nam et
al (27), homovanillate,
4-hydroxyphenylacetate, 5-hydroxyindoleacetate and urea are all
found in the urine samples of patients with BC.
The main contributing compounds in the urinary
metabolomics for BC include formate, succinate and nucleoside
uracil. Succinate, a metabolite of the tricarboxylic acid (TCA)
cycle and a marker for the Warburg effect, is also highlighted by
another MS investigation (28).
With reasonable specificity and sensitivity, the panel of succinic
acid and dimethyl-heptanoyl-carnitine is used to distinguish
between BC and healthy controls. According to research looking at
nucleosides in urine, 5-hydroxymethyl-2'-deoxyuridine,
8-hydroxy-2-deoxyguanosine and succinyl adenosine are all shown to
be more common in patients with BC (29-31).
Patients with BC have higher amounts of glucose,
creatinine, glutamine, glutamate, arginine, lysine and valine than
healthy controls. These metabolisms are closely linked to a higher
risk of BC. Moreover, it has been found that those with greater
levels of 5-amino valeric acid, tryptophan, phenylalanine,
y-glutamyl threonine, valine, or iso-glutamine are more likely to
be diagnosed with BC (23,33). A recent study predicted that
2-o-methylcytidine and 5-methylthioadenosine levels in patients
with BC will rise (34). Based on
the same research, hierarchical analysis reveals 71 out of 168
differentially expressed metabolites.
Analysing urine metabolomics biomarkers often uses
analytical techniques such as NMR and MS (35). By identifying the distinctive
electrochemical environment of each constituent proton, the urine
NMR readings of molecules can be identified using NMR. Low levels
of several metabolites including succinate have been found in the
urine of patients with epithelial ovarian cancer and BC according
to research on urinary-metabolite modifications (36,37).
A total of nine metabolites significantly differ in a study
comparing the urinary proton NMR metabolomic profiles of BC (n=48)
and ovarian cancer (n=50) based on Wilcoxon's rank-sum test. The
metabolites involved are acetone, allantoin, carnitine, urea,
1-methyl nicotinamide and levoglucosan. Slupsky et al
(39) discovered that the amount
of several high-level metabolites including glucose and creatine,
which are high in cancer tissue, decreases in the urine of patients
with BC (38).
Moreover, patients with BC have lower urine
succinate levels compared with healthy controls (39). The urine samples of patients with
BC have decreased glutamine level, which is typically high in
breast tissue. This discovery is also validated by additional
research that produces comparable outcomes (38). Urine of patients with BC has lower
threonine levels than controls as well (40). Changes can further be observed in
metabolites such as choline and 2-hydroxybutyrate. These two
metabolites have higher levels in BC samples than in healthy
control samples (41,42). Valine and lysine also rise
(43). Furthermore, patients with
BC have lower amounts of melatonin and indole-3-acetate in their
urine tests.
A study on BC indicators in urine examines metabolic
differences between patients with BC and healthy volunteers. The
investigation identified12 metabolites including amino acids,
organic acids and nucleosides as possible biomarkers (30). In a separate study, (27) used a LC-ion trap MS to analyse
urine samples from 85 patients with BC and corresponding controls.
A total of 44 pairwise ratios of metabolite characteristics were
effectively examined by computational analysis, with a sensitivity
and specificity of 83.5 and 90.6%, respectively, for the best BC
prediction. S-Adenosylhomocysteine and a few other methylated
nucleosides significantly dominate the classification performance.
In another study, a capillary electrophoresis (CE) MS was used to
examine urine samples from 21 patients with advanced BC before and
after receiving chemotherapy, as well as samples from the general
population (44). The
aforementioned study found that metabolite levels decrease by 30%
in chemotherapy-sensitive patients compared with the control group.
Specifically, glycine, cysteine, histidine, cysteine, and
tryptophan levels are affected. Those who are resistant to
treatment have 9% changes in metabolite levels. Meanwhile, the
amounts of succinate increases and the levels of chromium
considerably drop, whereas most amino and organic acids do not show
any apparent alterations. In another study, urine samples from 22
healthy controls were compared with those from 10 patients with BC,
9 with ovarian cancer and 12 with cervical cancer. The cancer
biomarkers were found to comprise 5-hydroxymethyl-2-deoxyuridine
and 8-hydroxy-2-deoxyguanosine (45).
The identification of BC biomarkers in urine samples
of patients with BC is also influenced by environmental factors.
Cadmium is markedly more prevalent in urine of patients with BC
(46). The same applies to
increasing chromium and arsenic. Moreover, it was revealed that
patients with BC have a general decrease in amino acids,
nucleotides and TCA cycle intermediates (40). The marker results from previous
studies based on different sample types, such as tissue, serum,
plasma and urine samples, are included in Table I.
 | Table IMetabolomics in studies of human BC:
Comparison between blood, tissue and urine samples. |
Table I
Metabolomics in studies of human BC:
Comparison between blood, tissue and urine samples.
| First
author/year | Sample type and
sample size | Method |
Results/Markers | Population | (Refs.) |
|---|
| Asiago et
al, 2010 | 257 serial blood
serum samples: 116 samples with recurrent BC, 141 samples with no
sign of recurrence | NMR and GC-MS | 11 markers (NMR:
formate, His, Pro, Cho, Tyr, 3-HB, Lact; GC-MS: Glu, N-acetyl-Gly,
nonanedioic acid, 3-hydroxy-2-methyl-butanoic acid | Houston, Texas | (48) |
| Oakman et
al, 2011 | Pre- and
post-operative blood serum samples from 44 patients with early BC
and 51 meta-static patients | NMR | Metastatic samples:
Higher values of Pro, Phe, Gluc, Lys and N-acetyl-Cys and lower
values of lipids. | Prato, Italy | (49) |
| Tenori et
al, 2012 | Blood serum samples
from 579 women with metastatic BC randomized to paclitaxel plus
either anti-HER2 (lapatinib) or placebo | NMR | Gluc higher in the
patients with longer time to progression. Glutamate and Phe higher
in patients with shorter time to progression in on-treatment
samples | Italy | (50) |
| Wei et al,
2013 | Serum samples from
28 patients with different response rates to NAC | NMR/LC-MS | Three metabolites
(Ile, Thr, Gln) from NMR and linolenic acid from LC-MS were
significantly different when comparing response to
chemotherapy | Germany | (51) |
| Jobard et
al, 2014 | Blood serum from
197 patients with early BC and 90 metastatic patients | NMR | Ala, His and
betaine were higher in the serum of patients with early BC; end
products of lipid degradation and β-oxidation (Acac and 3-HB)
(glycerol), Pyr, NAC glycoproteins, lipids, or Phe, Glu and mannose
concentrations increased for metastatic BC | France | (52) |
| Tenori et
al, 2015 | Blood serum from 80
patients with early-stage BC and 95 patients with metastatic
BC | NMR | Significantly lower
levels of His and higher serum levels of Gluc, Tyr, Lact and lipids
in metastatic patients | New York and
Italy | (53) |
| Henneges et
al, 2009 | Urine samples from
85 patients with BC and 85 HC | LC-MS | 44 pairwise ratios
of metabolite features had distinct predictive capacity;
S-adenosylhomo-cysteine as main identifiers; various methylated
nucleosides | Germany | (28) |
| Nam et al,
2009 | Urine samples from
50 patients with BC and 50 HC | GC-MS | Homovanillate,
5-hydroxyindoleacetate, 4-hydroxyphenylacetate and urea were
identified to be different in normal subjects and cancer | Korea | (27) |
| Woo et al,
2009 | Urine samples from
10 patients with BC, 9 patients with OV, 12 patients with cervical
cancer and 22 normal controls | GC-MS/LC-MS | BC samples contain
2-hydroxymethyl-2-deoxyuridine and 8-hydroxy-2-deoxyguanosine | Korea | (46) |
| Kim et al,
2010 | Urine samples from
50 patients with BC and 50 controls | GC-MS | Five potential
urinary biomarkers for BC; meta-bolites were not identified | Korea | (54) |
| Slupsky et
al, 2010 | Urine samples from
48 patients with BC, 50 patients with OV and 73 healthy
volunteers | NMR | 67 metabolites
identified; amino acids, tricarboxylic acid cycle and metabolites
relating to energy metabolism, and gut microbial metabolism | Edmonton,
Canada | (39) |
| Yu et al,
2013 | Urine samples from
21 patients with advanced or locally advanced BC before and after
chemotherapy and 21 healthy volunteers | CE-MS | In
chemotherapy-sensitive patients: Cys, Gly, cystine, His and Trp
were significantly decreased after chemotherapy; In
chemotherapy-insensitive patients: few obvious differences between
patients before and after chemotherapy (Succ increased while Cr
decreased) | China | (45) |
| Chen et al,
2009 | Urine samples from
20 patients withBC and 18 HC | LC-MS | 12 metabolites as
potential biomarkers including organic acids, amino acids, and
nucleosides; elevated Trp and nucleosides metabolism and protein
degradation in patients with BC | China | (31) |
| Bathen et
al, 2013 | 228 BC tissues | NMR | The loading
profiles from both PCA and PLS-DA analyses revealed
choline-containing compounds as the key indicators for tumour
content, with phosphocholine being more abundant in tumour tissue.
Glycine, taurine and glucose are also suggestive metabolites. | Trondheim
(Norway) | (55) |
| Borgan et
al, 2010 | 46 BC tissues | NMR | One of the
categories, A2, had samples with markedly lower glucose and higher
alanine levels than the other luminal A samples, indicating that
these tumours were more glycolytically active. Additionally, this
group was enriched for genes having Gene Ontology concepts
associated with DNA repair and cell cycle. | Trondheim
(Norway) | (56) |
| Debik et al,
2019 | 118 BC tissues and
serum | NMR | PLS-DA multilevel
analysis revealed significant changes in blood metabolite levels
following therapy (P=0.001), including unfavorable alterations in
lipid levels. PLS-DA detected metabolic differences between
survivors and non-survivors in tissue samples received 12 weeks
into therapy with an accuracy of 72% (P=0.005), but not in serum
samples | Oslo (Norway) | (57) |
| Haukaas et
al, 2016 | 228BC tissues | NMR | Among the most
notable changes were Mc1's high amounts of GPC and phosphocholine
(PCho), Mc2's high levels of glucose, and Mc3's high levels of
lactate and alanine | Oslo (Norway) | (58) |
| Chae et al,
2016 | 60 BC tissues | NMR | The GPC/PC ratio,
as well as the concentrations of myo-inositol and succinate, were
greater in the pure DCIS group than in the DCIS with invasive
cancer group (P=0.004, Bonferroni-corrected P=0.064). The OPLS-DA
models generated using HRMAS MR metabolic profiles could clearly
distinguish between pure DCIS and DCIS of myo-inositol and
succinate with concomitant invasive cancer using multivariate
analysis. | Seoul (South
Korea) | (59) |
| Euceda et
al, 2019 | 122 BC tissues | NMR | Linear
mixed-effects models revealed a significant interaction between
time and bevacizumab for glutathione, indicating higher levels of
this antioxidant in chemotherapy-only patients than in bevacizumab
receivers after treatment | Trondheim | (60) |
| Cala et al,
2019 | Plasma 58 (29BC;
29HC) | NMR | Particularly, the
understanding of the up regulation of long chain fatty acyl
carnitines and the downregulation of cyclic phosphatidic acid. In
addition, the mapped metabolic signatures in BC were similar but
not identical to those reported for non-Hispanic women, despite
racial differences. | Bogotà
(Colombia) | (61) |
| Lécuyer et
al, 2018 | Plasma 602 (206BC;
396HC) | NMR | Women characterized
by higher fasting plasma levels of valine, lysine, arginine,
glutamine, creatine, creatinine and glucose, and lower plasma
levels of lipoproteins, lipids, glycoproteins, acetone,
glycerol-derived compounds, and unsaturated lipids had a higher
risk of developing BC. | France | (62) |
| Suman et al,
2018 | Plasma 122
(72BC;50HC) | NMR | The levels of
hydroxybutyrate, lysine, glutamate, glucose, NAC glycoprotein and
lactate were highly distinguished in BC stages and showed a
favorable biomarker potential using receiver-operating curves based
diagnostic models. Furthermore, the significant modulation and
favorable diagnostic performances of glutamate, NAC glycoprotein
and Lactate in LBC as compared with EBC give their significance in
the BC progression. | Lucknow
(India) | (63) |
| Louis et al,
2015 | Plasma 145
(73BC;72HC) | NMR | The levels of
hydroxybutyrate, lysine, gluta-mate, glucose, NAC glycoprotein and
lactate were highly distinguished in BC stages and showed a
favorable biomarker potential using receiver-operating curves based
diagnostic models. Furthermore, the significant modulation and
favorable diagnostic performances of glutamate, NAC glycoprotein
and lactate in LBC as compared with EBC give their significance in
the BC progression. | Hasselt
(Belgium) | (64) |
| Vignoli et
al, 2020 | Plasma 43 BC | NMR | ER status in
patients with HER2-positive BC was found to induce significant
changes in the host circulatory metabolome with important
implications for the pCR to NACT and for the overall clinical
outcome | Aviano (Italy) | (65) |
| Jobard et
al, 2021 | Plasma 1582
(791BC;791HC) | NMR | The concentration
of NAC glycoproteins, ethanol, hypoxanthine and dimethylamine, were
positively associated with BC. The concentration of 10 metabolites
were increase in premenopausal group after FDR adjustment. The
strongest association: histidine. Borderline inversely associated
with BC are LDL and VLDL (fatty acids) | Lyon (France) | (1) |
| McCartney et
al, 2019 | 115 Serum BC | NMR | Metabolomic
signature between patients with early BC and metastatic BC is
similar and would be predictive of cancer recurrence. Low Random
Forest score: Disease free at follow-up. High Random Forest score:
One relapse case among seven patients. | NewYork (USA) | (66) |
| Jiang et al,
2018 | 29 Serum BC | NMR | NMR spectra
containing signal from a variety of amino acids (isoleucine,
valine, leucine, alanine, threonine, lysine, glutamine, glycine,
ornithine, phenylalanine, tyrosine, histidine), amino acid
derivative (creatinine, creatine, betaine), variety moieties of
lipids, ketone bodies (acetone, 3-D-hydroxybutyrate, acetoacetate),
choline metabolites, carbohydrate metabolism related metabolites,
NAC glycoproteins and organic acids. | Singapore | (67) |
| Wojtowicz et
al, 2020 | Serum 95
(9BC;86HC) | NMR | 31 metabolites,
four unknown signals, and nine ranges of chemical shift regions
assigned to different lipid types. Levels of citrate, glutamine,
creatinine, acetoacetate, acetate, glucose, betaine, glycerol,
leucine, choline and lysine were upregulated in TNBC Lipid levels,
lactate, acetone, alanine, glutamate, tyrosine, pyruvate and
isoleucine were down regulated in TNBC. | Wroclaw
(Poland) | (68) |
| Men et al,
2020 | Urine 144
(106BC;38HC) | NMR | Heavy metals in
urine samples. Cd has been detected in BC tissue at high
concentrations. The Cd was markedly increased in the urine of
patients with BC compared with the control population (~2-fold).
Numerous small molecule metabolites were altered in the urine of
patients with BC compared with the control population. | Tengzhou
(China) | (69) |
| Wang et al,
2017 | Urine 78
(40BC;38HC) | NMR | A total of 10
metabolites exhibited the highest contribution towards
discriminating patients with BC from HXs (variable importance in
projection (VIP) >1, P<0.05). The metabolomic pathway
analysis indicated several metabolism pathway disruptions,
including amino acid and carbohydrate metabolisms, in patients with
BC, namely, glycine and butanoate metabolisms. | Funchal
(Portugal) | (2) |
| Slupsky et
al, 2010 | Urine 170
(48BC;50OC;72 HC) | NMR | All metabolites
that were significantly different between the cancers and normal
controls were lower in concentration in both the EOC and BC groups
as compared with normal. Intermediates of the tricarboxylic acid
cycle and metabolites relating to energy metabolism, amino acids
and gut microbial metabolism were perturbed. | Edmonton
(Canada) | (71) |
Role of metabolites in cancer
development
A complex network of chemical processes is
responsible for metabolism within cells, which supports healthy
development and reproduction. Metabolism involves catabolism and
anabolism. The former provides energy and generates the cellular
building blocks required for cell division. Uncontrolled cell
proliferation and a diverse microenvironment are characteristics of
cancer. According to Cairns et al (71), cancer cells alter their preferred
metabolic pathway to balance their energy requirements with their
need to produce biosynthesis precursors for development (69) and to survive in low nutrient areas
and low oxygen concentrations (72). By changing the functions of current
metabolic pathways or rewiring new connections, cancer cells
experience widespread metabolic modifications, notably in
glycolysis, mitochondrial biogenesis, lipid metabolism and the
pentose phosphate pathway (73).
Through various processes, metabolic reprogramming in cancer cells
causes the accumulation or depletion of intermediate metabolites
(74). The first and foremost one
is an alteration in the activity of metabolic enzymes. Since the
1920s, the Warburg effect has been recognised as a distinctive
feature of cancer. It is a change in metabolic state wherein cells
show an enhanced conversion of glucose into lactate even in highly
oxygenated areas (75-77).
For instance, activating glycolysis-related enzymes results in the
build-up of several glycolytic intermediates during glycolysis, the
preferred method by which cancer cells receive energy and
biosynthesis building blocks. Conversely, the build-up of succinate
and fumarate is caused by a decrease in succinate dehydrogenase and
fumarate hydratase activities, respectively.
Since the discovery of oncogenic functions of
various mitochondrial metabolites such as 2-HG, succinate and
fumarate, researchers have become increasingly interested in the
functions of these ‘oncometabolites’ in cancer. Oncometabolites
affect signal transduction, post-transcriptional modifications, and
epigenetic changes. The inactivation of tumour-suppressor genes and
the promotion of carcinogenesis are caused by metabolic
remodelling, which can encourage DNA hypermethylation and histone
hyperacetylation (78,79). Numerous intermediate metabolites,
in addition to oncometabolites, can bind directly to proteins or
nucleotides and cause them to malfunction. These intermediate
metabolites can also function as transmembrane receptor ligands,
triggering subsequent signalling cascades.
The phenomenon in which cancer cells enhance their
intake of glucose and the formation of lactate with a significant
reliance on aerobic glycolysis is described as the Warburg effect
(75). Cancer cells can produce
only minimal ATP during this metabolic state, and they may start to
rely on glutamine as a fuel source (80). Thus, cancer therapies are
intensively researching the suppression of glucose and glutamine
metabolism (80,81). Under normoxic conditions, the
contribution of lactate to oxidative respiration has attracted
newfound attention (82). The
finding that lactate is a waste product and a crucial energy source
for tumours raises the possibility that metabolites other than
glucose and glutamine may support an environment favourable for the
proliferation and multiplication of cancer cells. Asparagine,
arginine, cysteine, serine and glycine are examples of downstream
amino acid by-products that have been studied for their role in the
survival of cancer cells. Further research into medicines targeting
each metabolic pathway is required, even if the deprivation of
these nutrients is beneficial in some situations. As an
alternative, several amino acids and essential vitamins such as
vitamins A, B, C, D, E and K function as antitumorigenic agents and
slow the spread of cancer. The same study emphasises the roles of
lactate, vitamins and amino acids in advancing, inhibiting, and
preventing cancer by drawing attention to these generally
underestimated metabolites (Table
II).
 | Table IIMetabolite contribution to tumour
survival according to cancer types. |
Table II
Metabolite contribution to tumour
survival according to cancer types.
| First author,
year | Metabolites | Cancer type | Role in tumour
progression | (Refs.) |
|---|
| Wang et al,
2021 | Vitamin A | Breast | 4-HPR induces cell
death | (83) |
| Sullivan et
al, 2016 | | | Vitamin A and
retinol reduce risk | (75) |
| Wang et al,
2021 | | Colon/Colorectal
Prostate | 4-HPR induces cell
death 4-HPR induces cell death | (83) |
| Sullivan et
al, 2016 | Vitamin
B1 | Breast | Intermediate
concentrations promote Ehrlich's ascites proliferation in
thiamine-deficient patients; high concentrations inhibit
proliferation Patients exhibit decreased expression of SLC9A3
transporter gene | (75) |
| Doldo et al,
2015 | Vitamin C | Breast | Low concentrations
induce cell invasiveness; high doses restrict EMT | (84) |
| Sullivan et
al, 2016 | Vitamin D | Breast
Colon/Colorectal | Calcitriol and D3
analogs suppress MMP-2 and -9 and VCAM-1; low serum D3 levels are
associated with high incidence Low serum D3 levels are associated
with high incidence | (75) |
| Zeng et al,
2019 | Vitamin E | Breast
Colon/Colorectal Prostate | Tocotrienols
exhibit chemotherapeutic and antitumour properties Tocotrienols
exhibit antitumour properties Tocotrienols exhibit chemotherapeutic
properties | (85) |
| Miyazawa et
al, 2020 | Vitamin K | Breast | K2
induces nonapoptotic cell death | (87) |
| | Arginine | Breast | Low plasma levels
act as a prognostic biomarker | (87) |
| Miyazawa et
al, 2020; Qiu et al, 2014 | | | Arginine starvation
is used to treat arginosuccinate synthase-deficient patients | (87,88) |
| Cheng et al,
2018 | | Ovarian | Cancer cells are
deficient in arginosuccinate synthase-1; ADI-PED-20 is used to
degrade arginine | (89) |
| Ji et al,
2020 | Asparagine | Breast | Maintains health of
glutamine-independent cells | (90) |
| Sullivan et
al, 2016 | Cysteine Lactate
Serine | Breast
Colon/Colorectal Breast Breast | Inhibition of
histone deacetylase-6 sensitizes TNBC cells to cysteine deprivation
via cystine/glutamate antiporter-targeted therapies Starvation
induces a reduction in liver-metastatic cell proliferation 10 mM
L-lactate acts as chemoattractant and facilitates migration Cells
prefer serine over glycine and exhibit a decrease in nucleic acid
synthesis when starved of serine | (75) |
Metabolomic techniques
Under certain specific circumstances, any metabolite
can be broken down into smaller product ions. Specific pressure,
temperature and collisional energy are required to break down the
metabolites. These processes of breaking down produce a distinctive
pattern of fragmentation used as identification information. Each
chemical has a unique fragmentation pattern crucial to determining
a compound's retention time for intensity quantification. The
methodology used in metabolomics is unique and has its own set of
procedures. Each approach has a similar set-up procedure.
Importantly, the samples needed must be prepared according to the
desired test. Following the metabolic extraction, the collected
samples are sent to metabolomics equipment for compound separation,
detection and analysis (91).
Fresh tissue and cells from in vitro cultures
are the two common sample types used to extract metabolomics data.
For fresh tissue models, the tissue is collected, immediately
snap-frozen in nitrogenous liquid N2, and then
homogenised in an identical mixture of solvents. This phase must
maintain the effectiveness of the extraction and the biochemical
integrity. Samples are centrifuged several times to guarantee that
all precipitated proteins and other macromolecules are wholly
removed using chemicals to help in this function. The pallets are
preserved for protein concentration analysis to normalise the
metabolite levels. These supernatants are collected, and then the
methanol-chloroform-water mixture is removed using a speed vacuum
and lyophilisation. The result is the formation of the powdered
metabolites. Then, prior to metabolomics acquisition with
metabolomics devices, metabolites are resuspended in a solvent
combination (92-95).
Numerous options are available for selecting
metabolomic equipment. Separation, detection and hyphenated
techniques are the three common strategies used for categorising
the instruments. Techniques including GC, CE, HPLC,
ultra-performance LC and ion chromatography can be used to separate
distinct metabolites that elute at varying retention durations.
Regarding the method of detection, MS equipment is frequently
utilised. MS equipment includes quadrupole time-of-flight (TOF)
chromatography, triple quadrupole and Fourier transforms (FT)
orbitrap. NMR spectroscopy is another detection method not
requiring separation techniques. It is also commonly used to
determine the structures of organic compounds. HPLC-MS, FT, ion
cyclotron resonance (ICR)-MS and GC-MS (91).
Software programs for metabolomic analysis are
required to analyse experimental metabolomic data. MS-based
equipment can identify metabolites by using an internal compound
standard database and MS/MS fragmentation capture under the same
conditions. The sample's fragmentation should match the database's
fragmentation to verify one's structure. NMR-based methods can be
used to investigate the structure of compounds and isotopomers.
Analyses based on NMR and MS can cross-validate and cover more
metabolites overall. Planning and conducting a metabolomics study
involves four significant steps. These steps include sample
collection or generation, data acquisition, bioinformatics and
interpretation. Based on the results, it is recommended that a
hypothesis be formed or the newly discovered biomarkers to be
tested in further studies. Adding quality control to obtain
reproducible outcomes and generate meaningful metabolomics data
during data acquisition is optimal.
MS-based metabolomics. One of the most
popular analytical tools used in metabolomics applications is the
MS. The primary goal of MS is the structural characterisation of
significant metabolites in the search for biomarkers (96). Metabolic fingerprinting can be
acquired by MS direct injection, although it has several
limitations such as co-suppression and low ionisation efficiency.
To avoid these issues, MS-based metabolomic techniques such as
CE-MS, GC-MS, LC-MS (97) and CE
are used. These tools can eliminate co-suppression whilst reducing
the complexity of biological material. Adding MS to these methods
increases the accuracy of compound identification, detection and
quantification and shows great sensitivity, selectivity, speed and
efficiency (42). The samples
prepared are infused directly or by chromatography before being
analysed in a MS. Data are recorded, analysed, processed and
interpreted before being compared with the theoretical data.
GC-MS. GC-MS has emerged as a crucial and
trusted analytical technique for the metabolomic study of
separation, detection and identification (98,99).
The collected samples are subjected to metabolite extraction before
being injected in split-less mode. Afterwards, the high-resolution
capillary column is used to propel and release the carrier gas
through the sample (42). GC
analysis must be performed under certain circumstances (for
example, high temperatures and in an oven), and the metabolites
must be volatile and thermally stable (for example, metabolites
such as alkenes, organic acids, ketones and aldehydes).
Non-volatile metabolites including lipids, amines, amino acids,
phosphorylated metabolites and sugars must first go through
derivatization (42). The samples
can be ionised by electro-impact (EI) or chemical ionisation for MS
detection. The EI approach is frequently used in ionisation. The
mass spectra can be revealed by molecular-ion fragmentation, which
EI can offer. The three techniques most frequently used in
metabolomics are quadrupole, TOF and ion trap.
Salivary volatiles are screened for potential BC by
GC-quadrupole MS (qMS) as part of an exploratory investigation;
geographically remote communities are included (100). It has been claimed that the
metabolomic signature of human BC cell lines can be established
using GC-qMS (98). Based on the
urinary volatomic biosignature, it can also be utilised to
distinguish amongst various cancer types (101). In contrast to GC-qMS, GC-TOFMS
can assess glutamate enrichment as a potential new method of
diagnosing BC. Patients with BC with oestrogen receptor
(ER)-positive (ER+) and ER-negative (ER-)
cells can be compared metabolically using a GC-TOF-MS framework
(102). In a pilot investigation
on patients with BC, it was found that GC-MS can be used to assess
the detectability, reliability and distribution of metabolites
obtained in pre-diagnostic plasma samples (103). The sensitivity, specificity,
reproducibility and high-throughput technology of GC-MS-based
metabolomics to handle a huge volume of samples renders it
preferable to use. However, GC-MS is limited in its mass range, and
because of fragmentation, molecule ions are frequently undetected.
Determining unknown metabolites is difficult because of these
limitations. Additionally, the required metabolites must be
thermally stable and volatile (104).
HPLC-MS. HPLC-MSis a simple method of
separating and characterising various metabolites, including acids,
bases, salts and hydrophobic and hydrophilic metabolites. Owing to
its capability to accommodate separation processes and various mass
analysers, LC-MS or HPLC-MS is preferred over MS-based metabolomics
because it is not restricted to volatile and thermally stable
metabolites (105). Ahad and
Nissar (106) used the
fundamental principles of HPLC-MS, eluting the metabolites through
a column based on the partition between a stationary phase and
mobile liquid phase. The kind of stationary phase that the
metabolites should elute through depends on their charge, size,
hydrophobicity and molecular weight (106). To achieve a quicker separation of
metabolites, the current HPLC technology focuses on smaller
columns, miniaturisation and low solvent volumes. Thus,
ultra-high-performance LC (UHPLC) replaces HPLC. UHPLC does not
require large amounts of solvent and speeds up resolution within
short analysis times.
NMR-based metabolomics. NMR-based
metabolomics is an alternative to MS-based metabolomics. NMR
spectroscopy, commonly known as NMR, is acknowledged as a promising
metabolomic approach. Despite having lesser intrinsic sensitivity
than MS, NMR offers a thorough metabolite fingerprinting, profiling
and metabolic study under particular conditions. This drawback has
limited its ability to deal with metabolites at the trace level.
NMR-based metabolomics has the benefits of automation, minor or no
sample preparation requirements, non-destructive, non-selectivity
in metabolite detection, excellent repeatability, and the capacity
to quantify numerous classes of metabolites simultaneously
(106). The foundation of NMR
spectroscopy is the radiation that numerous isotopes' nuclei absorb
at a particular frequency when exposed to a magnetic field
(104).
An NMR spectrum has been demonstrated to correspond
with a particular metabolite pattern. Additionally, it offers
structural details to enable easier identification of unknown
metabolites, which can be accelerated by combining spin-spin
coupling, chemical shift and relaxation or diffusion data. In
contrast to localised early disease (EBC), a 1H
NMR-based metabolic phenotyping study to identify metabolic serum
abnormalities connected with advanced metastatic BC (MBC) is
conducted (51). The MBC and EBC
groups are distinguished by the metabolite's acetoacetate,
histidine, pyruvate, glutamate, glycoproteins (N-acetylcysteine),
mannose, glycerol and phenylalanine.
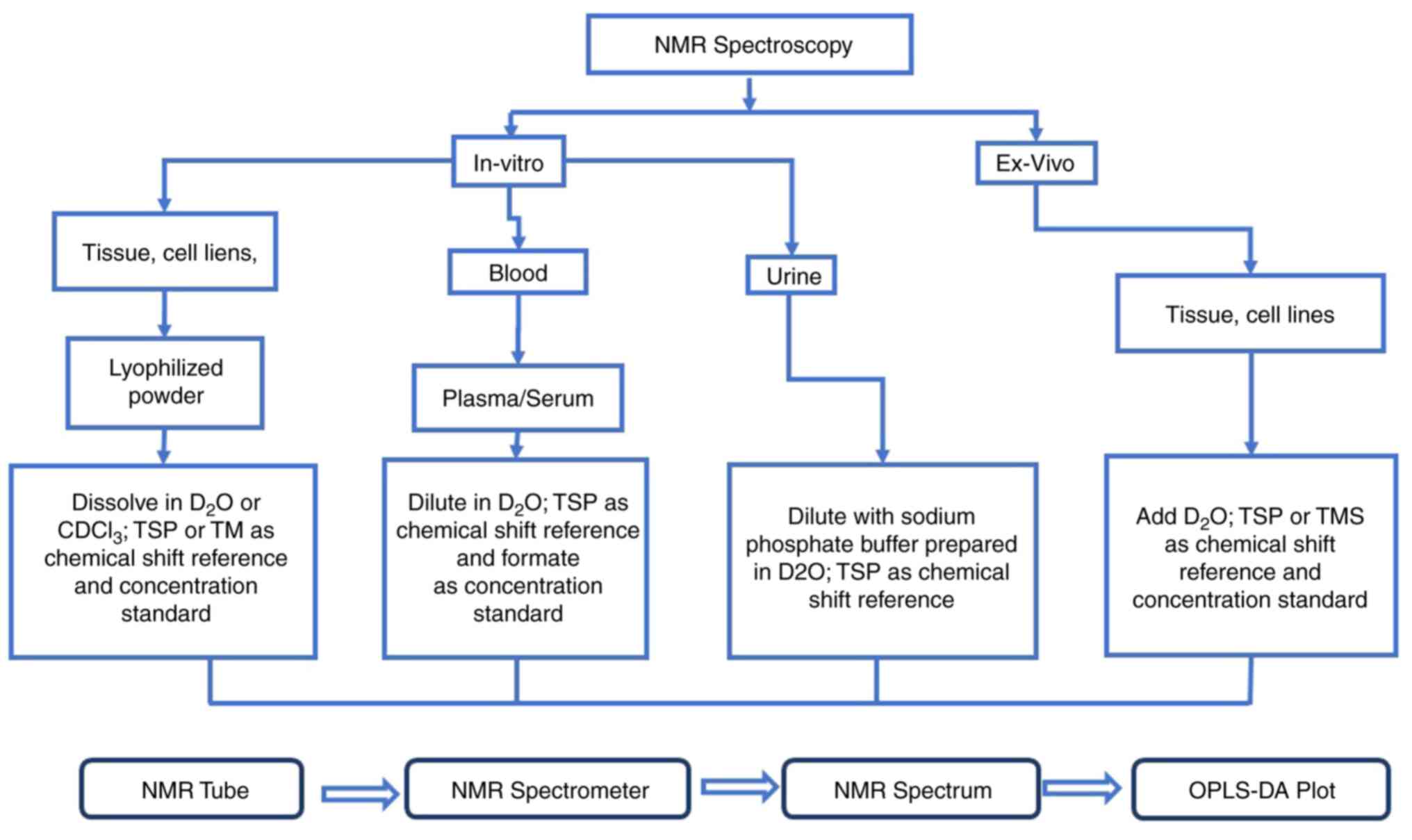
The general flowchart of the details of the in
vitro and ex vivo NMR spectroscopy methodology in BC
study is shown in Fig. 1. The
samples obtained from the patients and controls can be analysed and
studied through in vitro or ex vivo NMR spectroscopy
based on the suitability of the samples. This research primarily
focuses on urinary samples, thus the method used is in
vitro. Based on previous studies, if the samples used are
urine, they should be collected in the morning pre-prandial period
under sterile conditions after overnight fasting (107). Then, the samples should be placed
on ice and frozen in liquid nitrogen before being stored at -40˚C
or lower. Next, to perform NMR analysis, the samples must be
diluted with sodium phosphate buffer prepared in ddH2O
at 1:2 (prepared buffer/sample). The pH of the urine sample needs
to be adjusted to 7.4 constantly because it can lead to changes in
the chemical shift of the samples. A total of ~3 mM sodium azide is
added to prevent bacterial growth in the solution. Then, 0.5 mM TSP
is added for chemical-shift referencing and concentration
quantification. TSP is an internal reference for metabolites'
chemical-shift calibration and quantification in tissue and
urine.
Hyphenated techniques metabolomics.
Hyphenated approaches, along with MS-based and NMR-based
metabolomics, are eliciting attention in metabolomic investigations
owing to their ability to simultaneously detect hundreds of
metabolites. This is because this method can simultaneously detect
hundreds of metabolites. GC-GC-MS, LC-LC-MS, LC-FT-ICR-MS,
LC-MS-NMR and MALDI-FT-ICR-MS are a few examples of analytical
techniques. Two-dimensional liquid-LC and gas-GC are gaining
increased attention in the metabolomics field because metabolite
overlapping can be avoided by redirecting each peak from one GC or
LC column to a second column. These methods also increase
sensitivity and complementary selectivity (108).
Comparison between MS-based and
NMR-based metabolomics study
NMR and MS are the most often applied metabolomic
techniques for metabolomic profiling. NMR and MS may be utilised to
detect and identify metabolites whilst precisely measuring the
concentration, regardless of whether the study focuses on targeted
or untargeted analysis. However, each method has advantages and
disadvantages. Using several complementary technology platforms to
obtain the best results is optimal.
NMR is quantitative and reproducible and does not
require extensive sample preparation procedures such as separation
or derivation (109-111).
This method supports the simultaneous measurement of routine
lipids, lipoprotein subclass profiling with lipid concentrations
within 14 subclasses, fatty acid composition, and various
low-molecular metabolites, including amino acids, ketone bodies and
metabolites related to gluconeogenesis, in molar concentration
units. Considering that no sample preparation is required, it is a
quick analysis that requires ~5 min. The outcomes can be enhanced
by running more scans and using a stronger magnetic field (111). Additionally, NMR requires a
larger sample volume than MS analysis. However, the high
scalability and thorough coverage of numerous chemical pathways of
NMR render it ideal for the biomarker detection for chronic
diseases.
A small sample quantity can be used to evaluate
numerous metabolites through the compassionate MS technique.
Additionally, it can quantify molecular concentrations as low as
nanomolar and picomolar (109).
MS can be utilised to find hundreds of metabolites in a sample when
used in conjunction with chromatography, including GC and LC. If
combined with chromatography (109), MS can investigate secondary
metabolites even when the detection level is lower. However, a
sample in MS cannot be recovered after analysis (111). In addition to requiring sample
preparation and separation, MS is more expensive than NMR (112). MS is a favourable option for
achieving comprehensive metabolome coverage in metabolomic
profiling.
Biomarker identification.
Biomarkers
A biomarker is a term that refers to a trait that is
objectively assessed as an indication of normal biological
processes, pathological processes, or pharmacological reactions to
a therapeutic intervention (113), anticipating sickness occurrence
or outcome (114). Biomarkers are
used to convey information about human biology, and the discovery
of new oncological biomarkers is at the top of the list of
translation research goals. Diagnostic biomarkers are used to
differentiate sick from healthy persons. Conversely, predictive,
prognostic and therapeutic biomarkers may affect therapeutic
decision-making and management techniques with the goal of
personalising illness therapy (115). Prognostic biomarkers aim to
predict the likelihood of a clinical event in the context of
illness. Unfortunately, prognostic biomarkers are occasionally a
blunt measure of stratifying outcomes, and their reliability is
limited by interindividual variability (that is, varying values for
a range of patients), intraindividual variability (that is, varying
scoring by histopathologists providing Ki-67 measurement), and
sensitivity and specificity implications (116).
BC biomarkers. Currently, biomarkers are
crucial to managing patients with BC, particularly when choosing
the kind of systemic treatment to be used (117). Cell receptors, one of the several
varieties of biomarkers, show significant value as diagnostic,
prognostic and predictive biomarkers in cancer research and
therapy. Accordingly, they are incorporated into drug-development
trials (118). ER, PR and
HER2/neu receptors are two excellent examples of biomarkers that
are prognostic of outcomes and predictive of responsiveness to
specific therapy in BC (8). ERs
and progesterone receptors (PR) should be assessed on all newly
diagnosed invasive BCs to select patients likely to respond to
endocrine therapy (117).
ER and PR. PR is a steroid receptor
superfamily member that mediates progesterone's action in its
target tissues. Particularly, in the mammary gland, the luminal
epithelial cell compartment is the only place where PR is expressed
(118). The development of sex
organs, pregnancy, bone density, cholesterol mobilisation, brain
function, cardiovascular system and other biological processes are
only a few of the functions regulated by steroid hormones and their
receptors (119). They are
essential for the development and spread of BC. Hormone receptors
exist in >70% of breast tumours (120). Their cells exhibit positive ER
and PR expression, which is linked to the development and spread of
cancer cells. The development and spread of BC are significantly
influenced by oestrogen and its receptor, ER. PR can influence how
ER functions because it is an ER-upregulated target gene whose
expression is regulated by oestrogen (119). In BC, PR is a useful predictive
indicator of overall survival or disease-free survival (121).
The primary physiological actions of progesterone, a
21-carbon steroid, are mediated by binding to PRs A and B (PR-A and
PR-B), which trigger the transcription of specific genes and change
proliferative endometrium in an oestrogen-primed uterus into
secretory endometrium (121).
Progesterone's physiological function is essentially limited to
pregnancy, the peri- and post-ovulatory periods of the menstrual
cycle. The corpus luteum starts producing progesterone in the early
post-ovulatory phase of the menstrual cycle (119). In the later stages of breast
growth, side branching and amelogenesis, the receptor activator of
nuclear factor kappa B ligand (RANKL) acts as a paracrine mediator
of PR-B (119). By autocrine
activation through the RANKL pathway and the activation of the
downstream target Cyclin D1, the intrinsic proliferation of
PR-negative luminal epithelial cells of the breast can be induced
by progesterone and PR (121).
Experiments on a breast mouse model, normal human
breast tissue, and clinical trials have all shown that progesterone
and oestrogen are the two main proliferative steroid hormones in
the mammary epithelium that signal mammary gland development
(119). Early puberty requires
ductal elongation but not progesterone/PR; it requires oestradiol
and epithelial ER signalling (122). PR signalling is necessary for
ductal elongation and side branching in the epithelial compartment
in response to elevated oestrogen levels (8). Early in pregnancy, PR signalling can
cause the epithelial compartment to expand rapidly. In mid-to-late
pregnancy, progesterone is necessary for alveolar differentiation
(117). Progesterone changes from
promoting terminal differentiation to inhibiting it at term, and it
must be withdrawn for lactation (119).
Progesterone has been linked to the development of
BC in mechanistic investigations. However, weak epidemiologic
evidence does not indicate a link between circulating levels and
the risk of the disease (119).
Progesterone metabolites may exert pro- and anti-carcinogenic
effects, and the balance amongst these factors may affect BC risk
according to data primarily from the Wiebe laboratory (120). However, population-based research
pays little attention to this hypothesis primarily because assays
are insufficient (117). Lastly,
research links progesterone signalling to the development of BC in
BRCA1 mutation carriers, raising the possibility that using
chemotherapy to block downstream signalling can be beneficial
(120).
3. Conclusion
According to previous studies in the manuscripts and
their associations with cancer pathways and treatment, metabolomics
can be used to identify new biomarkers or be one for cancer
diagnosis and treatment stages and the effectivity of the
medications. By comparing metabolites in patients with BC and
healthy individuals, researchers may identify metabolites with high
associations unique to cancer in general and specific for BC. In
this review, we study the association of non-targeted and targeted
metabolites pathway with patients with BC and healthy controls in
numerous places and numerous publications as mentioned before. A
high chance of identifying biomarkers from metabolites by
conducting more studies was found.
Metabolomics face a number of challenges. i) Data
analysis: Metabolomics results contain vast and complex data to
analyse, which is one of the challenges. Computational tools and
expertise such as websites and programs are needed to analyse the
data. ii) Standardization: Metabolomic analysis involves multiple
steps, including data analysis, sample preparation, and data
acquisition. These steps need to be optimised to give us protocols
to produce the same results accurately. iii) Analytical
variability: Metabolomics is a susceptible technique, and slight
variations in sample preparation or data acquisition can lead to
significant differences in results. This variability can confer
difficulty in reproducing results between laboratories and in
developing robust and reliable biomarkers. Despite these
challenges, metabolomics has the potential to revolutionise cancer
research and improve patient outcomes. According to the valuable
data released from the original work, it will help in accurate
diagnosis and early detection of the BC. The future plan of this
article aim to produce an exact phenotype for BC detection
tool.
Acknowledgements
Not applicable.
Funding
Funding: The present study was supported by the Fundamental
Research Grant Scheme (grant no. 203/PPSP/6171345) of the Ministry
of Higher Education.
Availability of data and materials
Not applicable.
Authors' contributions
MMBY, MM, WNBWA, RAR, WFWAR and TADAATD
conceptualized the study. OMA, SSBM, NARBMR, NFABBH and LHY
prepared the original draft. OMA, MMBY, MM, WNBWA, RAR, WFWAR,
SSBM, NARBMR, NFABBH, LHY and TADAATD wrote, reviewed and edited
the manuscript. All authors revised the manuscript. All authors
read and approved the final version of the manuscript. Data
authentication is not applicable.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Jobard E, Dossus L, Baglietto L, Fornili
M, Lécuyer L, Mancini FR, Gunter MJ, Trédan O, Boutron-Ruault MC,
Elena-Herrmann B, et al: Investigation of circulating metabolites
associated with breast cancer risk by untargeted metabolomics: A
case-control study nested within the French E3N cohort. Br J
Cancer. 124:1734–1743. 2021.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Wang Q and Xu R: MetabolitePredict: A de
novo human metabolomics prediction system and its applications in
rheumatoid arthritis. J Biomed Inform. 71:222–228. 2017.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Silva CL, Olival A, Perestrelo R, Silva P,
Tomás H and Câmara JS: Untargeted urinary1H NMR-based
metabolomic pattern as a potential platform in breast cancer
detection. Metabolites. 9(269)2019.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Morad HM, Abou-Elzahab MM, Aref S and
El-Sokkary AMA: Diagnostic value of 1H NMR-based
metabolomics in acute lymphoblastic leukemia, acute myeloid
leukemia, and breast cancer. ACS Omega. 7:8128–8140.
2022.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Patel A: Benign vs malignant tumors. JAMA
Oncol. 6(1488)2020.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Britt KL, Cuzick J and Phillips KA: Key
steps for effective breast cancer prevention. Nat Rev Cancer.
20:417–436. 2020.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Ferlay J, Colombet M, Soerjomataram I,
Parkin DM, Piñeros M, Znaor A and Bray F: Cancer statistics for the
year 2020: An overview. Int J Cancer. 149:778–789. 2021.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Anastasiadi Z, Lianos GD, Ignatiadou E,
Harissis HV and Mitsis M: Breast cancer in young women: An
overview. Updates Surg. 69:313–317. 2017.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Nicolini A, Ferrari P and Duffy MJ:
Prognostic and predictive biomarkers in breast cancer: Past,
present and future. Semin Cancer Biol. 52:56–73. 2018.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Li J, Guan X, Fan Z, Ching LM, Li Y, Wang
X, Cao WM and Liu DX: Non-invasive biomarkers for early detection
of breast cancer. Cancers (Basel). 12(2767)2020.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Barzaman K, Karami J, Zarei Z,
Hosseinzadeh A, Kazemi MH, Moradi-Kalbolandi S, Safari E and
Farahmand L: Breast cancer: Biology, biomarkers, and treatments.
Int Immunopharmacol. 84(106535)2020.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Barzaman K, Karami J, Zarei Z,
Hosseinzadeh A, Kazemi MH, Moradi-Kalbolandi S, Safari E and
Farahmand L: Breast cancer: Biology, biomarkers, and treatments.
Int Immunopharmacol. 84(106535)2020.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Waks AG and Winer EP: Breast cancer
treatment: A review. JAMA. 321:288–300. 2019.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Sharma GN, Dave R, Sanadya J, Sharma P and
Sharma KK: Various types and management of breast cancer: An
overview. J Adv Pharm Technol Res. 1:109–126. 2010.PubMed/NCBI
|
|
15
|
Akram M, Iqbal M, Daniyal M and Khan AU:
Awareness and current knowledge of breast cancer. Biol Res.
50(33)2017.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Feng Y, Spezia M, Huang S, Yuan C, Zeng Z,
Zhang L, Ji X, Liu W, Huang B, Luo W, et al: Breast cancer
development and progression: Risk factors, cancer stem cells,
signaling pathways, genomics, and molecular pathogenesis. Genes
Dis. 5:77–106. 2018.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Thirumurugan D, Cholarajan A, Raja SSS and
Vijayakumar R: An introductory chapter: Secondary metabolites. In:
Vijayakumar R, Raja SSS (edis). Secondary Metabolites-Sources and
Applications. Crotia: InTech-Open Science, pp138, 2018.
|
|
18
|
Chen H and Wang L: Sugar strategies for
biomass biochemical conversion. Technologies for Biochemical
Conversion of Biomass. Metallurgical Industry Press, pp137-164,
2017.
|
|
19
|
Abdel-Aziz SM, Abo Elsoud MM and Anise
AAH: Microbial biosynthesis: A repertory of vital natural products.
Food Biosynthesis. Elsevier Inc., 25-54, 2017.
|
|
20
|
Tiwari R and Rana CS: Plant secondary
metabolites: A review. Int J Eng Res Gen Sci. 3:661–670. 2015.
|
|
21
|
Chandran H, Meena M, Barupal T and Sharma
K: Plant tissue culture as a perpetual source for production of
industrially important bioactive compounds. Biotechnol Rep (Amst).
26(e00450)2020.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Abdullah MA, Bahamid AAA, Alshajrawi OMS,
Nazir MS and Tahir Z: Integrated biomaterials engineering of oil
palm fibres and microalgae for bioenergy, environmental
remediation, and conversion into value-added-products. IOP Conf Ser
Earth Environ Sci. 448(012091)2020.
|
|
23
|
Jones DP, Park Y and Ziegler TR:
Nutritional metabolomics: Progress in addressing complexity in diet
and health. Annu Rev Nutr. 32:183–202. 2012.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Hanna VS and Hafez EAA: Synopsis of
arachidonic acid metabolism: A review. J Adv Res. 11:23–32.
2018.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Pezzuto F, Buonaguro L, Buonaguro FM and
Tornesello ML: The role of circulating free DNA and MicroRNA in
non-invasive diagnosis of HBV- and HCV-related hepatocellular
carcinoma. Int J Mol Sci. 19(1007)2018.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Lu T and Li J: Clinical applications of
urinary cell-free DNA in cancer: Current insights and promising
future. Am J Cancer Res. 7:2318–2332. 2017.PubMed/NCBI
|
|
27
|
Nam H, Chung BC, Kim Y, Lee KY and Lee D:
Combining tissue transcriptomics and urine metabolomics for breast
cancer biomarker identification. Bioinformatics. 25:3151–3157.
2009.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Henneges C, Bullinger D, Fux R, Friese N,
Seeger H, Neubauer H, Laufer S, Gleiter CH, Schwab M, Zell A and
Kammerer B: Prediction of breast cancer by profiling of urinary RNA
metabolites using Support Vector Machine-based feature selection.
BMC Cancer. 9(104)2009.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Dinges SS, Hohm A, Vandergrift LA, Nowak
J, Habbel P, Kaltashov IA and Cheng LL: Cancer metabolomic markers
in urine: Evidence, techniques and recommendations. Nat Rev Urol.
16:339–362. 2019.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Frickenschmidt A, Frohlich H, Bullinger D,
Zell A, Laufer S, Gleiter CH, Liebich H and Kammerer B:
Metabonomics in cancer diagnosis: Mass spectrometry-based profiling
of urinary nucleosides from breast cancer patients. Biomarkers.
13:435–449. 2008.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Chen Y, Zhang R, Song Y, He J, Sun J, Bai
J, An Z, Dong L, Zhan Q and Abliz Z: RRLC-MS/MS-based metabonomics
combined with in-depth analysis of metabolic correlation network:
Finding potential biomarkers for breast cancer. Analyst.
134:2003–2011. 2009.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Cho SH, Jung BH, Lee SH, Lee WY, Kong G
and Chung BC: Direct determination of nucleosides in the urine of
patients with breast cancer using column-switching liquid
chromatography-tandem mass spectrometry. Biomed Chromatogr.
20:1229–1236. 2006.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Lécuyer L, Victor Bala A, Deschasaux M,
Bouchemal N, Nawfal Triba M, Vasson MP, Rossary A, Demidem A, Galan
P, Hercberg S, et al: NMR metabolomic signatures reveal predictive
plasma metabolites associated with long-term risk of developing
breast cancer. Int J Epidemiol. 47:484–494. 2018.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Lecuyer L, Dalle C, Lyan B, Demidem A,
Rossary A, Vasson MP, Petera M, Lagree M, Ferreira T, Centeno D, et
al: Plasma metabolomic signatures associated with long-term breast
cancer risk in the SU.VI.MAX prospective cohort. Cancer Epidemiol
Biomarkers Prev. 28:1300–1307. 2019.PubMed/NCBI View Article : Google Scholar
|
|
35
|
An R, Yu H, Wang Y, Lu J, Gao Y, Xie X and
Zhang J: Integrative analysis of plasma metabolomics and proteomics
reveals the metabolic landscape of breast cancer. Cancer Metab.
10(13)2022.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Gasparri ML, Casorelli A, Bardhi E,
Besharat AR, Savone D, Ruscito I, Farooqi AA, Papadia A, Mueller
MD, Ferretti E and Benedetti Panici P: Beyond circulating microRNA
biomarkers: Urinary microRNAs in ovarian and breast cancer. Tumour
Biol. 39(1010428317695525)2017.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Ilyas MN, Ab A, Al-Hatamleh MAI,
Al-Shajrawi OM, Ariff TM and Simbak N: Rising trends of obesity in
Malaysia; role of inflammation and inflammatory markers in obesity
related insulin resistance: A nuclear factor kappa B (Nfkb)
perspective. Exp Clin Endocrinol Diabetes. 109:S135–S148. 2017.
|
|
38
|
Rudnicka E, Suchta K, Grymowicz M,
Calik-Ksepka A, Smolarczyk K, Duszewska AM, Smolarczyk R and
Meczekalski B: Chronic low grade inflammation in pathogenesis of
PCOS. Int J Mol Sci. 22(3789)2021.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Slupsky CM, Steed H, Wells TH, Dabbs K,
Schepansky A, Capstick V, Faught W and Sawyer MB: Urine metabolite
analysis offers potential early diagnosis of ovarian and breast
cancers. Clin Cancer Res. 16:5835–5841. 2010.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Bax C, Lotesoriere BJ, Sironi S and
Capelli L: Review and comparison of cancer biomarker trends in
urine as a basis for new diagnostic pathways. Cancers (Basel).
11(1244)2019.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Cala M, Aldana J, Sánchez J, Guio J and
Meesters RJW: Urinary metabolite and lipid alterations in Colombian
Hispanic women with breast cancer: A pilot study. J Pharm Biomed
Anal. 152:234–241. 2018.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Pasikanti KK, Esuvaranathan K, Hong Y, Ho
PC, Mahendran R, Raman Nee Mani L, Chiong E and Chan EC: Urinary
metabotyping of bladder cancer using two-dimensional gas
chromatography time-of-flight mass spectrometry. J Proteome Res.
12:3865–3873. 2013.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Silva C, Perestrelo R, Silva P, Tomás H
and Câmara JS: Breast cancer metabolomics: From analytical
platforms to multivariate data analysis. A review. Metabolites.
9(102)2019.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Putluri N, Shojaie A, Vasu VT, Vareed SK,
Nalluri S, Putluri V, Thangjam GS, Panzitt K, Tallman CT, Butler C,
et al: Metabolomic profiling reveals potential markers and
bioprocesses altered in bladder cancer progression. Cancer Res.
71:7376–7386. 2011.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Yu L, Jiang C, Huang S, Gong X, Wang S and
Shen P: Analysis of urinary metabolites for breast cancer patients
receiving chemotherapy by CE-MS coupled with on-line concentration.
Clin Biochem. 46:1065–1073. 2013.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Woo HM, Kim KM, Choi MH, Jung BH, Lee J,
Kong G, Nam SJ, Kim S, Bai SW and Chung BC: Mass spectrometry based
metabolomic approaches in urinary biomarker study of women's
cancers. Clin Chim Acta. 400:63–69. 2009.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Men Y, Li L, Zhang F, Kong X, Zhang W, Hao
C and Wang G: Evaluation of heavy metals and metabolites in the
urine of patients with breast cancer. Oncol Lett. 19:1331–1337.
2020.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Asiago VM, Alvarado LZ, Shanaiah N, Gowda
GAN, Owusu-Sarfo K, Ballas RA and Raftery D: Early detection of
recurrent breast cancer using metabolite profiling. Cancer Res.
70:8309–8318. 2010.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Oakman C, Tenori L, Claudino WM, Cappadona
S, Nepi S, Battaglia A, Bernini P, Zafarana E, Saccenti E, Fornier
M, et al: Identification of a serum-detectable metabolomic
fingerprint potentially correlated with the presence of
micrometastatic disease in early breast cancer patients at varying
risks of disease relapse by traditional prognostic methods. Ann
Oncol. 22:1295–1301. 2011.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Tenori L, Oakman C, Claudino WM, Bernini
P, Cappadona S, Nepi S, Biganzoli L, Arbushites MC, Luchinat C,
Bertini I and Di Leo A: Exploration of serum metabolomic profiles
and outcomes in women with metastatic breast cancer: A pilot study.
Mol Oncol. 6:437–444. 2012.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Wei S, Liu L, Zhang J, Bowers J, Gowda
GAN, Seeger H, Fehm T, Neubauer HJ, Vogel U, Clare SE and Raftery
D: Metabolomics approach for predicting response to neoadjuvant
chemotherapy for breast cancer. Mol Oncol. 7:297–307.
2013.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Jobard E, Pontoizeau C, Blaise BJ,
Bachelot T, Elena-Herrmann B and Trédan O: A serum nuclear magnetic
resonance-based metabolomic signature of advanced metastatic human
breast cancer. Cancer Lett. 343:33–41. 2014.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Tenori L, Oakman C, Morris PG, Gralka E,
Turner N, Cappadona S, Fornier M, Hudis C, Norton L, Luchinat C and
Di Leo A: Serum metabolomic profiles evaluated after surgery may
identify patients with oestrogen receptor negative early breast
cancer at increased risk of disease recurrence. Results from a
retrospective study. Mol Oncol. 9:128–139. 2015.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Kim Y, Koo I, Jung BH, Chung BC and Lee D:
Multivariate classification of urine metabolome profiles for breast
cancer diagnosis. BMC Bioinformatics. 11 (Suppl
2)(S4)2010.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Bathen TF, Geurts B, Sitter B, Fjøsne HE,
Lundgren S, Buydens LM, Gribbestad IS, Postma G and Giskeødegård
GF: Feasibility of MR metabolomics for immediate analysis of
resection margins during breast cancer surgery. PLoS One.
8(e61578)2013.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Borgan E, Sitter B, Lingjærde OC, Johnsen
H, Lundgren S, Bathen TF, Sørlie T, Børresen-Dale AL and Gribbestad
IS: Merging transcriptomics and metabolomics-advances in breast
cancer profiling. BMC Cancer. 10(628)2010.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Debik J, Euceda LR, Lundgren S, Gythfeldt
HDVL, Garred Ø, Borgen E, Engebraaten O, Bathen TF and Giskeødegård
GF: Assessing treatment response and prognosis by serum and tissue
metabolomics in breast cancer patients. J Proteome Res.
18:3649–3660. 2019.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Haukaas TH, Euceda LR, Giskeødegård GF,
Lamichhane S, Krohn M, Jernström S, Aure MR, Lingjærde OC,
Schlichting E, Garred Ø, et al: Metabolic clusters of breast cancer
in relation to gene- and protein expression subtypes. Cancer Metab.
4(12)2016.PubMed/NCBI View Article : Google Scholar
|
|
59
|
Chae EY, Shin HJ, Kim S, Baek HM, Yoon D,
Kim S, Shim YE, Kim HH, Cha JH, Choi WJ, et al: The role of
high-resolution magic angle spinning 1H nuclear magnetic resonance
spectroscopy for predicting the invasive component in patients with
ductal carcinoma in situ diagnosed on preoperative biopsy. PLoS
One. 11(e0161038)2016.PubMed/NCBI View Article : Google Scholar
|
|
60
|
Euceda LR, Haukaas TH, Giskeødegård GF,
Vettukattil R, Engel J, Silwal-Pandit L, Lundgren S, Borgen E,
Garred Y, Garred G, et al: Evaluation of metabolomic changes during
neoadjuvant chemotherapy combined with bevacizumab in breast cancer
using MR spectroscopy. Metabolomics. 13(37)2017.
|
|
61
|
Cala MP, Aldana J, Medina J, Sánchez J,
Guio J, Wist J and Meesters RJW: Multiplatform plasma metabolic and
lipid fingerprinting of breast cancer: A pilot control-case study
in Colombian Hispanic women. PLoS One. 13(e0190958)2018.PubMed/NCBI View Article : Google Scholar
|
|
62
|
Lécuyer L, Victor Bala A, Deschasaux M,
Bouchemal N, Nawfal Triba M, Vasson MP, Rossary A, Demidem A, Galan
P, Hercberg S, et al: NMR metabolomic signatures reveal predictive
plasma metabolites associated with long-term risk of developing
breast cancer. Int J Epidemiol. 47:484–494. 2018.PubMed/NCBI View Article : Google Scholar
|
|
63
|
Suman S, Sharma RK, Kumar V, Sinha N and
Shukla Y: Metabolic fingerprinting in breast cancer stages through
1H NMR spectroscopy-based metabolomic analysis of
plasma. J Pharm Biomed Anal. 160:38–45. 2018.PubMed/NCBI View Article : Google Scholar
|
|
64
|
Louis E, Bervoets L, Reekmans G, De Jonge
E, Mesotten L, Thomeer M and Adriaensens P: Phenotyping human blood
plasma by 1H-NMR: A robust protocol based on metabolite
spiking and its evaluation in breast cancer. Metabolomics.
11:225–236. 2015.
|
|
65
|
Vignoli A, Muraro E, Miolo G, Tenori L,
Turano P, Di Gregorio E, Steffan A, Luchinat C and Corona G: Effect
of estrogen receptor status on circulatory immune and metabolomics
profiles of HER2-positive breast cancer patients enrolled for
neoadjuvant targeted chemotherapy. Cancers (Basel).
12(314)2020.PubMed/NCBI View Article : Google Scholar
|
|
66
|
McCartney A, Vignoli A, Tenori L, Fornier
M, Rossi L, Risi E, Luchinat C, Biganzoli L and Di Leo A:
Metabolomic analysis of serum may refine 21-gene expression assay
risk recurrence stratification. NPJ Breast Cancer.
5(26)2019.PubMed/NCBI View Article : Google Scholar
|
|
67
|
Jiang L, Lee SC and Ng TC:
Pharmacometabonomics analysis reveals serum formate and acetate
potentially associated with varying response to
gemcitabine-carboplatin chemotherapy in metastatic breast cancer
patients. J Proteome Res. 17:1248–1257. 2018.PubMed/NCBI View Article : Google Scholar
|
|
68
|
Wojtowicz W, Wróbel A, Pyziak K, Tarkowski
R, Balcerzak A, Bębenek M and Młynarz P: Evaluation of MDA-MB-468
cell culture media analysis in predicting triple-negative breast
cancer patient sera metabolic profiles. Metabolites.
10(173)2020.PubMed/NCBI View Article : Google Scholar
|
|
69
|
Men Y, Li L, Zhang F, Kong X, Zhang W, Hao
C, et al: Evaluation of heavy metals and metabolites in the urine
of patients with breast cancer. Oncol Lett. 19:1331–1337.
2020.PubMed/NCBI View Article : Google Scholar
|
|
70
|
Li N, Deng Y, Zhou L, Tian T, Yang S, Wu
Y, Zheng Y, Zhai Z, Hao Q, Song D, et al: Global burden of breast
cancer and attributable risk factors in 195 countries and
territories, from 1990 to 2017: Results from the Global Burden of
Disease Study 2017. J Hematol Oncol. 12(140)2019.PubMed/NCBI View Article : Google Scholar
|
|
71
|
Slupsky CM, Steed H, Wells TH, Dabbs K,
Schepansky A, Capstick V, Faught W and Sawyer MB: Urine metabolite
analysis offers potential early diagnosis of ovarian and breast
cancers. Clin Cancer Res. 16:5835–5841. 2010.PubMed/NCBI View Article : Google Scholar
|
|
72
|
Cairns RA, Harris IS and Mak TW:
Regulation of cancer cell metabolism. Nat Rev Cancer. 11:85–95.
2011.PubMed/NCBI View Article : Google Scholar
|
|
73
|
Lau AN and Vander Heiden MG: Metabolism in
the tumor microenvironment. Annu Rev Cancer Biol. 4:17–40.
2020.
|
|
74
|
Phan LM, Yeung SCJ and Lee MH: Cancer
metabolic reprogramming: Importance, main features, and potentials
for precise targeted anti-cancer therapies. Cancer Biol Med.
11:1–19. 2014.PubMed/NCBI View Article : Google Scholar
|
|
75
|
Sullivan LB, Gui DY and Van Der Heiden MG:
Altered metabolite levels in cancer: Implications for tumour
biology and cancer therapy. Nat Rev Cancer. 16:680–693.
2016.PubMed/NCBI View Article : Google Scholar
|
|
76
|
Gu I, Gregory E, Atwood C, Lee SO and Song
YH: Exploring the role of metabolites in cancer and the associated
nerve crosstalk. Nutrients. 14(1722)2022.PubMed/NCBI View Article : Google Scholar
|
|
77
|
Potter M, Newport E and Morten KJ: The
Warburg effect: 80 Years on. Biochem Soc Trans. 44:1499–1505.
2016.PubMed/NCBI View Article : Google Scholar
|
|
78
|
Nalbantoglu S and Karadag A: Metabolomics
bridging proteomics along metabolites/oncometabolites and protein
modifications: Paving the way toward integrative multiomics. J
Pharm Biomed Anal. 199(114031)2021.PubMed/NCBI View Article : Google Scholar
|
|
79
|
Kinnaird A, Zhao S, Wellen KE and
Michelakis ED: Metabolic control of epigenetics in cancer. Nat Rev
Cancer. 16:694–707. 2016.PubMed/NCBI View Article : Google Scholar
|
|
80
|
Al-Shajrawi OM, Basit E and Baig AA: HIF1
(rs11549465) and NFKB1 (rs28362491) variants association with
obesity in Malaysia. Meta Gene. 25(100753)2020.
|
|
81
|
Choi YK and Park KG: Targeting glutamine
metabolism for cancer treatment. Biomol Ther (Seoul). 26:19–28.
2018.PubMed/NCBI View Article : Google Scholar
|
|
82
|
Butler M, van der Meer LT and van Leeuwen
FN: Amino acid depletion therapies: Starving cancer cells to death.
Trends Endocrinol Metab. 32:367–381. 2021.PubMed/NCBI View Article : Google Scholar
|
|
83
|
Wang ZH, Peng WB, Zhang P, Yang XP and
Zhou Q: Lactate in the tumour microenvironment: From immune
modulation to therapy. EBioMedicine. 73(103627)2021.PubMed/NCBI View Article : Google Scholar
|
|
84
|
Doldo E, Costanza G, Agostinelli S,
Tarquini C, Ferlosio A, Arcuri G, Passeri D, Scioli MG and Orlandi
A: Vitamin A, cancer treatment and prevention: The new role of
cellular retinol binding proteins. Biomed Res Int.
2015(624627)2015.PubMed/NCBI View Article : Google Scholar
|
|
85
|
Zeng LH, Wang QM, Feng LY, Ke YD, Xu QZ,
Wei AY, Zhang C and Ying RB: High-dose vitamin C suppresses the
invasion and metastasis of breast cancer cells via inhibiting
epithelial-mesenchymal transition. Onco Targets Ther. 12:7405–7413.
2019.PubMed/NCBI View Article : Google Scholar
|
|
86
|
Sailo BL, Banik K, Padmavathi G, Javadi M,
Bordoloi D and Kunnumakkara AB: Tocotrienols: The promising
analogues of vitamin E for cancer therapeutics. Pharmacol Res.
130:259–272. 2018.PubMed/NCBI View Article : Google Scholar
|
|
87
|
Miyazawa S, Moriya S, Kokuba H, Hino H,
Takano N and Miyazawa K: Vitamin K2 induces
non-apoptotic cell death along with autophagosome formation in
breast cancer cell lines. Breast Cancer. 27:225–235.
2020.PubMed/NCBI View Article : Google Scholar
|
|
88
|
Qiu F, Chen YR, Liu X, Chu CY, Shen LJ, Xu
J, Gaur S, Forman HJ, Zhang H, Zheng S, et al: Arginine starvation
impairs mitochondrial respiratory function in ASS1-deficient breast
cancer cells. Sci Signal. 7(ra31)2014.PubMed/NCBI View Article : Google Scholar
|
|
89
|
Cheng CT, Qi Y, Wang YC, Chi KK, Chung Y,
Ouyang C, Chen YR, Oh ME, Sheng X, Tang Y, et al: Arginine
starvation kills tumor cells through aspartate exhaustion and
mitochondrial dysfunction. Commun Biol. 1(178)2018.PubMed/NCBI View Article : Google Scholar
|
|
90
|
Ji JX, Cochrane DR, Tessier-Cloutier B,
Chen SY, Ho G, Pathak KV, Alcazar IN, Farnell D, Leung S, Cheng A,
et al: Arginine depletion therapy with ADI-PEG20 limits tumor
growth in argininosuccinate synthase-deficient ovarian cancer,
including small-cell carcinoma of the ovary, hypercalcemic type.
Clin Cancer Res. 26:4402–4413. 2020.PubMed/NCBI View Article : Google Scholar
|
|
91
|
Krall AS, Xu S, Graeber TG, Braas D and
Christofk HR: Asparagine promotes cancer cell proliferation through
use as an amino acid exchange factor. Nat Commun.
7(11457)2016.PubMed/NCBI View Article : Google Scholar
|
|
92
|
Hoang G, Udupa S and Le A: Application of
metabolomics technologies toward cancer prognosis and therapy. Int
Rev Cell Mol Biol. 347:191–223. 2019.PubMed/NCBI View Article : Google Scholar
|
|
93
|
Elgogary A, Xu Q, Poore B, Alt J,
Zimmermann SC, Zhao L, Fu J, Chen B, Xia S, Liu Y, et al:
Combination therapy with BPTES nanoparticles and metformin targets
the metabolic heterogeneity of pancreatic cancer. Proc Natl Acad
Sci USA. 113:E5328–E5336. 2016.PubMed/NCBI View Article : Google Scholar
|
|
94
|
Lane AN, Fan TWM and Higashi RM:
Isotopomer-based metabolomic analysis by NMR and mass spectrometry.
Methods Cell Biol. 84:541–588. 2008.PubMed/NCBI View Article : Google Scholar
|
|
95
|
Le A, Lane AN, Hamaker M, Bose S, Gouw A,
Barbi J, Tsukamoto T, Rojas CJ, Slusher BS, Zhang H, et al:
Glucose-independent glutamine metabolism via TCA cycling for
proliferation and survival in B cells. Cell Metab. 15:110–121.
2012.PubMed/NCBI View Article : Google Scholar
|
|
96
|
Beger R: A review of applications of
metabolomics in cancer. Metabolites. 3:552–574. 2013.PubMed/NCBI View Article : Google Scholar
|
|
97
|
Zhang A, Sun H, Wang P, Han Y and Wang X:
Modern analytical techniques in metabolomics analysis. Analyst.
137:293–300. 2012.PubMed/NCBI View Article : Google Scholar
|
|
98
|
Lyon DE, Starkweather A, Yao Y, Garrett T,
Kelly DL, Menzies V, Dereziński P, Datta S, Kumar S and
Jackson-Cook C: Pilot study of metabolomics and psychoneurological
symptoms in women with early stage breast cancer. Biol Res Nurs.
20:227–236. 2018.PubMed/NCBI View Article : Google Scholar
|
|
99
|
Silva CL, Perestrelo R, Silva P, Tomás H
and Câmara JS: Volatile metabolomic signature of human breast
cancer cell lines. Sci Rep. 7(43969)2017.PubMed/NCBI View Article : Google Scholar
|
|
100
|
Cífková E, Lísa M, Hrstka R, Vrána D,
Gatěk J, Melichar B and Holčapek M: Correlation of lipidomic
composition of cell lines and tissues of breast cancer patients
using hydrophilic interaction liquid chromatography/electrospray
ionization mass spectrometry and multivariate data analysis. Rapid
Commun Mass Spectrom. 31:253–263. 2017.PubMed/NCBI View Article : Google Scholar
|
|
101
|
Cavaco C, Pereira JAM, Taunk K, Taware R,
Rapole S, Nagarajaram H and Câmara JS: Screening of salivary
volatiles for putative breast cancer discrimination: An exploratory
study involving geographically distant populations. Anal Bioanal
Chem. 410:445–468. 2018.PubMed/NCBI View Article : Google Scholar
|
|
102
|
Porto-Figueira P, Pereira JAM and Câmara
JS: Exploring the potential of needle trap microextraction combined
with chromatographic and statistical data to discriminate different
types of cancer based on urinary volatomic biosignature. Anal Chim
Acta. 1023:53–63. 2018.PubMed/NCBI View Article : Google Scholar
|
|
103
|
Budczies J, Brockmöller SF, Müller BM,
Barupal DK, Richter-Ehrenstein C, Kleine-Tebbe A, Griffin JL,
Orešič M, Dietel M, Denkert C and Fiehn O: Comparative metabolomics
of estrogen receptor positive and estrogen receptor negative breast
cancer: Alterations in glutamine and beta-alanine metabolism. J
Proteomics. 94:279–288. 2013.PubMed/NCBI View Article : Google Scholar
|
|
104
|
Dougan MM, Li Y, Chu LW, Haile RW,
Whittemore AS, Han SS, Moore SC, Sampson JN, Andrulis IL, John EM
and Hsing AW: Metabolomic profiles in breast cancer: A pilot
case-control study in the breast cancer family registry. BMC
Cancer. 18(532)2018.PubMed/NCBI View Article : Google Scholar
|
|
105
|
Sas KM, Karnovsky A, Michailidis G and
Pennathur S: Metabolomics and diabetes: Analytical and
computational approaches. Diabetes. 64:718–732. 2015.PubMed/NCBI View Article : Google Scholar
|
|
106
|
Ahad T and Nissar J: Fingerprinting in
determining the adultration of food. J Pharmacogn Phytochem.
6:1543–1553. 2017.
|
|
107
|
Claudino WM, Goncalves PH, di Leo A,
Philip PA and Sarkar FH: Metabolomics in cancer: A bench-to-bedside
intersection. Crit Rev Oncol Hematol. 84:1–7. 2012.PubMed/NCBI View Article : Google Scholar
|
|
108
|
De Castro F, Benedetti M, Del Coco L and
Fanizzi FP: NMR-based metabolomics in metal-based drug research.
Molecules. 24(2240)2019.PubMed/NCBI View Article : Google Scholar
|
|
109
|
Cacciola F, Farnetti S, Dugo P, Marriott
PJ and Mondello L: Comprehensive two-dimensional liquid
chromatography for polyphenol analysis in foodstuffs. J Sep Sci.
40:7–24. 2017.PubMed/NCBI View Article : Google Scholar
|
|
110
|
Emwas AHM: The strengths and weaknesses of
NMR spectroscopy and mass spectrometry with particular focus on
metabolomics research. Methods Mol Biol. 1277:161–193.
2015.PubMed/NCBI View Article : Google Scholar
|
|
111
|
Beltran A, Suarez M, Rodríguez MA, Vinaixa
M, Samino S, Arola L, Correig X and Yanes O: Assessment of
compatibility between extraction methods for NMR- and LC/MS-based
metabolomics. Anal Chem. 84:5838–5844. 2012.PubMed/NCBI View Article : Google Scholar
|
|
112
|
Emwas AH, Roy R, McKay RT, Tenori L,
Saccenti E, Gowda GAN, Raftery D, Alahmari F, Jaremko L, Jaremko M
and Wishart DS: Nmr spectroscopy for metabolomics research.
Metabolites. 9(123)2019.PubMed/NCBI View Article : Google Scholar
|
|
113
|
Dunn WB, Broadhurst DI, Atherton HJ,
Goodacre R and Griffin JL: Systems level studies of mammalian
metabolomes: The roles of mass spectrometry and nuclear magnetic
resonance spectroscopy. Chem Soc Rev. 40:387–426. 2011.PubMed/NCBI View Article : Google Scholar
|
|
114
|
Qiu S, Cai Y, Yao H, Lin C, Xie Y, Tang S
and Zhang A: Small molecule metabolites: Discovery of biomarkers
and therapeutic targets. Signal Transduct Target Ther.
8(132)2023.PubMed/NCBI View Article : Google Scholar
|
|
115
|
Burke HB: Predicting clinical outcomes
using molecular biomarkers. Biomark Cancer. 8:89–99.
2016.PubMed/NCBI View Article : Google Scholar
|
|
116
|
Carlomagno N, Incollingo P, Tammaro V,
Peluso G, Rupealta N, Chiacchio G, Sandoval Sotelo ML, Minieri G,
Pisani A, Riccio E, et al: Diagnostic, predictive, prognostic, and
therapeutic molecular biomarkers in third millennium: A
breakthrough in gastric cancer. Biomed Res Int.
2017(7869802)2017.PubMed/NCBI View Article : Google Scholar
|
|
117
|
Mayeux R: Biomarkers: Potential uses and
limitations. NeuroRx. 1:182–188. 2004.PubMed/NCBI View Article : Google Scholar
|
|
118
|
Duffy MJ, Harbeck N, Nap M, Molina R,
Nicolini A, Senkus E and Cardoso F: Clinical use of biomarkers in
breast cancer: Updated guidelines from the European Group on Tumor
Markers (EGTM). Eur J Cancer. 75:284–298. 2017.PubMed/NCBI View Article : Google Scholar
|
|
119
|
Brennan M and Lim B: The actual role of
receptors as cancer markers, biochemical and clinical aspects:
Receptors in breast cancer. Adv Exp Med Biol. 867:327–337.
2015.PubMed/NCBI View Article : Google Scholar
|
|
120
|
Li Z, Wei H, Li S, Wu P and Mao X: The
role of progesterone receptors in breast cancer. Drug Des Devel
Ther. 16:305–314. 2022.PubMed/NCBI View Article : Google Scholar
|
|
121
|
Brisken C, Hess K and Jeitziner R:
Progesterone and overlooked endocrine pathways in breast cancer
pathogenesis. Endocrinology. 156:3442–3450. 2015.PubMed/NCBI View Article : Google Scholar
|
|
122
|
Trabert B, Sherman ME, Kannan N and
Stanczyk FZ: Progesterone and breast cancer. Endocr Rev.
41:320–344. 2020.PubMed/NCBI View Article : Google Scholar
|