1. Introduction
In recent years, due to factors such as increased
environmental pollution, the incidence of lung cancer has been
increasing, and it now occupies first place in cancer incidence
(1). According to its pathological
type, lung cancer can be divided into categories of small cell lung
cancer and non-small cell lung cancer (NSCLC). NSCLC accounts for
85% of lung cancers and is the most common type of lung cancer. It
mainly includes lung squamous cell carcinoma (LUSC) and lung
adenocarcinoma (LUAD) (2,3). Among NSCLCs, LUAD is the main
manifestation, and accounts for >85% of NSCLCs (4). It is known that tumor markers are
helpful for detecting the early onset of cancer and treating the
disease. Existing serum tumor molecular markers, including
carcinoembryonic antigen and neuron specific enolase have been
widely used in the diagnosis of lung cancer. However, their
diagnostic sensitivity and specificity in lung cancer are very
limited, and especially for early-stage lung cancer. Therefore,
early detection and control of the occurrence, invasion and
metastasis of LUAD are of great significance for improving the
survival of patients with that disease. With the advancement of
molecular medicine, our understanding of lung cancer now extends to
the genetic level, and especially our understanding of LUAD, for
which treatment plans can be detailed to specific target genes (for
example, EGFR, ROS1 and ALK). However,
numerous patients with advanced LUAD still lack detectable genetic
mutations, and some develop drug resistance or experience side
effects following targeted therapy. Therefore, exploring novel
genes associated with the occurrence and progression of LUAD and
identifying tumor markers with greater specificity and sensitivity
are crucial for optimizing treatment strategies for this
disease.
Glycosylation is a widespread type of protein
post-translational modification, of which N-glycosylation and
O-glycosylation are the two most important examples (5). N-glycosylation regulates protein
folding, secretion and stability. When N-glycosylation is abnormal,
the endoplasmic reticulum stress response and apoptosis become
activated. O-glycosylated proteins are found on the cell surface,
serum and extracellular matrix (ECM). Changes in O-glycoproteins on
the cell surface are usually associated with uncontrolled
proliferation, invasion and metastasis. O-glycosylated ECM proteins
are associated with various pathological changes (6). Abnormal forms of O-glycans are found
in different solid tumors, including ovarian, bladder, breast,
cervical, colon and lung cancers (7-9).
O-glycosylation is initiated by
polypeptide-N-acetyl-galactosamine-transferase (GALNT enzyme
family), which covalently combines the GalNAc group of the
glycoside donor UDP-GalNAc with the side chain hydroxyl group of a
protein serine or threonine residue (10). The GalNAc-α-Ser/Thr structure is
formed, also known as the Tn antigen structure. Members of the
GALNT enzyme family are type II transmembrane proteins. During
development and differentiation, the expression of various GALNT
genes is highly restricted to cells and tissues (11). In recent years, numerous studies
have shown that GALNT family proteins play an important role in the
development of lung cancer. Therefore, the mechanism by which
abnormal expression of GALNT family proteins regulates the
occurrence and development of lung cancer is reviewed.
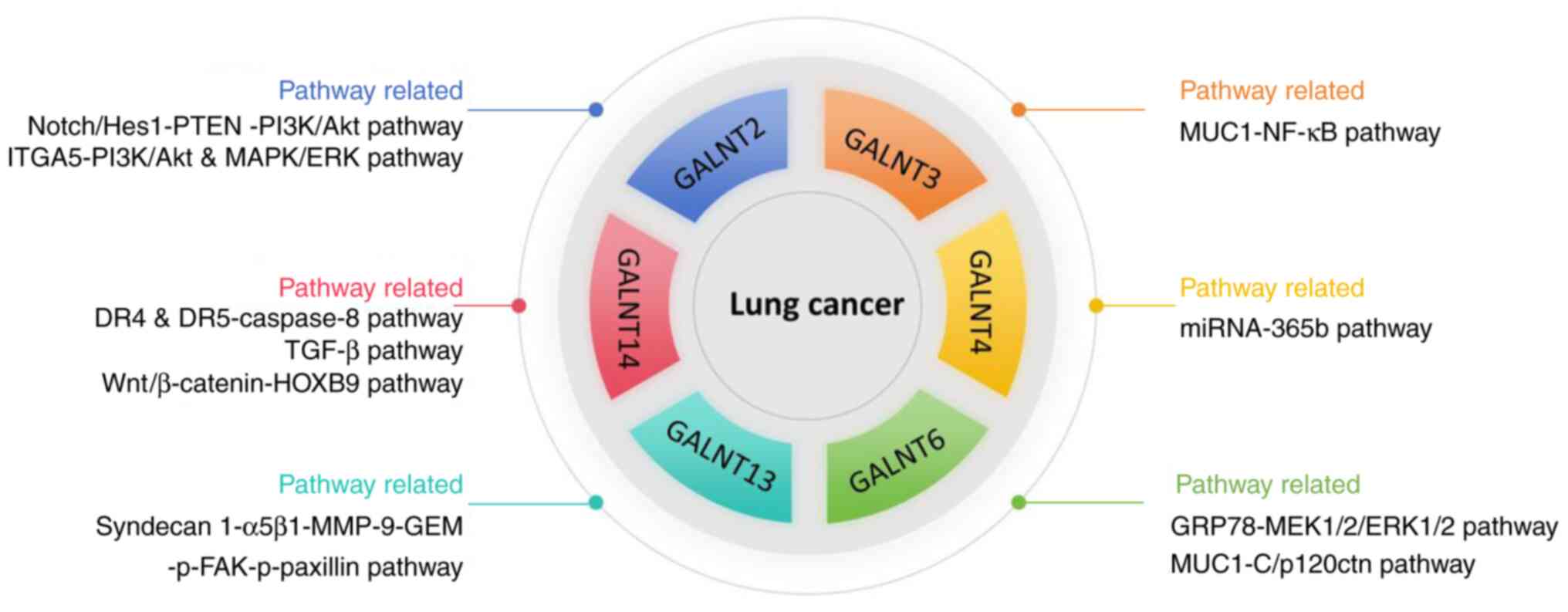
2. GALNT proteins
GALNT2 protein
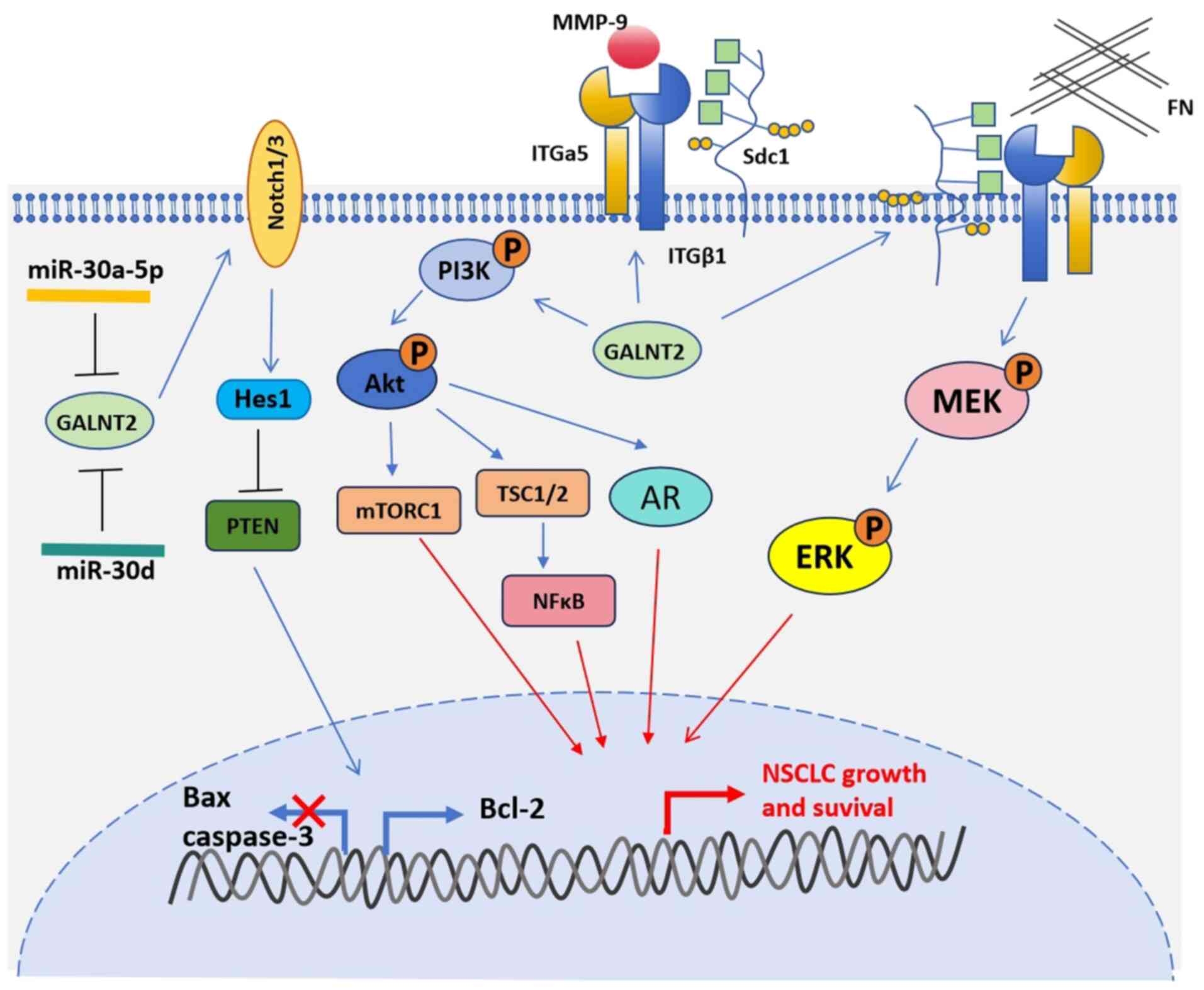
The GALNT2 protein is overexpressed in lung cancer
and regulated by microRNA (miR)-30a-5p (Fig. 1). A proteomics search for
differentially expressed proteins in lung cancer tissues and normal
lung tissues, plus an analysis of RNA sequencing data for lung
cancer tissues and corresponding paracancerous tissues in The
Cancer Genome Atlas (TCGA; https://www.cancer.gov/ccg/research/genome-sequencing/tcga),
Gene Expression Omnibus (https://www.ncbi.nlm.nih.gov/geo/) and Oncomine
(https://www.oncomine.com/) databases,
among others, revealed that GALNT2 protein is involved in NSCLC.
GALNT2 expression is significantly increased in NSCLC, and GALNT2
is positively correlated with the poor prognosis for NSCLC
(12-15).
A high level of GALNT2 expression in lung cancer is related to the
low methylation level in the promoter region of the gene.
Expression of the GALNT2 protein is also regulated by miR-30a-5p
(16). Induction of miR-30a-5p
expression in lung cancer cell lines was found to reduce GALNT2
mRNA and protein levels in LUAD.
It has been reported that GALNT2 promotes cell
proliferation, migration and invasion by activating the
Notch/Hes1-PTEN-PI3K/Akt signaling pathway (Fig. 1) (12). Using small interfering RNA (siRNA)
to interfere with GALNT2 protein expression in lung cancer cells
can lead to decreased levels of Notch1/3, Hes1, p-AKT, t-AKT and
p-mToR, and increased expression of the tumor suppressor gene,
PTEN. The Notch signaling pathway regulates several
cancer-related processes, including proliferation, metastasis,
epithelial-to-mesenchymal transition (EMT), angiogenesis, and the
stemness of cancer cells (17,18).
Notch1 and Notch3 promote LUAD, while Notch2 inhibits LUAD. A
meta-analysis (including 19 articles with a total of 3,663 cases)
showed that overexpression of Notch1 or Notch3 significantly
reduced the overall survival (OS) of patients with NSCLC (19). Results of another NSCLC study
showed that GALNT2 mediates the development of lung cancer by
regulating the O-glycosylation of ITGA5, leading to activation of
the PI3K/Akt and MAPK/ERK pathways (Fig. 1) (14). ITGA5 belongs to the integrin alpha
chain family. Integrins are heterodimeric integral membrane
proteins composed of α and β subunits that play roles in cell
surface adhesion and signaling. The ITGA5 subunit and ITGB1 subunit
combine to form fibronectin receptors. This integrin may promote
tumor invasion, and high expression of this gene may be associated
with a shorter survival time for patients with lung cancer
(20). The AKT and ERK signaling
pathways are closely related to the occurrence and development of
tumors. It has been reported that ERK1/2 and p-ERK1/2 expression
are associated with low survival rates among patients with lung
cancer (21).
The GALNT2 protein is also a key regulator of
radioresistance in n NSCLC, and the insulin-like growth factor 1
receptor (IGF1R) may be an important effector protein downstream of
the GALNT2 signaling pathway (16). IGF1R is a ubiquitously expressed
membrane-bound tyrosine kinase receptor that recognizes its two
major ligands, IGF1 and IGF2, and controls a variety of basic
cellular functions (22). IGF1R
signaling is a key factor in cancer cell proliferation, survival,
migration and resistance to anticancer therapy, and plays an
important role in lung cancer (23).
In addition, GALNT2 can also regulate immune cell
infiltration in lung cancer (13,15).
Single sample Gene Set Enrichment Analysis (ssGSEA) was used to
analyze the correlation between GALNT2 expression and immune cell
infiltration in LUAD, and it was found that GALNT2 expression was
negatively correlated with activated B cells, activated CD8 T
cells, immature B cells and eosinophil infiltration (all
P<0.001). Further research found that PD-L1 expression was
positively correlated with GALNT2 expression, suggesting that
GALNT2 could serve as a predictive biomarker for the therapeutic
effect of immunotherapy drugs.
GALNT3 protein
When compared with normal lung tissues, the levels
of GALNT3 mRNA and protein in NSCLC tissues are significantly
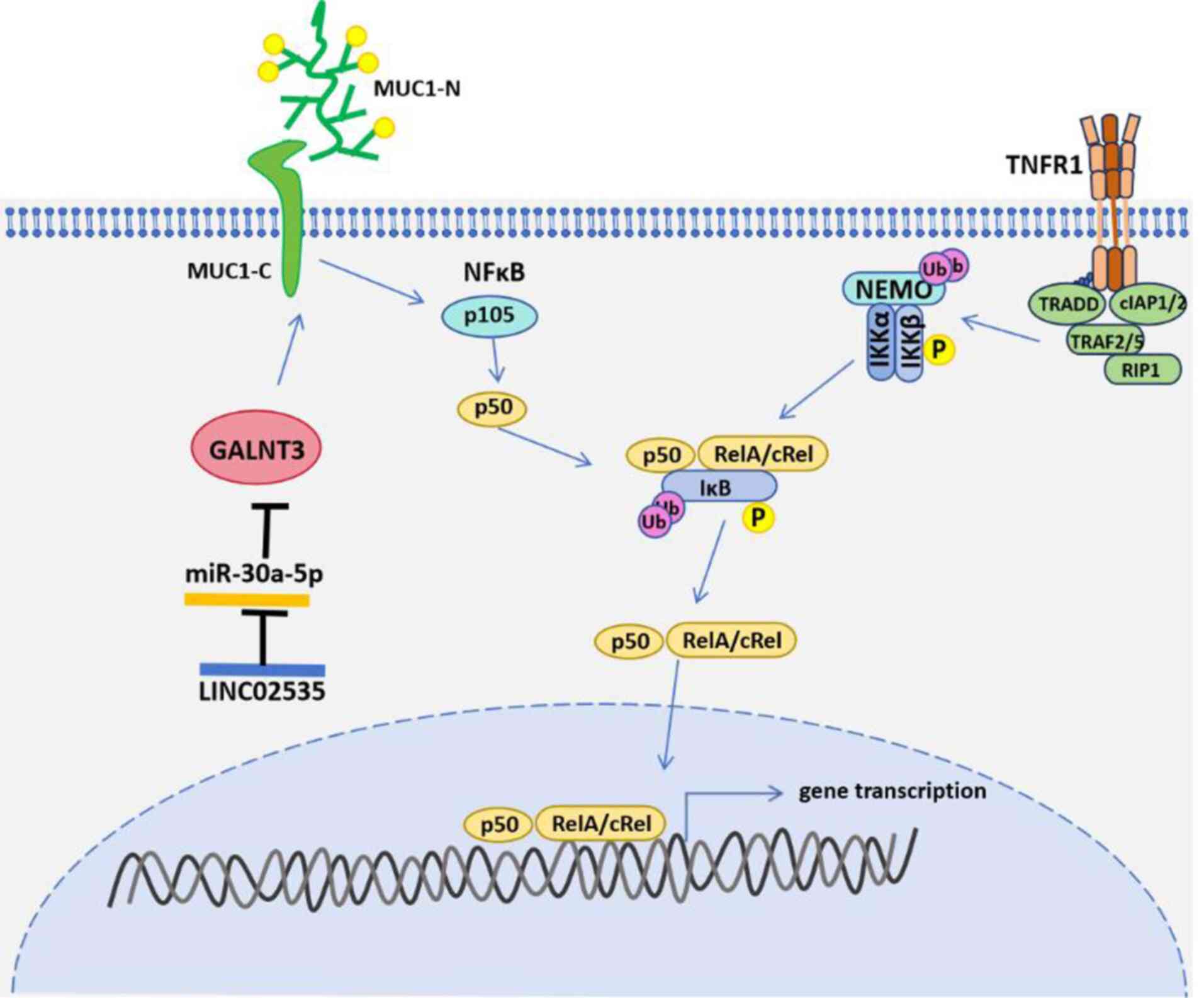
increased (24,25). GALNT3 protein expression in LUAD is
regulated by the LINC02535/miR-30a-5p axis (Fig. 2) (26). LINC02535 is significantly
upregulated in LUAD, resulting in a downregulation of miR-20a-5p.
Downregulation of miR-20a-5p results in increased GALNT3 protein
expression. In addition, it has been revealed that abnormal
expression of the GALNT3 protein is related to abnormal promoter
methylation (27).
GALNT3 protein can promote the proliferation,
migration and invasion of LUAD cells, activate the NF-κB signaling
pathway by affecting glycosylation of the MUC1 protein, and promote
the malignant progression of LUAD cells (Fig. 2). MUC1 is a mucin-like type I
transmembrane glycoprotein (28)
that plays an important role in the renewal and differentiation of
epithelial cells, maintenance of epithelial cell integrity, as well
as tumor occurrence and metastasis (29). In numerous malignant tumors,
changes in MUC1 glycosylation lead to its highly abnormal
expression (30). The
transmembrane C-terminus of MUC1 (MUC1-C), as an oncoprotein, can
affect the NF-κB signaling pathway and promote the occurrence and
development of various malignant tumors (31).
Overexpression of GALNT3 mRNA is associated with
immune cell infiltration and a poor prognosis in cases of LUAD, and
patients with high GALNT3 mRNA expression have a higher survival
rate (24). The level of GALNT3
expression in LUAD is closely related to the Th2 cell immune marker
genes STAT6, CCR8 and HAVCR1. The
aforementioned study also found that in LUAD cases with high levels
of macrophages, regulatory T cells, and type 2 T helper cell
infiltration, high GALNT3 expression was most closely related to a
poor prognosis. There are also studies showing completely
contradictory conclusions regarding the role of GALNT3 in lung
cancer. A previous study showed that GALNT3 inhibited the
development of lung cancer in both xenograft and syngeneic mouse
models, where it inhibited lung cancer by reducing myeloid-derived
suppressor cell infiltration and angiogenesis in a TNFR- and c-MET
pathway-dependent manner (32).
Additionally, it has been shown that the abnormally high expression
of GALNT3 in LUAD is promoting tumor growth. However, it has also
been reported that abnormally high expression of GALNT3 can
decelerate lung cancer growth by disrupting the tumor
microenvironment (TME), which may be achieved through different
signaling pathways. Notably, these results need to be verified by
more powerful biological experiments. Therefore, a hypothesis was
proposed by the authors: Abnormally high expression of GALNT3
protein in the early stage of lung cancer promotes tumor growth,
and when the expression reaches a certain threshold, it destroys
the TME and thus decelerates the tumor progression, and serves to
inhibit tumor growth.
GALNT4 protein
GALNT4 protein can promote the proliferation and
invasion of NSCLC cells and inhibit tumor cell apoptosis (33). In the H1299 cell line, using siRNA
to reduce the expression level of GALNT3 protein also reduced the
proliferation rate of tumor cells, the number of clones formed, and
increased the proportion of apoptotic cells. In NSCLC, GALNT4
protein expression is regulated by miR 365b. The levels of miR-365b
are significantly downregulated in NSCLC cells, and the reduced
binding to the GALNT4 mRNA 3'-untranslated region induces a high
level of GALNT4 mRNA expression, thereby promoting the development
of lung cancer.
GALNT4 protein can be used to predict the prognosis
of LUAD. One group of researchers constructed a model to predict
the prognosis of patients with LUAD. The model included SMCO2,
SATB2, HAVCR1, GRIA1 and GALNT4 protein expression, as well as TP53
mutations (34). A patient's risk
score was derived from those indicators, and calculated as follows:
Risk score=0.737888 x Exp SMCO2 + 0.357248 x Exp SATB2 + 0.061489 x
Exp HAVCRI-0.72351 x Exp GRIA1 + 0.43393 x Exp GALNT4 + 0.14491 x
mut TP53. Patients with high risk scores had lower survival rates,
while patients with low risk scores had a greater likelihood of
survival.
GALNT6 protein
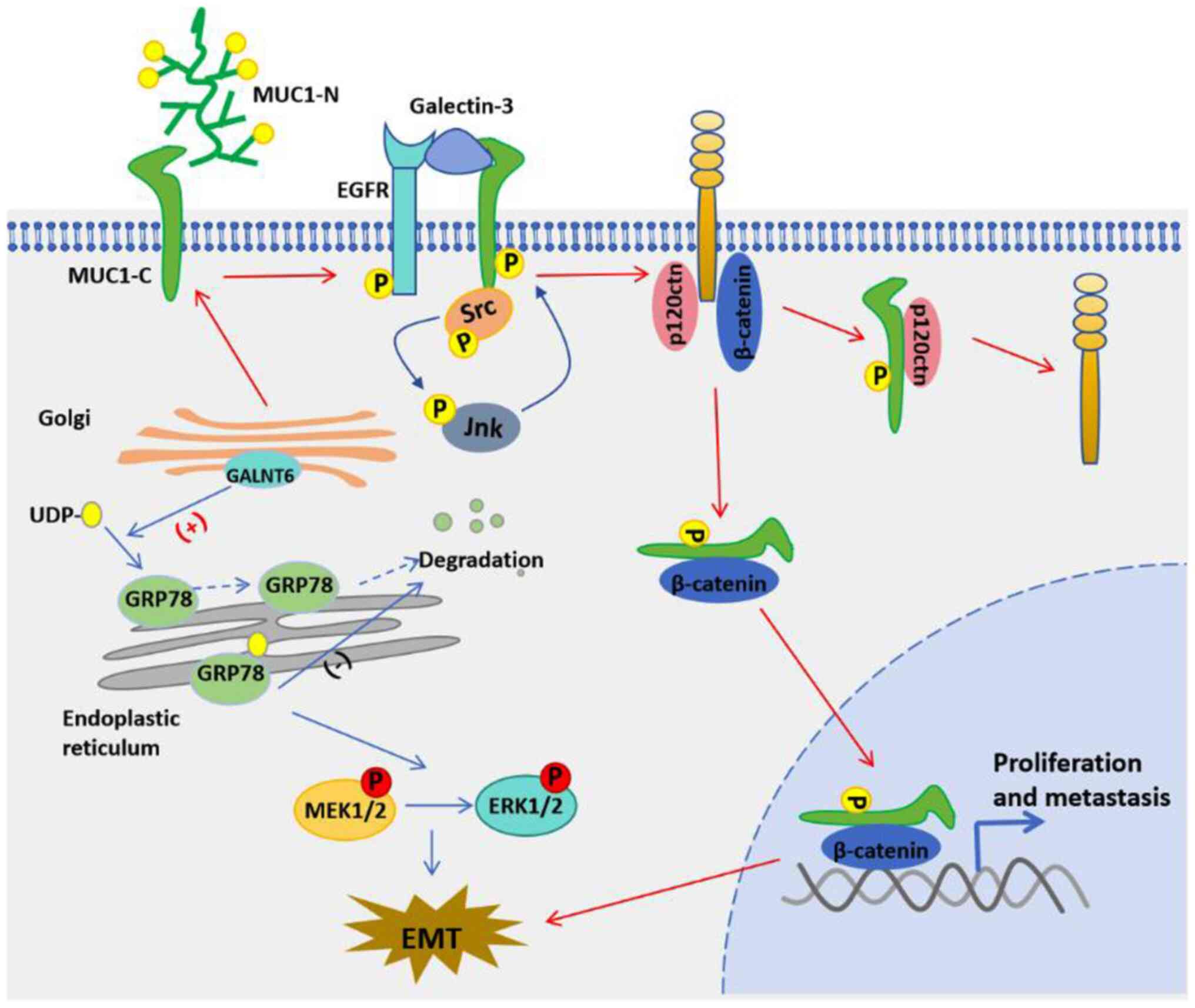
The upregulated expression of GALNT6 protein in LUAD
is associated with lymph node metastasis and a poor prognosis
(35). In LUAD cells, GALNT6
overexpression was found to promote EMT, wound healing and
invasion, while reducing GALNT6 protein expression significantly
reversed those effects. Furthermore, reducing GALNT6 expression
alleviated LUAD metastasis and prolonged the survival times of mice
with xenograft tumors. Mechanistic studies indicate that GALNT6
directly interacts with the glycosylation chaperone GRP78, and
GRP78 promotes EMT by enhancing the MEK1/2/ERK1/2 signaling pathway
in lung cancer cells (Fig. 3).
GALNT6-induced O-glycosylation is critical for the stability of
GRP78, its subcellular localization in the endoplasmic reticulum,
and its anti-apoptotic function. O-glycosylation stabilizes GRP78
protein, and high levels of GRP78 protein can drive the relocation
of GALNT6 from the Golgi apparatus to the endoplasmic reticulum.
Loss of O-glycosylation also results in the translocation of GRP78
protein from the endoplasmic reticulum to the cytoplasm.
Furthermore, O-glycosylation of the GRP78 protein is critical for
cell survival under conditions of nutrient deprivation. Therefore,
GALNT6 is becoming recognized as a new type of positive regulatory
factor for human LUAD malignancy, and targeting of
GALNT6-GRP78-MEK1/2/ERK1/2 may become a new strategy for preventing
lung cancer metastasis. Similar to GALNT3 protein, GALNT6 protein
can also regulate the glycosylation of MUC1 protein. Blocking the
O-glycosylation of MUC1-N and its subsequent MUC1-C/p120ctn
interaction with GALNT6 shRNA can prevent the initiation of EMT and
reduce the viability of lung cancer cells (36).
GALNT13 protein
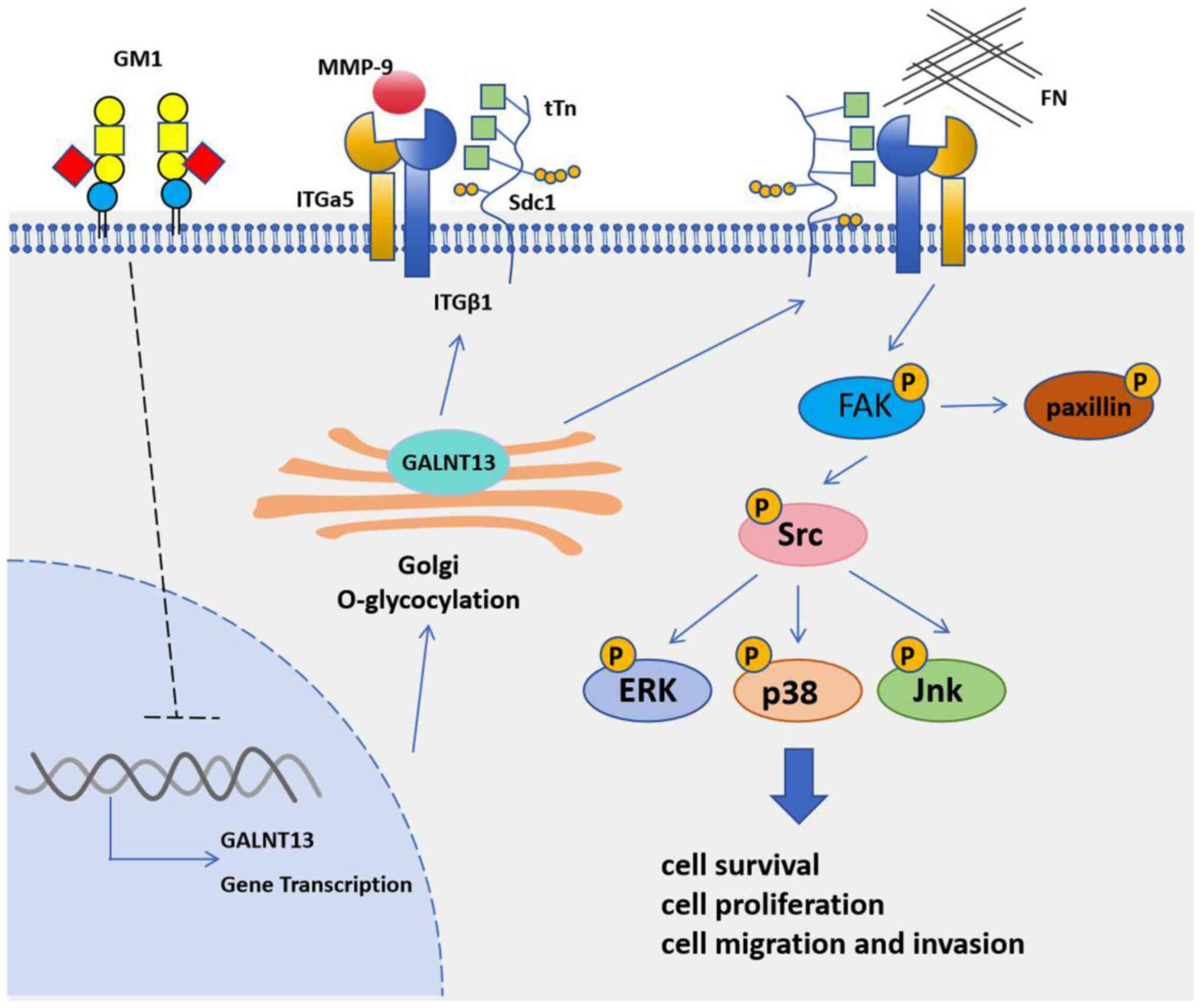
GALNT13 protein can promote lung cancer metastasis.
It was first discovered that the GALNT13 protein was upregulated in
the highly metastatic subline of the mouse Lewis lung cancer cell
line (37). In an analysis of the
mechanism of cancer metastasis, it was found that reduced levels of
ganglioside GM1 lead to increased invasion and metastasis potential
(38). In the mouse Lewis lung
cancer cell line, reduced GM1 protein expression induces an
upregulation of GALNT13 expression (Fig. 4). Stable overexpression of the
GALNT13 protein enhances the invasiveness and motility of Lewis
lung cancer cells and induces the formation of trimeric Tn antigen
on Syndecan 1. In C57BL/6 mice, injection of the GALNT13-silenced
Lewis lung cancer cell line produced primary tumors that were less
integrated with fascia and peritoneum and had significantly fewer
lung metastases. Further research on the mechanism of action of the
trimeric Tn antigen formed by Syndecan 1 during lung cancer
metastasis revealed that it enhances cell adhesion to fibronectin
by forming a complex with integrin α 5β1. It also enhances invasion
and metastasis activity by recruiting MMP-9 to GEM/rafts, and
increases the phosphorylation levels of FAK and paxillin (39). These results provide a convincing
basis for explaining the mechanistic role of GALNT13 protein in
lung cancer metastasis.
Overexpression of GALNT13 protein is associated with
a poor prognosis in patients with lung cancer. Reverse
transcription-quantitative PCR was used to analyze the expression
levels of GALNT13 mRNA and the use of its variant exons in 91
surgical specimens of lung cancer; after which, the correlation
with clinical data was evaluated. It was found that a high level of
GALNT13 mRNA significantly shortened the recurrence-free survival
(RFS) time of patients with lung cancer (P=0.045) (40). There are different transcriptional
variants of GALNT13 mRNA; the E13 positive expression group is
significantly correlated with a poor OS prognosis. On the contrary,
when compared with a negative group, the E14 positive expression
group had a significantly longer RFS time. In the aforementioned
study, immunohistochemistry was used to detect GM1 and evaluate the
expression of ppGalNAc-T13 and trimeric Tn antigen as prognostic
factors (40). The results
identified that among 35 patients with lung cancer, GALNT13 and
trimeric Tn antigens were associated with a poor prognosis.
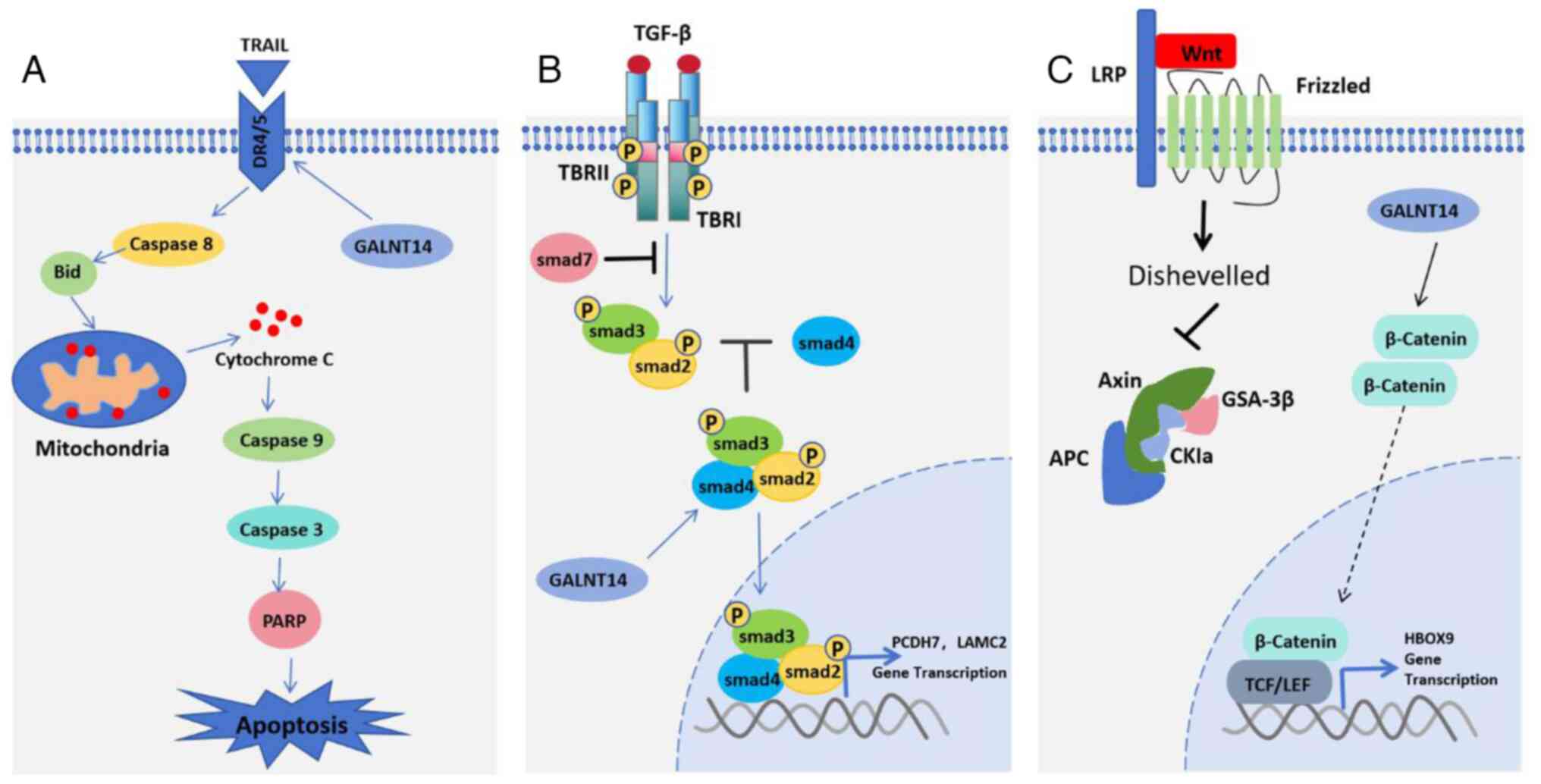
GALNT14 protein
GALNT14 can promote the sensitivity of lung cancer
cells to Apo2L/TRAIL ligands by regulating the glycosylation of
apoptosis receptors, DR4 and DR5 (Fig.
5A). Apo2L/TRAIL stimulates cancer cell death via the pro
apoptotic receptors, DR4 and DR5. When studying the mechanism of
tumor cell susceptibility to this ligand, researchers found that
the levels of GALNT14 mRNA were correlated with the sensitivity of
Apo2L/TRAIL in pancreatic cancer, NSCLC and melanoma (41). RNA interference with GALNT14
expression reduces cell sensitivity to Apo2L/TRAIL, while
overexpression produces the opposite effect. GALNT14 mRNA is
overexpressed in >30% of NSCLC cells. Further research has shown
that GALNT14 can regulate the O-glycosylation of DR4 and DR5
proteins. O-glycosylation promotes the aggregation of
ligand-stimulated DR4 and DR5, which mediates the recruitment and
activation of apoptosis initiated by protease caspase-8. GALNT14
expression in NSCLC cell lines can strongly predict the efficacy of
the pro-apoptotic receptor agonist dulanermin, the in vitro
sensitivity of rhApo2L/TRAIL, and the efficacy of drozitumab
monoclonal antibodies (DR5 agonist antibodies) (42). Those findings revealed a new
connection between death receptor O-glycosylation and apoptosis
signaling pathways, and suggest predictive biomarkers for cancer
treatment strategies based on Apo2L/TRAIL.
Overexpression of GALNT14 is associated with a poor
prognosis for patients with NSCLC. In a clinical genomics study
involving 138 patients with NSCLC, GALNT14 expression was highly
correlated with shorter RFS times (43). GALNT14 can also regulate lung
cancer metastasis. An analysis of RFS and differentially expressed
genes in 516 cases of LUAD in the TCGA database revealed 7 genes
(GALNT14, 9COL7A1, GPR115, C1QTNF6,
KRT16, INHA and TNFSF11) that were
significantly correlated with cancer progression and recurrence,
suggesting those genes as predictive factors for a poor prognosis
(44). In a group of patients that
highly expressed all 7 genes, both the metastatic and tumor
features showed positive enrichment, which was very significant in
the high GALNT14 gene expression group. In patients with lung
cancer, GALNT14 expression is significantly and negatively
correlated with local RFS, distant metastasis-free survival and OS
rate.
GALNT14 protein regulates the occurrence and
development of lung cancer via multiple pathways (Fig. 5B and C) (44,45).
GALNT14 protein promotes tumor metastasis and SOX4 expression. The
expression levels of AREG and VCAN proteins are related. In lung
cancer, the GALNT14 protein also affects the TGF-β signaling
pathway, which has been widely studied as a tumor suppressor, tumor
promoter and promoter of metastasis (46-48).
GALNT14 can also enhance the sensitivity to WNT signals, increase
the stability of β-catenin, and thereby induce the expression of
HOXB9 and promote development of an invasive phenotype (47). A meta-analysis of clinical genomics
data showed that overexpression of GALNT14 or HOXB9 was closely
related to a decrease in RFS time and an increase in HR, suggesting
that targeting the GALNT14/WNT/HOXB9 axis may be a new therapeutic
approach for inhibiting NSCLC metastasis.
The GALNT14 gene is associated with
paclitaxel resistance in lung cancer (48). Gene methylation and expression
differences between paclitaxel resistant and sensitive lung cancer
cells have been studied using methylation chip analysis and
transcriptome sequencing. A total of 43,426 differentially
methylated genes and 2,870 differentially expressed genes were
identified, including 6 genes (KANK1 ALDH3A1,
GALNT14, PIK3R3, LRG1 and WEE2) that
may be related to paclitaxel resistance in LUAD. GALNT14 was
one of those genes.
In addition, GALNT14 can also regulate immune cell
infiltration in lung cancer (13).
The correlation between GALNT14 expression and immune cell
infiltration in LUAD was analyzed using ssGSEA, and it was observed
that f GALNT14 expression was negatively correlated with immature B
cells and eosinophil infiltration (all P<0.01). Further research
has found that PD-L1 expression is positively correlated with
GALNT14 expression, which suggests that GALNT14 protein expression
could be used for predicting the efficacy of immunotherapy.
Other GALNT family proteins
Researchers have conducted studies using Oncomine
database. The NSCLC data from TCGA, UALCAN (https://ualcan.path.uab.edu/), GTEx (https://www.gtexportal.org/home/) and Kaplan
Meier plotter (https://kmplot.com/analysis/) databases were analyzed,
and the differential expression of mRNA for GANLT family proteins
in NSCLC was systematically examined (14). In addition to the GALNT family
proteins related to lung cancer aforementioned, the expression
levels of GALNT7 mRNA in LUAD and LUSC were higher than those in
normal tissues. Furthermore, the expression levels of
GALNT5/15/16/18/20 mRNA in LUAD and LUSC were lower than those in
normal tissues; the expression levels of GALNT8/9/11/17/19 in LUAD
and LUSC were basically consistent with those in normal tissues;
the expression levels of GALNT10/12 mRNA in LUSC were lower than
those in normal tissues, and the expression levels in LUAD were
consistent with those in normal tissues. A high level of GALNT 9
expression was associated with a decrease in the OS rate of
patients with lung cancer, and a high level of GALNT16 expression
was associated with poor disease-free survival in patients with
lung cancer. At present, the role of these GALNT family members in
lung cancer has not been reported.
Limitations and outlook
It is necessary to acknowledge the limitations of
the present review. The generalizability of our findings may have
been affected by the small sample size of the study, which sought
to investigate the mechanisms of lung cancer occurrence and
development in a specific population. Additional studies with
larger sample sizes will be needed to confirm the present results.
Moreover, the similar mechanism of action of the important members
of the GALNT protein family and the interaction network between the
proteins need to be explored in depth in the future.
Despite some limitations, we remain confident that
we can carry out ambitious research in the field. Regarding the
GALNT family proteins, it is hoped that progress can be made in the
following areas: i) determine whether GALNT family proteins can be
used as predictive markers for early-stage lung cancer; ii) explore
GALNT family proteins as biomarkers for predicting the efficacy of
lung cancer drugs; iii) investigate the role of other GALNT family
proteins in lung cancer; iv) investigate the relationship between
members of the GALNT family and the immune microenvironment, as
well as the efficacy of immunotherapy; v) investigate the
regulatory mechanism of GALNT family proteins in lung cancer, with
the goal of developing new strategies for lung cancer treatment
based on O-GalNAc glycosylation.
3. Summary
In summary, the high expression of numerous members
of the GALNT family in lung cancer is closely related to the
occurrence, development, and a poor prognosis for those tumors. The
abnormal expression of GALNT family members is usually caused by
abnormal methylation of gene promoters, and upstream regulatory
gene changes caused by changes in miRs. GALNT family proteins
generally exert their effects by regulating the O-glycosylation of
proteins that play a crucial role in the occurrence and development
of lung cancer; however, the mechanisms of action of different
members vary. The main signaling pathways involved in regulating
lung cancer occurrence and development by members of the GALNT
family include Notch/Hes1-PTEN-PI3K/Akt, GRP78-MEK1/2/ERK1/2 and
TGF-β (Fig. 6). Another study has
found that GALNT3 exerts inhibitory effects in lung cancer, and
indicate that the mechanism by which the GALNT proteins regulate
the occurrence and development of lung cancer is complex (36). The positive and negative regulation
of GALNT3 protein in lung cancer requires further exploration.
Among the GALNT family proteins, GALNT2/3/14 can affect immune cell
infiltration in lung cancer, suggesting that members of the GALNT
family may serve as biomarkers for predicting the therapeutic
effect of immunotherapy drugs. The GALNT family proteins are also
used to construct predictive models for patient prognosis, but they
are usually combined with other indicators to construct predictive
models. A single GALNT family protein indicator predictive model
has not yet emerged. There is relatively little research on the
relationship between GALNT family proteins and the drug sensitivity
of tumors. Only GALNT 14 has been found to be associated with lung
cancer resistance to paclitaxel, and be a potential predictive
biomarker for Apo2L/TRAIL-based cancer treatment strategies.
Briefly, the present review summarizes the abnormal
expression and important roles of GALNT family protein members
(2-4,6,13,14)
in lung cancer. The sensitivity and specificity of GALNT family
proteins will be an important direction for future exploration, and
the correlation between the expression of GALNT family proteins for
drug efficacy monitoring in lung cancer and immunotherapy is also
worth exploring. Moreover, the role of other GALNT family proteins
(except 2-4,6,13,14) in lung cancer and the research on the
molecular mechanisms by which GALNT family proteins regulate the
development of lung cancer will provide new ideas for the diagnosis
and treatment of lung cancer.
Acknowledgements
The authors would like to thank Dr Xiaofeng Wan from
the Hefei Cancer Hospital of the Chinese Academy of Sciences for
her pertinent comments on this manuscript.
Funding
Funding: The present study was supported by the Chuzhou Health
Research Program (grant no. 2022008).
Availability of data and materials
Not applicable.
Authors' contributions
CM wrote the original draft, conducted investigation
and methodology. RS conceptualized the study. DJ performed software
analysis. LM conducted visualization. QF performed literature
review. MW revised the manuscript. ZW supervised the study and
polished the manuscript. All authors read and approved the final
version of the manuscript. Data authentication is not
applicable.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
DeSantis CE, Ma J, Gaudet MM, Newman LA,
Miller KD, Goding Sauer A, Jemal A and Siegel RL: Breast cancer
statistics, 2019. CA Cancer J Clin. 69:438–451. 2019.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Jiang JJ, Li YQ and Gu YY: Clinical and
histopathological features of immune checkpoint inhibitor-related
myositis in patients with advanced non-small cell lung cancer.
Zhonghua Jie He He Hu Xi Za Zhi. 45:47–52. 2022.PubMed/NCBI View Article : Google Scholar : (In Chinese).
|
|
3
|
Duma N, Santana-Davila R and Molina JR:
Non-small cell lung cancer: Epidemiology, screening, diagnosis, and
treatmen. Mayo Clin Proc. 94:1623–1640. 2019.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Broderick SR: Adjuvant and neoadjuvant
immunotherapy in non-small cell lung cancer. Thorac Surg Clin.
30:215–220. 2020.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Hussain MR, Hoessli DC and Fang M:
N-acetylgalactosaminyltransferases in cancer. Oncotarget.
7:54067–54081. 2016.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Hollingsworth MA and Swanson BJ: Mucins in
cancer: Protection and control of the cell surface. Nat Rev Cancer.
4:45–60. 2004.PubMed/NCBI View
Article : Google Scholar
|
|
7
|
Ohyama C: Glycosylation in bladder cancer.
Int J Clin Oncol. 13:308–313. 2008.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Langbecker D and Janda M: Systematic
review of interventions to improve the provision of information for
adults with primary brain tumors and their caregivers. Front Oncol.
5(1)2015.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Stowell SR, Ju T and Cummings RD: Protein
glycosylation in cancer. Annu Rev Pathol. 10:473–510.
2015.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Peng C, Togayachi A, Kwon YD, Xie C, Wu G,
Zou X, Sato T, Ito H, Tachibana K, Kubota T, et al: Identification
of a novel human UDP-GalNAc transferase with unique catalytic
activity and expression profile. Biochem Biophys Res Commun.
402:680–686. 2010.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Gomes J, Mereiter S, Magalhães A and Reis
CA: Early GalNAc O-glycosylation: Pushing the tumor boundaries.
Cancer Cell. 32:544–545. 2017.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Wang W, Sun R, Zeng L, Chen Y, Zhang N,
Cao S, Deng S, Meng X and Yang S: GALNT2 promotes cell
proliferation, migration, and invasion by activating the
Notch/Hes1-PTEN-PI3K/Akt signaling pathway in lung adenocarcinoma.
Life Sci. 276(119439)2021.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Yu Y, Wang Z, Zheng Q and Li J: GALNT2/14
overexpression correlate with prognosis and methylation: Potential
therapeutic targets for lung adenocarcinoma. Gene.
790(145689)2021.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Hu Q, Tian T, Leng Y, Tang Y, Chen S, Lv
Y, Liang J, Liu Y, Liu T, Shen L and Dong X: The O-glycosylating
enzyme GALNT2 acts as an oncogenic driver in non-small cell lung
cancer. Cell Mol Biol Lett. 27(71)2022.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Alghamdi RA and Al-Zahrani MH: Integrated
bioinformatics analyses identifying key transcriptomes correlated
with prognosis and immune infiltrations in lung squamous cell
carcinoma. Saudi J Biol Sci. 30(103596)2023.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Dong X, Leng Y, Tian T, Hu Q, Chen S, Liu
Y and Shen L: GALNT2, an O-glycosylating enzyme, is a critical
regulator of radioresistance of non-small cell lung cancer:
Evidence from an integrated multi-omics analysis. Cell Biol
Toxicol. 39:3159–3174. 2023.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Baumgart A, Mazur PK, Anton M, Rudelius M,
Schwamborn K, Feuchtinger A, Behnke K, Walch A, Braren R, Peschel
C, et al: Opposing role of Notch1 and Notch2 in a Kras(G12D)-driven
murine non-small cell lung cancer model. Oncogene. 34:578–588.
2015.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Zheng Y, de la Cruz CC, Sayles LC,
Alleyne-Chin C, Vaka D, Knaak TD, Bigos M, Xu Y, Hoang CD, Shrager
JB, et al: A rare population of CD24(+)ITGB4(+)Notch(hi) cells
drives tumor propagation in NSCLC and requires Notch3 for
self-renewal. Cancer Cell. 24:59–74. 2013.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Yuan X, Wu H, Xu H, Han N, Chu Q, Yu S,
Chen Y and Wu K: Meta-analysis reveals the correlation of Notch
signaling with non-small cell lung cancer progression and
prognosis. Sci Rep. 5(10338)2015.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Zheng W, Jiang C and Li R: Integrin and
gene network analysis reveals that ITGA5 and ITGB1 are prognostic
in non-small-cell lung cancer. Onco Targets Ther. 9:2317–2327.
2016.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Zhang YL, Wang RC, Cheng K, Ring BZ and Su
L: Roles of Rap1 signaling in tumor cell migration and invasion.
Cancer Biol Med. 14:90–99. 2017.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Girnita L, Worrall C, Takahashi S,
Seregard S and Girnita A: Something old, something new and
something borrowed: emerging paradigm of insulin-like growth factor
type 1 receptor (IGF-1R) signaling regulation. Cell Mol Life Sci.
71:2403–2427. 2014.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Alfaro-Arnedo E, López IP, Piñeiro-Hermida
S, Canalejo M, Gotera C, Sola JJ, Roncero A, Peces-Barba G,
Ruíz-Martínez C and Pichel JG: IGF1R acts as a cancer-promoting
factor in the tumor microenvironment facilitating lung metastasis
implantation and progression. Oncogene. 41:3625–3639.
2022.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Luo D, Fang M, Shao L, Wang J, Liang Y,
Chen M, Gui X, Yan J, Wang W, Yu L, et al: The EMT-related genes
GALNT3 and OAS1 are associated with immune cell infiltration and
poor prognosis in lung adenocarcinoma. Front Biosci (Landmark Ed).
28(271)2023.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Pucci M, Duca M, Malagolini N and
Dall'Olio F: Glycosyl-transferases in cancer: Prognostic biomarkers
of survival in patient cohorts and impact on malignancy in
experimental models. Cancers (Basel). 14(2128)2022.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Li Y, Zhao J, Zhang W, Wang A, Jiao M, Cai
X, Zhu J, Liu Z and Huang JA: LINC02535/miR-30a-5p/GALNT3 axis
contributes to lung adenocarcinoma progression via the NF-κB
signaling pathway. Cell Cycle. 21:2455–2470. 2022.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Vojta A, Samaržija I, Bočkor L and Zoldoš
V: Glyco-genes change expression in cancer through aberrant
methylation. Biochim Biophys Acta. 1860:1776–1785. 2016.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Chen W, Zhang Z, Zhang S, Zhu P, Ko JK and
Yung KK: MUC1: Structure, function, and clinic application in
epithelial cancers. Int J Mol Sci. 22(6567)2021.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Supruniuk K, Czarnomysy R, Muszyńska A and
Radziejewska I: Combined action of anti-MUC1 monoclonal antibody
and pyrazole-platinum(II) complexes reveals higher effectiveness
towards apoptotic response in comparison with monotherapy in AGS
gastric cancer cells. Pharmaceutics. 13(968)2021.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Hagiwara M, Fushimi A, Yamashita N,
Bhattacharya A, Rajabi H, Long MD, Yasumizu Y, Oya M, Liu S and
Kufe D: MUC1-C activates the PBAF chromatin remodeling complex in
integrating redox balance with progression of human prostate cancer
stem cells. Oncogene. 40:4930–4940. 2021.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Ahmad R, Raina D, Trivedi V, Ren J, Rajabi
H, Kharbanda S and Kufe D: MUC1 oncoprotein activates the IkappaB
kinase beta complex and constitutive NF-kappaB signalling. Nat Cell
Biol. 9:1419–1427. 2007.PubMed/NCBI View
Article : Google Scholar
|
|
32
|
Park MS, Yang AY, Lee JE, Kim SK, Roe JS,
Park MS, Oh MJ, An HJ and Kim MY: GALNT3 suppresses lung cancer by
inhibiting myeloid-derived suppressor cell infiltration and
angiogenesis in a TNFR and c-MET pathway-dependent manner. Cancer
Lett. 521:294–307, Aug 17. 2021.PubMed/NCBI View Article : Google Scholar : (Epub ahead of
print).
|
|
33
|
Xing L, Hong X, Chang L, Ren P and Zhang
H: miR-365b regulates the development of non-small cell lung cancer
via GALNT4. Exp Ther Med. 20:1637–1643. 2020.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Chen X, Yu L, Zhang H and Jin H:
Identification of new prognostic genes and construction of a
prognostic model for lung adenocarcinoma. Diagnostics (Basel).
13(1914)2023.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Song J, Liu W, Wang J, Hao J, Wang Y, You
X, Du X, Zhou Y, Ben J, Zhang X, et al: GALNT6 promotes invasion
and metastasis of human lung adenocarcinoma cells through
O-glycosylating chaperone protein GRP78. Cell Death Dis.
11(352)2020.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Zhang L, Gallup M, Zlock L, Chen YT,
Finkbeiner WE and McNamara NA: Pivotal role of MUC1 glycosylation
by cigarette smoke in modulating disruption of airway adherens
junctions in vitro. J Pathol. 234:60–73. 2014.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Matsumoto Y, Zhang Q, Akita K, Nakada H,
Hamamura K, Tokuda N, Tsuchida A, Matsubara T, Hori T, Okajima T,
et al: pp-GalNAc-T13 induces high metastatic potential of murine
Lewis lung cancer by generating trimeric Tn antigen. Biochem
Biophys Res Commun. 419:7–13. 2012.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Zhang Q and Furukawa K, Chen HH,
Sakakibara T, Urano T and Furukawa K: Metastatic potential of mouse
Lewis lung cancer cells is regulated via ganglioside GM1 by
modulating the matrix metalloprotease-9 localization in lipid
rafts. J Biol Chem. 281:18145–18155. 2006.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Matsumoto Y, Zhang Q, Akita K, Nakada H,
Hamamura K, Tsuchida A, Okajima T and Furukawa K, Urano T and
Furukawa K: Trimeric Tn antigen on syndecan 1 produced by
ppGalNAc-T13 enhances cancer metastasis via a complex formation
with integrin α5β1 and matrix metalloproteinase 9. J Biol Chem.
288:24264–24276. 2013.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Nogimori K, Hori T, Kawaguchi K, Fukui T,
Mii S, Nakada H, Matsumoto Y, Yamauchi Y, Takahashi M, Furukawa K,
et al: Increased expression levels of ppGalNAc-T13 in lung cancers:
Significance in the prognostic diagnosis. Int J Oncol.
49:1369–1376. 2016.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Wagner KW, Punnoose EA, Januario T,
Lawrence DA, Pitti RM, Lancaster K, Lee D, von Goetz M, Yee SF,
Totpal K, et al: Death-receptor O-glycosylation controls tumor-cell
sensitivity to the proapoptotic ligand Apo2L/TRAIL. Nat Med.
13:1070–1077. 2007.PubMed/NCBI View
Article : Google Scholar
|
|
42
|
Stern HM, Padilla M, Wagner K, Amler L and
Ashkenazi A: Development of immunohistochemistry assays to assess
GALNT14 and FUT3/6 in clinical trials of dulanermin and drozitumab.
Clin Cancer Res. 16:1587–1596. 2010.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Lee ES, Son DS, Kim SH, Lee J, Jo J, Han
J, Kim H, Lee HJ, Choi HY, Jung Y, et al: Prediction of
recurrence-free survival in postoperative non-small cell lung
cancer patients by using an integrated model of clinical
information and gene expression. Clin Cancer Res. 14:7397–7404.
2008.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Kwon OS, Lee H, Kong HJ, Kwon EJ, Park JE,
Lee W, Kang S, Kim M, Kim W and Cha HJ: Connectivity map-based drug
repositioning of bortezomib to reverse the metastatic effect of
GALNT14 in lung cancer. Oncogene. 39:4567–4580. 2020.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Derynck R, Akhurst RJ and Balmain A:
TGF-beta signaling in tumor suppression and cancer progression. Nat
Genet. 29:117–129. 2001.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Padua D and Massagué J: Roles of TGFbeta
in metastasis. Cell Res. 19:89–102. 2009.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Kwon OS, Oh E, Park JR, Lee JS, Bae GY,
Koo JH, Kim H, Choi YL, Choi YS, Kim J and Cha HJ: GalNAc-T14
promotes metastasis through Wnt dependent HOXB9 expression in lung
adenocarcinoma. Oncotarget. 6:41916–41928. 2015.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Pu J, Shen J, Zhong Z, Yanling M and Gao
J: KANK1 regulates paclitaxel resistance in lung adenocarcinoma
A549 cells. Artif Cells Nanomed Biotechnol. 48:639–647.
2020.PubMed/NCBI View Article : Google Scholar
|