Introduction
Intravascular large B-cell lymphoma (IVLBCL), which
has an incidence rate of ~0.095/100,000 individuals (1) and accounts for 1% of B-cell lymphomas
(2), is characterized by confining
within the lumina of small to medium-sized blood vessels,
particularly capillaries (3). It
can involve all organs, particularly the central nervous system,
skin, kidney, lung and adrenal glands, but rarely involves the
thyroid (4). The pathological
mechanism underlying development of IVLBCL involves B-cell
receptor/nuclear factor-κB signaling, which leads to inhibition of
apoptosis (5). Hemophagocytic
syndrome (HPS)-associated IVLBCL usually portends a poor prognosis
and is necessary for diagnosis early. However, IVLBCL presents with
numerous non-specific signs and symptoms which makes it difficult
to diagnose. Most of the patients were assessed as stage IV at the
time of diagnosis and a long-term survival fraction between 20-40%
due to the aggressive (6).
Rituximab, cyclophosphamide, hydroxydaunorubicin, vincristine,
prednisone and high-dose methotrexate plus intrathecal chemotherapy
are a safe and active treatment for patients with IVLBCL without
apparent central nervous system involvement at diagnosis (7). In the present report, such a case is
documented and the possible pathogenesis is explored.
Case presentation
A 63-year-old-woman presented to the Haematology
department of Hebei General Hospital (Shijiazhuang, China) in
September 2022, with a persistent high fever lasting a month, dry
mouth and eyes, and partial tooth loss. A detailed examination
showed body temperature 35.7-40˚C, pulse 112/min, respiration
21/min, blood pressure 123/64 mmHg, clear consciousness, small,
palpable, red spots in the neck, chest and both lower
extremities.
The main laboratory findings are shown in Table I and suggest Epstein-Barr virus
(EBV) infection. An ophthalmologist diagnosed xerophthalmia. Bone
marrow biopsy samples were positive for CD20 (Fig. 1) and BCL2 and C-myc large cells
were identified within vascular spaces, resulting in a diagnosis of
double-expression IVLBCL. Immunostaining also revealed that the
tumour cells were positive for PAX-5, CD31, CD34, CyclinD1 and Mum1
(Fig. S1), and negative for CD10,
BCL6 and CD30; the Ki-67 index was 80%. Flow cytometric analyses of
bone marrow revealed positivity for CD20, CD22, CD79b and lambda
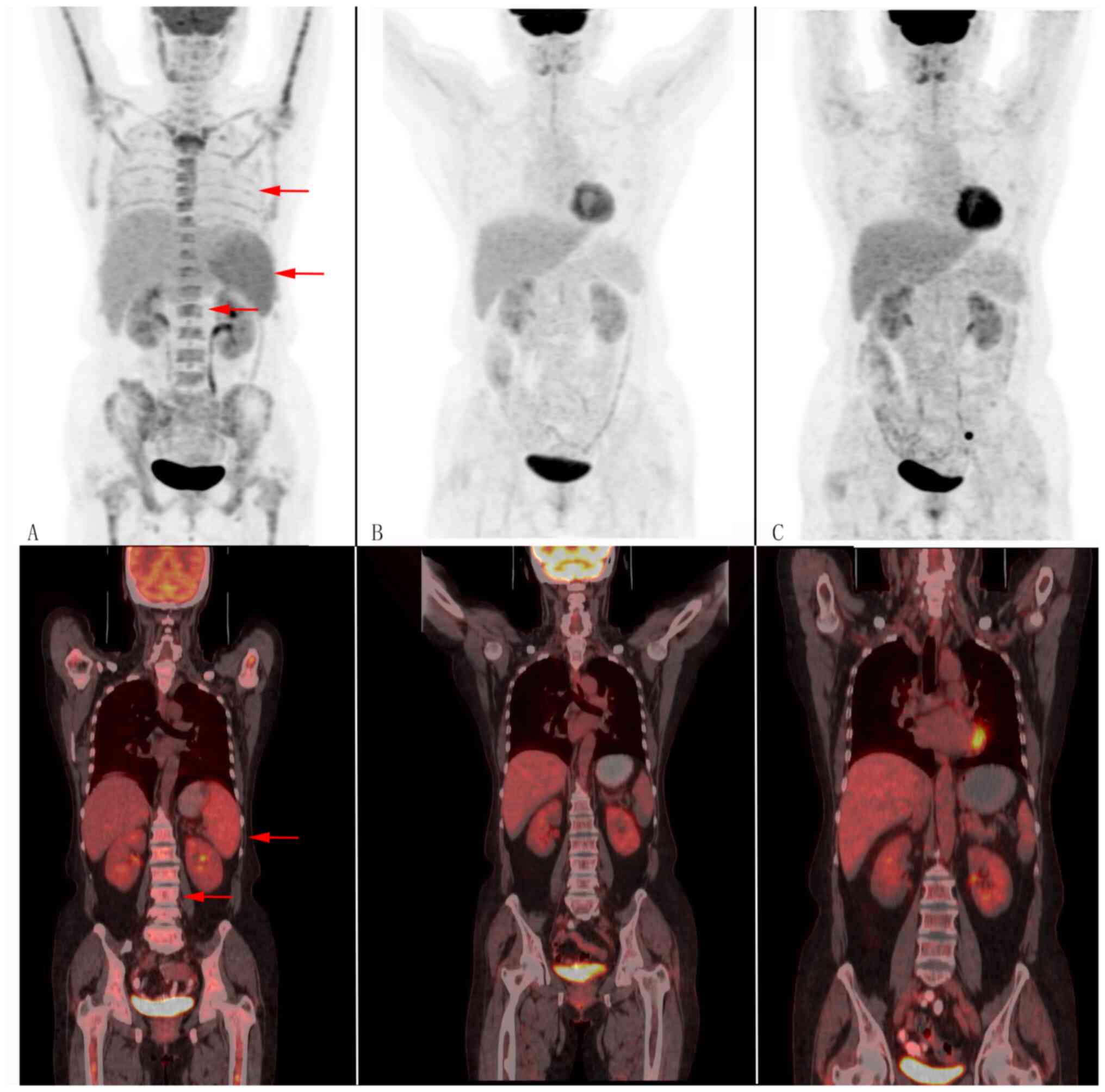
chains and were negative for CD38, CD138 and kappa (Fig. S2). In addition, positron emission
tomography/computed tomography (PET/CT) showed splenomegaly and
hypermetabolism, and bilateral lung and bone marrow diffuse
hypermetabolism, resulting in a diagnosis of IVLBCL before bone
marrow biopsy (Fig. 2). Our
patient thus met the five diagnostic criteria for hemophagocytic
lympho-histiocytosis (HLH), namely fever, splenomegaly, cytopenia,
ferritin ≥500 ng/ml, sCD 25 ≥2,400 U/ml and a bone marrow sample
showing hemophagocytes (Fig. 1)
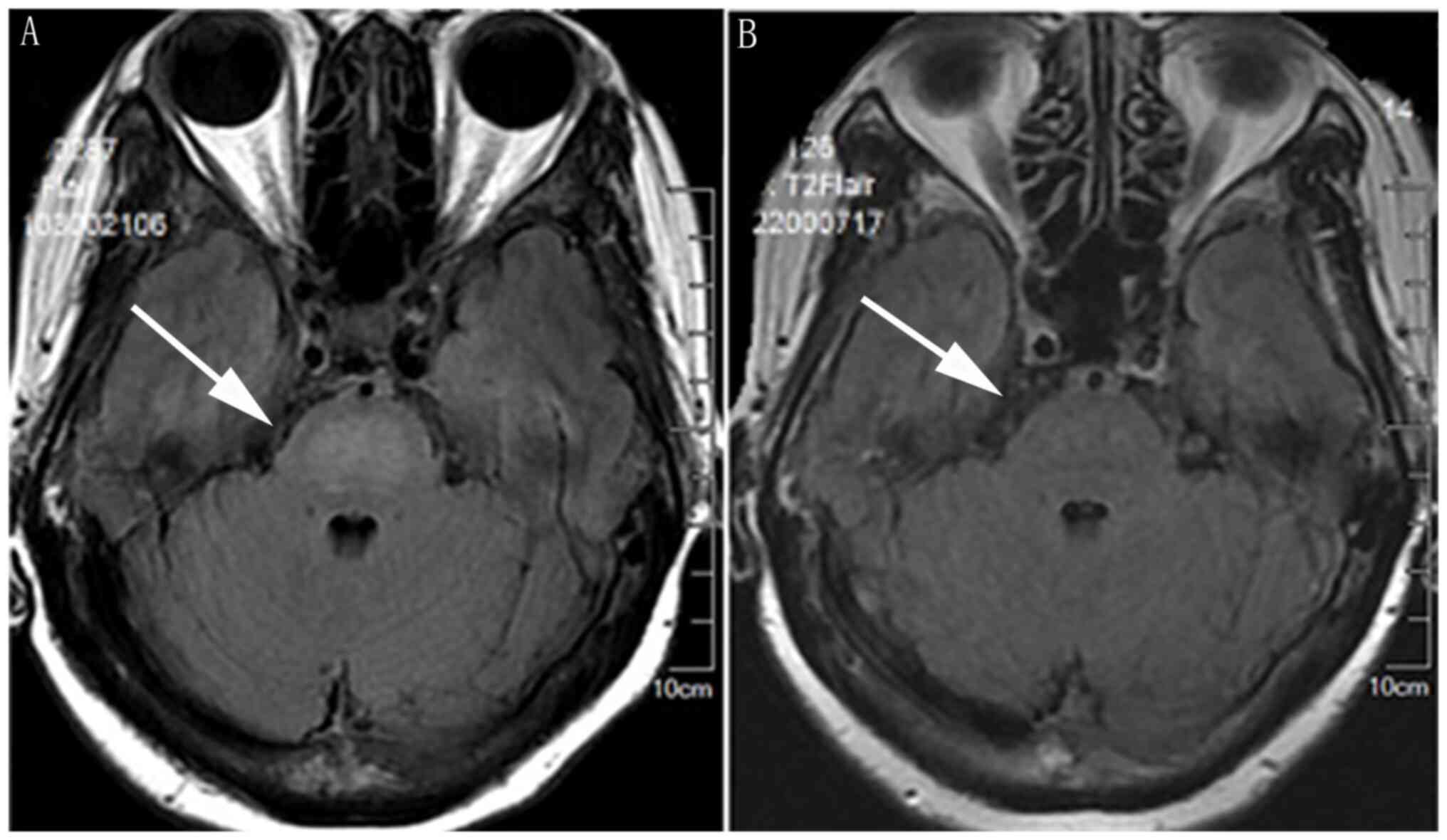
(8). Cranial magnetic resonance
imaging (MRI) revealed a hyperintense lesion in the central pons
(Fig. 3). Thyroid ultrasound
indicated hypoechoic thyroid nodules. Papillary thyroid carcinoma
(PTC) was diagnosed by fine needle aspiration biopsy (Fig. 1). Immunofixation electrophoresis
displayed M proteins positive and IgG kappa light chains (Fig. S3). Next-generation sequencing of
bone marrow yielded mutations for the genes of DNMT3A exon13,
DNMT3A exon8, FAT1 exon6 and CCND1 exon3. Nucleic acid extraction
was performed using the Blood Genomic DNA Extraction Kit (0.1-1 ml;
cat. no. YDP348-03; Tiangen Biotech Co., Ltd.). Nucleic acid
quality inspection was performed using a NANODROP ONE (Thermo
Fisher Scientific, Inc.) to measure the preliminary DNA
concentration, A260/280 and A260/230, where A260 is the absorption
wavelength of the highest absorption peak of nucleic acid and A280
is the absorption wavelength of the highest absorption peak of
protein. A230 is the absorption wavelength of the highest
absorption peak of carbohydrates.
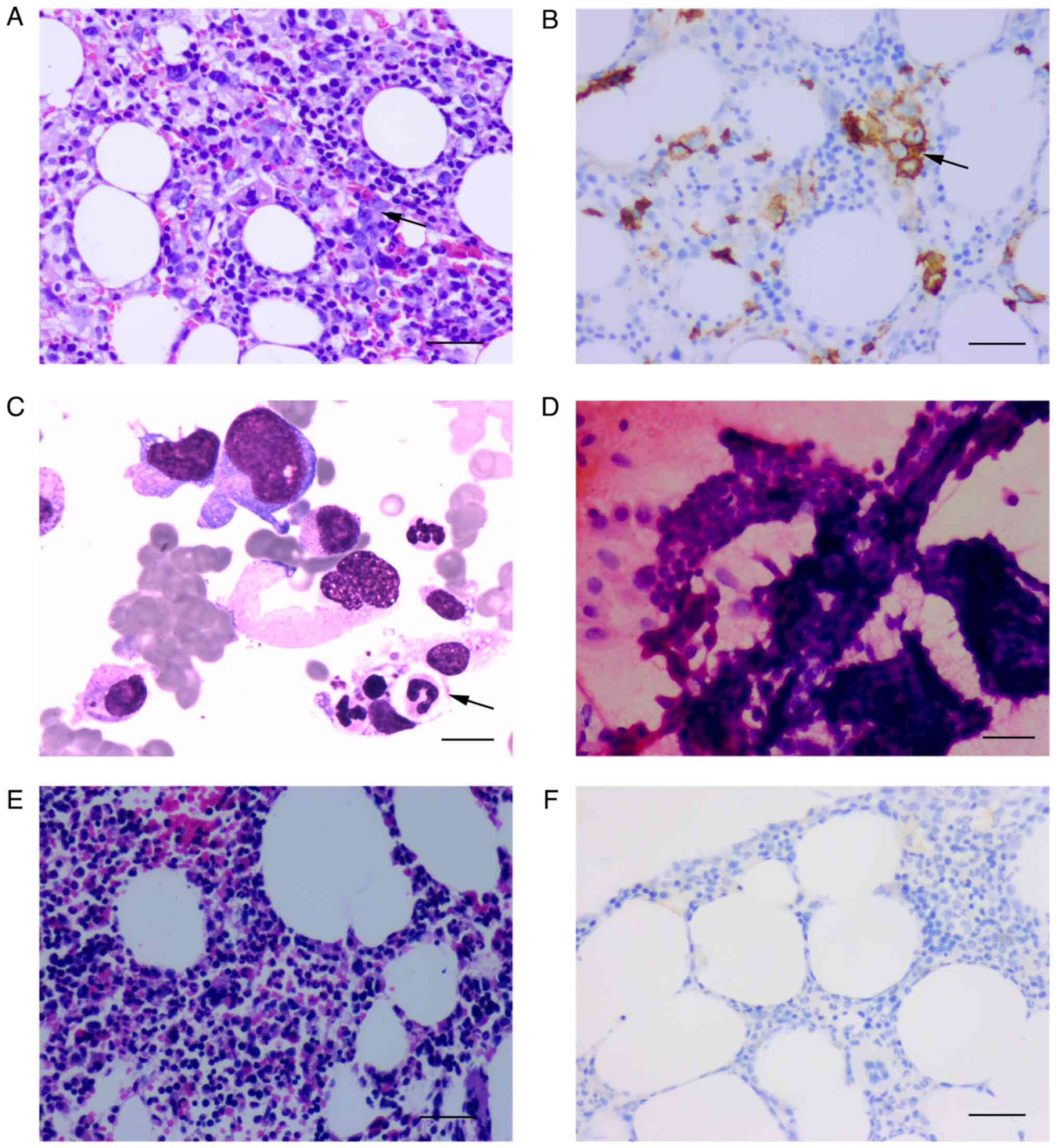 | Figure 1Photomicrographs samples of bone
marrow and thyroid. (A) Haematoxylin and eosin sample of bone
marrow before treatment (magnification, x200; scale bar, 100 µm).
(B) Immunostained sample of bone marrow showing CD20 positive
before treatment (magnification, x200; scale bar 100 µm). (C)
Photomicrograph sample of bone marrow showing neutrophils engulfed
by macrophages (black arrow) (scale bar, 20 µm). (D)
Photomicrograph of fine needle aspiration biopsy sample suggesting
papillary thyroid carcinoma (magnification, x400; scale bar, 20
µm). (E) Haematoxylin and eosin sample of bone marrow after four
cycles of treatment (magnification, x200; scale bar, 100 µm). (F)
Immunostained sample of bone marrow showing CD20 negative after
four cycles of treatment (magnification, x200, scale bar 100
µm). |
 | Table ILaboratory findings. |
Table I
Laboratory findings.
| | Biomarker | Value | Normal
range/limit |
|---|
| CRP | CRP | 223.7 mg/λ↑ | 0-10 |
| Complete blood
count | White blood
cells |
5.12x109/l | 3.5-9.5 |
| | Neutrophils |
3.86x109/l | 1.8-6.3 |
| | Mononuclear |
0.25x109/l↑ | 0.1-0.6 |
| | Eosinophils | 0↓ | 0.02-0.52 |
| | Red blood cells |
3.43x1012/l | 3.8-5.1 |
| | Haemoglobin | 79 g/l↓ | 115-150 |
| | Peripheral T
cell |
54x109/l↓ | 125-350 |
| Biochemistry | Lactate
dehydrogenase | 2,323.5 U/l↑ | 120-250 |
| Immunoglobulin | Complement C1q | 310.9 mg/l↑ | 159-233 |
| Cytokines | IL-6 | 309.3 pg/ml↑ | ≤7 |
| | IL-8 | 70.4 pg/ml↑ | ≤62 |
| | IL-10 | 107.2 pg/ml↑ | ≤9.1 |
| | IFN-γ | 116.5 pg/ml↑ | ≤13.2 |
| | TNF-α | 62.7 pg/ml↑ | ≤8 |
| EBV PCR | - |
8.09x103↑ | 0 |
| sCD25, NK cell
activity | - | 17.634 pg/ml↑ | 0-6,400 |
| | - | 19.12% | ≥15.11% |
| Serum iron
β2 microglobulin | - | 1,366 ng/ml | 13-150 |
| | - | 3.726 µg/ml↑ | 0.9-2.7 |
| Antinuclear
antibodies | - | 1:1,000 | <1:100 |
A260/280 and A260/230 are indicative values of
nucleic acid purity. An A260/280 ratio of 1.8-2.0 and an A260/230
ratio of 2.0-2.2 indicate that the purity of DNA is favourable.
Qubit 4.0 fluorometer (cat. no. Q33238; Thermo Fisher Scientific,
Inc.) detection was used as the standard for library construction
input. Raw samples were analyzed by electrophoresis (2.5% agarose
gels) using DNA marker (BM401-01; TransGen Biotech Co., Ltd.) to
initially confirm DNA integrity.
The sequencing protocol was paired-end 150 bp
sequencing. The DNA library was constructed with a commercial kit
(cat. no. 20025524; Illumina DNA Prep with Enrichment). The library
quality control was performed using a Qubit 4.0 to detect the
library concentration, and Agilent DNF-915 Reagent Kits (Agilent
Technologies, Inc.) were used to perform fragment analysis quality
control on the Agilent 5200 platform (Agilent Technologies, Inc.).
The average fragments ranged between 330 and 390 bp [dilution
conversion formula for the concentration of the library: 1.25 nm x
average molar mass of bases (660) x average fragment length of
library (330-390 bp)/106=0.27-0.32 ng/µl (0.4-0.48 nM)].
Hybridization was performed using the Next era DNA Flex
Pre-Enrichment Library Prep and Enrichment Reagents 96 samples, and
targeted capture amplification of the target region was performed
with probes manufactured by Tianjin Xiehe Bojing Medical Diagnostic
Technology Co., Ltd. The sequencing platform was Illumina
novaseq6000 (Illumina, Inc.) with the NovaSeq6000 S1 Reagents Kit
v1.5 (300 cycles; cat. no. 20028318). The sequencing input protocol
was as follows: Samples to be uploaded were prepared and their
molar concentrations were calculated, then the libraries were
denatured and samples were detected at a concentration of 250
pM(1.25 nM). The molarity was calculated as follows: (ng/µl
x105)/[660 g/mol x average library size (bp)]=Molarity
(nM).
Next-generation sequencing
analysis
The quality control process Fastp (version 0.23.2;
https://github.com/OpenGene/fastp) was
applied for FASTQ data by removing the terminal adaptor sequences
and low-quality reads from the raw data. Dragen (version 3.10.4;
Illumina, Inc.), a local installation hardware-accelerated
sequencing processing pipeline, was used to perform data alignment
and mutation calling. The lower limit of detection for variant
allele frequency was 0.5%, and then an in-house algorithm was used
to review hotspot variants. The final candidate variants were all
manually verified in the Integrative Genomics Viewer (IGV;
https://www.igv.org/). Copy number alterations
were identified using CNVkit (version 0.9.10; https://cnvkit.readthedocs.io/en/stable/) with default
parameters. The insertion and deletion caller Pindel (version
v0.2.5b8; http://gmt.genome.wustl.edu/pack-ages/pindel/) and
FLT3_ITD_ext (26) (version 1.1;
https://github.com/ht50/FLT3_ITD_ext)
were run on exon 13-15 to identify FMS-like tyrosine kinase 3
internal tandem duplication alleles.
IgH gene monoclonal rearrangement was identified by
polymerase chain reaction-based Genescan analysis of bone marrow.
Chromosome immunofluorescence in situ hybridisation analysis
revealed that the BCL2, BCL6 and MYC genes had
no rearrangement (Fig. S4). A
total of 2 ml of bone marrow were drawn and added to a 15-ml
centrifuge tube. A total of 5 ml of saline was injected and
thoroughly mixed. Then the samples were centrifuged and hypotonic
solution was added.
The samples were prefixed and fixed (methanol:
glacial acetic acid 3:1) three times successively. After each
fixation, the supernatant was centrifuged at 1,800 rpm and
discarded. The fixed liquid was composed of methanol and glacial
acetic acid at 3:1. The sample concentration was adjusted, the
cells dropped on the slide and air dried. Microscopic examination
was conducted to find the marks of well-dispersed cells. Then the
slides were washed with 2X SSC solution, rinsed with deionized
water, dehydrated with gradient ethanol, and then air-dried.
A total of 2 µl probe solution was added to 8x80-mm
cover glass and the edge was sealed with film sealant. In the
hybridizer, denatured at 88˚C for 2 min and hybridized at 45˚C for
>2 h. The sealing glue and cover glass were carefully removed,
and washed in 0.3% NP-40/0.4X SSC solution at 73˚C and 0.1%
NP-40/2X SSC solution at room temperature. A total of 2 µl DAPI was
added to a 12x12-mm cover slide, and the slide with cells was
inverted on the cover slide, stored away from light at 20˚C, and
finally observed under a fluorescence microscope.
Based on the aforementioned findings, the patient
was diagnosed with HPS-associated IVLBCL (Ann Arbor IVB, IPI 3
scores) and PTC. On the second day of treatment with the HLH-1994
and ruxolitinib treatment protocol, the patient's high fever, which
had consistently been present for more than a month, normalised.
Tests for EBV were negative after treatment with acyclovir and one
course of R-CHOPE therapy comprising rituximab, cyclophosphamide,
hydroxydaunorubicin, vincristine, prednisone and etoposide
[rituximab 600 mg, intravenous guttae (ivgtt): day 0 (D0);
etoposide 0.1 g ivgtt: D1-2; cyclophosphamide 1,000 mg ivgtt: D1;
doxorubicin liposomes 40 ms ivgtt: D1; vincristine 2 mg ivgtt: D1;
and prednisone 100 mg p.o: D1-5]. After 4 courses of chemotherapy,
a bone marrow biopsy showed an absence of tumour cells (Fig. 1). Throughout these 4 courses of
treatment, PET/CT images showed complete remission (Fig. 2) and serum titres of cytokines
normalised. The patient refused to consent for treatment of her PTC
by thyroidectomy. At the time of submitting this article, the
patient had undergone 8 courses of chemotherapy and 4 courses of
intrathecal chemotherapy (methotrexate 10 mg) and PET/CT had shown
a complete response (Fig. 2). She
has been taking zanubrutinib 80 mg orally twice daily for 1.5 years
and remains in remission.
Discussion
A literature search revealed 8 previously reported
cases of IVLBCL that involved the thyroid (9-16)
(Table II). These patients
presented with diverse symptoms and had poor prognoses. IVLBCL and
PTC occurred concurrently in only 1 of these patients (10). In that patient, IVLBCL was
initially diagnosed by PET/CT and subsequently confirmed by bone
marrow biopsy. In our patient, fine needle aspiration biopsy of the
thyroid revealed only PTC. PET/CT showed thyroid and lung
hypermetabolism. The decrease in PET/CT metabolism after
chemotherapy suggested that IVLBCL may had invaded the thyroid and
lung. It was nto possible to confirm this because the patient did
not undergo total thyroidectomy or lung biopsy. It has been
reported that, even in the absence of neurological symptoms, ~90%
of patients with IVLBCL have abnormalities on pre-treatment brain
MRI and that hyperintense lesion in the pons on T2WI are
potentially of diagnostic value (17). No brain lesions were definitively
diagnosed because brain biopsy is difficult. In our patient, the
hyperintense lesion revealed on a pre-treatment MRI had completely
resolved by four courses of treatment.
 | Table IIPreviously reported cases of IVLBCL
involved the thyroid. |
Table II
Previously reported cases of IVLBCL
involved the thyroid.
| First author/s,
year | Age | Sex | Diagnosis | Treatment | Outcome | (Refs.) |
|---|
| Stonecypher et
al, 2014 | 68 | Male | IVLBCL + PTC | Total thyroidectomy
+ methotrexate + R-CHOP | In favourable
condition | (10) |
| Linnik et
al, 2018 | 79 | Female | IVLBCL | 3 courses of
R-CHOP | No recurrence after
follow-up | (14) |
| Saleem et
al, 2023 | 70 | Female | IVLBCL | Total
thyroidectomy | Disease-free after
2 years | (11) |
| Luo et al,
2017 | 68 | Male | IVLBCL | Total
thyroidectomy, but declined chemotherapy | Died in the fifth
month after operation | (13) |
| Rea et al,
2020 | 70 | Female | IVLBCL | Total
thyroidectomy | - | (12) |
| Teo et al,
2022 | 65 | Male | IVLBCL + thyroid
mucormycosis | 3 courses of R-CHOP
+ total thyroidectomy | Succumbed to
lymphoma relapse 2 years later | (9) |
| Gaul et al,
2006 | 68 | Male | IVL+ autopsy
revealed tumor in heart and thyroid | - | Died before a
definitive diagnosis | (15) |
| Darko et al,
2006 | 69 | Male | IVLBCL | - | Died because of
heart failure | (16) |
M-protein, usually associated with plasma-cell
dyscrasias, is also often reported in other B-cell malignancies,
and is reportedly always an independent prognostic marker of poor
outcomes (18). However, M-protein
is rarely found in patients with IVLBCL. Findings on immunofixation
electrophoresis in our patient suggested that the differential
diagnosis should have included both multiple myeloma and IVLBCL.
Flow cytometric analyses were negative for plasma cell labelling
and bone marrow biopsy identified CD20- and PAX-5-positive large
cells within vascular spaces, both of which are distinguishing
features.
Studies have shown that patients with IVLBCL with
double expression have lower rates of complete remission and higher
mortality rates than those without double expression (19). It has been found that deficiency of
BCL2, which is an anti-apoptotic proto-oncoprotein, is associated
with thyroid tumour dedifferentiation (20). Expression of BCL2 is reportedly
significantly stronger in patients with PTC than in healthy
individuals (21). Differences in
effects of BCL2 between IVLBCL and PTC need further research.
In individuals with autoimmune diseases, the
incidence of cancers such as non-Hodgkin's lymphoma and thyroid
cancer increase (22).
Xerophthalmia, antinuclear antibody titre of our patient was
1:1,000 and the titres of various cytokines were higher than
normal, prompting consideration of autoimmune diseases, such as
Sjogren's syndrome. Sjogren's syndrome American College of
Rheumatology/European Alliance of Association for Rheumatology
criteria are based on the weighted sum of 5 items: anti-SSA
antibody positivity, focal lymphocytic sialadenitis, an abnormal
ocular staining score ≥5, a Schirmer test ≤5 mm/5 min and an
unstimulated salivary flow rate ≤0.1 ml/min (23), which were not met in our
patient.
HLH, triggered by malignancies, consists of
uncontrolled activated lymphocytes and macrophages that secrete
excessive cytokines (24). The
HLH-2004 protocol still forms the basis of the diagnosis of HLH in
adults and treatment of HLH is primarily based on the HLH-94
protocol. Our patient was considered for HLH secondary to IVLBCL,
met the HLH-2004 and was treated with HLH-94 protocol (8). It has been reported that serum
interleukin-10 (IL-10) concentration has a diagnostic sensitivity
and specificity for IVLBCL of 80 and 100%, respectively, when the
cutoff value is 95.65 pg/ml (25).
Our patient's IL-10 concentration decreased to <30 pg/ml. The
present findings support the role of serum IL-10 as a valuable
biomarker for early diagnosis and monitoring of treatment in
patients with IVLBCL.
The pathological mechanism underlying simultaneous
IVLBCL and PTC is unknown. Second-generation sequencing in our
patient showed a high frequency of CCND1 mutation. The
CCND1 gene encodes the cyclin D1 oncogene product, a major
regulator of G1-S transition in the cell cycle. Aberrant expression
of cyclin D1 protein has been implicated in the pathogenesis of
several types of human neoplasms (26). Pathological examination of a sample
of our patient's marrow revealed aberrant cyclin D1 expression.
DNMT3A encodes an enzyme of DNA methylation.
DNA methylation is involved in both lymphomagenesis and progression
and relapse of lymphomas (27).
Our patient was 63 years old, infected with EBV, and had
DNMT3A mutations. Clonal haematopoiesis, which is associated
with DNMT3A mutation, leads to systemic inflammation
(28). A previous study regarding
the pathological mechanisms underlying PTC found an association
between chronic inflammation and risk of developing PTC (29).
In the present study, an interesting case that
extends our understanding of the simultaneous presence of
HPS-associated IVLBCL and PTC was presented. The mechanisms for
this association are complex, possibly including having a high
frequency of CCND1 mutation, or EBV infection, or Sjögren's
syndrome. It is therefore feasible that a combination of
ruxolitinib and chemotherapy would be an effective treatment for
HPS-associated IVLBCL and an autoimmune disease. In conclusion,
because our experience is limited to one patient, further research
is needed to explore the potential pathogenesis of simultaneous
IVLBCL and PTC.
Supplementary Material
Immunostained sample of bone marrow
showing CyclinD1, Mum1, CD31, CD34 and PAX-5 positive before
treatment (magnification, x200; scale bar, 100 μm).
Flow cytometric analyses of bone
marrow showing positivity for CD20, CD22, CD79b and lambda chains,
and were negative for CD38, CD138 and kappa.
Immunofixation electrophoresis
displayed M proteins positive and IgG kappa light chains.
Chromosome immunofluorescence in situ
hybridisation analysis showing that the BCL2, BCL6 and MYC genes
had no rearrangement.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets generated in the present study may be
found in the National Center for Biotechnology Information database
under accession number PRJNA1205904 or at the following URL:
https://www.ncbi.nlm.nih.gov/sra/PRJNA1205904.
Authors' contributions
JieL and YL initiated and designed the study. JinL,
QW and BL collected data and performed the immunohistochemical
staining and histopathology. JinL, JY and JieL drafted the final
manuscript. YL and JY critically revised of the manuscript for key
intellectual content. All authors read and approved the final
version of the manuscript. JinL and YL confirm the authenticity of
all the raw data.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Consent for the publication of data and associated
images was provided by the patient.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Crilley P: Intravascular large B-cell
lymphoma: An elusive disease. Oncol Times. 40:16–17. 2018.
|
|
2
|
Shimada K and Kiyoi H: Current progress
and future perspectives of research on intravascular large B-cell
lymphoma. Cancer Sci. 112:3953–3961. 2021.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Sukswai N, Lyapichev K, Khoury JD and
Medeiros LJ: Diffuse large B-cell lymphoma variants: An update.
Pathology. 52:53–67. 2020.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Liu Y, Ma Y, Zhou H, Zhou X and Shao J:
Analysis of clinicopathological features and prognostic factors of
non-Hodgkin's intravascular large B-cell lymphoma. Oncol Lett.
20(43)2020.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Shimada K, Yoshida K, Suzuki Y, Iriyama C,
Inoue Y, Sanada M, Kataoka K, Yuge M, Takagi Y, Kusumoto S, et al:
Frequent genetic alterations in immune checkpoint-related genes in
intravascular large B-cell lymphoma. Blood. 137:1491–1502.
2021.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Geer M, Roberts E, Shango M, Till BG,
Smith SD, Abbas H, Hill BT, Kaplan J, Barr PM, Caimi P, et al:
Multicentre retrospective study of intravascular large B-cell
lymphoma treated at academic institutions within the United States.
Br J Haematol. 186:255–262. 2019.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Shimada K, Yamaguchi M, Atsuta Y, Matsue
K, Sato K, Kusumoto S, Nagai H, Takizawa J, Fukuhara N, Nagafuji K,
et al: Rituximab, cyclophosphamide, doxorubicin, vincristine, and
prednisolone combined with high-dose methotrexate plus intrathecal
chemotherapy for newly diagnosed intravascular large B-cell
lymphoma (PRIMEUR-IVL): A multicentre, single-arm, phase 2 trial.
Lancet Oncol. 21:593–602. 2020.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Ponnatt TS, Lilley CM and Mirza KM:
Hemophagocytic lymphohistiocytosis. Arch Pathol Laboratory Med.
146:507–519. 2022.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Teo SCF, Fu EWZ, Bundele MM, Hoe JKM, Ling
LM, Lim MY and Gan JYJ: Intravascular large B-cell lymphoma
associated with sudden stridor arising from thyroid mucormycosis
and concomitant bacterial infection. Ann Acad Med Singap.
51:189–191. 2022.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Stonecypher M, Yan Z, Wasik MA and LiVolsi
V: Intravascular large B cell lymphoma presenting as a thyroid
mass. Endocr Pathol. 25:359–360. 2013.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Saleem K, Nasrazadani A, Kuang C, Jaitly
V, Ho J, Raptis A, Smith R and Seaman C: Intravascular lymphoma-the
creepy crawler: A case series and brief literature review. Cancer
Diagn Progn. 3:31–37. 2022.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Rea B, Peel RL, Han M, Ohori NP and
Aggarwal N: Intravascular large B-cell lymphoma involving
multinodular goiter and mimicking carcinoma. Int J Surg Pathol.
28:517–518. 2019.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Luo B, Chen JM, Liu J, Li WH, Shi YX, Zeng
P, Xie YH and Zhang HF: A case of intravascular large B cell
lymphoma presenting as nodular goiter. Diagn Pathol.
12(64)2017.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Linnik Y, Nicka C, Lansigan F, Loo E and
Liu X: Intravascular Large B-cell lymphoma within a thyroid nodule:
A diagnostic pitfall. Int J Surg Pathol. 26:428–431.
2018.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Gaul C, Hanisch F, Neureiter D, Behrmann
C, Neundörfer B and Winterholler M: Intravascular lymphomatosis
mimicking disseminated encephalomyelitis and encephalomyelopathy.
Clin Neurol Neurosurg. 108:486–489. 2006.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Katalinić D, Valković T, Lucin K and Rudez
J: Intravascular lymphoma and thyroid gland. Coll Antropol.
30:239–241. 2006.PubMed/NCBI
|
|
17
|
Abe Y, Narita K, Kobayashi H, Kitadate A,
Takeuchi M, Kikuchi Y, Ouchi T, Takeuchi K and Matsue K: Clinical
value of abnormal findings on brain magnetic resonance imaging in
patients with intravascular large B-cell lymphoma. Ann Hematol.
97:2345–2352. 2018.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Cox MC, Esposito F, Postorino M, Venditti
A and Di Napoli A: Serum paraprotein is associated with adverse
prognostic factors and outcome, across different subtypes of mature
B-cell malignancies-A systematic review. Cancers (Basel).
15(4440)2023.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Boonsakan P, Iamsumang W,
Chantrathammachart P, Chayavichitsilp P, Suchonwanit P and Rutnin
S: Prognostic value of concurrent expression of C-MYC and BCL2 in
intravascular large B-cell lymphoma: A 10-year retrospective study.
Biomed Res Int. 2020(1350820)2020.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Gupta A, Jain S, Khurana N and Kakar AK:
Expression of p63 and Bcl-2 in malignant thyroid tumors and their
correlation with other diagnostic immunocytochemical markers. J
Clin Diagn Res. 10:EC04–EC08. 2016.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Zhang C, Bo C, Guo L, Yu P, Miao S and Gu
X: BCL2 and hsa-miR-181a-5p are potential biomarkers associated
with papillary thyroid cancer based on bioinformatics analysis.
World J Surg Oncol. 17(221)2019.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Zhou Z, Liu H, Yang Y, Zhou J, Zhao L,
Chen H, Fei Y, Zhang W, Li M, Zhao Y, et al: The five major
autoimmune diseases increase the risk of cancer: Epidemiological
data from a large-scale cohort study in China. Cancer Commun
(Lond). 42:435–446. 2022.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Shiboski CH, Shiboski SC, Seror R,
Criswell LA, Labetoulle M, Lietman TM, Rasmussen A, Scofield H,
Vitali C, Bowman SJ, et al: 2016 American College of
Rheumatology/European League against rheumatism classification
criteria for primary Sjögren's syndrome: A consensus and
data-driven methodology involving three international patient
cohorts. Arthritis Rheumatol. 69:35–45. 2016.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Kaçar AG and Celkan TT: Hemophagocytic
Lymphohistiocytosis. Balkan Med J. 39:309–317. 2022.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Zhang Y, Wang L, Sun J, Wang W, Wei C,
Zhou D and Zhang W: Serum interleukin-10 as a valuable biomarker
for early diagnosis and therapeutic monitoring in intravascular
large B-cell lymphoma. Clin Transl Med. 10(e131)2020.PubMed/NCBI View
Article : Google Scholar
|
|
26
|
Vela-ChÁvez T, Adam P, Kremer M, Bink K,
Bacon CM, Menon G, Ferry JA, Fend F, Jaffe ES and
Quintanilla-Martínez L: Cyclin D1 positive diffuse large B-cell
lymphoma is a post-germinal center-type lymphoma without
alterations in theCCND1gene locus. Leuk Lymphoma. 52:458–466.
2011.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Vanessa S, Caroline B, Alboukadel K, Devin
J, Cartron G, Costes-Martineau V and Moreaux J: An epigenetic
regulator-related score (EpiScore) predicts survival in patients
with diffuse large B cell lymphoma and identifies patients who may
benefit from epigenetic therapy. Oncotarget. 9:19079–19099.
2018.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Venanzi A, Marra A, Schiavoni G, Milner
SG, Limongello R, Santi A, Pettirossi V, Ultimo S, Tasselli L,
Pucciarini A, et al: Dissecting clonal hematopoiesis in tissues of
patients with classic hodgkin lymphoma. Blood Cancer Discov.
2:216–225. 2021.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Pagano L, Mele C, Sama MT, Zavattaro M,
Caputo M, De Marchi L, Paggi S, Prodam F, Aimaretti G and Marzullo
P: Thyroid cancer phenotypes in relation to inflammation and
autoimmunity. Front Biosci (Landmark Ed). 23:2267–2282.
2018.PubMed/NCBI View
Article : Google Scholar
|