1. Introduction
Histone modifications are crucial mechanisms in the
regulation of gene expression (1).
Lysine acylation is a form of post-translational modification (PTM)
in which an acyl group is covalently attached to a lysine residue
of a protein (2). Lysine acylation
is a broad term that encompasses several different types of
modifications that depend on the nature of the acyl group added.
Since the development of highly sensitive mass spectrometry (MS)
technology, various metabolites have been shown to covalently
modify proteins via different forms of lysine acylation, including
lysine acetylation, crotonylation, lactylation, succinylation,
propionylation, butyrylation, malonylation, glutarylation,
2-hydroxyisobutyrylation and β-hydroxybutyrylation (3). Lysine acylations are a versatile and
complex family of PTMs that play essential roles in the regulation
of cellular processes. These modifications are involved in
metabolic regulation, epigenetic regulation, and signal
transduction (4,5). In addition to traditional histone
modifications such as acetylation, methylation, and phosphorylation
(6), a new modification called
crotonylation has recently been discovered (7). Crotonylation is a short-chain fatty
acid modification that was initially identified in yeast and was
later confirmed to occur in human cells. Lysine, an amphiphilic
residue with a hydrophobic side chain, can undergo acylation, which
neutralizes the positive charge of the amino group and potentially
alters protein conformation. Lysine crotonylation (Kcr) refers to
the modification of lysine residues by histone crotonyltransferases
(HCTs). Kcr reportedly plays a role in several physiological and
pathological processes. In histones, crotonylation modifications
have been shown to be closely associated with biological processes
such as gene transcription regulation (7), spermatogenesis (8), acute kidney injury (9), depression (10) and HIV latency (11).
Increasing evidence has shown that histone
crotonylation plays a critical role in tumorigenesis and tumor
progression (12-15).
The present review aims to systematically summarize the research
progress on histone crotonylation, explore its specific roles in
tumors, and discuss its potential therapeutic applications, which
may provide novel insights into cancer pathogenesis and therapeutic
targets.
2. Discovery and mechanism of
crotonylation
MS has become an ideal analytical tool for the
qualitative and quantitative analyses of protein modifications, due
to its unparalleled sensitivity and specificity (16). Currently, four strategies have been
developed for the characterization of PTM sites via (liquid
chromatography) LC-MS/MS: i) Tandem MS using one or more of several
available fragmentation mechanisms; ii) removal of the modification
between consecutive mass spectrometric analyses; iii) selective
enrichment of modified proteins or peptides on the basis of the
modified functional group prior to MS/MS; and iv) PTM-specific
multistage MS strategies (16).
LC-MS/MS has revolutionized the field of proteomics by providing a
powerful platform for the identification and characterization of
PTMs. The ability of MS to provide detailed insights into protein
modifications underpins its critical role in advancing the
understanding of cellular mechanisms and disease pathways. With the
development and improvement of MS detection technology, increasing
numbers of PTMs have been identified. These include acetylation,
crotonylation, butyrylation, propionylation, and succinylation
modifications, among others (Table
I) (7,17-25).
In 2011, Tan et al (7) from
the University of Chicago discovered Kcr modification for the first
time through an integrated MS-based proteomics approach and
confirmed that it represents an evolutionarily conserved PTM of
histone proteins. In 2017, Xu et al (26) at Peking University discovered that
non-histone proteins can also be modified by crotonylation. Some
acetyltransferases and deacetylases have also been shown to exhibit
crotonyltransferase and deprotonylase activities (26). Kcr is closely related to but
distinctly different from acetylation. Kcr occurs primarily on the
ε-amino group of lysine, but its planar orientation and four-carbon
length distinguish it from lysine acetylation (26). The results of quantitative
proteomics also revealed that only 43% of the sites targeted by
crotonylation overlap with those targeted by acetylation, which
suggests differences in the substrate proteins targeted by these
two modifications (27).
 | Table IList of acylation modifications. |
Table I
List of acylation modifications.
| Modification | Abbreviation | Reported year | (Refs.) |
|---|
| Acetylation | Kac | 1962 | (17) |
| Propionylation | Kpr | 2007 | (18) |
| Butyrylation | Kbu | 2007 | (18) |
| Crotonylation | Kcr | 2011 | (7) |
| Succinylation | Ksucc | 2012 | (19) |
| Malonylation | Kma | 2012 | (19) |
| Glutarylation | Kglu | 2014 | (20) |
|
2-Hydroxyisobutyrylation | Khib | 2014 | (21) |
|
β-hydroxybutyrylation | Kbhb | 2016 | (22) |
| Benzoylation | Kbz | 2018 | (23) |
| Lactylation | Kla | 2019 | (24) |
|
Isonicotinylation | Kinic | 2021 | (25) |
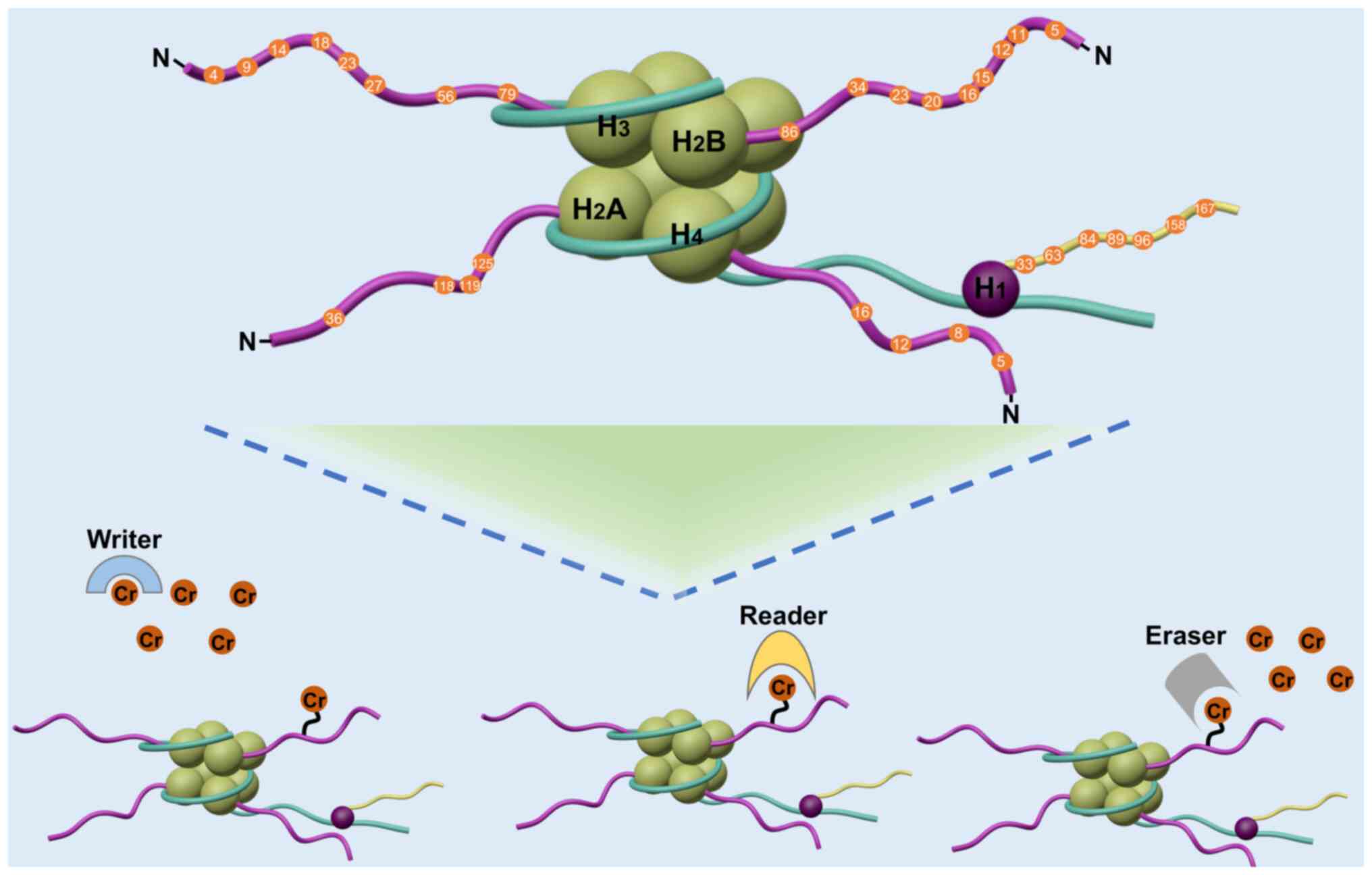
Crotonylation modifications are classified as
reversible acylation modifications and are regulated by a variety
of acylases and deacylases as well as by intracellular crotonyl-CoA
substrate concentrations. The process and sites of histone
crotonylation are shown in Fig. 1.
Some studies have revealed that the process of intracellular
crotonylation is in dynamic equilibrium, which is attributed to the
presence of multiple regulatory proteins, such as
crotonyltransferases, decrotonylases, and crotonylation recognition
proteins (28,29). Research has found that the addition
of exogenous crotonate significantly increased the abundance of
crotonyl-CoA in cells, and the levels of crotonyl modification on
global histones were also significantly upregulated, especially
H3K18, confirming the close relationship between intracellular
crotonyl modification levels and crotonyl-CoA (30). Sabari et al (30) found that H3K18 is the dominant site
of both p300-catalyzed histone crotonylation.
Kcr writers
Crotonyltransferase, also known as a writer protein,
can add crotonyl groups to substrate proteins. Sabari et al
(30) reported that p300, a member
of the lysine acetyltransferase family, can also catalyze Kcr via
the use of crotonyl-CoA as a donor. p300 has been demonstrated to
catalyze histone Kcr, which in turn stimulates transcription to a
greater degree than does histone lysine acetylation (30). Liu et al (31) subsequently focused on the enzymes
that catalyze histone crotonylation and demonstrated that among
known histone acetyltransferases, in addition to CREB-binding
protein (CBP) and p300, males absent on the first (MOF) proteins
possess HCT activity and that this activity has been evolutionarily
conserved (31). In addition, a
previous study revealed that the acetyltransferases, CBP,
p300/CBP-associated factor and human MOF act as
crotonyltransferases for non-histone proteins (26). Kollenstart et al (32) identified the Gcn5- and
Esa1-containing ADA and Piccolo NuA4 complexes as bona fide
crotonyltransferases that promote crotonylation-dependent
transcription in budding yeast (32).
Kcr erasers
Decrotonylases, also known as erasers, can remove
crotonyl groups from proteins. Histone deacetylases (HDACs)
comprise two main families: i) Zinc (Zn)+-dependent HDAC
family members, including class I HDACs (HDAC1-3, HDAC8), which are
localized in the nucleus; class II HDACs (HDAC4-7, HDAC9-10); and
class IV HDACs (HDAC11), which are localized in the nucleus and
cytoplasm; and ii) The NAD+-dependent deacetylase family
including class III HDACs [sirtuin (SIRT)1-7]. HDACs have also been
reported to exhibit histone decrotonylase activity (28). HDAC3 in complex with nuclear
receptor corepressor 1 was shown to exhibit decrotonylase activity
in vitro by systematic screening of the activities of eleven
human Zn-dependent lysine deacylases (33). Bao et al (34) used a chemical proteomics approach
to comprehensively profile ‘eraser’ enzymes that recognize a
lysine-4 crotonylated histone H3 (H3K4Cr) mark and reported that
SIRT1, SIRT2 and SIRT3 can catalyze the hydrolysis of lysine
crotonylated histone peptides and proteins. However, among these
three selective H3K4Cr binders, SIRT3 is likely a selective and
relatively tight binding partner of H3K4Cr (34). Wei et al (35) presented evidence that class I
HDACs, but not SIRT family deacetylases, are the major HDACs
(35). Kelly et al
(36) reported that genetic
deletion of HDAC1/2 in embryonic stem cells increases the overall
levels of histone crotonylation and results in an 85% decrease in
total deprotonase activity and that HDAC1/2 regulates H3K18cr
levels at active gene loci. However, its physiological effects have
not been reported. In addition, it was revealed that the
crotonylation level was increased by HDAC knockdown or by the
addition of the HDAC inhibitor, TSA, which inhibited hepatoma cell
motility and proliferation (12).
Kcr readers
Crotonylation recognition proteins, also known as
readers, can recognize crotonylation sites on proteins. Certain
specific structural domains were found to be involved in the
transcriptional regulation process induced by crotonylation.
Researchers have reported that YEATS, bromodomain, and double PHD
finger (DPF) are important readers of Kcr modifications (37). The YEATS structural domain proteins
constitute the first identified family of reader proteins that
recognize crotonylation modifications. Researchers have shown that
the Taf14 YEATS domain engages crotonyllysine via a unique
πππ-stacking mechanism and that other YEATS domains have
crotonyllysine-binding activity (38). Li et al (39) reported that the AF9 YEATS domain
selectively displays increased binding affinity for crotonyllysine
over acetyllysine. Structural studies revealed an extended aromatic
sandwiching cage with crotonyl specificity that arises from
π-aromatic and hydrophobic interactions between the crotonyl group
and the aromatic rings (27,39).
The YEATS2 protein efficiently reads H3K27cr and is essential for
Kcr-mediated active transcription (40). The bromodomain has been reported to
be involved in acetyllysine modification of histones, and it has
also been shown to be involved in crotonyllysine modification. The
second bromodomain of TATA-box binding protein associated factor 1
(TAF1) can bind crotonyl marks (41). Xiong et al (42) demonstrated that the histone
acetylation-binding DPF domains of human monocytic leukemia zinc
finger protein (MOZ) and D4 zinc finger domain containing protein 2
(DPF2) accommodate a wide range of histone lysine acylations with
the strongest preference for Kcr. Crystal structures of the DPF
domain of MOZ in a complex with H3K14cr, H3K14bu, and H3K14pr
peptides reveal that these nonacetyl acylations are anchored in a
hydrophobic ‘dead-end’ pocket with selectivity for crotonylation
arising from intimate encapsulation and an amide-sensing hydrogen
bonding network (42). A summary
of regulatory factors involved in histone crotonylation is provided
in Table II.
 | Table IIRegulatory factors involved in
histone crotonylation modification. |
Table II
Regulatory factors involved in
histone crotonylation modification.
| Enzyme family | Regulatory
molecules | Crotonylation
site | Reported year | (Refs.) |
|---|
| Writer | p300 | H3K18 | 2015 | (30) |
| | MOF | H3K4, H3K9, H3K18,
H3K23, H4K8 and H4K12 | 2017 | (31) |
| | GCN5 | H3K9, H3K14, H3K18,
H3K23 and H3K27 | 2019 | (32) |
| | Esa1 | H4K5, H4K8, H4K12
and H4K16 | 2019 | (32) |
| Eraser | | | | |
|
Zn2+-dependent HDACs | HDAC1,2,3,8 | H3K4, H3K9, H3K23,
H4K8, H4K12 and H3K23 | 2017 | (35) |
|
NAD+-dependent sirtuins | SIRT1,2,3 | H3K4 | 2014 | (34) |
| Reader | Taf14 | H3K9 | 2016 | (38) |
| | AF9 | H3K9, H3K18 and
H3K27 | 2016 | (39) |
| | MOZ | H3K14 | 2016 | (42) |
3. Crotonylation and malignant tumors
Protein PTM is a regulatory mechanism for activity
modulation, localization, expression, and interactions of proteins
with other cellular molecules (43). The PTMs of histones likely play
pivotal roles in cancer development and progression, as they
influence gene transcription, chromatin remodeling, and the
organization of the nuclear architecture (44). Histone crotonylation modifications
are also closely related to oncogenesis (12,27).
The continuous advancement of various detection methods also
provides strong support for the identification of crotonylation
sites related to tumors (45). For
the first time, Wan et al (28) suggested that the state of Kcr may
be an important type of PTM that explains cancer progression. Using
quantitative proteomics, researchers have found that p300-mediated
Kcr and p300-targeted Kcr substrates are involved in the regulation
of cancer (27). Huang et
al (27) reported that 4.5%
(20 out of 443) of the cancer protein biomarkers in the EDRN
database are crotonylated. In addition, 32 Kcr proteins are related
to cancer genes and account for 5.9% of all genes in the COSMIC
cancer gene database. Notably, six p300 target proteins were
identified as cancer gene-related proteins. It was revealed that
some p300-targeted Kcr substrates are potentially linked to
diseases such as cancer (27). A
series of cancer samples was collected from patients with either
liver, stomach, kidney, thyroid, esophageal, colon, pancreatic, or
lung cancer. Wan et al (12) performed immunohistochemical
staining with a pan anti-Kcr antibody. Kcr was detected in both the
cytoplasm and nucleus, and its expression was downregulated in
liver, stomach, and kidney carcinomas and upregulated in thyroid,
esophageal, colon, pancreatic, and lung carcinomas (12). These findings suggest that Kcr may
play diverse roles during cancer progression through the modulation
of different key cancer-related proteins. In addition, Wan et
al (12) collected 68
hepatocellular carcinoma (HCC) samples and performed
immunohistochemical staining. The staining scores of Kcr expression
levels were dichotomized into two groups, low and high. The
correlations between Kcr expression and clinicopathological
characteristics of HCC were investigated. The results revealed that
Kcr is associated with the tumor, node, metastasis (TNM) stage.
Through Transwell assays and WST-1 assays, it was found that the
cell migration and proliferation abilities of Huh-7 cells decreased
when HDAC1 or HDAC3 were knocked out or HDAC inhibitor TSA was
added. Further in vivo xenograft tumor growth experiments
revealed that the tumor growth rate and tumor weight in the TSA
treatment group were lower than those in the control group. These
findings indicated that crotonylation is also correlated with tumor
progression (12).
Liver cancer
HCC is a common liver malignancy with high lethality
and poor overall patient prognosis (46,47).
Early-stage HCC is typically treated through liver resection and
other forms of surgical intervention. For advanced HCC, treatment
options include chemotherapy, immunotherapy, and oncolytic
virotherapy. With the rise of nanotechnology-based drug delivery
systems, these treatment approaches can be combined with
nanotechnology to increase therapeutic efficacy and reduce side
effects. Additionally, the combination of chemotherapy and
immunotherapy can further improve treatment outcomes and overcome
resistance (47). Gene Ontology
and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway analyses
revealed that in HCC tissues, Kcr proteins are extensively involved
in various cellular processes, including signaling, metabolism,
translation, acylation, and carcinogenesis (48). Zhang et al (49) investigated the correlation between
crotonylation and HCC in 100 tumor tissues. Using amino acid
analysis and LC-MS/MS for stable isotope labeling of HCC cells, it
was reported that crotonylation was positively correlated with HCC
metastasis and that high levels of crotonylation in HCC cells
promoted cellular invasiveness (49). Researchers have revealed that the
level of Kcr is correlated with TNM stage in HCC (12). Additionally, in a study by Zhang
et al (48), Kcr protein
levels were found to be positively correlated with HIF1α in tissue
microarrays derived from a cohort of patients with liver cancer.
These findings suggest that Kcr promotes liver cancer cell
proliferation (48). Lamin A was
previously reported to be an oncogenic protein that enhances the
proliferation of HCC (50). Zhang
et al (48) even
demonstrated that lamin A is a key Kcr protein that regulates the
proliferation of HCC cells and that the crotonylation of lamin A
occurs at K265 and K270. Zhang et al (51) reported that Acyl-CoA oxidase 2
(Acox2) expression levels are significantly lower in human HCC
tissues than in normal liver tissues. In Acox2-knockout C57BL/6n
mice (Acox2-/- mice), Acox2 loss
damaged metabolic homeostasis by downregulating the level of
crotonylation of several metabolic enzymes and peroxidases, which
ultimately induced hepatocarcinogenesis in these mice. In that
study, non-histone Kcr was partially downregulated in the liver
tissues of Acox2-/- mice; however,
histone Kcr was mildly upregulated, which suggests that histone and
non-histone Kcr are differentially regulated in HCC (51). The potential mechanisms of Kcr in
HCC progression remain unclear. Therefore, future research should
focus on understanding how Kcr influences HCC progression and
explore whether this process could provide a theoretical foundation
for innovative treatments for liver cancer.
Glioblastoma (GBM)
GBM is the most common and aggressive primary brain
tumor in adults and has the highest grade according to the World
Health Organization classification of brain tumors (52). Histone Kcr and lysine lactylation
(Kla) are widely present in the brain and undergo significant
changes during neural development. Furthermore, the dynamic
genome-wide changes in H3K9cr and H3K18la are extensively involved
in neural differentiation and cell proliferation, which highlights
how the remodeling of histone acetylation coordinates changes in
gene expression and cell fate transitions (53). Fellows et al (13) reported that proteins weighing ~70
kDa in brain extracts are recognized by antibodies against crotonyl
lysine, which indicates the presence of crotonylated non-histone
proteins in the brain (13). Yuan
et al (54) discovered that
glioblastoma stem cells (GSCs) reprogram lysine catabolism to
propagate and transform into an immunosuppressive state and
reported that reducing histone Kcr via genetic manipulation or
lysine restriction impaired tumor growth. Lysine-restricted diets
are more effective at slowing tumor growth and improving survival
in immunologically active hosts. It has also been revealed that
although Kcr is usually associated with increased gene
transcription, H4 Kcr, rather than the well-characterized H3 Kcr,
is enriched due to reprogrammed lysine catabolism in GSCs (55).
Lung cancer
Lung cancer is the leading cause of cancer-related
deaths worldwide (56). Non-small
cell lung cancer (NSCLC) is the most common type of lung cancer and
accounts for ~85% of all lung cancer cases. Of the NSCLC cases,
lung adenocarcinoma (LUAD) and lung squamous cell carcinoma (LUSC)
are the most prevalent subtypes (57). With continuous advancements in
medicine, early diagnosis and personalized treatment are key to
improving the survival rate of patients with NSCLC. The development
of molecular targeted therapy, immune checkpoint inhibitors, and
anti-angiogenic drugs has significantly improved patient prognosis
(58). After A549 cells (NSCLC)
were treated with suberoylanilide hydroxamic acid, an HDAC family
inhibitor, 10,163 Kcr sites were identified on 2,445 proteins.
Subcellular localization revealed that the sites were located
mainly in proteins in the cytoplasm, nucleus and mitochondria
(59). Proteomic analysis of H1299
lung adenocarcinoma cells revealed 2,696 crotonylation sites on
1,024 proteins (26). Recently,
brain-expressed X-linked gene 2 (BEX2) was found to be localized in
the cytosol and/or mitochondria and to regulate the apoptosis of
cancer cells and tumor growth. Mu et al (60) reported that BEX2 is overexpressed
in lung adenocarcinoma and is associated with poor prognosis in
lymph node metastasis-free patients and clinical stage (I + II)
patients (60). In addition, it
was revealed that crotonylated BEX2 plays an important role in
inhibiting chemotherapeutic agent-induced apoptosis by enhancing
mitophagy in NSCLC cells. Combination treatment with mitophagy
inhibitors and anticancer drugs that target BEX2 represent a
potential strategy for NSCLC treatment (60). The advantage of using drugs to
control protein crotonylation is that crotonylation is a reversible
modification, which means that its levels can be dynamically
regulated through drug intervention. Therefore, flexible
therapeutic effects can be achieved. The disadvantage is that
crotonylation plays a role in various cellular processes and
different tissues, and drug regulation may lead to nonspecific
effects and cause adverse side effects. In the future, drugs that
target specific crotonylation enzymes (such as crotonyltransferases
or decrotonylation enzymes) should be developed to achieve
increased therapeutic specificity and reduce interference with
other biological processes. The effective delivery of drugs to
target cells or tissues also remains a technical challenge.
Colorectal cancer
Crotonylation is abnormally abundant in the
epithelial tissues of the human small intestine, particularly in
the crypts of the small intestine, and in the colon (13). This may be due to the production of
crotonic acid (CA) resulting from the fermentation and degradation
of food by the gut microbiota (13). Fellows et al (13) reported that histone H3K18cr is the
most abundant histone crotonylation mark in the intestine. This
site was characterized through chromatin immunoprecipitation
sequencing (ChIP-seq). The analysis indicated that H3K18cr is
associated with transcription start sites (TSSs). KEGG pathway
analysis of genes with high levels of H3K18cr at their TSS
highlighted several cancer-related pathways, which suggests that
histone crotonylation may be involved in cancer (13). Further research revealed that the
addition of short-chain fatty acids to the culture medium of human
colon cancer cells (HCT116) and mouse small intestine organoids
promotes the crotonylation of H3 and H4 histones (13). DNA damage plays a crucial role in
the development and progression of colon cancer. Researchers have
reported that H3K27cr levels are reduced in the setting of DNA
damage in colon cancer and that changes in these levels may be
mediated by SIRT6(61). The
regulatory mechanism of histone crotonylation in tumors with DNA
damage should be further investigated. Liao et al (62) reported that H3K27cr expression is
upregulated in metastatic colorectal cancer tissues and is
positively correlated with clinical advanced stage disease. In this
study it was reported that LINC00922 interacts with the protein
SIRT3 and hinders its binding to the ETS1 promoter region, which
leads to an increase in the level of H3K27cr in this promoter
region and the subsequent activation of ETS1 transcription
(62). These findings revealed a
novel regulatory function of H3K27cr in colorectal cancer
metastasis and facilitate the discovery of new therapeutic
strategies. The level of crotonylation of H2BK12 (H2BK12cr) was
revealed to be significantly increased in peripheral blood
mononuclear cells (PBMCs) from patients with colorectal cancer and
was strongly associated with distant metastasis and advanced TNM
stage (63). The H2BK12cr level
provides a novel method for the diagnosis of colorectal cancer. Hou
et al (14) reported that
the Kcr of enolase (ENO1) is significantly elevated in human
colorectal cancer tissues compared with that in paraneoplastic
tissues and further identified K420 as the major Kcr site of ENO1;
crotonylation at this site regulated the expression of
tumor-associated genes and promoted the growth, migration, and
invasion of colorectal cancer cells in vitro (14). Notably, researchers have reported
that crotonylation occurs on a serine residue rather than on the
more well-known lysine residue. CA was demonstrated to reduce p53
levels in human cells by inducing Ser46 crotonylation. CA increased
p53-dependent glycolytic activity and promoted the proliferation of
colorectal cancer cells (64).
These findings provide a new perspective on the role of histone
crotonylation in tumors.
Prostate cancer (PCa)
The level of crotonylation modification was revealed
to be greater in PCa tissues than in adjacent tissues, and the
level of modification gradually increased with increasing PCa
malignancy (15). This study also
revealed that BRD4 inhibitors (I-BET762, I-BET726, and CPI-203)
inhibit the migration and invasiveness of PCa cells, whereas
histone crotonylation promotes the migration and invasiveness of
PCa cell lines (15). Following
BRD4 inhibition, the expression level of p300 and the overall
crotonylation level within the cells decreased, which indicates
that BRD4 may influence crotonylation via p300. The expression
level of the HDAC family proteins was not significantly altered,
which suggests that crotonylation in PCa is not regulated by
HDACs.
Cervical cancer
Human papillomavirus (HPV) is the primary etiologic
factor of cervical cancer (65),
which is the leading cause of cancer-related deaths among women
worldwide (66). Han et al
(67) reported increased
expression levels of heterogeneous nuclear ribonucleoprotein A1
(HNRNPA1) in HPV-associated cervical cancer cells, including HeLa,
Caski, and SiHa cells, but especially in HeLa cells (67). In addition, HeLa cell proteomics
revealed that 14,311 sites of 3,734 proteins could be modified by
crotonylation (68). HNRNPA1 is a
p300-regulated Kcr protein (27).
In the study by Han et al (67) it was demonstrated that
p300-mediated Kcr enhances HNRNPA1 expression, which promotes the
proliferation, invasiveness and migration of HeLa cells (67). These findings revealed the
therapeutic potential of controlling crotonylation in cervical
cancer. Several common crotonylation-regulated proteins, such as
SIRT2(69) and SIRT3(70), have been confirmed to play
regulatory roles in cervical cancer. However, the specific
mechanisms through which crotonylation modifications contribute to
the regulation of these proteins remain unknown. Therefore, further
experimental validation is needed to explore the regulatory
mechanisms of crotonylation in cervical cancer.
Head and neck squamous cell carcinoma
(HNSCC)
Most head and neck cancers are derived from the
mucosal epithelium in the oral cavity, pharynx and larynx and are
known collectively as HNSCC (71).
Jiang et al (72) revealed
that the expression of Kcr regulators is associated with the
tumorigenesis and progression of HNSCC. Compared with early T-stage
tumors, lysine acetyltransferase 2B (KAT2B) was downregulated in
advanced T-stage tumors (72).
Additionally, HDAC2 was upregulated in patients with HNSCC with
lymph node metastasis compared with those without lymph node
metastasis (72). Furthermore,
most Kcr regulators, including DPF2, HDAC2, HDAC3, HDAC8, KAT8,
MLLT3, SIRT1, TAF1, and YEATS2, were significantly upregulated in
patients with HNSCC with high histological grades. Notably, in the
aforementioned study (72),
several independent interaction groups were detected among the
‘writers’, ‘readers’, and ‘erasers’ which indicates the existence
of different functional pathways of various regulators. The study
identified and validated a nine-gene signature for HNSCC on the
basis of Kcr regulators (72).
These results may contribute to prognostic stratification and
treatment escalation in patients with HNSCC.
4. Prospects for tumor treatment
Although the mechanism of crotonylation in tumors
requires further research, the known findings still offer hope for
the development of new targeted cancer therapies. Reducing the
level of crotonylation modification by inhibition of HDAC family
proteins has become a concept for the clinical treatment of tumors.
In addition, a series of specific inhibitors of crotonylated reader
proteins have been used in clinical practice. The application of
B029-2, a novel p300 inhibitor, has shown significant antitumor
effects on HCC cells both in vitro and in vivo
(73). Lao et al (74) reported that glutaryl-CoA
dehydrogenase (GCDH) inhibits HCC progression through
crotonylation-induced suppression of the pentose phosphate pathway
and glycolysis, which leads to HCC cell senescence. Senescent cells
further shape the antitumor microenvironment through the
senescence-associated secretory cell phenotype. Due to the increase
in PD-1+CD8+ T cells, the GCDH low-expression
group exhibited a better response to anti-PD-1 therapy compared
with the GCDH high-expression group (74). The YEATS domain is associated with
the progression of various malignancies (75) and serves as a key domain for
recognizing crotonylation modifications. Several studies have
demonstrated the application of inhibitors targeting the YEATS
domain in the treatment of cancers such as lung cancer (76) and leukemia (77), which provides the potential for
further development of crotonylation-related cancer therapies.
5. Conclusion
Since the discovery of crotonylation, numerous
studies have demonstrated its significant role. This process is
involved in the regulation of a wide range of biological processes
and diseases. As detection technologies advance, the impact of
histone crotonylation on tumors will continue to be revealed.
Histone crotonylation in tumors, an emerging epigenetic
modification, is still in its early stages of research. Future
studies should focus on and elucidate the following: i) The
specific mechanisms of crotonylation in gene regulation and its
interactions with other histone modifications; ii) the specificity
and universality of crotonylation markers in different types of
tumors determined using large-scale clinical sample analysis; iii)
the development of efficient crotonylation inhibitors and the
assessment of their efficacy and safety in cancer treatment; and
iv) the combination of MS analysis and gene editing techniques to
promote multidisciplinary research on the function and therapeutic
potential of crotonylation in tumors. Future investigations will
help us better understand the mechanisms of malignant tumor
development and provide a theoretical foundation for the
development of new targeted cancer therapies. As research continues
to expand, histone crotonylation is expected to become an important
field in cancer treatment, which will offer more therapeutic
options and hope for patients.
Acknowledgements
Not applicable.
Funding
Funding: The present study was supported by grants from the
Youth Science Foundation of Shandong First Medical University
(grant no. 202201-066), the China Postdoctoral Science Foundation
(grant no. 2023M741507), and the Shandong Provincial Natural
Science Foundation (grant nos. ZR2017MH091, ZR2020MH228,
ZR2022MH095 and ZR2024QH004).
Availability of data and materials
Not applicable.
Authors' contributions
All authors (XW, YQ, ZL and QX) contributed to the
study conception and design, as well as performed the literature
search and interpretation of the relevant literature. The first
draft of the manuscript was written by XW and YQ and all authors
commented on previous versions of the manuscript. All authors read
and approved the final manuscript. Data authentication is not
applicable.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Zaib S, Rana N and Khan I: Histone
modifications and their role in epigenetics of cancer. Curr Med
Chem. 29:2399–2411. 2022.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Wang M and Lin H: Understanding the
function of mammalian sirtuins and protein lysine acylation. Annu
Rev Biochem. 90:245–285. 2021.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Fu Y, Yu J, Li F and Ge S: Oncometabolites
drive tumorigenesis by enhancing protein acylation: From
chromosomal remodelling to nonhistone modification. J Exp Clin
Cancer Res. 41(144)2022.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Sabari BR, Zhang D, Allis CD and Zhao Y:
Metabolic regulation of gene expression through histone acylations.
Nat Rev Mol Cell Biol. 18:90–101. 2017.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Shvedunova M and Akhtar A: Modulation of
cellular processes by histone and non-histone protein acetylation.
Nat Rev Mol Cell Biol. 23:329–349. 2022.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Zhang Y, Sun Z, Jia J, Du T, Zhang N, Tang
Y, Fang Y and Fang D: Overview of histone modification. Adv Exp Med
Biol. 1283:1–16. 2021.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Tan M, Luo H, Lee S, Jin F, Yang JS,
Montellier E, Buchou T, Cheng Z, Rousseaux S, Rajagopal N, et al:
Identification of 67 histone marks and histone lysine crotonylation
as a new type of histone modification. Cell. 146:1016–1028.
2011.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Goudarzi A, Shiota H, Rousseaux S and
Khochbin S: Genome-scale acetylation-dependent histone eviction
during spermatogenesis. J Mol Biol. 426:3342–3349. 2014.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Berger K and Moeller MJ: Mechanisms of
epithelial repair and regeneration after acute kidney injury. Semin
Nephrol. 34:394–403. 2014.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Liu Y, Li M, Fan M, Song Y, Yu H, Zhi X,
Xiao K, Lai S, Zhang J, Jin X, et al: Chromodomain Y-like
protein-mediated histone crotonylation regulates stress-induced
depressive behaviors. Biol Psychiatry. 85:635–649. 2019.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Jiang G, Nguyen D, Archin NM, Yukl SA,
Méndez-Lagares G, Tang Y, Elsheikh MM, Thompson GR III,
Hartigan-O'Connor DJ, Margolis DM, et al: HIV latency is reversed
by ACSS2-driven histone crotonylation. J Clin Invest.
128:1190–1198. 2018.PubMed/NCBI View
Article : Google Scholar
|
|
12
|
Wan J, Liu H and Ming L: Lysine
crotonylation is involved in hepatocellular carcinoma progression.
Biomed Pharmacother. 111:976–982. 2019.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Fellows R, Denizot J, Stellato C, Cuomo A,
Jain P, Stoyanova E, Balázsi S, Hajnády Z, Liebert A, Kazakevych J,
et al: Microbiota derived short chain fatty acids promote histone
crotonylation in the colon through histone deacetylases. Nat
Commun. 9(105)2018.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Hou JY, Cao J, Gao LJ, Zhang FP, Shen J,
Zhou L, Shi JY, Feng YL, Yan Z, Wang DP and Cao JM: Upregulation of
α enolase (ENO1) crotonylation in colorectal cancer and its
promoting effect on cancer cell metastasis. Biochem Biophys Res
Commun. 578:77–83. 2021.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Xu X, Zhu X, Liu F, Lu W, Wang Y and Yu J:
The effects of histone crotonylation and bromodomain protein 4 on
prostate cancer cell lines. Transl Androl Urol. 10:900–914.
2021.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Johnson H and Eyers CE: Analysis of
post-translational modifications by LC-MS/MS. Methods Mol Biol.
658:93–108. 2010.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Allfrey VG, Faulkner R and Mirsky AE:
Acetylation and methylation of histones and their possible role in
the regulation of RNA synthesis. Proc Natl Acad Sci USA.
51:786–794. 1964.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Chen Y, Sprung R, Tang Y, Ball H, Sangras
B, Kim SC, Falck JR, Peng J, Gu W and Zhao Y: Lysine propionylation
and butyrylation are novel post-translational modifications in
histones. Mol Cell Proteomics. 6:812–819. 2007.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Xie Z, Dai J, Dai L, Tan M, Cheng Z, Wu Y,
Boeke JD and Zhao Y: Lysine succinylation and lysine malonylation
in histones. Mol Cell Proteomics. 11:100–107. 2012.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Tan M, Peng C, Anderson KA, Chhoy P, Xie
Z, Dai L, Park J, Chen Y, Huang H, Zhang Y, et al: Lysine
glutarylation is a protein posttranslational modification regulated
by SIRT5. Cell Metab. 19:605–617. 2014.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Dai L, Peng C, Montellier E, Lu Z, Chen Y,
Ishii H, Debernardi A, Buchou T, Rousseaux S, Jin F, et al: Lysine
2-hydroxyisobutyrylation is a widely distributed active histone
mark. Nat Chem Biol. 10:365–370. 2014.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Xie Z, Zhang D, Chung D, Tang Z, Huang H,
Dai L, Qi S, Li J, Colak G, Chen Y, et al: Metabolic regulation of
gene expression by histone lysine β-hydroxybutyrylation. Mol Cell.
62:194–206. 2016.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Huang H, Zhang D, Wang Y, Perez-Neut M,
Han Z, Zheng YG, Hao Q and Zhao Y: Lysine benzoylation is a histone
mark regulated by SIRT2. Nat Commun. 9(3374)2018.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Zhang D, Tang Z, Huang H, Zhou G, Cui C,
Weng Y, Liu W, Kim S, Lee S, Perez-Neut M, et al: Metabolic
regulation of gene expression by histone lactylation. Nature.
574:575–580. 2019.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Jiang Y, Li Y, Liu C, Zhang L, Lv D, Weng
Y, Cheng Z, Chen X, Zhan J and Zhang H: Isonicotinylation is a
histone mark induced by the anti-tuberculosis first-line drug
isoniazid. Nat Commun. 12(5548)2021.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Xu W, Wan J, Zhan J, Li X, He H, Shi Z and
Zhang H: Global profiling of crotonylation on non-histone proteins.
Cell Res. 27:946–949. 2017.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Huang H, Wang DL and Zhao Y: Quantitative
crotonylome analysis expands the roles of p300 in the regulation of
lysine crotonylation pathway. Proteomics.
18(e1700230)2018.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Wan J, Liu H, Chu J and Zhang H: Functions
and mechanisms of lysine crotonylation. J Cell Mol Med.
23:7163–7169. 2019.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Ntorla A and Burgoyne JR: The regulation
and function of histone crotonylation. Front Cell Dev Biol.
9(624914)2021.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Sabari BR, Tang Z, Huang H, Yong-Gonzalez
V, Molina H, Kong HE, Dai L, Shimada M, Cross JR, Zhao Y, et al:
Intracellular crotonyl-CoA stimulates transcription through
p300-catalyzed histone crotonylation. Mol Cell. 58:203–215.
2015.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Liu X, Wei W, Liu Y, Yang X, Wu J, Zhang
Y, Zhang Q, Shi T, Du JX, Zhao Y, et al: MOF as an evolutionarily
conserved histone crotonyltransferase and transcriptional
activation by histone acetyltransferase-deficient and
crotonyltransferase-competent CBP/p300. Cell Discov.
3(17016)2017.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Kollenstart L, de Groot AJL, Janssen GMC,
Cheng X, Vreeken K, Martino F, Côté J, van Veelen PA and van
Attikum H: Gcn5 and Esa1 function as histone crotonyltransferases
to regulate crotonylation-dependent transcription. J Biol Chem.
294:20122–20134. 2019.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Madsen AS and Olsen CA: Profiling of
substrates for zinc-dependent lysine deacylase enzymes: HDAC3
exhibits decrotonylase activity in vitro. Angew Chem Int Ed Engl.
51:9083–9087. 2012.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Bao X, Wang Y, Li X, Li XM, Liu Z, Yang T,
Wong CF, Zhang J, Hao Q and Li XD: Identification of ‘erasers’ for
lysine crotonylated histone marks using a chemical proteomics
approach. Elife. 3(e02999)2014.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Wei W, Liu X, Chen J, Gao S, Lu L, Zhang
H, Ding G, Wang Z, Chen Z, Shi T, et al: Class I histone
deacetylases are major histone decrotonylases: Evidence for
critical and broad function of histone crotonylation in
transcription. Cell Res. 27:898–915. 2017.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Kelly RDW, Chandru A, Watson PJ, Song Y,
Blades M, Robertson NS, Jamieson AG, Schwabe JWR and Cowley SM:
Histone deacetylase (HDAC) 1 and 2 complexes regulate both histone
acetylation and crotonylation in vivo. Sci Rep.
8(14690)2018.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Zhao S, Zhang X and Li H: Beyond histone
acetylation-writing and erasing histone acylations. Curr Opin
Struct Biol. 53:169–177. 2018.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Andrews FH, Shinsky SA, Shanle EK,
Bridgers JB, Gest A, Tsun IK, Krajewski K, Shi X, Strahl BD and
Kutateladze TG: The Taf14 YEATS domain is a reader of histone
crotonylation. Nat Chem Biol. 12:396–398. 2016.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Li Y, Sabari BR, Panchenko T, Wen H, Zhao
D, Guan H, Wan L, Huang H, Tang Z, Zhao Y, et al: Molecular
Coupling of histone crotonylation and active transcription by AF9
YEATS domain. Mol Cell. 62:181–193. 2016.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Zhao D, Guan H, Zhao S, Mi W, Wen H, Li Y,
Zhao Y, Allis CD, Shi X and Li H: YEATS2 is a selective histone
crotonylation reader. Cell Res. 26:629–632. 2016.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Flynn EM, Huang OW, Poy F, Oppikofer M,
Bellon SF, Tang Y and Cochran AG: A subset of human bromodomains
recognizes butyryllysine and crotonyllysine histone peptide
modifications. Structure. 23:1801–1814. 2015.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Xiong X, Panchenko T, Yang S, Zhao S, Yan
P, Zhang W, Xie W, Li Y, Zhao Y, Allis CD and Li H: Selective
recognition of histone crotonylation by double PHD fingers of MOZ
and DPF2. Nat Chem Biol. 12:1111–1118. 2016.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Li Y, Zhang R and Hei H: Advances in
post-translational modifications of proteins and cancer
immunotherapy. Front Immunol. 14(1229397)2023.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Li Y and Seto E: HDACs and HDAC inhibitors
in cancer development and therapy. Cold Spring Harb Perspect Med.
6(a026831)2016.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Chen YZ, Wang ZZ, Wang Y, Ying G, Chen Z
and Song J: nhKcr: A new bioinformatics tool for predicting
crotonylation sites on human nonhistone proteins based on deep
learning. Brief Bioinform. 22(bbab146)2021.PubMed/NCBI View Article : Google Scholar
|
|
46
|
European Association for the Study of the
Liver. EASL clinical practice guidelines: Management of
hepatocellular carcinoma. J Hepatol. 69:182–236. 2018.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Alawyia B and Constantinou C:
Hepatocellular carcinoma: A narrative review on current knowledge
and future prospects. Curr Treat Options Oncol. 24:711–724.
2023.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Zhang D, Tang J, Xu Y, Huang X, Wang Y,
Jin X, Wu G and Liu P: Global crotonylome reveals hypoxia-mediated
lamin A crotonylation regulated by HDAC6 in liver cancer. Cell
Death Dis. 13(717)2022.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Zhang XY, Liu ZX, Zhang YF, Xu LX, Chen
MK, Zhou YF, Yu J, Li XX and Zhang N: SEPT2 crotonylation promotes
metastasis and recurrence in hepatocellular carcinoma and is
associated with poor survival. Cell Biosci. 13(63)2023.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Liu H, Li D, Zhou L, Kan S, He G, Zhou K,
Wang L, Chen M and Shu W: LMNA functions as an oncogene in
hepatocellular carcinoma by regulating the proliferation and
migration ability. J Cell Mol Med. 24:12008–12019. 2020.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Zhang Y, Chen Y, Zhang Z, Tao X, Xu S,
Zhang X, Zurashvili T, Lu Z, Bayascas JR, Jin L, et al: Acox2 is a
regulator of lysine crotonylation that mediates hepatic metabolic
homeostasis in mice. Cell Death Dis. 13(279)2022.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Wirsching HG, Galanis E and Weller M:
Glioblastoma. Handb Clin Neurol. 134:381–397. 2016.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Dai SK, Liu PP, Li X, Jiao LF, Teng ZQ and
Liu CM: Dynamic profiling and functional interpretation of histone
lysine crotonylation and lactylation during neural development.
Development. 149(dev200049)2022.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Yuan H, Wu X, Wu Q, Chatoff A, Megill E,
Gao J, Huang T, Duan T, Yang K, Jin C, et al: Lysine catabolism
reprograms tumour immunity through histone crotonylation. Nature.
617:818–826. 2023.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Dai SK, Liu PP, Du HZ, Liu X, Xu YJ, Liu
C, Wang YY, Teng ZQ and Liu CM: Histone crotonylation regulates
neural stem cell fate decisions by activating bivalent promoters.
EMBO Rep. 22(e52023)2021.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Molina JR, Yang P, Cassivi SD, Schild SE
and Adjei AA: Non-small cell lung cancer: Epidemiology, risk
factors, treatment, and survivorship. Mayo Clin Proc. 83:584–594.
2008.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Alexander M, Kim SY and Cheng H: Update
2020: Management of non-small cell lung cancer. Lung. 198:897–907.
2020.PubMed/NCBI View Article : Google Scholar
|
|
59
|
Wu Q, Li W, Wang C, Fan P, Cao L, Wu Z and
Wang F: Ultradeep lysine crotonylome reveals the crotonylation
enhancement on both histones and nonhistone proteins by SAHA
treatment. J Proteome Res. 16:3664–3671. 2017.PubMed/NCBI View Article : Google Scholar
|
|
60
|
Mu N, Wang Y, Li X, Du Z, Wu Y, Su M, Wang
Y, Sun X, Su L and Liu X: Crotonylated BEX2 interacts with NDP52
and enhances mitophagy to modulate chemotherapeutic agent-induced
apoptosis in non-small-cell lung cancer cells. Cell Death Dis.
14(645)2023.PubMed/NCBI View Article : Google Scholar
|
|
61
|
Liao M, Chu W, Sun X, Zheng W, Gao S, Li D
and Pei D: Reduction of H3K27cr modification during DNA damage in
colon cancer. Front Oncol. 12(924061)2022.PubMed/NCBI View Article : Google Scholar
|
|
62
|
Liao M, Sun X, Zheng W, Wu M, Wang Y, Yao
J, Ma Y, Gao S and Pei D: LINC00922 decoys SIRT3 to facilitate the
metastasis of colorectal cancer through up-regulation the H3K27
crotonylation of ETS1 promoter. Mol Cancer. 22(163)2023.PubMed/NCBI View Article : Google Scholar
|
|
63
|
Hou JY, Li N, Wang J, Gao LJ, Chang JS and
Cao JM: Histone crotonylation of peripheral blood mononuclear cells
is a potential biomarker for diagnosis of colorectal cancer.
Epigenetics Chromatin. 16(35)2023.PubMed/NCBI View Article : Google Scholar
|
|
64
|
Liao P, Bhattarai N, Cao B, Zhou X, Jung
JH, Damera K, Fuselier TT, Thareja S, Wimley WC, Wang B, et al:
Crotonylation at serine 46 impairs p53 activity. Biochem Biophys
Res Commun. 524:730–735. 2020.PubMed/NCBI View Article : Google Scholar
|
|
65
|
Kusakabe M, Taguchi A, Sone K, Mori M and
Osuga Y: Carcinogenesis and management of human
papillomavirus-associated cervical cancer. Int J Clin Oncol.
28:965–974. 2023.PubMed/NCBI View Article : Google Scholar
|
|
66
|
Rahangdale L, Mungo C, O'Connor S,
Chibwesha CJ and Brewer NT: Human papillomavirus vaccination and
cervical cancer risk. BMJ. 379(e070115)2022.PubMed/NCBI View Article : Google Scholar
|
|
67
|
Han X, Xiang X, Yang H, Zhang H, Liang S,
Wei J and Yu J: p300-catalyzed lysine crotonylation promotes the
proliferation, invasion, and migration of HeLa cells via
heterogeneous nuclear ribonucleoprotein A1. Anal Cell Pathol
(Amst). 2020(5632342)2020.PubMed/NCBI View Article : Google Scholar
|
|
68
|
Yu H, Bu C, Liu Y, Gong T, Liu X, Liu S,
Peng X, Zhang W, Peng Y, Yang J, et al: Global crotonylome reveals
CDYL-regulated RPA1 crotonylation in homologous
recombination-mediated DNA repair. Sci Adv.
6(eaay4697)2020.PubMed/NCBI View Article : Google Scholar
|
|
69
|
Kuhlmann N, Chollet C, Baldus L, Neundorf
I and Lammers M: Development of substrate-derived sirtuin
inhibitors with potential anticancer activity. ChemMedChem.
12:1703–1714. 2017.PubMed/NCBI View Article : Google Scholar
|
|
70
|
Xu LX, Hao LJ, Ma JQ, Liu JK and Hasim A:
SIRT3 promotes the invasion and metastasis of cervical cancer cells
by regulating fatty acid synthase. Mol Cell Biochem. 464:11–20.
2020.PubMed/NCBI View Article : Google Scholar
|
|
71
|
Johnson DE, Burtness B, Leemans CR, Lui
VWY, Bauman JE and Grandis JR: Head and neck squamous cell
carcinoma. Nat Rev Dis Primers. 6(92)2020.PubMed/NCBI View Article : Google Scholar
|
|
72
|
Jiang L, Yin X, Zhang H, Zhang X, Cao Z,
Zhou M and Xu W: Development and validation of a prognostic
signature based on the lysine crotonylation regulators in head and
neck squamous cell carcinoma. Biomed Res Int.
2023(4444869)2023.PubMed/NCBI View Article : Google Scholar
|
|
73
|
Cai LY, Chen SJ, Xiao SH, Sun QJ, Ding CH,
Zheng BN, Zhu XY, Liu SQ, Yang F, Yang YX, et al: Targeting
p300/CBP attenuates hepatocellular carcinoma progression through
epigenetic regulation of metabolism. Cancer Res. 81:860–872.
2021.PubMed/NCBI View Article : Google Scholar
|
|
74
|
Lao Y, Cui X, Xu Z, Yan H, Zhang Z, Zhang
Z, Geng L, Li B, Lu Y, Guan Q, et al: Glutaryl-CoA dehydrogenase
suppresses tumor progression and shapes an anti-tumor
microenvironment in hepatocellular carcinoma. J Hepatol.
81:847–861. 2024.PubMed/NCBI View Article : Google Scholar
|
|
75
|
Zeng Z, Lei S, He Z, Chen T and Jiang J:
YEATS2 is a target of HIF1α and promotes pancreatic cancer cell
proliferation and migration. J Cell Physiol. 236:2087–2098.
2021.PubMed/NCBI View Article : Google Scholar
|
|
76
|
Listunov D, Linhares BM, Kim E, Winkler A,
Simes ML, Weaver S, Cho HJ, Rizo A, Zolov S, Keshamouni VG, et al:
Development of potent dimeric inhibitors of GAS41 YEATS domain.
Cell Chem Biol. 28:1716–1727.e6. 2021.PubMed/NCBI View Article : Google Scholar
|
|
77
|
Ma XR, Xu L, Xu S, Klein BJ, Wang H, Das
S, Li K, Yang KS, Sohail S, Chapman A, et al: Discovery of
selective small-molecule inhibitors for the ENL YEATS domain. J Med
Chem. 64:10997–11013. 2021.PubMed/NCBI View Article : Google Scholar
|