1. Introduction
Diagnosis and treatment of advanced solid tumors,
among which lung and breast cancers feature the most rapid
development, have been greatly improved owing to the advances of
modern oncology. Over the past two decades, the discovery of
treatable driver mutations and the development of immune checkpoint
inhibitors (ICIs) have led to various breakthroughs in treating
non-small-cell lung cancer (NSCLC). One such breakthrough is the
development of targeted therapies based on treatable driver
mutations, while a second one is the employment of immunotherapies
based on ICIs. Unlike anti-angiogenic therapeutic drugs that have
their unique mechanisms of action and are widely used in clinical
practice, vascular-targeted drugs still cannot be used as first- or
second-line treatments alone. Vascular-targeted drugs are used in
combination with other treatments for added benefit, thereby they
are associated with a modest breakthrough. The next breakthrough in
advanced lung cancer treatment could be the adoption of precise and
multidisciplinary combination therapies.
Through a rigorous investigation and summary of
current NSCLC treatments, the aim of the present study is to
provide clinicians with a comprehensive reference on current and
upcoming treatment options, focusing on some key mutations and
promising emerging treatments, as well as providing an outlook on
the future direction of lung cancer treatment.
2. Comprehensive management of NSCLC
Disease staging and incidence frequency of lung
cancer are critical research topics, particularly because the
current trends indicate a shift in demographics and disease
management. Lung cancer staging is based on the TNM staging system,
which classifies tumors according to their size (T), lymph node
involvement (N) and distant metastasis (M). The newly published
ninth edition of the TNM staging system for lung cancer is an
important guide for the diagnosis, treatment and prognostic
assessment of lung cancer (1). The
major changes to the TNM staging system include further subdivision
of stage N2 into N2a and N2b, and further subdivision of stage M1c
into M1c1 and M1c2. These changes are intended to more accurately
predict patient prognosis and guide treatment decisions. Current
statistics indicate that stage I lung cancer (early stage, tumor is
small and has not spread) accounts for ~48.6%, stage II (locally
progressive) accounts for 12.2%, stage III (regionally advanced,
extended to adjacent lymph nodes) accounts for 11.2%, while stage
IV (advanced or has developed distant metastasis) accounts for 28%
(2). Due to the lack of early
symptoms of lung cancer, several patients at the time of their
diagnosis are already in the advanced stage, resulting in a lost
opportunity for surgery and poor overall prognosis (3).
Patients with lung cancer can have activating
mutations that may drive tumor growth. These mutations can be
identified through a range of molecular diagnostic techniques.
These methods include traditional tissue biopsy-based approaches
such as Sanger sequencing, fluorescence in situ
hybridization, reverse-transcription quantitative PCR,
immunohistochemistry, as well as more comprehensive next-generation
sequencing (4). Additionally,
liquid biopsy techniques, which analyze circulating tumor DNA or
circulating tumor cells in the blood, provide a minimally invasive
and repeatable option for patients unable to undergo tissue biopsy
(5,6). Among these methods, PCR-based
technologies such as the amplification refractory mutation system
and digital PCR are frequently used to detect specific gene
mutations due to their high sensitivity and specificity. Upon
advances in technology, emerging methods such as exosome-based
detection are also offering new perspectives for genetic testing in
lung cancer. Physicians select the most appropriate testing method
based on the specific condition of the patient, sample
availability, testing sensitivity and specificity requirements, as
well as economic considerations, to guide treatment strategies
effectively (7).
In addition, with the continuous advancements in
lung cancer treatment options, interdisciplinary collaboration has
become increasingly important. Advanced lung cancer involves
numerous complexities and patients often exhibit varying responses
to different treatment options. By assembling a multidisciplinary
team, including oncologists, radiologists, surgeons, pathologists
and other healthcare professionals, not only can the accuracy of
diagnosis be improved, but a more comprehensive perspective can be
offered in the development of treatment strategies. This approach
addresses the individual needs of patients more thoroughly;
therefore, establishing an efficient multidisciplinary team is
crucial for improving the survival rates and quality of life for
patients with lung cancer (8,9).
3. Existing treatment paradigm for
NSCLC
Over the past two decades, breakthroughs in the
treatment of NSCLC have included the widespread adoption of
targeted therapy, immunotherapy, anti-vascular therapy and
personalized treatment strategies, significantly improving patient
survival and quality of life (Fig.
1). Among all the treatments, chemotherapy is the most toxic
and even paclitaxel, which has significantly improved tumor
treatment, can cause adverse reactions such as allergic reactions
(10). The advent of albumin-bound
paclitaxel and paclitaxel polymer micelles are advancements in the
field of chemotherapy; however, they are also associated with
adverse effects such as alopecia, peripheral nerve toxicity and
impaired cardiac function (11).
Subsequent clinical selection of chemotherapeutic agents is based
on case types for individualized treatment.
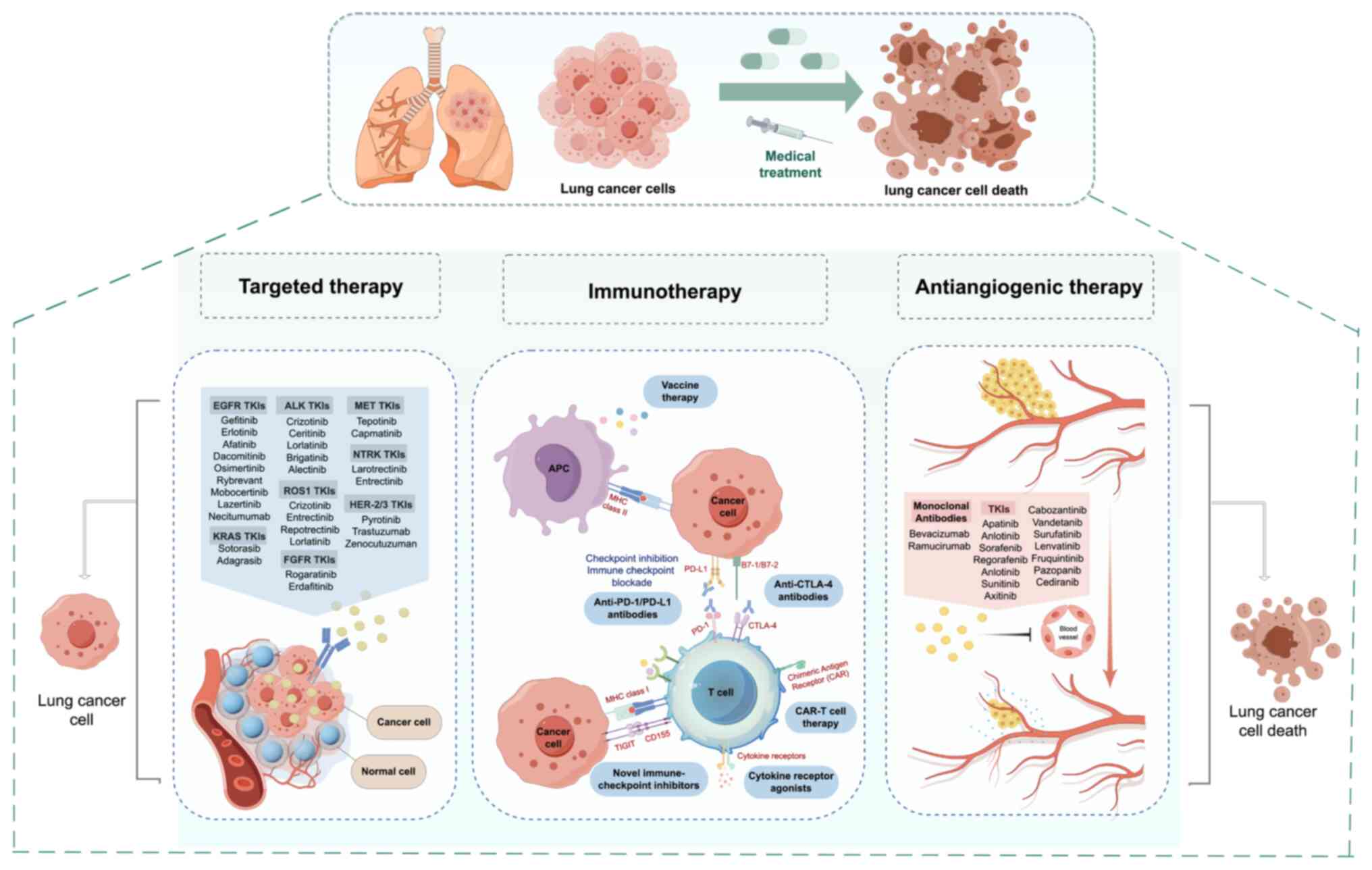 | Figure 1Breakthroughs in the treatment of
non-small cell lung cancer in the past two decades. TKI, tyrosine
kinase inhibitor; EGFR, epidermal growth factor receptor; ALK,
anaplastic lymphoma kinase; MET, mesenchymal-epithelial transition
factor; KRAS, kirsten rat sarcoma viral oncogene homolog; ROS1, ROS
proto-oncogene 1, receptor tyrosine kinase; NTRK, neurotrophic
tyrosine receptor kinase; HER-2/3, human epidermal growth factor
receptor 2/3; FGFR, fibroblast growth factor receptor; APC,
antigen-presenting cell; MHC, major histocompatibility complex;
PD-1/PD-L1, programmed cell death protein 1/programmed death-ligand
1; CTLA-4, cytotoxic T-lymphocyte-associated protein 4; CAR-T,
chimeric antigen receptor T-cell; TIGIT, T-cell immune receptor
with Ig and ITIM domains. |
Since 2004, lung cancer treatment has entered the
era of targeted therapy. Targeted therapy is a precise treatment
that focuses on specific targets, allowing patients with treatable
target mutations to achieve high efficacy and relatively low
toxicity. Gefitinib is the first tyrosine kinase inhibitor (TKI)
approved for use in advanced NSCLC, marking the beginning of the
era of targeted therapy (12). At
that time, clinicians discovered that higher efficacy could be
achieved in specific populations by administering oral doses of a
targeted drug with much lower toxicity than traditional
chemotherapy. This efficient and less toxic treatment option
quickly replaced chemotherapy. Since then, nearly 10 pathways,
including anaplastic lymphoma kinase (ALK) fusion, ROS
proto-oncogene 1, receptor tyrosine kinase (ROS1) fusion and
rearranged during transfection (RET) fusion, have been discovered
and new drugs have been developed, bringing survival benefits to
several patients (13).
A decade ago, for patients with negative-driving
genes, long-term survival was in stark contrast with those with
positive mutations due to a lack of effective treatment options.
Focusing on the tumor itself, the development of kinase inhibitors
has reached a bottleneck, and even neurotrophic tyrosine receptor
kinase (NTRK) fusion with a mutation rate of only ~3% has been
widely studied (14). Finding
effective therapeutic targets, such as EGFR, that benefit a large
proportion of the population is challenging. However, ICIs focusing
on the tumor microenvironment (TME) have significantly changed the
treatment landscape for patients with driver-negative genes
(15). In 2013, Nivolumab, as the
first ICI, was approved for marketing. Since then, immunotherapy
has experienced a development path from backline to first-line,
from advanced to locally advanced to early, and from single agent
to combination.
Bevacizumab is the first drug developed based on the
concept of antiangiogenesis. The ECOG-4599 study and BEYOND study
have successfully established bevacizumab combined with
chemotherapy as a first-line treatment (16,17).
In addition to bevacizumab, anlotinib is another antiangiogenic
drug widely used in clinical settings. The mechanism of action of
anlotinib is dissimilar in some respects to bevacizumab and it is
the only antiangiogenic drug that is effective as a single drug
treatment at present, although it is a late-line drug (18). In clinical practice, antiangiogenic
drugs need to be used in combination with other treatments in most
cases and have the disadvantages of relatively high toxicity and
lack of established biomarkers. Considering these factors,
antiangiogenic targeted therapy represents an innovation, although
it is not as significant as targeted therapy and immunotherapy.
In addition to considering the disease stage,
pathologic type, driver gene mutation status and expression of
immunotherapy predictors of the patient, treatment selection for
NSCLC must also consider patient frailty and comorbidities. Frail
patients and those with significant comorbidities, such as
cardiovascular disease or diabetes, often have limited tolerance
for standard therapies, necessitating a more tailored approach.
Tools such as ECOG performance status and the Charlson Comorbidity
Index can guide clinicians in optimizing treatment regimens, which
may include dose reduction, alternative drug choices or supportive
care strategies. For instance, in clinical practice, for elderly
patients (aged >70 years), with comorbidities, such as severe
hepatic or renal dysfunction, poor performance status (PS score
≥2), lack of sensitive driver gene mutations and with programmed
death-ligand 1 (PD-L1) expression <50%, single-agent
chemotherapy (administered intravenously or orally) is often used
as a first-line treatment instead of standard platinum-based
doublet chemotherapy with or without immunotherapy (19). Such personalized approaches are
critical in improving outcomes while minimizing treatment-related
adverse effects.
4. Progress and prospects of treatment for
NSCLC
Targeted therapy
In previous years, the field of targeted therapy for
lung cancer has witnessed significant advancements. Various
targets, such as EGFR, mesenchymal-epithelial transition factor
(MET), v-raf murine sarcoma viral oncogene homolog B1 (BRAF), the
proto-oncogene RET, Kirsten rat sarcoma viral oncogene homolog G12C
(KRAS G12C) and HER2, have been identified and corresponding drugs
are continuously being developed or improved. In addition to
well-known mutations such as EGFR, ALK and ROS1 fusions,
researchers are paying increasing attention to rare mutations and
the development of drugs targeting these mutations (12,20,21).
For patients with typical EGFR mutations,
osimertinib remains the standard first-line therapy; however,
combination therapies such as osimertinib with chemotherapy or
amivantamab (a bispecific antibody targeting EGFR and c-MET) plus
lazertinib (a third-generation EGFR TKI), have exhibited improved
progression-free survival (PFS), although with increased toxicity
(22). For EGFR exon 20 insertion
mutations, amivantamab plus chemotherapy is a first-line option
(23). Sunvozertinib, a novel EGFR
TKI, was approved in China and shows promise for exon 20
insertions, Thr790Met and uncommon mutations (24). For patients with ALK
rearrangements, the third-generation ALK-TKI, lorlatinib, has
exhibited exceptional efficacy in treatment-naïve patients and
demonstrates strong central nervous system (CNS) activity. In
recent years, both crizotinib, repotrectinib and entrectinib were
proven to be effective in treating ROS1 fusion-positive NSCLC
(25-27).
The objective response rate (ORR) of entrectinib in patients with
ROS1 fusion-positive reached 67.1%, with a CNS response rate of
79.2% (26). For patients with RET
fusion-positive NSCLC, selpercatinib and pralsetinib demonstrated
improved prognosis (28,29); however, it is crucial to monitor
for pulmonary infections while using RET inhibitors.
In lung cancer, the BRAF and HER2 genes are involved
in regulating the cell cycle. Mutations in the BRAF gene,
particularly the BRAF V600E mutation, have been extensively studied
and are known to be closely associated with the initiation and
progression of various cancers. The BRAF protein is a key signaling
molecule regulating cell proliferation and survival. Its activation
leads to the activation of the downstream MAPK signaling pathway,
promoting cell cycle progression and enhancing cellular
proliferation capacity (30). BRAF
mutations have become a research hotspot in the past 2 years,
especially after the approval of combination targeted therapies.
Using dabrafenib in combination with trametinib has become a new
paradigm in treating BRAF V600E-mutated NSCLC (26). In addition, the combination of
encorafenib with binimetinib is also a viable option for patients
with advanced NSCLC carrying the BRAF V600E mutation (31). For patients carrying MET Exon 14
skipping mutations, capmatinib, tepotinib and savolitinib were
demonstrated to be effective therapeutic options (32).
The role of the HER2 gene is crucial in lung cancer.
HER2 is a receptor tyrosine kinase and its overexpression or
mutation can lead to aberrant increases in proliferative signaling.
Upon activation, HER2 not only promotes cell cycle progression but
also interacts with the BRAF signaling pathway, which further aids
tumor development and metastasis (33). In the case of HER2-mutated NSCLC,
both trastuzumab and pyrotinib have demonstrated effective
treatment performance (34).
Additionally, other drugs such as the antibody-drug conjugates
(ADCs), DS8201 and RC48, have shown promising results (34,35).
For patients harboring KRAS G12C mutations, results
from the CodeBreaK 100 study revealed that previously treated
patients with NSCLC and with the KRAS G12C mutation had an ORR of
40.7% with a median PFS (mPFS) and a median overall survival (mOS)
of 6.3 and 12.5 months, respectively, following treatment with
sotorasib (36). On this basis,
CodeBreak 200 second-line comparison of the difference in efficacy
between sotorasib and docetaxel in patients with KRAS G12C
mutations revealed an mPFS of 5.6 vs. 4.5 months, respectively.
Concurrently, OS did not reach a statistical difference. Although
the CodeBreaK 200 trial met its primary endpoint, the mPFS benefit
in the sotorasib arm was 5 weeks, less than the imaging follow-up
interval. Thus, the Food and Drug Administration panel ultimately
concluded that the primary study endpoint of CodeBreaK 200 could
not be reliably interpreted given the inherent error in assessing
PFS during the imaging follow-up interval (37). The efficacy of another small
molecule KRAS G12C inhibitor, adagrasib, was evaluated in the
KRYSTAL-1 study as a second-line treatment for KRAS G12C-mutated
NSCLC, with an ORR of 42.9%, mPFS of 6.5 months and mOS of 12.6
months. The same study revealed some efficacy of adagrasib against
intracranial lesions (38). At
present, sotorasib and adagrasib are approved as second-line
treatment. Ongoing studies are exploring their efficacy as
first-line combinations with chemotherapy and immunotherapy. KRAS
G12C is currently a hot research topic and, in addition to
sotorasib and adagrasib, new drugs such as JDQ443, D-1553 and
JAB-21822 are in early clinical trial stages and breakthroughs in
their efficacy are anticipated (39,40).
NTRK fusion is a driver mutation in various tumors
and can be detected in multiple types of cancer, including NSCLC
with an incidence rate of 0.1-3.3% (41). NTRK inhibitors, larotrectinib and
entrectinib, exhibited favorable systemic and intracranial efficacy
(42,43). MET exon 14 skipping mutations, an
important driver gene in NSCLC, have an incidence rate of 3-4%.
Several MET-TKIs, such as savolitinib, are approved treatments for
various cancers, including NSCLC, with MET exon 14 skipping
mutations (44). In addition to
the aforementioned rare targets in NSCLC, research on other targets
such as neuregulin 1 (NRG1), cytoplasmic linker protein 1-leukocyte
receptor tyrosine kinase fusion and KRAS G12D is also progressing
rapidly (45-47).
These targets may also result in breakthroughs and contribute to
advancing precision treatment for NSCLC. Current and clinically
available targeted agents for NSCLC and other investigational
agents are summarized in Table
I.
 | Table ISummary of drugs currently available
in the clinic and under investigation for treatable driver gene
targets in non-small cell lung cancer. |
Table I
Summary of drugs currently available
in the clinic and under investigation for treatable driver gene
targets in non-small cell lung cancer.
| Gene | Target point | Available
drugs | Clinical trial
drugs | Clinical trial
numbers |
|---|
| EGFR | Exon 19
deletion | Erlotinib (first
generation) | HSK40118 | NCT06050980 |
| | Exon 21 L858R
mutation | Gefitinib (first
generation) | JNJ6372 | NCT02609776 |
| | Exon 20 T790M
mutation | Icotinib (first
generation) | DAJH-1050766 | CTR20221031 |
| | | Afatinib (second
generation) | U31402 | NCT04965766 |
| | | Dacomitinib (second
generation) | | |
| | | Osimertinib (third
generation) | | |
| | | Furmonertinib
(third generation) | | |
| | | Almonertinib (third
generation) | | |
| | | Befotertinib (third
generation) | | |
| | Exon 20 insertion
mutation | Mobocertinib
(TAK788) | Furmonertinib | NCT05607550 |
| | | Amivantamab
(JNJ6372) | PLB1004 | NCT06015503 |
| | | Sunvozertinib
(DZD9008) | YK029A | NCT05767892 |
| | | | FWD1509 | NCT05068024 |
| | Exon 18 G719X point
mutation | Afatinib (second
generation) | Mefatinib | CTR2000029058 |
| | Exon 21 L861Q point
mutation | Osimertinib (third
generation) | HTMC0503 | CTR20212743 |
| | Exon 20 S768I point
mutation | | Sutetinib | NCT05168566 |
| ALK | EML4-ALK | Crizotinib (first
generation) | TGRX-326 | NCT06082635 |
| | | Alectinib (second
generation) | CT3505 | NCT05257512 |
| | | Ceritinib (second
generation) | TL139 | CTR20202551 |
| | | Brigatinib (second
generation) | | |
| | | Ensartinib (second
generation) | | |
| | | Iruplinalkib
(second generation) | | |
| | | Lorlatinib (third
generation) | | |
| ROS1 | CD74-ROS1 | Crizotinib | TPX-0005 | NCT03093116 |
| | | Entrectinib | AB-106 | NCT04395677 |
| BRAF | V600E | Dabrafenib +
Trametinib | ABM-1310 | NCT05501912 |
| | | Encorafnib +
Binimetinib | KIN2787 | NCT04913285 |
| MET | METex14 | Tepotinib | Glesatinib | NCT02954991 |
| | | Capmatinib | APL-101 | NCT03175224 |
| | | Savolitinib | Cabozantinib | NCT03911193 |
| | | Glumetinib | | |
| | | Bozitinib | | |
| RET | Rearrangement | Selpercatinib | SY-5007 | NCT06031558 |
| | | Pralsetinib | HS-10365 | NCT06147570 |
| | | | APS03118 | CTR20222441 |
| KRAS | G12C | Sotorasib | D1553 | NCT06300177 |
| | | Adagrasib | Divarasib | NCT06497556 |
| | | | GH35 | CTR20222296 |
| | | | HJ891 | CTR20212195 |
| | | | Olomorasib | NCT06119581 |
| HER2 | HER2
amplification | DS8201 | PLB1004 | NCT0601550 |
| | Exon 20
insertions | | FS-1502 | NCT03944499 |
| NTRK | 1-3 | Larotrectinib | TL118 | CTR20191622 |
| | | Entrectinib | HG030 | CTR20202020 |
| | | | VC004 | NCT06658353 |
In the treatment of lung cancer, drug resistance is
a major obstacle leading to therapeutic failure and tumor
recurrence, particularly in targeted therapies where diverse
resistance mechanisms may be active. In EGFR-mutated lung cancer,
the most common resistance mechanisms include secondary T790M
mutations, C797S mutations and bypass pathway activation (such as
MET or HER2 amplification). In patients with ALK fusion-positive,
resistance can arise from secondary mutations (such as L1196M and
G1269A) or bypass pathway activation (such as EGFR, KIT or MET
amplification). For KRAS-mutated cancers, resistance mechanisms
involve secondary mutations or activation of other signaling
pathways (such as PI3K, MAPK or HER2 amplification) (48). Additionally, resistance to
immunotherapy is often linked to changes in the TME or immune
escape, such as loss of antigen presentation. During treatment,
some patients with NSCLC may also undergo phenotypic transformation
into SCLC, which further contributes to resistance. Tumor cells may
also evade drug effects by enhancing DNA damage repair mechanisms
(49). Overcoming these resistance
mechanisms encounters the following challenges: i) Difficulty in
identifying heterogeneity; the resistance mechanisms within
different patients and their tumors are highly heterogeneous and
need to be monitored dynamically relying on highly sensitive gene
sequencing technology; ii) lack of efficient drugs; for example,
the efficacy of existing third-generation TKIs for the C797S
mutation is insufficient and the development of fourth-generation
TKIs is still in the early stage; iii) difficulty in optimizing the
combination strategy; although the combination of chemotherapy,
radiotherapy and targeting agents can delay resistance, the
accumulation of toxicity has reduced its application (50). Future directions include the
development of specialized inhibitors targeting drug-resistant
mutations (such as fourth-generation EGFR-TKIs and KRAS
multi-mutant targeted drugs), the advancement of liquid biopsy
technology to monitor the dynamic changes of drug-resistant genes
and the precise design of combination therapies with controllable
toxicity.
Overall, recent advancements in targeted therapy for
NSCLC have led to the development of treatments targeting mutations
such as EGFR, MET, BRAF, RET, KRAS G12C and HER2. For EGFR
mutations, osimertinib remains as standard treatment, with
combination therapies exhibiting improved PFS. Amivantamab is a
first-line option for EGFR exon 20 insertions. Lorlatinib is
effective for ALK-rearranged NSCLC, and drugs such as crizotinib
and entrectinib are used for ROS1-positive cases. Selpercatinib and
pralsetinib are effective for RET fusions. BRAF mutations are
treated with dabrafenib plus trametinib, while MET exon 14
mutations are targeted by capmatinib and tepotinib. HER2 mutations
are addressed with trastuzumab, pyrotinib and ADCs such as DS8201.
KRAS G12C mutations respond to sotorasib and adagrasib, with
studies exploring their use in first-line combinations. NTRK
fusions are treated with larotrectinib and entrectinib. Despite
these advancements, resistance mechanisms remain a major challenge,
including secondary mutations and bypass pathways, highlighting the
need for new inhibitors, improved liquid biopsies and optimized
combination therapies.
Immunotherapy
Immunotherapy primarily works by stimulating the
immune system to enhance immune cell recognition and antitumor
activities, enabling the immune system to attack and eliminate
tumor cells. Tumor immunotherapy encompasses various approaches,
including the use of ICIs, adoptive cell therapy, cancer vaccines,
monoclonal antibodies, oncolytic virus therapy, cytokine therapy,
Toll-like receptor agonists and chimeric antigen receptor T-cell
(CAR-T) therapy, leveraging the immune system of the body to target
and destroy cancer cells (51).
Among these, ICIs such as programmed cell death protein 1 (PD-1),
PD-L1 and cytotoxic t-lymphocyte-associated protein 4 (CTLA-4)
antibodies (ipilimumab and tremelimumab) demonstrated advanced and
widespread clinical research and utilization in lung cancer. For
NSCLC without actionable mutations, ICIs targeting PD-1/PD-L1 and
CTLA-4 are the standard treatment. For high PD-L1 expression
(≥50%), anti-PD-1 or anti-PD-L1 monotherapy, or their combination
with chemotherapy, are established as effective. For intermediate
PD-L1 expression (1-49%), the standard treatment combines ICIs with
chemotherapy. In cases with low PD-L1 expression (<1%),
combinations of anti-PD-1/PD-L1 and anti-CTLA-4 inhibitors are
commonly used (52). ICI
monotherapy has exhibited limited efficacy in patients with EGFR,
ALK, RET, ROS1 and NTRK mutations. However, KRAS G12C mutations are
associated with a stronger response to ICI therapy. In addition,
re-treatment with ICIs may be considered for patients who initially
responded but have not been treated for an extended period,
specifically >6 months (53).
In recent years, inhibitors targeting other immune checkpoints in
addition to PD-1/PD-L1, have demonstrated potential in the
treatment of NSCLC, offering a promising new direction for therapy.
CTLA-4 inhibitors, such as ipilimumab, did not achieve significant
advancements in the treatment of various solid tumors, including
NSCLC, however, the combination of CTLA-4 inhibitors with
PD-1/PD-L1 inhibitors revealed some progress in NSCLC treatment. In
the CheckMate 227 trial, the combination of ipilimumab with
nivolumab significantly improved PFS and OS compared with
chemotherapy (54). However, the
KEYNOTE-598 study found that in patients with high PD-L1
expression, combining CTLA-4 inhibitors with pembrolizumab did not
provide significant benefits over monotherapy and was associated
with higher side effects (55).
This finding highlights the need for further exploration to
identify suitable patient populations for such combinations.
Previous research has increasingly focused on some novel ICIs,
including T-cell immunoreceptors with Ig and ITIM domains (TIGIT),
lymphocyte-activation gene 3 (LAG-3) and T-cell immunoglobulin and
mucin-domain containing-3 (TIM-3) targets. In the CITYSCAPE trial,
TIGIT inhibitors (such as tiragolumab) combined with atezolizumab
significantly improved the PFS and remission rate in PD-L1-positive
patients, and other drugs (including vibostolimab and ociperlimab)
also exhibited potential in this area (56). The soluble LAG-3 protein,
eftilagimod α, showed positive results in treating NSCLC in
combination with immunotherapy and chemotherapy by enhancing the
function of T cells and antigen-presenting cells (57). TIM-3 target inhibitors (such as
sabatolimab) demonstrated enhanced antitumor immune responses in
preclinical research (58). Novel
ICIs and dual-immunization strategies have shown promising results
in selected patients. However, efficacy and safety vary from person
to person, thereby the beneficiary populations should be further
clarified. Moreover, optimization of treatment regimens is needed
to improve efficacy and reduce the risk of adverse events in
advanced NSCLC.
Tumor-specific vaccine therapy aims to stimulate the
host immune system to generate tumor antigen-specific effector T
cells and memory T cells by introducing tumor antigens, thereby
initiating or amplifying adaptive antitumor immune responses.
Although NSCLC vaccines demonstrated promising results in
preliminary phase II clinical studies, there are still challenges
in their administration, including limited penetration into tumors,
weakened immune responses and resistance (51). Current vaccine research primarily
focuses on antigen-specific vaccines and whole-cell vaccines.
Antigen-specific vaccines include peptide/protein vaccines, DNA
vaccines, and vector vaccines, while whole-cell vaccines comprise
allogeneic vaccines and autologous dendritic cell vaccines.
Adaptive cell therapy utilizes immune cells from patients that have
tumor-reactive properties. These cells are cultured, genetically
engineered and reinfused into the patients to recognize and target
cancer cells. The most commonly used approaches in this field are
CAR-T, tumor-infiltrating lymphocyte (TIL), T-cell receptor and
natural killer cell therapies (51,59,60).
The remission rate of CAR-T therapy in hematological malignancies
can reach 80-90% (51). However,
the efficacy of CAR-T therapy in NSCLC still requires further
exploration.
ICIs have reshaped the treatment landscape of
advanced NSCLC however, only ~20% of patients achieve durable
responses to immunotherapy and some patients may experience severe
adverse reactions or are unresponsive. Therefore, identifying
biomarkers that can predict the efficacy and prognosis of
immunotherapy is of paramount importance. The only validated
predictive biomarker is PD-L1, but its predictive value is not
absolute. With a deeper understanding of molecular biology, genomic
sequencing technologies, and the immune microenvironment, the
identification of new molecular characteristics has led to the
discovery of additional potential biomarkers. PD-L1 expression is a
key biomarker for predicting the efficacy of immunotherapy;
however, even without PD-L1 expression, some cases can achieve
favorable responses to immunotherapy. The heterogeneity of tumors
in terms of time and space can partially explain this phenomenon.
In this regard, TILs are currently a hot topic (61). Studies have found that analyzing
tumor infiltration characteristics at diagnosis can predict the
efficacy of immunotherapy and guide treatment decisions. Human
leukocyte antigen class I (HLA-I) exhibits polymorphism in patients
with NSCLC (62). For example, a
study by Chowell et al (63) revealed that the HLA B44 supertype
is associated with improved OS, while the HLA B62 supertype is
associated with low OS. Currently, several systematic reviews
confirmed the prognostic value of the neutrophil-to-lymphocyte
ratio (NLR) in various types of cancer and this easily obtainable
and cost-effective index may become the next widely used predictive
biomarker for ICIs based on PD-L1(64).
Tumor mutational burden (TMB) refers to the number
of somatic mutations in the tumor genome after excluding germline
mutations. These mutations can generate neoantigens that can be
recognized by the immune system, leading to antitumor immune
responses. Therefore, an elevated TMB value (≥10 mut/Mb) may be a
predictive factor for the efficacy of ICIs (65). Additionally, studies indicated that
the gut microbiome can regulate adaptive and innate immunity,
influencing the antitumor immune response in the TME. However,
despite the significant benefits of immunotherapy, patients with
NSCLC inevitably experience disease progression due to resistance
to the therapy. Resistance to ICIs arises from immune escape, the
TME and tumor heterogeneity, which together limit long-lasting
efficacy and exacerbate treatment challenges (66). Resistance to ICIs is manifested
through: i) Antigen loss or downregulation of immune recognition
such as reduced PD-L1 expression in cancer cells or mutated major
histocompatibility complex molecules, which weaken T-cell
recognition (67); ii)
immunosuppressive TME-regulatory T cells, myeloid-derived
suppressor cells and M2-type macrophages which are increased, and
factors such as TGF-β, IL-10 and VEGF are upregulated (68); and iii) genetic and functional
heterogeneity through which subpopulations of drug-resistant T
cells expand under pressure of therapeutic selection (69). Addressing ICI resistance faces
several challenges including: i) The lack of reliable methods for
early identification of resistance mechanisms; and ii) the limited
efficacy of existing TGF-β or colony stimulating factor 1 receptor
inhibitors due to the complexity of TME dynamic regulation.
Response strategies include combination-targeted TME therapies
(such as TGF-β inhibitors in combination with PD-1 inhibitors),
exploration of antitumor vaccines to restore antigen-presenting
function and development of multi-targeted immunomodulatory agents
to address the treatment of patients with drug resistance (15,66,70).
In summary, immunotherapy has transformed advanced
NSCLC treatment, primarily through ICIs targeting PD-1/PD-L1 and
CTLA-4. For patients with high PD-L1 expression, monotherapy or
combination with chemotherapy is effective, while intermediate and
low PD-L1 cases often require combined ICI therapies. KRAS G12C
mutations exhibit stronger responses, but ICIs are less effective
in EGFR, ALK and other mutations. New ICIs targeting TIGIT, LAG-3
and TIM-3 are emerging, alongside combination therapies to improve
efficacy. However, resistance mechanisms such as immune escape and
tumor heterogeneity remain major challenges. Ongoing research is
focused on identifying reliable biomarkers and developing
strategies to overcome these resistance issues.
ADCs
Traditional lung cancer treatments such as
chemotherapy, targeted therapy and immunotherapy face challenges of
systemic toxicity and drug resistance, while the emergence and
rapid development of ADCs have brought new hope to patients with
NSCLC. ADC drugs combine the targeting properties of monoclonal
antibodies with the potent cytotoxicity of chemotherapeutic drugs,
using antibodies to specifically recognize targeted antigens on the
surface of tumor cells, and then delivering the cytotoxic agent
precisely inside the tumor cells (71). This ‘targeted bomb’ mode of action
not only improves the tumor-killing ability of the drug but also
minimizes the damage to normal cells. With the continuous
advancement of ADC technology, including the improvement of linker
technology, optimization of antibody selection and toxin design,
ADC drug research in lung cancer is gaining more and more
attention.
For lung cancers carrying specific biomarkers such
as HER2 or TROP2, ADC drugs such as DS-8201 (detrastuzumab, HER2
ADC, T-DXd) and DS-1062 (datopotamab deruxtecan, TROP2 ADC,
dato-DXd) demonstrated high antitumor activity, offering new
therapeutic options for patients who previously experienced
treatment failure (72,73).
As a HER2-targeted ADC drug, trastuzumab deruxtecan
achieved positive results in pivotal clinical trials such as
DESTINY-Lung01, DESTINY-Lung02 and DESTINY-Lung05 (74-76).
Trastuzumab deruxtecan was approved for marketing in the United
States, Europe and China, becoming the world's first ADC drug for
the treatment of adult patients with unresectable locally advanced
or metastatic NSCLC with HER2-activating mutations.
Telisotuzumab vedotin was revealed to be effective
in pretreated patients (who had received ≤2 lines of prior systemic
therapies, including ≤1 line cytotoxic chemotherapy, immunotherapy,
and targeted therapy if eligible) with c-Met-positive NSCLC,
especially those with high MET protein expression and EGFR
wild-type NSCLC (77).
In EGFR-mutated NSCLC, patritumab deruxtecan
(HER3-DXd) demonstrated efficacy in patients who progressed after
EGFR TKI treatment (78).
Additionally, BL-B01D1, a bispecific ADC targeting both EGFR and
HER3, is showing early promise in a clinical trial (79).
ADC drug development also faces some difficulties,
such as the optimization of the drug-antibody ratio, the stability
and release characteristics of the linker, the complexity of the
TME, and the double-edged sword represented by the bystander effect
(71). Current clinical studies
focus on the design and optimization of ADCs and investigators are
exploring ways to improve the efficacy and safety of ADCs.
Researchers have been summarizing clinical trial data in real time,
allowing them to speculate about the design direction of future
ADCs. They anticipate developing ADCs that will have more promising
clinical applications. In addition, as the understanding of the
molecular biology of NSCLC deepens, future ADCs may incorporate new
targets and therapeutic strategies to further improve therapeutic
efficacy. In short, the research of ADC drugs not only fills the
gap in the field of lung cancer treatment, but also represents the
future direction of precision tumor therapy.
ADCs offer a promising solution to the challenges of
traditional lung cancer treatments by targeting tumor cells more
precisely with minimal damage to healthy tissue. Drugs, such
trastuzumab deruxtecan (HER2) and DS-1062 (TROP2) have strong
activity in biomarker-positive NSCLC, while others including
telisotuzumab vedotin (MET-positive) and patritumab deruxtecan
(EGFR-mutant) are also promising. Despite some challenges in ADC
optimization, they represent a key future direction in lung cancer
therapy.
Challenges in NSCLC combination
therapies
Recent advances in combination therapies for
advanced-stage NSCLC highlighted the potential of integrating
different therapeutic modalities to enhance treatment efficacy. For
example, the combination of ICIs with chemotherapy demonstrated
improved survival outcomes in patients with high TMB (80). Similarly, targeted therapies such
as EGFR inhibitors are being combined with anti-angiogenic agents
or chemotherapy to overcome resistance mechanisms and extend PFS
(81). Novel approaches
integrating immunotherapy with radiotherapy or epigenetic
modulators are also being explored in preclinical and clinical
settings, aiming to exploit synergistic effects (70). Despite these promising advances,
significant challenges remain in optimizing these combination
strategies for widespread clinical use.
The development of combination therapies for
advanced-stage NSCLC faces significant challenges, including the
complexity of biological mechanisms, the risk of inducing new
resistance pathways and the difficulty of managing cumulative
toxicity (70). Clinically, the
lack of robust predictive biomarkers complicates patient selection,
while trial designs for multi-drug regimens remain challenging due
to the need to separate the contributions of each component
(80). Additionally, combination
therapies can significantly increase the economic burden, and their
limited accessibility in resource-constrained settings poses a
challenge to their worldwide adoption and application (82). Addressing these challenges will
require a multidisciplinary approach, combining advances in
molecular biology, innovative trial methodologies and collaborative
frameworks to ensure the efficacy, safety and affordability of
combination strategies.
5. Discussion
The treatment landscape for advanced NSCLC has
witnessed notable advancements in recent years. The introduction of
ICIs, targeted therapies and personalized medicine approaches has
significantly improved outcomes for patients with NSCLC. These
novel therapeutic modalities have expanded treatment options,
offering greater efficacy and reduced toxicity compared with
conventional chemotherapy regimens.
Despite these advancements, challenges remain,
including the development of resistance to targeted therapies, the
identification of predictive biomarkers for treatment response and
the optimization of combination strategies to overcome resistance
mechanisms. Additionally, the exploration of novel
immunotherapeutic approaches such as cytokine receptor agonists,
tumor vaccines, CAR-T-cell therapy and new ICIs hold promise for
further improving patient outcomes.
Multiple challenges are encountered in the treatment
of advanced NSCLC, including drug resistance, therapeutic toxicity,
high cost and underrepresentation of minorities in clinical trials.
These issues not only affect patient outcomes but also limit the
development and application of new therapies. Drug resistance is a
major obstacle in the treatment of advanced NSCLC. Although the
emergence of targeted therapies and immunotherapies has provided
new hope, several patients still develop drug resistance after
receiving treatment, which increases the risk of disease
progression (83). In addition,
toxicity of treatment is a problem that cannot be ignored;
especially in elderly patients or patients with comorbidities,
treatment-related adverse effects may be more severe (84). The high cost of treatment is also
an important factor influencing the willingness of patients to
undergo treatment. The extremely high cost of several novel drugs
and therapies renders them unaffordable for some patients, which in
turn affects their treatment choices and survival prognosis
(85). At the same time, the
underrepresentation of minorities in clinical trials further
exacerbates this problem. Research has revealed that the
participation of minorities in cancer clinical trials is markedly
lower than their proportion of cancer incidence, which leaves
treatment programs for these groups without adequate clinical data
to support them (86). To address
these challenges, steps must be taken to improve the design and
conduct of clinical trials, to make them more inclusive and
patient-centered. Clinical trial participation rates can be
improved by optimizing trial enrollment criteria, decreasing
participation thresholds and increasing recruitment of minorities,
thereby providing more patients with effective treatment options
(87). Concurrently, new
strategies are needed to expand treatment options and comprehensive
approaches are needed to ensure sustainability and global
accessibility of innovations. The scientific community needs to
strengthen collaboration to stimulate innovative solutions in order
to advance the field of NSCLC treatment (88).
Immunotherapy, with ICIs as the representative,
greatly improve the prognosis of patients who are negative for
driver mutations. Currently, the most common immunotherapy
combinations involve the use of immunotherapies with chemotherapy.
Additionally, immunotherapy drugs have the potential to be combined
with several different medications, including anti-angiogenic
drugs, targeted therapies, Janus kinase inhibitors, poly ADP-ribose
polymerase inhibitors, ADCs, TIGIT monoclonal antibodies, VEGFR
inhibitors and bispecific antibodies. However, predicting the
efficacy and prognosis of immunotherapy remains a challenge.
Promising biomarkers such as TILs, HLA-I polymorphism, NLR and the
gut microbiome are being investigated to improve patient selection
and guide treatment decisions. In addition, it is necessary to
explore the best treatment modality of immunotherapy combined with
other drugs to maximize the benefits of immunotherapy.
6. Conclusion
In summary, recent advances in NSCLC treatment,
including ICIs, targeted therapies and ADCs, have significantly
improved patient outcomes. However, several challenges remain such
as drug resistance, limited efficacy in certain patient subgroups,
and the need for reliable predictive biomarkers. Emerging therapies
such as ADCs are promising but require further investigation to
assess long-term efficacy and safety. Moving forward, research
should focus on optimizing combination therapies, overcoming
resistance and identifying new therapeutic targets, with a deeper
understanding of NSCLC biology being key to future innovations.
7. Expert opinion
Looking ahead, the future of NSCLC treatment is
promising, with ongoing research efforts focused on unraveling the
complexities of tumor biology, identifying novel therapeutic
targets and developing innovative treatment strategies. The advent
of precision medicine, liquid biopsy techniques and
immunotherapy-based combination regimens herald a new era in NSCLC
management, offering hope for improved survival and quality of life
for patients with advanced disease. In the future, there will be an
increasing number of targeted drugs and combination treatment
strategies, leading to the advancement of precision treatment for
lung cancer. With the increasing availability of targeted therapy
drugs, the re-biopsy of resistant tumors and identification of rare
mutations will facilitate the continuation of personalized
treatment for patients who develop resistance to targeted therapy,
thereby prolonging patient survival and transitioning lung cancer
into a manageable chronic condition. The maturation of kinase
inhibitor technology is evident and the field is projected to
concentrate on drug developments and broadening the scope of
existing drugs over the next decade. The emergence of
next-generation kinase inhibitors to supplant earlier medications
has been observed in various signaling pathways. For example, in
the EGFR and ALK pathways, fourth-generation drugs are already in
preclinical development. Furthermore, exploring different treatment
modalities, such as combination therapies with different mechanisms
of action, adjuvant use of kinase inhibitors, and neoadjuvant
therapies, is another area of research.
8. Review article highlights
The highlights of the present review article are
summarized as follows: i) Advances in oncology have revolutionized
lung cancer treatment with breakthroughs in targeted therapies and
immunotherapy, emphasizing personalized approaches; ii) over the
past two decades, breakthroughs in NSCLC treatment, including
targeted therapies, immunotherapy and personalized strategies, have
significantly improved patient survival and quality of life, while
also emphasizing the need for tailored approaches based on disease
stage, driver mutations and patient comorbidities; iii) recent
advancements in targeted therapy for lung cancer have focused on
identifying rare mutations and developing corresponding drugs, with
ongoing updates enhancing treatment options; iv) ADCs have emerged
as a promising class of drugs, combining targeted delivery with
potent cytotoxic effects and representing a significant step
forward in precision treatment strategies; and v) immunotherapy has
transformed treatment paradigms, although challenges remain in
predicting responses and managing side effects.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
Not applicable.
Authors' contributions
ZW, GC, JZ, and JW contributed to drafting the
manuscript, while ZW and YZ critically reviewed it. All authors
were involved in significant aspects of the present study,
including conception, study design, research acquisition, analysis,
and interpretation. All authors have participated in drafting,
revising, or critically reviewing the manuscript, read and approved
the final version for publication, agreed on the selected journal,
and accepted accountability for all aspects of the work. Data
authentication is not applicable.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Asamura H, Nishimura KK, Giroux DJ,
Chansky K, Hoering A, Rusch V and Rami-Porta R: Members of the
IASLC Staging and Prognostic Factors Committee and of the Advisory
Boards, Participating Institutions. IASLC lung cancer staging
project: The new database to inform revisions in the ninth edition
of the TNM classification of lung cancer. J Thorac Oncol.
18:564–575. 2023.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Rami-Porta R, Nishimura KK, Giroux DJ,
Detterbeck F, Cardillo G, Edwards JG, Fong KM, Giuliani M, Huang J,
Kernstine KH Sr, et al: The international association for the study
of lung cancer lung cancer staging project: Proposals for revision
Of the TNM stage groups in the forthcoming (Ninth) edition of the
TNM classification for lung cancer. J Thorac Oncol. 19:1007–1027.
2024.
|
|
3
|
Lu S, Lu C, Xiao Y, Zhu W, He Q, Xie B,
Zhou J, Tao Y, Liu S and Xiao D: Comparison of EML4-ALK fusion gene
positive rate in different detection methods and samples of
non-small cell lung cancer. J Cancer. 11:1525–1531. 2020.
|
|
4
|
Imyanitov EN, Iyevleva AG and Levchenko
EV: Molecular testing and targeted therapy for non-small cell lung
cancer: Current status and perspectives. Crit Rev Oncol Hematol.
157(103194)2021.
|
|
5
|
Shields MD, Chen K, Dutcher G, Patel I and
Pellini B: Making the rounds: Exploring the role of circulating
tumor DNA (ctDNA) in non-small cell lung cancer. Int J Mol Sci.
23(9006)2022.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Li W, Liu JB, Hou LK, Yu F, Zhang J, Wu W,
Tang XM, Sun F, Lu HM, Deng J, et al: Liquid biopsy in lung cancer:
Significance in diagnostics, prediction, and treatment monitoring.
Mol Cancer. 21(25)2022.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Hussain MS, Gupta G, Ghaboura N, Moglad E,
Almalki WH, Alzarea SI, Kazmi I, Ali H, MacLoughlin R, Loebenberg
R, et al: Exosomal ncRNAs in liquid biopsies for lung cancer. Clin
Chim Acta. 565(119983)2024.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Pezzuto A, Terzo F, Graziani ML, Ricci A,
Bruno P and Mariotta S: Lung cancer requires multidisciplinary
treatment to improve patient survival: A case report. Oncol Lett.
14:3035–3038. 2017.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Hardavella G, Chorostowska-Wynimko J and
Blum TG: Lung cancer: An update on the multidisciplinary approach
from screening to palliative care. Breathe (Sheff).
20(240117)2024.PubMed/NCBI View Article : Google Scholar
|
|
10
|
McCurdy M, McAleer MF, Wei W, Ezhil M,
Johnson V, Khan M, Baker J, Luo D, Ajani J and Guerrero T:
Induction and concurrent taxanes enhance both the pulmonary
metabolic radiation response and the radiation pneumonitis response
in patients with esophagus cancer. Int J Radiat Oncol Biol Phys.
76:816–823. 2010.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Duan Y, Wang Y, Lu S, Zeng M, Liu L, Dai Q
and Yin R: Adverse event profile of albumin-bound paclitaxel: A
real-world pharmacovigilance analysis. Front Pharmacol.
15(1448144)2024.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Minguet J, Smith KH and Bramlage P:
Targeted therapies for treatment of non-small cell lung
cancer-recent advances and future perspectives. Int J Cancer.
138:2549–2561. 2016.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Wagner SA, Szczesniak PP, Voigt A, Gräf JF
and Beli P: Proteomic analysis of tyrosine phosphorylation induced
by exogenous expression of oncogenic kinase fusions identified in
lung adenocarcinoma. Proteomics. 21(e2000283)2021.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Jiang T, Wang G, Liu Y, Feng L, Wang M,
Liu J, Chen Y and Ouyang L: Development of small-molecule
tropomyosin receptor kinase (TRK) inhibitors for NTRK fusion
cancers. Acta Pharm Sin B. 11:355–372. 2021.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Schoenfeld AJ and Hellmann MD: Acquired
resistance to immune checkpoint inhibitors. Cancer Cell.
37:443–455. 2020.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Lopez-Chavez A, Young T, Fages S, Leon L,
Schiller JH, Dowlati A, Brahmer JR, Johnson DH and Sandler A:
Bevacizumab maintenance in patients with advanced non-small-cell
lung cancer, clinical patterns, and outcomes in the Eastern
cooperative oncology group 4599 study: Results of an exploratory
analysis. J Thorac Oncol. 7:1707–1712. 2012.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Zhou C, Wu YL, Chen G, Liu X, Zhu Y, Lu S,
Feng J, He J, Han B, Wang J, et al: BEYOND: A randomized,
double-blind, placebo-controlled, multicenter, phase III study of
first-line carboplatin/paclitaxel plus bevacizumab or placebo in
Chinese patients with advanced or recurrent nonsquamous
non-small-cell lung cancer. J Clin Oncol. 33:2197–2204.
2015.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Chu T, Zhang W, Zhang B, Zhong R, Zhang X,
Gu A, Shi C, Wang H, Xiong L, Lu J, et al: Efficacy and safety of
first-line anlotinib-based combinations for advanced non-small cell
lung cancer: A three-armed prospective study. Transl Lung Cancer
Res. 11:1394–1404. 2022.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Camerini A, Del Conte A, Pezzuto A, Scotti
V, Facchinetti F, Ciccone LP, Perna M, Sartori G, Puccetti C, Ricci
A, et al: Selection criteria and treatment outcome for advanced
non-small cell lung cancer (NSCLC) patients unfit for
platinum-based first-line therapy: Results of the MOON-OSS
observational trial. Cancers (Basel). 14(6074)2022.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Chan BA and Hughes BG: Targeted therapy
for non-small cell lung cancer: Current standards and the promise
of the future. Transl Lung Cancer Res. 4:36–54. 2015.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Marmarelis ME and Langer CJ: Treatment of
patients with non-small-cell lung cancer harboring rare oncogenic
mutations. Clin Lung Cancer. 21:395–406. 2020.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Cho BC, Lu S, Felip E, Spira AI, Girard N,
Lee JS, Lee SH, Ostapenko Y, Danchaivijitr P, Liu B, et al:
Amivantamab plus lazertinib in previously untreated EGFR-mutated
advanced NSCLC. N Engl J Med. 391:1486–1498. 2024.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Zhou C, Tang KJ, Cho BC, Liu B, Paz-Ares
L, Cheng S, Kitazono S, Thiagarajan M, Goldman JW, Sabari JK, et
al: Amivantamab plus chemotherapy in NSCLC with EGFR Exon 20
insertions. N Engl J Med. 389:2039–2051. 2023.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Wang M, Yang JC, Mitchell PL, Fang J,
Camidge DR, Nian W, Chiu CH, Zhou J, Zhao Y, Su WC, et al:
Sunvozertinib, a selective EGFR inhibitor for previously treated
non-small cell lung cancer with EGFR exon 20 insertion mutations.
Cancer Discov. 12:1676–1689. 2022.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Wu YL, Yang JC, Kim DW, Lu S, Zhou J, Seto
T, Yang JJ, Yamamoto N, Ahn MJ, Takahashi T, et al: Phase II study
of crizotinib in east asian patients with ROS1-positive advanced
non-small-cell lung cancer. J Clin Oncol. 36:1405–1411.
2018.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Dziadziuszko R, Krebs MG, De Braud F,
Siena S, Drilon A, Doebele RC, Patel MR, Cho BC, Liu SV, Ahn MJ, et
al: Updated integrated analysis of the efficacy and safety of
entrectinib in locally advanced or metastatic ROS1 fusion-positive
non-small-cell lung cancer. J Clin Oncol. 39:1253–1263.
2021.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Drilon A, Camidge DR, Lin JJ, Kim SW,
Solomon BJ, Dziadziuszko R, Besse B, Goto K, de Langen AJ, Wolf J,
et al: Repotrectinib in ROS1 fusion-positive non-small-cell lung
cancer. N Engl J Med. 390:118–131. 2024.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Drilon A, Subbiah V, Gautschi O, Tomasini
P, de Braud F, Solomon BJ, Tan DSW, Alonso G, Wolf J, Park K, et
al: Selpercatinib in patients With RET fusion-positive
non-small-cell lung cancer: Updated safety and efficacy from the
registrational LIBRETTO-001 phase I/II trial. J Clin Oncol.
41:385–394. 2023.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Griesinger F, Curigliano G, Thomas M,
Subbiah V, Baik CS, Tan DSW, Lee DH, Misch D, Garralda E, Kim DW,
et al: Safety and efficacy of pralsetinib in RET fusion-positive
non-small-cell lung cancer including as first-line therapy: Update
from the ARROW trial. Ann Oncol. 33:1168–1178. 2022.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Li C, Nguyen V, Clark KN, Zahed T, Sharkas
S, Filipp FV and Boiko AD: Down-regulation of FZD3 receptor
suppresses growth and metastasis of human melanoma independently of
canonical WNT signaling. Proc Natl Acad Sci USA. 116:4548–4557.
2019.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Riely GJ, Smit EF, Ahn MJ, Felip E,
Ramalingam SS, Tsao A, Johnson M, Gelsomino F, Esper R, Nadal E, et
al: Phase II, open-label study of encorafenib plus binimetinib in
patients with BRAF(V600)-Mutant metastatic non-small-cell lung
cancer. J Clin Oncol. 41:3700–3711. 2023.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Rocco D, Gravara LD, Palazzolo G and
Gridelli C: The treatment of a new entity in advanced non-small
cell lung cancer: MET exon 14 skipping mutation. Curr Med Chem.
31:3043–3056. 2024.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Bourhis A, Remoué A and Uguen A: KRAS and
BRAF double mutations and functional classes of BRAF mutations in
non-small-cell lung cancers. Clin Lung Cancer. 21:e240–e242.
2020.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Yang G, Xu H, Yang Y, Zhang S, Xu F, Hao
X, Li J, Xing P, Hu X, Liu Y, et al: Pyrotinib combined with
apatinib for targeting metastatic non-small cell lung cancer with
HER2 alterations: A prospective, open-label, single-arm phase 2
study (PATHER2). BMC Med. 20(277)2022.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Sheng X, Yan X, Wang L, Shi Y, Yao X, Luo
H, Shi B, Liu J, He Z, Yu G, et al: Open-label, multicenter, phase
II study of RC48-ADC, a HER2-Targeting antibody-drug conjugate, in
patients with locally advanced or metastatic urothelial carcinoma.
Clin Cancer Res. 27:43–51. 2021.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Dy GK, Govindan R, Velcheti V, Falchook
GS, Italiano A, Wolf J, Sacher AG, Takahashi T, Ramalingam SS,
Dooms C, et al: Long-Term outcomes and molecular correlates of
sotorasib efficacy in patients with pretreated KRAS G12C-mutated
non-small-cell lung cancer: 2-Year analysis of CodeBreaK 100. J
Clin Oncol. 41:3311–3317. 2023.PubMed/NCBI View Article : Google Scholar
|
|
37
|
de Langen AJ, Johnson ML, Mazieres J,
Dingemans AC, Mountzios G, Pless M, Wolf J, Schuler M, Lena H,
Skoulidis F, et al: Sotorasib versus docetaxel for previously
treated non-small-cell lung cancer with KRAS(G12C) mutation: A
randomised, open-label, phase 3 trial. Lancet. 401:733–746.
2023.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Jänne PA, Riely GJ, Gadgeel SM, Heist RS,
Ou SI, Pacheco JM, Johnson ML, Sabari JK, Leventakos K, Yau E, et
al: Adagrasib in non-small-cell lung cancer harboring a KRAS(G12C)
mutation. N Engl J Med. 387:120–131. 2022.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Lorthiois E, Gerspacher M, Beyer KS,
Vaupel A, Leblanc C, Stringer R, Weiss A, Wilcken R, Guthy DA,
Lingel A, et al: JDQ443, a structurally novel, pyrazole-based,
covalent inhibitor of KRAS(G12C) for the treatment of solid tumors.
J Med Chem. 65:16173–16203. 2022.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Shi Z, Weng J, Niu H, Yang H, Liu R, Weng
Y, Zhu Q, Zhang Y, Tao L, Wang Z, et al: D-1553: A novel KRAS(G12C)
inhibitor with potent and selective cellular and in vivo antitumor
activity. Cancer Sci. 114:2951–2960. 2023.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Lassen U, Bokemeyer C, Garcia-Foncillas J,
Italiano A, Vassal G, Paracha N, Marian M, Chen Y, Linsell L and
Abrams K: prognostic value of neurotrophic tyrosine receptor kinase
gene fusions in solid tumors for overall survival: A systematic
review and meta-analysis. JCO Precis Oncol.
7(e2200651)2023.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Doz F, van Tilburg CM, Geoerger B,
Højgaard M, Øra I, Boni V, Capra M, Chisholm J, Chung HC, DuBois
SG, et al: Efficacy and safety of larotrectinib in TRK
fusion-positive primary central nervous system tumors. Neuro Oncol.
24:997–1007. 2022.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Paz-Ares L, Barlesi F, Siena S, Ahn MJ,
Drilon A, Conley A, Rolfo C, Wolf J, Seto T, Doebele R, et al:
Patient-reported outcomes from STARTRK-2: A global phase II basket
study of entrectinib for ROS1 fusion-positive non-small-cell lung
cancer and NTRK fusion-positive solid tumours. ESMO Open.
6(100113)2021.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Xu L, Wang F and Luo F: MET-targeted
therapies for the treatment of non-small-cell lung cancer: A
systematic review and meta-analysis. Front Oncol.
12(1013299)2022.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Yang SR, Schultheis AM, Yu H, Mandelker D,
Ladanyi M and Büttner R: Precision medicine in non-small cell lung
cancer: Current applications and future directions. Semin Cancer
Biol. 84:184–198. 2022.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Izumi H, Matsumoto S, Liu J, Tanaka K,
Mori S, Hayashi K, Kumagai S, Shibata Y, Hayashida T, Watanabe K,
et al: The CLIP1-LTK fusion is an oncogenic driver in
non-small-cell lung cancer. Nature. 600:319–323. 2021.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Poole A, Karuppiah V, Hartt A, Haidar JN,
Moureau S, Dobrzycki T, Hayes C, Rowley C, Dias J, Harper S, et al:
Therapeutic high affinity T cell receptor targeting a KRAS(G12D)
cancer neoantigen. Nat Commun. 13(5333)2022.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Zhang H, Zhang Y, Zhu Y, Dong T and Liu Z:
Understanding the treatment response and resistance to targeted
therapies in non-small cell lung cancer: Clinical insights and
perspectives. Front Oncol. 14(1387345)2024.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Sini C, Tuzi A, Rossi G, Russo A and
Pezzuto A: Acquired resistance in oncogene-addicted non-small-cell
lung cancer. Future Oncol. 14:29–40. 2018.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Wu J and Lin Z: Non-Small cell lung cancer
targeted therapy: Drugs and mechanisms of drug resistance. Int J
Mol Sci. 23(15056)2022.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Lahiri A, Maji A, Potdar PD, Singh N,
Parikh P, Bisht B, Mukherjee A and Paul MK: Lung cancer
immunotherapy: Progress, pitfalls, and promises. Mol Cancer.
22(40)2023.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Hendriks LE, Kerr KM, Menis J, Mok TS,
Nestle U, Passaro A, Peters S, Planchard D, Smit EF, Solomon BJ, et
al: Non-oncogene-addicted metastatic non-small-cell lung cancer:
ESMO Clinical practice guideline for diagnosis, treatment and
follow-up. Ann Oncol. 34:358–376. 2023.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Levra MG, Cotté FE, Corre R, Calvet C,
Gaudin AF, Penrod JR, Grumberg V, Jouaneton B, Jolivel R, Assié JB
and Chouaïd C: Immunotherapy rechallenge after nivolumab treatment
in advanced non-small cell lung cancer in the real-world setting: A
national data base analysis. Lung Cancer. 140:99–106.
2020.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Brahmer JR, Lee JS, Ciuleanu TE, Caro RB,
Nishio M, Urban L, Audigier-Valette C, Lupinacci L, Sangha R,
Pluzanski A, et al: Five-Year survival outcomes with nivolumab plus
ipilimumab versus chemotherapy as first-line treatment for
metastatic non-small-cell lung cancer in CheckMate 227. J Clin
Oncol. 41:1200–1212. 2023.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Boyer M, Şendur MAN, Rodríguez-Abreu D,
Park K, Lee DH, Çiçin I, Yumuk PF, Orlandi FJ, Leal TA, Molinier O,
et al: Pembrolizumab plus ipilimumab or placebo for metastatic
non-small-cell lung cancer with PD-L1 tumor proportion score ≥50%:
Randomized, double-blind phase III KEYNOTE-598 study. J Clin Oncol.
39:2327–2338. 2021.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Cho BC, Abreu DR, Hussein M, Cobo M, Patel
AJ, Secen N, Lee KH, Massuti B, Hiret S, Yang JCH, et al:
Tiragolumab plus atezolizumab versus placebo plus atezolizumab as a
first-line treatment for PD-L1-selected non-small-cell lung cancer
(CITYSCAPE): Primary and follow-up analyses of a randomised,
double-blind, phase 2 study. Lancet Oncol. 23:781–792.
2022.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Genova C, Dellepiane C, Carrega P,
Sommariva S, Ferlazzo G, Pronzato P, Gangemi R, Filaci G, Coco S
and Croce M: Therapeutic implications of tumor microenvironment in
lung cancer: Focus on immune checkpoint blockade. Front Immunol.
12(799455)2021.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Datar I, Sanmamed MF, Wang J, Henick BS,
Choi J, Badri T, Dong W, Mani N, Toki M, Mejías LD, et al:
Expression analysis and significance of PD-1, LAG-3, and TIM-3 in
Human non-small cell lung cancer using spatially resolved and
multiparametric single-cell analysis. Clin Cancer Res.
25:4663–4673. 2019.PubMed/NCBI View Article : Google Scholar
|
|
59
|
Qu J, Mei Q, Chen L and Zhou J: Chimeric
antigen receptor (CAR)-T-cell therapy in non-small-cell lung cancer
(NSCLC): Current status and future perspectives. Cancer Immunol
Immunother. 70:619–631. 2021.PubMed/NCBI View Article : Google Scholar
|
|
60
|
Pockley AG, Vaupel P and Multhoff G: NK
cell-based therapeutics for lung cancer. Expert Opin Biol Ther.
20:23–33. 2020.PubMed/NCBI View Article : Google Scholar
|
|
61
|
Paijens ST, Vledder A, de Bruyn M and
Nijman HW: Tumor-infiltrating lymphocytes in the immunotherapy era.
Cell Mol Immunol. 18:842–859. 2021.PubMed/NCBI View Article : Google Scholar
|
|
62
|
Sadagopan A, Michelakos T, Boyiadzis G,
Ferrone C and Ferrone S: Human leukocyte antigen class I
antigen-processing machinery upregulation by anticancer therapies
in the era of checkpoint inhibitors: A review. JAMA Oncol.
8:462–473. 2022.PubMed/NCBI View Article : Google Scholar
|
|
63
|
Chowell D, Morris LGT, Grigg CM, Weber JK,
Samstein RM, Makarov V, Kuo F, Kendall SM, Requena D, Riaz N, et
al: Patient HLA class I genotype influences cancer response to
checkpoint blockade immunotherapy. Science. 359:582–587.
2018.PubMed/NCBI View Article : Google Scholar
|
|
64
|
Templeton AJ, McNamara MG, Šeruga B,
Vera-Badillo FE, Aneja P, Ocaña A, Leibowitz-Amit R, Sonpavde G,
Knox JJ, Tran B, et al: Prognostic role of neutrophil-to-lymphocyte
ratio in solid tumors: A systematic review and meta-analysis. J
Natl Cancer Inst. 106(dju124)2014.PubMed/NCBI View Article : Google Scholar
|
|
65
|
Lee KW, Van Cutsem E, Bang YJ, Fuchs CS,
Kudaba I, Garrido M, Chung HC, Lee J, Castro HR, Chao J, et al:
Association of tumor mutational burden with efficacy of
pembrolizumab±chemotherapy as first-line therapy for gastric cancer
in the phase III KEYNOTE-062 study. Clin Cancer Res. 28:3489–3498.
2022.PubMed/NCBI View Article : Google Scholar
|
|
66
|
Marei HE, Hasan A, Pozzoli G and
Cenciarelli C: Cancer immunotherapy with immune checkpoint
inhibitors (ICIs): Potential, mechanisms of resistance, and
strategies for reinvigorating T cell responsiveness when resistance
is acquired. Cancer Cell Int. 23(64)2023.PubMed/NCBI View Article : Google Scholar
|
|
67
|
Dotsu Y, Muraoka D, Ogo N, Sonoda Y, Yasui
K, Yamaguchi H, Yagita H, Mukae H, Asai A and Ikeda H: Chemical
augmentation of mitochondrial electron transport chains tunes T
cell activation threshold in tumors. J Immunother Cancer.
10(e003958)2022.PubMed/NCBI View Article : Google Scholar
|
|
68
|
Hong Y, Robbins Y, Yang X, Mydlarz WK,
Sowers A, Mitchell JB, Gulley JL, Schlom J, Gameiro SR, Sievers C
and Allen CT: Cure of syngeneic carcinomas with targeted IL-12
through obligate reprogramming of lymphoid and myeloid immunity.
JCI Insight. 7(e157448)2022.PubMed/NCBI View Article : Google Scholar
|
|
69
|
Liu Y, Feng C, Zhou Y, Shao X and Chen M:
Simulating the dynamic intra-tumor heterogeneity and therapeutic
responses. Cancers (Basel). 14(1645)2022.PubMed/NCBI View Article : Google Scholar
|
|
70
|
Sharma P, Hu-Lieskovan S, Wargo JA and
Ribas A: Primary, adaptive, and acquired resistance to cancer
immunotherapy. Cell. 168:707–723. 2017.PubMed/NCBI View Article : Google Scholar
|
|
71
|
Zhao C, Zhang R, Yang H, Gao Y, Zou Y and
Zhang X: Antibody-drug conjugates for non-small cell lung cancer:
Advantages and challenges in clinical translation. Biochem
Pharmacol. 226(116378)2024.PubMed/NCBI View Article : Google Scholar
|
|
72
|
Meric-Bernstam F, Makker V, Oaknin A, Oh
DY, Banerjee S, González-Martín A, Jung KH, Ługowska I, Manso L,
Manzano A, et al: Efficacy and safety of trastuzumab deruxtecan in
patients With HER2-expressing solid tumors: Primary results from
the DESTINY-PanTumor02 phase II Trial. J Clin Oncol. 42:47–58.
2024.PubMed/NCBI View Article : Google Scholar
|
|
73
|
Shimizu T, Sands J, Yoh K, Spira A, Garon
EB, Kitazono S, Johnson ML, Meric-Bernstam F, Tolcher AW, Yamamoto
N, et al: First-in-human, phase I dose-escalation and
dose-expansion study of trophoblast cell-surface antigen 2-directed
antibody-drug conjugate datopotamab deruxtecan in non-small-cell
lung cancer: TROPION-PanTumor01. J Clin Oncol. 41:4678–4687.
2023.PubMed/NCBI View Article : Google Scholar
|
|
74
|
Smit EF, Felip E, Uprety D, Nagasaka M,
Nakagawa K, Rodríguez LPA, Pacheco JM, Li BT, Planchard D, Baik C,
et al: Trastuzumab deruxtecan in patients with metastatic
non-small-cell lung cancer (DESTINY-Lung01): Primary results of the
HER2-overexpressing cohorts from a single-arm, phase 2 trial.
Lancet Oncol. 25:439–454. 2024.PubMed/NCBI View Article : Google Scholar
|
|
75
|
Goto K, Goto Y, Kubo T, Ninomiya K, Kim
SW, Planchard D, Ahn MJ, Smit EF, de Langen AJ, Pérol M, et al:
Trastuzumab deruxtecan in patients with HER2-mutant metastatic
non-small-cell lung cancer: Primary results from the randomized,
phase II DESTINY-lung02 Trial. J Clin Oncol. 41:4852–4863.
2023.PubMed/NCBI View Article : Google Scholar
|
|
76
|
Cheng Y, Wu L, Fang Y, Fan Y, Li X, Zhang
M, Yu Y, Yao Y, Xu R, Guo J, et al: Trastuzumab deruxtecan (T-DXd)
in Chinese patients (pts) with previously treated HER2 mutant
non-small cell lung cancer (NSCLC): Primary analysis from the Phase
2 DESTINY-Lung05 (DL-05) trial. Cancer Res. 84(CT248)2024.
|
|
77
|
Camidge DR, Bar J, Horinouchi H, Goldman
J, Moiseenko F, Filippova E, Cicin I, Ciuleanu T, Daaboul N, Liu C,
et al: Telisotuzumab vedotin monotherapy in patients with
previously treated c-Met Protein-overexpressing advanced
nonsquamous EGFR-wildtype non-small cell lung cancer in the phase
II LUMINOSITY trial. J Clin Oncol. 42:3000–3011. 2024.PubMed/NCBI View Article : Google Scholar
|
|
78
|
Yu HA, Goto Y, Hayashi H, Felip E, Yang
JCH, Reck M, Yoh K, Lee SH, Paz-Ares L, Besse B, et al:
HERTHENA-Lung01, a phase II trial of patritumab deruxtecan
(HER3-DXd) in epidermal growth factor receptor-mutated
non-small-cell lung cancer after epidermal growth factor receptor
tyrosine kinase inhibitor therapy and platinum-based chemotherapy.
J Clin Oncol. 41:5363–5375. 2023.PubMed/NCBI View Article : Google Scholar
|
|
79
|
Ma Y, Huang Y, Zhao Y, Zhao S, Xue J, Yang
Y, Fang W, Guo Y, Han Y, Yang K, et al: BL-B01D1, a first-in-class
EGFR-HER3 bispecific antibody-drug conjugate, in patients with
locally advanced or metastatic solid tumours: A first-in-human,
open-label, multicentre, phase 1 study. Lancet Oncol. 25:901–911.
2024.PubMed/NCBI View Article : Google Scholar
|
|
80
|
Hellmann MD, Nathanson T, Rizvi H, Creelan
BC, Sanchez-Vega F, Ahuja A, Ni A, Novik JB, Mangarin LMB,
Abu-Akeel M, et al: Genomic features of response to combination
immunotherapy in patients with advanced non-small-cell lung cancer.
Cancer Cell. 33:843–852.e844. 2018.PubMed/NCBI View Article : Google Scholar
|
|
81
|
Soria JC, Ohe Y, Vansteenkiste J,
Reungwetwattana T, Chewaskulyong B, Lee KH, Dechaphunkul A, Imamura
F, Nogami N, Kurata T, et al: Osimertinib in untreated EGFR-mutated
advanced non-small-cell lung cancer. N Engl J Med. 378:113–125.
2018.PubMed/NCBI View Article : Google Scholar
|
|
82
|
Gordon LG, Merollini KMD, Lowe A and Chan
RJ: A systematic review of financial toxicity among cancer
survivors: We can't pay the co-pay. Patient. 10:295–309.
2017.PubMed/NCBI View Article : Google Scholar
|
|
83
|
Fukuda A and Okuma Y: From rarity to
reality: Osimertinib's promising horizon in treating uncommon EGFR
mutations in non-small cell lung cancer. Clin Cancer Res.
30:3128–3136. 2024.PubMed/NCBI View Article : Google Scholar
|
|
84
|
Bortolot M, Cortiula F, Fasola G, De
Ruysscher D, Naidoo J and Hendriks LEL: Treatment of unresectable
stage III non-small cell lung cancer for patients who are
under-represented in clinical trials. Cancer Treat Rev.
129(102797)2024.PubMed/NCBI View Article : Google Scholar
|
|
85
|
Mittal A, Moore S, Navani V, Jiang DM,
Stewart DJ, Liu G and Wheatley-Price P: What is ailing oncology
clinical trials? Can we fix them? Curr Oncol. 31:3738–3751.
2024.PubMed/NCBI View Article : Google Scholar
|
|
86
|
Chua AV Jr, Delmerico J, Sheng H, Huang
XW, Liang E, Yan L, Gandhi S, Puzanov I, Jain P, Sakoda LC, et al:
Under-Representation and under-reporting of minoritized racial and
ethnic groups in clinical trials on immune checkpoint inhibitors.
JCO Oncol Pract. 22(OP2400033)2024.PubMed/NCBI View Article : Google Scholar
|
|
87
|
Waqar SN and Govindan R: Novel therapies
in cancer: Trials and tribulations. Clin Cancer Res. 30:3655–3657.
2024.PubMed/NCBI View Article : Google Scholar
|
|
88
|
Meyer ML, Fitzgerald BG, Paz-Ares L,
Cappuzzo F, Jänne PA, Peters S and Hirsch FR: New promises and
challenges in the treatment of advanced non-small-cell lung cancer.
Lancet. 404:803–822. 2024.PubMed/NCBI View Article : Google Scholar
|















