Introduction
MicroRNAs (miRNAs/miRs) are non-coding RNAs which
consist of ~20 nucleotides and have been determined to be critical
regulators of eukaryotic gene expression such as in mammals, plants
and viruses (1,2). The first miRNA was found >30 years
ago in the nematode Caenorhabditis elegans, with the
detection of the developmental regulator, lin-4 (3). To date, >2,000 miRNAs have been
identified in humans governing one third of the genes in the genome
(4). During synthesis, RNA
polymerase II transcribes a miRNA gene to produce a long primary
miRNA (pri-miRNA), which is cleaved to generate precursor miRNA
(pre-miRNA) within the nucleus (5,6). The
pre-miRNA is then translocated to the cytoplasm (7-9)
where it is cleaved to generate a ~22 nt miRNA duplex (10,11). Of
note, one strand of this duplex generates the mature guide miRNA,
which binds to an Argonaute protein to become the core of the
miRNA-induced silencing complex (miRISC) (12,13). The
miRNA region then drives miRISC to complementary sites to govern
repression of the target mRNA, while another strand of the mature
miRNA duplex, as passenger (opposite) strand, degrades. In mammals,
the miRNA target sites, are usually localized in the
3'-untranslated region (3´UTR) of mRNAs, which are known as the
sites with the most complementarity to the seed region (14,15). The
determination of miRNA-mRNA target interactions is critical for
exploring the regulatory role of miRNAs, resulting in the discovery
of novel therapies (16).
In recent years, the regulatory roles of miRNAs in
various pathologies, ranging from cancer to autoimmune and
cardiovascular disease, have attracted major attention (17). By these means, miRNAs possess
promising potential for use as biomarkers in diagnostic
applications. For example, following the discovery of the
regulatory role of miRNAs in cancer, a number of investigations
have focused on exploring their potential therapeutic value
(18). Currently, the Food and Drug
Administration and the European Medicines Agency have accepted a
total of 11 RNA-based therapeutics involving small interfering RNA
and antisense oligonucleotide. Although miRNAs are not yet approved
as therapeutic agents for diseases, some miRNA mimics and anti-miR
oligonucleotides are undergoing or completed clinical phase I
(NCT01829971, NCT04675996, NCT02369198, NCT04045405) and II
(NCT03837457, NCT03373786, NCT02855268 and NCT03601052) trials. For
instance, an anti-miR-122 drug, miravirsen, developed for treatment
of hepatitis C, has successfully completed clinical phase I
(NCT01646489) and II (NCT02508090, NCT02508090, NCT02452814 and
NCT02452814) trials (18).
The present systematic review explored what is known
regarding miRNAs and hypospadias. Hypospadias is a malformation of
the male external genitalia (18.6-80 per 10,000 male births), and
it is characterized by a proximal dislocation of the urethral
meatus, ventrally split foreskin, and can be combined with penile
curvature and transposition of the scrotum (19,20). The
moderate to severe cases account for 30%, while the remaining 70%
are considered a mild form that is not linked with other urogenital
malformations (20).
The etiology of hypospadias is described as complex,
meaning that it occurs due to genetic factors in combination with
environmental factors. The most well-established risk factor is a
low birth weight and growth retardation during gestation (21). In ~10% of cases, hypospadias can be
found in an additional family member. Some level of genetic
predisposition is established in hypospadias (7% of cases having
affected first, second, or third-degree relatives) and hereditary
occurrence appears more frequent for distal and middle forms than
for posterior varieties (22). The
risk of a male sibling also having hypospadias is 9-17% and
hypospadias is evenly transmitted through the maternal and paternal
side, with an assessed heritability of 57-77% (23). In only 1/3 of hypospadias, an obvious
genetic reason is established; however, hypospadias has been
described in >200 syndromes, with the most well-known being
Wilms' tumor, aniridia, genitourinary malformations, mental
retardation (WAGR) and genitourinary malformations and
susceptibility to Wilms' tumor (Denys-Drash syndrome) (22,24).
In the present study, it was hypothesized that the
genetic predisposition to hypospadias may not be primarily related
to mutations in gene-coding regions, but may be caused by silencing
intracellular post-transcriptional factors, such as miRNAs. Thus, a
systematic review was performed to further elucidate what is known
on the subject.
Data and methods
Eligibility criteria
The present systematic review was registered in the
International Prospective Register of Systematic Reviews (PROSPERO;
no. CRD42024502746). The Preferred Reporting Items of Systematic
Reviews and Meta-Analyses (PRISMA) guidelines were used for the
organization and completion of the study. Only included studies
focusing on the association between miRNAs and hypospadias were
included, encompassing both human and animal studies. These
publications, indexed in formerly two identified databases, were
required to have available abstracts, fully accessible online.
Moreover, only articles written in the English language where
included.
Search strategy
The present systematic literature review was
conducted using the PubMed and Embase databases in September, 2024,
including both MeSH and free text terms, with search expression
hypospadias AND miRNA (Table I).
Furthermore, the reference lists of the identified articles where
searched. EndNote 21 (Clarivate) was utilized for the organization
and management of reference materials throughout the search
process.
 | Table IDescriptors used in the search
strategy. |
Table I
Descriptors used in the search
strategy.
| Topic | Descriptors |
|---|
| MicroRNAs | ‘MicroRNAs’[MeSH]
OR microRNA* OR miRNA* OR Micro RNA OR Micro RNAs OR MiR OR
‘Primary MicroRNA’ OR ‘Primary miRNA*’ OR
Pri-miRNA* OR Pri miRNA* OR ‘Temporal RNA,
small’ OR ‘RNA, small temporal’ OR stRNA* OR ‘Small temporal RNA’
OR Pre-miRNA* OR Pre miRNA* OR MicroRNA* OR miRNA* OR
‘Micro RNA’ OR ‘Micro RNAs’ OR MiR OR ‘Primary MicroRNA’ OR
‘Primary miRNA*’ OR Pri-miRNA* OR Pri miRNA* OR ‘Temporal RNA,
small’ OR ‘RNA, small temporal’ OR stRNA* OR ‘Small
temporal RNA’ OR Pre-miRNA* OR Pre miRNA* |
| Hypospadias | ‘Hypospadias’[MeSH]
OR Hypospadia* OR ‘Urethral development’ OR ‘Urogenital
malformation*’ OR ‘Disorder of sexual development’ OR ‘development
of urethra’ OR ‘Urethra development’ OR ‘Urethra malformation*’ OR
DSD OR ‘Genital malformation*’ OR Hypospadia* OR ‘Urethral
development’ OR ‘Urogenital malformation*’ OR ‘Disorder of sexual
development’ OR ‘development of urethra’ OR ‘Urethra development’
OR ‘Urethra malformation*’ OR DSD OR ‘Genital malformation*’ |
Literature selection
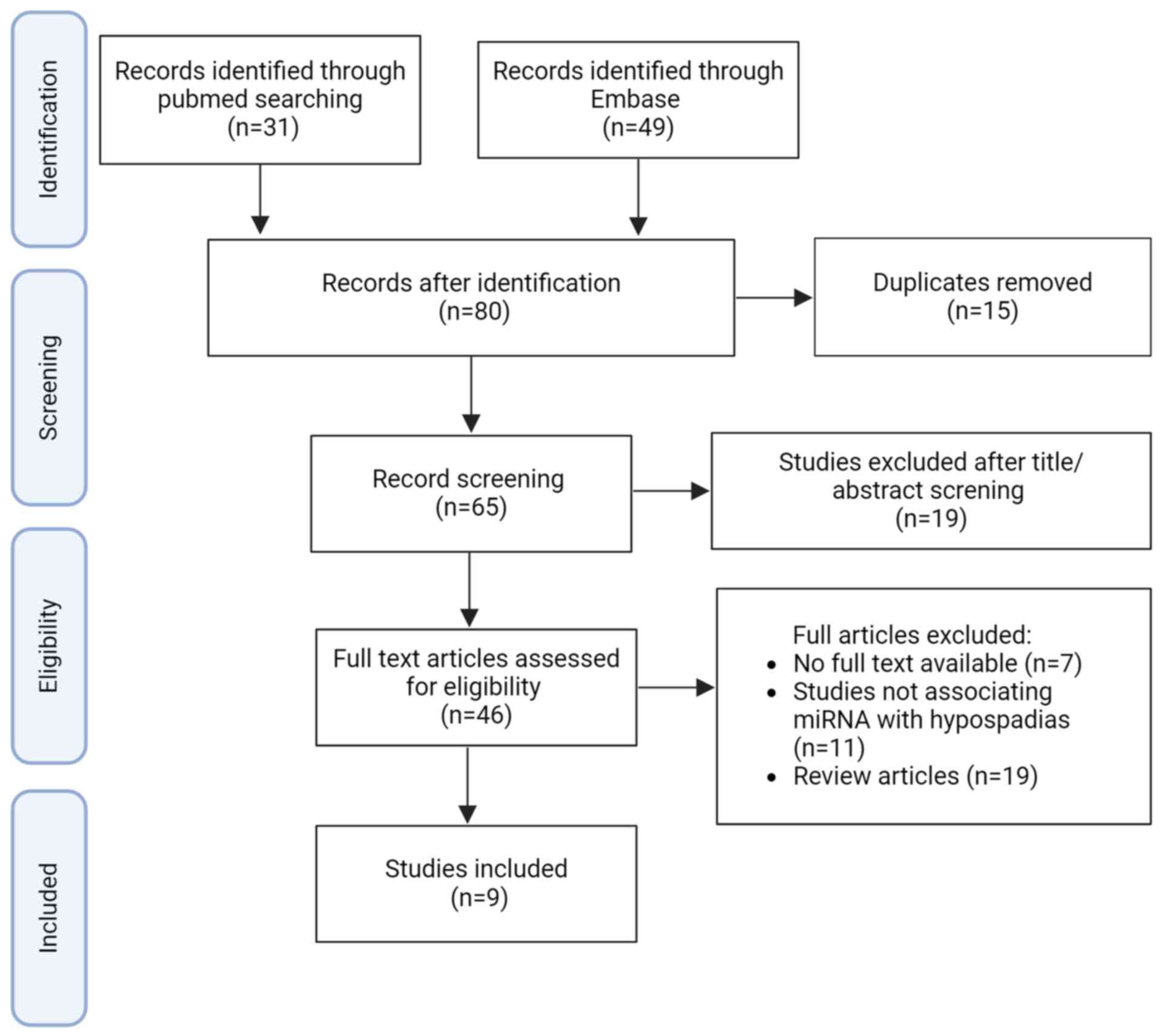
The literature search retrieved 65 studies (Fig. 1), whereof 9 studies were selected.
The titles and abstracts of all articles identified through the
search strategy were independently assessed by three authors
involved in the present study (MA, PHJ and MF). These authors also
conducted a thorough evaluation of the full articles, applying
predetermined eligibility criteria to make their selections. In
cases of disagreements the article was discussed, and a consensus
was reached.
The extracted data included publication details
(first author and year of publication), study design, participant
characteristics (mean age, disease type), miRNAs associated with
hypospadias, miRNA detection and analysis methods, and the role of
miRNAs, as discussed in the original article.
Results
To the best of our knowledge, no other systemic
review was found on the topic. In five studies, the sample species
were only human (25-29),
in one study, both human and rat samples (30) while in three studies, the sample
species were mice (31) and rats
(32,33). In total, the compiled studies
presented information on miR494, miR-145, miR-6756-5p, miR-182,
miR-210, miR-143-3p, miR-566 and miR200-c. The main findings of the
selected studies are compiled in Tables
II and III.
 | Table IISummary of literature search findings
in humans. |
Table II
Summary of literature search findings
in humans.
| Authors, year of
publication | Study design | Sample (size/cell
line number) | Age in human
samples | Type of
hypospadias | Type of
experiments | Key
results/comments | (Refs.) |
|---|
| Peng et al,
2023 |
Experimental/randomized parallel
group | Commercial HFF-1
cell line (CBP60935) | - | - | RT-qPCR, luciferase
assay and Transwell assay to investigate the involvement of
testosterone in hypospadias through miR-143-3p/IGFBP-3 axis. | miR-143-3p can bind
to IGFBP-3 by regulatory role of testosterone, activating HFF-1
cells growth and AR signaling, resulting in hypospadias
occurrence. | (28) |
| Elias et al,
2022 | Case-control
study | Blood samples of
patients with 46, XY DSD (n=18) and healthy (n=36) individuals | 1-55 years | Second- or
third-degree hypospadias | RT-qPCR to study
the relationship between miR-210 plasma level and atypical
genitalia at birth. | Plasma levels of
miR-210 expression may have a positive association with presence of
atypical genitalia in the patients with 46, XY DSD. | (27) |
| Deng et al,
2021 | Case-control
study | Venous blood
samples of hypospadias (n=557) and healthy (n=654) individuals | Unknown | Mild/moderate:
coronal or glanular or shaft penis hypospadias; Severe:
penoscrotal, scrotal and perineal hypospadias | Bioinformatic
analysis and luciferase assay to study the role of miR-182 and
GREM1 in development of hypospadias. | rs3743104 can
govern GREM1 expression by miR-182 leading to impact development of
hypospadias. | (26) |
| Chen et al,
2021 | Case-control
study | Penile skin tissue,
and 293 cell line | Unknown | Anterior:
glandular, coronal, and subcoronal, middle: penile, and posterior:
penoscrotal, scrotal, and perineal | SNP Selection and
genotyping, and luciferase assay to study the effect of GATA4
polymorphisms on hypospadias incidence. | miR-566 can be a
regulator of GATA4 expression, involving in development of
hypospadias. | (29) |
| Huang et al,
2023 |
Experimental/randomized parallel
group | Commercial HFF-1
cell line (ATCC number: SCRC-1041) | - | - | RT-qPCR, luciferase
assay, in situ hybridization and flow cytometry to explore the role
of hsa-circ-000417/miR-6756-5p/AR axis in penile mesenchymal cell
proliferation and apoptosis. |
hsa_circ_0000417/miR-6756-5p/AR/p-AKT axis
may play a regulatory role in the development of hypospadias by
reducing apoptosis of penile mesenchymal cells. | (25) |
| Shang et al,
2019 |
Experimental/randomized parallel
group | Hypospadias tissues
(n=208), preputial tissues (n=241) | 1-4 years | Mild/moderate:
Urethral opening between distal and middle penis. Severe: Urethral
opening between middle and radix penis, scrotum, or perineum | Study of miR-145
role in hypospadias development in human and rat hypospadias model
following by microarray, Luciferase assay, western blotting, and
flow cytometry. | miR-145 may have
regulatory role in the development of hypospadias through SOX9 and
MAPK signaling pathway. | (30) |
 | Table IIISummary of literature search findings
in animals. |
Table III
Summary of literature search findings
in animals.
| Authors, year of
publication | Study design | Species (sample
size) | Model
construction | Type of
hypospadias | Type of
experiments | Key
results/comments | (Refs.) |
|---|
| Chen et al.,
2024 |
Experimental/randomized parallel
group | Rat (n=unknown,
primary cells from normal and hypospadias urethral tissues) | Intragastric
administration of DBP in female pregnant rats at GD 14-18 | Unknown | RT-qPCR, luciferase
assay, and western blotting of primary cells isolated from normal
and hypospadias rat urethral tissues to determine the role of
SRD5A2 in the development of hypospadias through miR-1199-5p. | SRD5A2
downregulated miR-1199-5p and significantly influences key cellular
processes in hypospadias, underscoring the pivotal role of
miR-1199-5p in the development of this condition. | (33) |
| Tian et al.,
2020 |
Experimental/Randomized parallel
group | Mouse (n=17 in each
group) | Intragastric
administration of DEHP in female pregnant mice every day from GD12
to GD19 | i) A significantly
smaller genital protrusion, a clear fissure in the abdomen, not
prominent testis, female appearance; ii) the urethral orifice close
to side of the penis, and bent penis | Study of miR-494
role in mouse model of hypospadias by RT-qPCR, luciferase assay,
western blotting, and flow cytometry. | miR-494 can
progress hypospadias through binding to Nedd4L and activation of
TGF-β1/Smad signaling pathway. | (31) |
| Shang et
al., 2019 |
Experimental/randomized parallel
group | Rat (n=20 in each
group) | Intragastric
administration of DEHP in female pregnant rats at GD 14-18 | Mild/moderate:
Urethral opening between distal and middle penis; Severe: Urethral
opening between middle and radix penis, scrotum, or perineum | Study of miR-145
role in hypospadias development in human and rat hypospadias model
following by microarray, luciferase assay, western blotting, and
flow cytometry | miR-145 may have
regulatory role in development of hypospadias through SOX9 and MAPK
signaling pathway. | (30) |
| Qian et al.,
2016 |
Experimental/randomized parallel
group | Rat (n=20 in each
group) | Intragastric
administration of DEHP in female pregnant mice every day from GD12
to GD19 | Hypospadias of rats
were diagnosed based on length, curvature, and urethral opening
position of penis | RT-qPCR, luciferase
assay, immunohistochemistry and western blotting of rat hypospadias
model to explore the effect of miR-200c in development of
hypospadias. | MiR-200c can
promote hypospadias through TGF-β1/Smad3 signaling pathway by
increasing the expression of Zeb1. | (32) |
Human studies
The range of human sample sizes were from 54 to
1,211 individuals (Table II). The
samples were collected from patients with hypospadias of different
ages, ranging from 1 to 55 years, with an average age of 5 years.
The severity of hypospadias in participants in these studies ranged
from mild/moderate to severe. miRNA extraction had been performed
from blood samples, urethral epithelial tissues, foreskin
fibroblast cells and/or preputial tissues. These human studies
indicated the association between miR-6756-5p, miR-182, miR-210,
miR-143-3p, miR-556 and miR-145 and hypospadias (25-30).
The main analyses applied in these studies were reverse
transcription-quantitative polymerase chain reaction (RT-qPCR) and
dual luciferase reporter gene assay.
Huang et al (25) established a cell line model, using
mesenchymal-derived human foreskin fibroblasts (HFF-1), to
investigate the role of the hsa-circ-0000417/miR-6756-5p/androgen
receptor (AR) axis in PI3K/AKT signaling and in the apoptosis of
penile mesenchymal cells. The analysis was conducted using dual
luciferase assays, RT-qPCR, flow cytometry and western blot
analyses. Their findings indicated that high levels of miR-6756-5p
led to the increased proliferation and diminished apoptosis of
human foreskin fibroblasts (HFF-1), resulting in the development of
hypospadias (25).
Additionally, Deng et al (26) examined the impact of miR-182,
miR-212, miR-221 and miR-3128 on Gremlin1 (GREM1)
expression and the development of hypospadias using luciferase
assays. Their study involved 557 patients with hypospadias and 654
healthy controls. They extracted genomic DNA from patient blood
samples using TIANamp kits and analyzed the GREM1 rs3743104 SNP via
TaqMan genotyping. Bioinformatics tools, TargetScan and MirSNP,
were then used to identify potential miRNA binding sites in the
GREM1 3'-UTR. They reported that rs3743104 could affect the binding
of miR-182 to GREM1, potentially increasing the risk of developing
hypospadias (26).
Moreover, Elias et al (27) analyzed the expression of miR-210 in
plasma from patients with 46,XY investigated for differences in sex
development (DSD). Their study involved 36 male controls and 18
patients with 46,XY DSD, with controls divided into the
pre-pubertal and post-pubertal subgroups, and patients with DSD
categorized based on genitalia features and testicular position.
Blood samples were collected, processed and stored for RNA
extraction, and miRNA expression was analyzed using RT-qPCR. They
demonstrated that miR-210 expression was downregulated in the
plasma of patients with 46,XY DSD with hypospadias compared with
the control subjects (27).
Peng et al (28) demonstrated that miR-143-3p, under the
regulatory influence of testosterone, targeted insulin-like growth
factor-binding protein 3 (IGFBP-3). They treated HFF-1 cells with
testosterone, miR-143-3p mimics, or IGFBP-3 siRNA in various
experimental setups to investigate their roles. Transfections were
performed using Lipofectamine 2000, with RNA and protein
expressions analyzed using RT-qPCR and western blot analysis. Cell
viability, migration and luciferase reporter assays were conducted
to evaluate cellular responses using specific antibodies and
reagents. Their findings indicated that miR-143-3p, by targeting
IGFBP-3, inhibited cell growth and reduced AR signaling. This
action counteracted the effects of testosterone and may contribute
to the development of hypospadias (28).
Furthermore, Chen et al (29) conducted a case-control study
involving 410 boys with hypospadias and 520 healthy controls
between 2009 and 2017. They collected maternal and birth details
and excluded participants with a family history of hypospadias.
Genotyping of four SNPs (rs12825, rs12458, rs884662 and rs904018)
was conducted using TaqMan assays. A dual-luciferase reporter assay
revealed that the rs12458 variant could create a binding site for
miR-556, potentially influencing GATA binding protein 4
(GATA4) expression and increasing hypospadias risk. They
identified miR-556 as a regulator of GATA4 expression,
contributing to the development of hypospadias (29).
In the study by Shang et al (30), preputial tissue was collected from
pediatric patients following hypospadias repair surgery, and a rat
model of hypospadias was established with isolated spermatogonial
stem cells. Protein expression related to cell apoptosis and
oxidative stress using western blot analysis was analyzed.
Additionally, cell apoptosis, proliferation and viability were
assessed using flow cytometry,
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
assays and colony formation assays. Their results revealed that
miR-145 was highly expressed in hypospadias prepuce. Moreover,
their study revealed changes in the expression of miR-377, miR-497,
miR-37a and miR-424 in patients with hypospadias compared with
healthy controls (30).
Animal studies
In four identified studies, animal models were
applied to investigate the development of hypospadias (30-33).
The animal models of hypospadias were created by the daily
intragastric administration of Di (2-Ethylhexyl) phthalate (DEHP)
or dibutyl phthalate (DBP) to female pregnant mice (one study) or
rats (three studies), at different timepoints. The sample size in
the mouse study was 34 mice and in the two rat studies used 20 rats
in each (Table III). Moreover, in
one of the rat studies, the sample size was not specified. In these
studies, miRNA was extracted from penile tissue, urethral
epithelial tissue, and the genital tubercle. The main applied
analyses in the studies were RT-qPCR, dual luciferase reporter gene
assay, immunohistochemistry, and western blot analysis. These
animal studies demonstrated the association between miR-145,
miR-494, miR200-c and miR-1199-5p, and hypospadias. Shang et
al (30) administered DEHP to
female pregnant rats (Table III).
In their study, miR-145 expression differed between the hypospadias
and control groups.
In the study by Tian et al (31), DEHP was administered to female
pregnant mice. Following cesarean delivery, male pups with a
significantly smaller genital protrusion, urethral orifice at the
base of the penis, and bent penis were considered to present a
hypospadias phenotype (Table III).
Selected pups were euthanized, and the urethral tissues were
collected for further analyses, including histological staining and
immunohistochemistry to assess tissue morphology and cellular
changes. Their results revealed a higher expression of miR-494 in
urethral tissue from male mice with hypospadias compared with the
control mice (31).
Qian et al (32) also administered DEHP to female
pregnant rats. They selected newborn rats with hypospadias by
measuring the length, curvature and the position of the urethral
opening on the penis. Penile tissues were then collected, stored,
and analyzed for miR-200c and protein expression using RT-qPCR,
immunohistochemistry and western blot analysis techniques. They
demonstrated that the downregulation of miR-200c increased the
expression of zinc finger E-box binding homeobox 1 (Zeb1) in
rats, governing the TGF-β/suppressor of mothers against
decapentaplegic (Smad)3 pathway and resulting in the formation of
hypospadias (32).
Chen et al (33) investigated the role of steroid 5
alpha-reductase type 2 (SRD5A2) in cell proliferation, migration,
invasion and epithelial-mesenchymal transformation (EMT) in
hypospadias. They developed a model of hypospadias by administering
DBP daily to healthy pregnant female rats. Primary cells were then
isolated from normal and hypospadias rat urethral tissues and
cultured in a complete medium at 37˚C with 5% CO2. The
cells were infected with lentiviral vectors carrying either
silenced or overexpressed SRD5A2, selected using puromycin, and
further analyzed for gene expression using RT-qPCR and protein
expression using western blot analysis. Their findings indicated
that SRD5A2 negatively regulated miR-1199-5p and significantly
affected key cellular processes in hypospadias, highlighting the
critical role of miR-1199-5p in the development of the condition
(33).
Associations between clinical features
and miRNAs
To elucidate the role of miRNAs in the clinical
manifestation of hypospadias, the present study examined the
association between specific miRNAs and clinical features. As
regards the association of miRNAs with the severity of hypospadias,
miR-494 has been shown to be associated with severe forms of
hypospadias (31). This is
potentially due to its regulatory effects on the TGF-β1/Smad
signaling pathway, which is critical in tissue remodeling and
development. The increased expression of miR-494 may be linked to a
greater disruption of urethral development, being associated with
more severe anatomical presentations (31,34).
Conversely, the downregulation of miR-200c expression has been
detected in severe hypospadias cases (32). This miRNA is known to regulate EMT
processes, which are crucial for proper urethral fusion (35). Its reduced expression suggests a loss
of regulation in mesenchymal transition, contributing to severe
phenotypes. As regards the association of miRNAs with anatomical
characteristics, miR-145 has been implicated in regulating
apoptosis and oxidative stress in penile tissues (36,37),
suggesting an association with abnormal penile curvature and
urethral positioning observed in moderate to severe hypospadias.
Its high expression in these tissues indicates that it may
contribute to the structural characteristic of the condition.
Moreover, miR-6756-5p has been shown to be
associated with alterations in AR signaling, which plays a role in
penile and urethral development (25). Higher levels of miR-6756-5p have been
linked to the dysregulation of AR expression, indicating a
potential link between miRNA levels and specific anatomical
anomalies. Furthermore, the interaction of miR-182 with
GREM1 suggests its potential role in hypospadias progression
through the bone morphogenic protein (BMP) signaling pathway, which
is known to be involved in urethral development (26). Variants in miR-182 may be associated
with familial cases or those presenting with additional urogenital
anomalies. miR-210, a hypoxia-inducible miRNA, exhibits higher
levels in patients with 46,XY DSD and hypospadias, suggesting a
link to tissue oxygenation status and potential developmental
delays in genital formation (27).
Discussion
The main purpose of the present systematic review
was to explore what was already described regarding the role of
miRNAs in hypospadias. The literature was analyzed systematically
and a total of nine different miRNAs were determined in the nine
selected articles.
In one of the studies, Tian et al (31) indicated that miR-494 decreased the
expression of Neural precursor cell expressed developmentally
downregulated gene 4-like (Nedd4L), resulting in the activation of
the TGF-β1/Smad signaling pathway and the promotion of hypospadias.
Accordingly, Liu et al (38)
demonstrated that the higher expression of TGF-β1 promoted the
occurrence of hypospadias in DEHP-treated mice. Of note, other
studies have demonstrated the essential role of the TGF-β1
signaling pathway in urethral development (38-40).
Moreover, the TGF-β signaling pathway and its downstream genes,
including activating transcription factor 3, connective tissue
growth factor and cysteine-rich angiogenic inducer 61, have been
suggested as potential factors in the etiology of hypospadias
(38). TGF-β1 governs a vast range
of biological processes, such as proliferation, EMT and apoptosis
(41-43).
EMT is a critical event in urethral embryogenesis (44,45).
During this critical biological process, epithelial cells achieve
mesenchymal features, such as becoming non-polarized, losing
intercellular adhesions and moving throughout the extracellular
matrix for tissue generation (46).
Previous studies have suggested the transformation of urethral
epithelial cells into mesenchymal cells following urethral plate
fusion through EMT (44,46). The disruption of the EMT process can
cause a failure of the urethral plate fusion, leading to the
occurrence of hypospadias (46-48).
A recent study determined that miR-1199-5p targets SRD5A2,
influencing EMT transformation and promoting the development of
hypospadias, highlighting the crucial role of EMT through
miR-1199-5p in this condition (33).
Moreover, TGF-β1 signaling also plays a critical role in
hypospadias development by its regulation of EMT. Notably, recent
studies have demonstrated miR-494 as a key regulator of TGF-β1
signaling (49-51).
Previous studies have also demonstrated that miRNAs
play a critical role in the regulation of Nedd4L expression,
which mediates TGF-β1 signaling (52,53). It
has been shown that Nedd4L is a critical ubiquitin ligase which
selectively targets the activation of Smad2/3 for degradation,
resulting in the suppression of TGF-β1 signaling (52). Therefore, miR-494 can indirectly
regulate the occurrence of hypospadias. Accordingly, Qian et
al (32) confirmed the
importance of the TGF-β1 signaling pathway in the occurrence of
hypospadias through the regulatory role of miR-200c by comparing a
rat model of hypospadias induced by DEHP administration with
healthy rats. They indicated that there were low levels of miR-200c
expression in hypospadias penile tissues, resulting in the high
expression of the Zeb1 gene and protein, thereby activating the
TGF-b/Smad3 pathway (32). However,
both studies included a small sample size, and the degree of
hypospadias was not specified in their findings.
miR-145 has been suggested as another potential
regulator for the progression of hypospadias in the study by Shang
et al (30), one of the
studies selected for the present systematic review. Previous
studies have demonstrated the regulatory role of miR-145 in the
expression of pluripotency factors, leading to the reduction of
self-renewal and promoting the differentiation of human embryonic
cells (54,55). In addition, miR-145 has been
demonstrated to affect embryo attachment by reducing the expression
of maternal IGF1R. miR-145 has also been shown as a suppressor of
phosphorylated extracellular-regulated kinase expression by
targeting PAK4, resulting in the reduction of human colorectal cell
growth (56). The importance of
mitogen-activated protein kinase (MAPK) signaling expands to
spermatogenesis and dysgenesis activated by environmental
toxicities. This can be ascribed to the compromised blood-testis
barrier, leading to reduce semen quality, which could also serve as
a contributing factor to hypospadias (57). Of note, MAPK signaling can alter the
proper action of downstream SRY-Box transcription actor 9 (SOX9)
and the mutation of SOX9 can progress the development of
hypospadias (58). SOX9 was found to
increase testis development in transgenic mice with XX karyotype
(59). Furthermore, the mutation of
SOX9 can enhance the progression of campomelic syndrome and sex
reversal of XY (60). All the
effects of SOX9 on testis development, sex differentiation and
hormone secretion suggest a linkage between SOX9 and hypospadias
development. Of note, SOX9 has been demonstrated as a target gene
of miR-145 for regulating cell activities. Moreover, several
studies have shown the accelerating effects of miR-145 on cell
apoptosis (61-64).
These studies have revealed its inhibitory roles on the
proliferation, migration and invasion of the cells through multiple
targets, such as Mucin 1, c-Myc, Kirsten rat sarcoma virus, Bax and
caspase-3 (61,62).
Cell apoptosis has been demonstrated as a key
criterion for normal embryogenesis of male genital tubercles and
the anterior urethra. Furthermore, the abnormal alteration of
apoptosis in the urethra of mice has been reported in mouse models
of hypospadias. Therefore, miR-145 may progress the development of
hypospadias through aberrant cell apoptosis. Surprisingly, Shang
et al (30) suggested the
regulatory role of miR-145 in the development of hypospadias was
through MAPK signaling and SOX9 expression. They also demonstrated
the inhibitory role of miR-145 on nuclear factor erythroid
2-related factor 2-HO-1 signaling and downstream glutathione
peroxidase 1 and superoxide dismutase type 1 expression,
demonstrating the role of miR-145 as a contributor to hypospadias
through the suppression of the antioxidant system. A recent study
confirmed the theory of hypospadias development through apoptosis
by demonstrating the regulatory role of miR-556 in the GATA4
expression level (29). GATA4
has also been shown as a regulator of cell survival
(anti-apoptotic) signaling (65).
This is supported by findings from the study by Grepin et al
(66), where the depletion of GATA4
led to apoptosis. These studies demonstrate the involvement of both
miR-145 and miR-556 in the development of hypospadias through
apoptosis.
miR-6756-5p has been proposed as a potential
biomarker involved in the progression of hypospadias. Huang et
al (25) demonstrated that a
high level of miR-6756-5p led to AR suppression, enhanced the
activation of PI3K/AKT pathway, the proliferation of HFF-1 cells
and impaired apoptosis, potentially contributing to the development
of hypospadias. However, the precise functional impact of these
molecular events and cellular responses on the progression of
hypospadias was not examined in their study. This is a significant
limitation as without this information, a direct link between the
two cannot be definitively established. Of note, previous studies
have indicated that patients with severe hypospadias exhibit a
defect in the AR gene (67-70).
Specifically, there is evidence suggesting that a crucially reduced
level of AR mRNA expression could play a significant role in the
development of hypospadias at the midshaft (67). Therefore, miR-6756-5p may play a
pivotal role as a post-transcriptional regulator of AR in genital
mesodermal cells, which could potentially contribute to hypospadias
driven by AR loss in humans. Peng et al (28) treated HFF-1 cells with testosterone
and revealed that miR-143-3p, by targeting IGFBP-3, impede cell
growth and decrease AR signaling, thereby counteracting
testosterone, leading to the progression of hypospadias. Therefore,
miR-143-3p can be another potential regulator of AR signaling,
resulting in the regulation of hypospadias development. Notably, it
should be considered that their study did not explore the detailed
mechanism or the direct connection between AR signaling and the
progression of hypospadias. Furthermore, all their findings were at
the cellular level and require validation in human and animal
samples. miR-143-3p has also been shown to modulate specific target
genes associated with cancer, cardiovascular disease and other
health conditions, influencing the progression of these diseases in
numerous studies (71-74).
Furthermore, studies have indicated that IGFBP-3 plays a role in
influencing the progression of various diseases (75-77).
This is achieved by inhibiting receptor binding and impeding their
anti-apoptotic functions. Additionally, IGFBP-3 has been reported
to hinder angiogenesis and impact apoptosis (78-80).
Deng et al (26) also discovered that miR-182 can target
the seeding region encompassing rs3743104 within the 3'-UTR of
GREM1, which in turn inhibits GREM1 transcription. miR-182 has
previously been documented as a biomarker for kidney injury and
bladder cancer (81,82). It functions as a regulatory miRNA by
binding to the 3'-UTR of target genes, influencing the progression
of diseases (81). GREM1 is
recognized as part of the bone morphogenic protein antagonist
family (BMP) (82), and has been
linked to susceptibility to hypospadias. Although these findings
did not demonstrate a direct link between miR-182 and the
progression of hypospadias, miR-182 could potentially play a key
role in advancing the development of hypospadias through the BMP
signaling pathway.
miR-210 may serve as another regulatory key in the
onset of hypospadias. Elias et al (27) measured the plasma level of miR-210 in
patients with 46,XY DSD with atypical genitalia, demonstrating a
higher expression of miR-210 in hypospadias compared with the
control group. It has been shown that miR-210 directly targets IGF2
through in vitro experiments conducted in NT2 cells.
Consequently, these findings suggest a potential association
between miR-210 and Insulin/IGF signaling. However, it should be
considered that their study involved a small group of patients.
Prior research has demonstrated that miR-210 expression is elevated
in the testes of infertile men with maturation arrest (83,84).
Moreover, miR-210 exhibits a ubiquitous expression across a diverse
range of cells, playing physiological roles, such as suppressing
cell proliferation, mitochondrial respiration and DNA repair, and
affecting vascular biology and angiogenesis (85-87).
Recognized as a major hypoxia-inducible miRNA, its relevance is
noteworthy in conditions, such as placental hypertension and
preeclampsia, which can induce fetal hypoxia (88). A previous study demonstrated that
insulin/IGF and hypoxia signaling act in concert in
Caenorhabditis elegans (89).
Therefore, miR-210 can promote the development of hypospadias
through insulin/IGF1 and hypoxia signaling.
Surprisingly, several miRNAs in the reviewed
articles, including miR-494, miR-145, miR-6756-5p, miR-182, miR-210
and miR-200c, were found to be associated with key clinical
features of hypospadias, such as disease severity and specific
anatomical abnormalities. miR-494 was found to be associated with
more severe forms of the condition, potentially due to its impact
on TGF-β1/Smad signaling pathways. Moreover, the downregulation of
miR-200c was linked to impaired EMT processes in urethral
development. To investigate the role of miRNAs in the clinical
presentation of hypospadias, the association between specific
miRNAs and key clinical characteristics was analyzed. The analysis
suggested that specific miRNAs could serve as biomarkers for
predicting the severity and anatomical characteristics of
hypospadias. Further studies with larger sample sizes are required
however, to validate these findings and explore the direct
mechanistic roles of these miRNAs in hypospadias.
The present systematic review provides a
comprehensive analysis of the role of specific miRNAs in the
development of hypospadias, integrating evidence from both human
and animal studies. By highlighting miRNAs, such as miR-494,
miR-145, miR-6756-5p, miR-182, miR-210, miR-143-3p, miR-556 and
miR-200c, the present systematic review identifies key molecular
pathways, including TGF-β, MAPK, PI3K/AKT, BMP and AR signaling
(Fig. 2), that are crucial in
regulating cellular processes, such as proliferation, apoptosis and
differentiation resulting in hypospadias progression. The findings
suggest that miRNAs do not merely function as biomarkers, but
actively participate in disease progression, linking genetic
predispositions with environmental influences. This integrative
approach provides valuable insight into the molecular mechanisms
underlying hypospadias and underscores the potential of miRNAs as
therapeutic targets, setting the stage for future translational
research aimed at developing novel diagnostic and treatment
strategies.
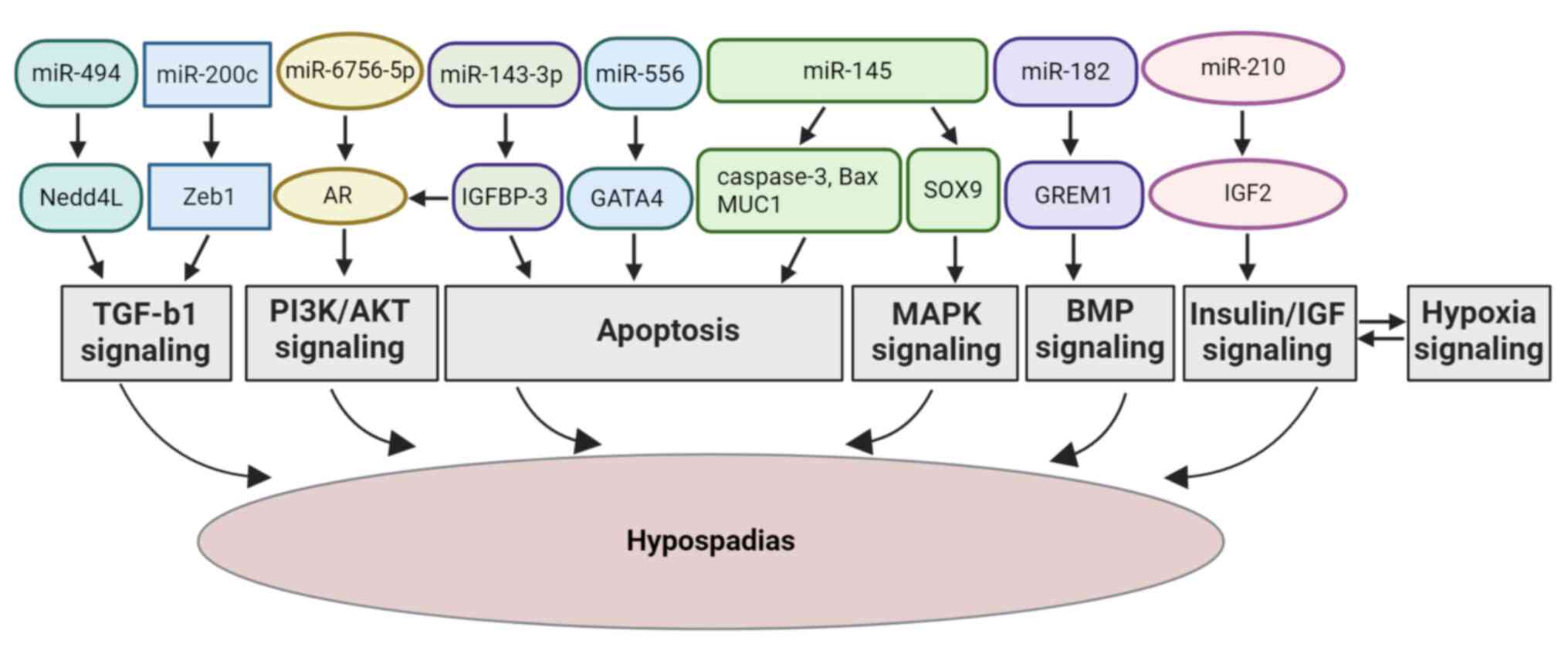 | Figure 2Schematic diagram presenting the
mechanisms through which the regulatory roles of miR-494, miR-200c,
miR-145, miR-6756-5p, miR-143-3p, miR-182, miR-210 and miR-556 can
lead to hypospadias through the TGF-β, MAPK, PI3K/AKT, AR, BMP,
insulin/IGF, hypoxia signaling pathways, and apoptosis,
respectively. MiR, microRNA; Nedd4L, neural precursor cell
expressed developmentally downregulated 4-like; TGF-β1,
transforming growth factor-β1; Smad, suppressor of mothers against
decapentaplegic; SOX9, sex-determining region Y box 9; MAPK,
mitogen-activated protein kinase; Zeb1, zinc finger E-box binding
homeobox 1; IGFBP-3, insulin-like growth factor binding protein;
AR, androgen receptor; PI3K/AKT, phosphoinositide 3-kinase/protein
kinase B; GREM1, Gremlin-1; GATA4, GATA binding protein 4; IGF2,
insulin-like growth factor 2. |
However, the included studies faced significant
limitations, including small sample sizes, a lack of stratified
analyses, the absence of specific treatment groups and findings
limited to cellular level, which restricted the understanding of
the molecular mechanisms and the direct links between miRNAs and
hypospadias progression. Further investigations are required to
validate these findings in human and animal samples and to explore
the detailed roles of miRNAs in the development of hypospadias. A
limitation of the present systematic review is that the literature
search was conducted using only two databases, which may have
restricted the comprehensiveness of the review and may have thus
potentially excluded relevant studies. Several potential biases in
the included studies have been highlighted throughout the present
systematic review. Small sample sizes reduce the statistical power
and limit generalizability, while selection bias arises from
focusing on specific populations, such as pediatric patients and
animal models. Language bias from including only studies in the
English language and publication bias favoring significant results
may skew the findings. Variability in experimental methods and the
lack of stratified analyses further complicate comparisons across
studies. The majority of the studies were observational, lacked
validation in human samples, and often relied on single time point
measurements, which may miss dynamic changes. Additionally,
confounding factors, such as environmental exposures and genetic
variability were not consistently controlled, limiting the ability
to establish the link between miRNAs and hypospadias.
In conclusion, the present systematic review
demonstrated the link between miR-494, miR-145, miR-6756-5p,
miR-182, miR-200c, miR-210 and miR-1199-5p, and the occurrence of
hypospadias. The majority of the included articles focused on
etiological factors. Notably, miR-494, miR-200c, miR-145,
miR-6756-5p, miR-182 and miR-210 could promote the development of
hypospadias through different signaling pathways, such as TGF-β,
MAPK, PI3K/AKT, BMP, insulin/IGF and hypoxia. These pathways are
also involved in tissue regeneration and wound healing. Therefore,
the present systematic review suggests that miRNAs may serve as
potential biomarkers related to hypospadias and may play a role in
potentiating wound healing following surgery. Further
investigations are required however, to shed light onto the
regulatory role of miRNAs in the development of hypospadias, which
can provide a novel direction for the understanding of the etiology
of hypospadias and improving treatment modalities.
Acknowledgements
The authors would like to thank the information
specialist, Mrs. Annemette Møller Hansen, at Copenhagen University,
Copenhagen, Denmark, for providing excellent advice on the search
strategies for the present systematic review.
Funding
Funding: The present study was supported by the Freemason's Fund
for Children's Health in Stockholm, the Promobilia Foundation, The
Danish Frie Forskningsfond (grant no. 2096-00151), Greater
Copenhagen Health Science Partners CAG-SURF (grant no.
3141-00016A), and the Novo Nordisk Foundation (NNFSA170030576).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
MF was involved in the conceptualization of the
study, as well as in funding acquisition and study supervision. MA
and PHJ were involved in the study methodology and in data
acquisition, as well as in the writing of the original draft of the
manuscript. MA was involved in data visualization. MA, PHJ and MF
were involved in the investigative aspects of the study. MA, PHJ,
EP, ABK and MF were involved in the data interpretation, writing,
review and editing of the manuscript. MA, PHJ and MF confirmed the
authenticity of the raw data. All authors have read and approved
the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Lee HJ: Additional stories of microRNAs.
Exp Biol Med (Maywood). 239:1275–1279. 2014.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Alles J, Fehlmann T, Fischer U, Backes C,
Galata V, Minet M, Hart M, Abu-Halima M, Grässer FA, Lenhof HP, et
al: An estimate of the total number of true human miRNAs. Nucleic
Acids Res. 47:3353–3364. 2019.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Horvitz HR and Sulston JE: Isolation and
genetic characterization of cell-lineage mutants of the nematode
Caenorhabditis elegans. Genetics. 96:435–454.
1980.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Hammond SM: An overview of microRNAs. Adv
Drug Deliv Rev. 87:3–14. 2015.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Denli AM, Tops BB, Plasterk RH, Ketting RF
and Hannon GJ: Processing of primary microRNAs by the
Microprocessor complex. Nature. 432:231–235. 2004.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Gregory RI, Yan KP, Amuthan G, Chendrimada
T, Doratotaj B, Cooch N and Shiekhattar R: The Microprocessor
complex mediates the genesis of microRNAs. Nature. 432:235–240.
2004.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Kim VN, Han J and Siomi MC: Biogenesis of
small RNAs in animals. Nat Rev Mol Cell Biol. 10:126–139.
2009.PubMed/NCBI View
Article : Google Scholar
|
|
8
|
Han J, Lee Y, Yeom KH, Nam JW, Heo I, Rhee
JK, Sohn SY, Cho Y, Zhang BT and Kim VN: Molecular basis for the
recognition of primary microRNAs by the Drosha-DGCR8 complex. Cell.
125:887–901. 2006.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Okada C, Yamashita E, Lee SJ, Shibata S,
Katahira J, Nakagawa A, Yoneda Y and Tsukihara T: A high-resolution
structure of the pre-microRNA nuclear export machinery. Science.
326:1275–1279. 2009.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Chendrimada TP, Gregory RI, Kumaraswamy E,
Norman J, Cooch N, Nishikura K and Shiekhattar R: TRBP recruits the
Dicer complex to Ago2 for microRNA processing and gene silencing.
Nature. 436:740–744. 2005.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Lee Y, Hur I, Park SY, Kim YK, Suh MR and
Kim VN: The role of PACT in the RNA silencing pathway. EMBO J.
25:522–532. 2006.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Khvorova A, Reynolds A and Jayasena SD:
Functional siRNAs and miRNAs exhibit strand bias. Cell.
115:209–216. 2003.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Schwarz DS, Hutvagner G, Du T, Xu Z,
Aronin N and Zamore PD: Asymmetry in the assembly of the RNAi
enzyme complex. Cell. 115:199–208. 2003.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Kilikevicius A, Meister G and Corey DR:
Reexamining assumptions about miRNA-guided gene silencing. Nucleic
Acids Res. 50:617–634. 2022.PubMed/NCBI View Article : Google Scholar
|
|
15
|
O'Brien J, Hayder H, Zayed Y and Peng C:
Overview of MicroRNA biogenesis, mechanisms of actions, and
circulation. Front Endocrinol (Lausanne). 9(402)2018.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Samad AFA and Kamaroddin MF: Innovative
approaches in transforming microRNAs into therapeutic tools. Wiley
Interdiscip Rev RNA. 14(e1768)2023.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Van Rooij E and Olson EN: MicroRNA
therapeutics for cardiovascular disease: Opportunities and
obstacles. Nat Rev Drug Discov. 11:860–872. 2012.PubMed/NCBI View
Article : Google Scholar
|
|
18
|
Forterre A, Komuro H, Aminova S and Harada
M: A comprehensive review of cancer MicroRNA therapeutic delivery
strategies. Cancers (Basel). 12(1852)2020.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Nordenvall AS, Frisen L, Nordenstrom A,
Lichtenstein P and Nordenskjold A: Population based nationwide
study of hypospadias in Sweden, 1973 to 2009: Incidence and risk
factors. J Urol. 191:783–789. 2014.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Van der Horst HJ and de Wall LL:
Hypospadias, all there is to know. Eur J Pediatr. 176:435–441.
2017.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Fredell L, Lichtenstein P, Pedersen NL,
Svensson J and Nordenskjold A: Hypospadias is related to birth
weight in discordant monozygotic twins. J Urol. 160:2197–2199.
1998.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Blaschko SD, Cunha GR and Baskin LS:
Molecular mechanisms of external genitalia development.
Differentiation. 84:261–268. 2012.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Van der Zanden LF, van Rooij IA, Feitz WF,
Franke B, Knoers NV and Roeleveld N: Aetiology of hypospadias: A
systematic review of genes and environment. Hum Reprod Update.
18:260–283. 2012.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Bouty A, Ayers KL, Pask A, Heloury Y and
Sinclair AH: The genetic and environmental factors underlying
hypospadias. Sex Dev. 9:239–259. 2015.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Huang J, Su C, Lu P, Zhao X, Liu Y, Xie Q
and Chen C: hsa_circ_0000417 downregulation suppresses androgen
receptor expression and apoptotic signals in human foreskin
fibroblasts via sponging miR-6756-5p. Mol Biol Rep. 50:6769–6781.
2023.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Deng F, Zhao J, Jia W, Fu K, Zuo X, Huang
L, Wang N, Xia H, Zhang Y, Fu W and Liu G: Increased hypospadias
risk by GREM1 rs3743104[G] in the southern Han Chinese population.
Aging (Albany NY). 13:13898–13908. 2021.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Elias FM, Nishi MY, Sircili MHP, Bastista
RL, Gomes NL, Ferrari MTM, Costa EMF, Denes FT, Mendonca BB and
Domenice S: Elevated plasma miR-210 expression is associated with
atypical genitalia in patients with 46,XY differences in sex
development. Mol Genet Genomic Med. 10(e2084)2022.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Peng QL, Zhao YW and Tian W: Testosterone
promotes human foreskin fibroblast growth through miR-143-3p
targeting IGFBP-3. J Men's Health. 19:15–25. 2023.
|
|
29
|
Chen J, Cui X, Li A, Li G and Sun F:
Association of a GATA Binding protein 4 polymorphism with the risk
of hypospadias in the Chinese children. Urol Int. 105:1018–1023.
2021.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Shang Y, Kang Y, Sun J, Wei P, Yang J and
Zhang H: MiR-145-modulated SOX9-mediated hypospadias through acting
on mitogen-activated protein kinase signaling pathway. J Cell
Physiol. 234:10397–10410. 2019.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Tian RH, Guo KM, Han GH and Bai Y:
Downregulation of MicroRNA-494 inhibits the TGF-beta1/Smads
signaling pathway and prevents the development of hypospadias
through upregulating Nedd4L. Exp Mol Pathol.
115(104452)2020.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Qian C, Dang X, Wang X, Xu W, Pang G, Chen
Y and Liu C: Molecular mechanism of MicroRNA-200c regulating
transforming growth factor-β (TGF-β)/SMAD family member 3 (SMAD3)
pathway by targeting zinc finger E-Box binding homeobox 1 (ZEB1) in
hypospadias in rats. Med Sci Monit. 22:4073–4081. 2016.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Chen Y, Hu J, Peng L and Zhao Y:
MicroR-1199-5p targeting SRD5A2 promotes the biological behavior
and EMT of hypospadias cells. Cell Mol Biol (Noisy-le-Grand).
70:122–128. 2024.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Lan YF, Chen HH, Lai PF, Cheng CF, Huang
YT, Lee YC, Chen TW and Lin H: MicroRNA-494 reduces ATF3 expression
and promotes AKI. J Am Soc Nephrol. 23:2012–2023. 2012.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Gollavilli PN, Parma B, Siddiqui A, Yang
H, Ramesh V, Napoli F, Schwab A, Natesan R, Mielenz D and Asangani
IA: The role of miR-200b/c in balancing EMT and proliferation
revealed by an activity reporter. Oncogene. 40:2309–2322.
2021.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Hu D, Ge Y, Xi Y, Chen J, Wang H, Zhang C,
Cui Y, He L, Su Y, Chen J, et al: MicroRNA-145 gene modification
enhances the retention of bone marrow-derived mesenchymal stem
cells within corpus cavernosum by targeting kruppel-like factor 4.
World J Mens Health. 42:638–649. 2024.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Zheng W, Li T, Wei J, Zhang Y, Zuo Q and
Lin Y: Identification of miR-145 as a regulator of the
cardiomyocyte inflammatory response and oxidative stress under
hyperglycemia. Exp Ther Med. 21(467)2021.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Liu X, He DW, Zhang DY, Lin T and Wei GH:
Di(2-ethylhexyl) phthalate (DEHP) increases transforming growth
factor-beta1 expression in fetal mouse genital tubercles. J Toxicol
Environ Health A. 71:1289–1294. 2008.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Willingham E and Baskin LS: Candidate
genes and their response to environmental agents in the etiology of
hypospadias. Nat Clin Pract Urol. 4:270–279. 2007.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Baskin LS, Hayward SW, Sutherland RA,
DiSandro MS, Thomson AA and Cunha GR: Cellular signaling in the
bladder. Front Biosci. 2:d592–d595. 1997.PubMed/NCBI View
Article : Google Scholar
|
|
41
|
Tomlinson DC, Freestone SH, Grace OC and
Thomson AA: Differential effects of transforming growth
factor-beta1 on cellular proliferation in the developing prostate.
Endocrinology. 145:4292–4300. 2004.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Tian YC, Chen YC, Chang CT, Hung CC, Wu
MS, Phillips A and Yang CW: Epidermal growth factor and
transforming growth factor-beta1 enhance HK-2 cell migration
through a synergistic increase of matrix metalloproteinase and
sustained activation of ERK signaling pathway. Exp Cell Res.
313:2367–2377. 2007.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Sanchez-Capelo A: Dual role for TGF-beta1
in apoptosis. Cytokine Growth Factor Rev. 16:15–34. 2005.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Zhou Y, Liu X, Huang F, Liu Y, Cao X, Shen
L, Long C, He D, Lin T and Wei G: Epithelial-mesenchymal
transformation and apoptosis in rat urethra development. Pediatr
Res. 82:1073–1079. 2017.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Baskin LS, Erol A, Jegatheesan P, Li Y,
Liu W and Cunha GR: Urethral seam formation and hypospadias. Cell
Tissue Res. 305:379–387. 2001.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Zhou Y, Huang F, Liu Y, Li D, Zhou Y, Shen
L, Long C, Liu X and Wei G: TGF-β1 relieves epithelial-mesenchymal
transition reduction in hypospadias induced by DEHP in rats.
Pediatr Res. 87:639–646. 2020.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Kubiczkova L, Sedlarikova L, Hajek R and
Sevcikova S: TGF-β-an excellent servant but a bad master. J Transl
Med. 10(183)2012.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Chen T, Li Q, Xu J, Ding K, Wang Y, Wang
W, Li S and Shen Y: Mutation screening of BMP4, BMP7, HOXA4 and
HOXB6 genes in Chinese patients with hypospadias. Eur J Hum Genet.
15:23–28. 2007.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Yang Y, Zhang Y, Lin Z, Wu K, He Z, Zhu D,
Zhao J, Zhang C and Fan Y: Silencing of histone deacetylase 3
suppresses the development of esophageal squamous cell carcinoma
through regulation of miR-494-mediated TGIF1. Cancer Cell Int.
22(191)2022.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Zhang J, Zhu Y, Hu L, Yan F and Chen J:
miR-494 induces EndMT and promotes the development of HCC
(Hepatocellular Carcinoma) by targeting SIRT3/TGF-β/SMAD signaling
pathway. Sci Rep. 9(7213)2019.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Maharati A, Akhlaghipour I, Taghehchian N,
Farshchian Yazdi Z and Moghbeli M: Role of microRNA-494 in tumor
progression. Am J Transl Res. 15:6342–6361. 2023.PubMed/NCBI
|
|
52
|
Gao S, Alarcon C, Sapkota G, Rahman S,
Chen PY, Goerner N, Macias MJ, Erdjument-Bromage H, Tempst P and
Massagué J: Ubiquitin ligase Nedd4L targets activated Smad2/3 to
limit TGF-beta signaling. Mol Cell. 36:457–468. 2009.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Chu MQ, Zhang LC, Yuan Q, Zhang TJ and
Zhou JD: Distinct associations of NEDD4L expression with genetic
abnormalities and prognosis in acute myeloid leukemia. Cancer Cell
Int. 21(615)2021.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Xu N, Papagiannakopoulos T, Pan G, Thomson
JA and Kosik KS: MicroRNA-145 regulates OCT4, SOX2, and KLF4 and
represses pluripotency in human embryonic stem cells. Cell.
137:647–658. 2009.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Kang YJ, Lees M, Matthews LC, Kimber SJ,
Forbes K and Aplin JD: MiR-145 suppresses embryo-epithelial
juxtacrine communication at implantation by modulating maternal
IGF1R. J Cell Sci. 128:804–814. 2015.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Wang Z, Zhang X, Yang Z, Du H, Wu Z, Gong
J, Yan J and Zheng Q: MiR-145 regulates PAK4 via the MAPK pathway
and exhibits an antitumor effect in human colon cells. Biochem
Biophys Res Commun. 427:444–449. 2012.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Yamaguchi K, Ishikawa T, Kondo Y and
Fujisawa M: Cisplatin regulates Sertoli cell expression of
transferrin and interleukins. Mol Cell Endocrinol. 283:68–75.
2008.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Wang Y, Li Q, Xu J, Liu Q, Wang W, Lin Y,
Ma F, Chen T, Li S and Shen Y: Mutation analysis of five candidate
genes in Chinese patients with hypospadias. Eur J Hum Genet.
12:706–712. 2004.PubMed/NCBI View Article : Google Scholar
|
|
59
|
Vidal VP, Chaboissier MC, de Rooij DG and
Schedl A: Sox9 induces testis development in XX transgenic mice.
Nat Genet. 28:216–217. 2001.PubMed/NCBI View
Article : Google Scholar
|
|
60
|
Olney PN, Kean LS, Graham D, Elsas LJ and
May KM: Campomelic syndrome and deletion of SOX9. Am J Med Genet.
84:20–24. 1999.PubMed/NCBI
|
|
61
|
Zeinali T, Karimi L, Hosseinahli N,
Shanehbandi D, Mansoori B, Mohammadi A, Hajiasgharzadeh K, Babaloo
Z, Majidi-Zolbanin J and Baradaran B: Overexpression of miRNA-145
induces apoptosis and prevents proliferation and migration of
MKN-45 gastric cancer cells. EXCLI J. 19:1446–1458. 2020.PubMed/NCBI View Article : Google Scholar
|
|
62
|
Ye D, Shen Z and Zhou S: Function of
microRNA-145 and mechanisms underlying its role in malignant tumor
diagnosis and treatment. Cancer Manag Res. 11:969–979.
2019.PubMed/NCBI View Article : Google Scholar
|
|
63
|
Manvati S, Mangalhara KC, Kalaiarasan P,
Chopra R, Agarwal G, Kumar R, Saini SK, Kaushik M, Arora A, Kumari
U, et al: miR-145 supports cancer cell survival and shows
association with DDR genes, methylation pattern, and epithelial to
mesenchymal transition. Cancer Cell Int. 19(230)2019.PubMed/NCBI View Article : Google Scholar
|
|
64
|
Zhang J, Guo H, Qian G, Ge S, Ji H, Hu X
and Chen W: MiR-145, a new regulator of the DNA fragmentation
factor-45 (DFF45)-mediated apoptotic network. Mol Cancer.
9(211)2010.PubMed/NCBI View Article : Google Scholar
|
|
65
|
Suzuki YJ: Cell signaling pathways for the
regulation of GATA4 transcription factor: Implications for cell
growth and apoptosis. Cell Signal. 23:1094–1099. 2011.PubMed/NCBI View Article : Google Scholar
|
|
66
|
Grepin C, Nemer G and Nemer M: Enhanced
cardiogenesis in embryonic stem cells overexpressing the GATA-4
transcription factor. Development. 124:2387–2395. 1997.PubMed/NCBI View Article : Google Scholar
|
|
67
|
Silva TS, Richeti F, Cunha DP, Amarante
AC, de Souza Leao JQ and Longui CA: Androgen receptor mRNA measured
by quantitative real time PCR is decreased in the urethral mucosa
of patients with middle idiopathic hypospadias. Horm Metab Res.
45:495–500. 2013.PubMed/NCBI View Article : Google Scholar
|
|
68
|
Pichler R, Djedovic G, Klocker H,
Heidegger I, Strasak A, Loidl W, Bektic J, Skradski V, Horninger W
and Oswald J: Quantitative measurement of the androgen receptor in
prepuces of boys with and without hypospadias. BJU Int.
112:265–270. 2013.PubMed/NCBI View Article : Google Scholar
|
|
69
|
Allera A, Herbst MA, Griffin JE, Wilson
JD, Schweikert HU and McPhaul MJ: Mutations of the androgen
receptor coding sequence are infrequent in patients with isolated
hypospadias. J Clin Endocrinol Metab. 80:2697–2699. 1995.PubMed/NCBI View Article : Google Scholar
|
|
70
|
Batista RL, Costa EMF, Rodrigues AS, Gomes
NL, Faria JA Jr, Nishi MY, Arnhold IJP, Domenice S and Mendonca BB:
Androgen insensitivity syndrome: A review. Arch Endocrinol Metab.
62:227–235. 2018.PubMed/NCBI View Article : Google Scholar
|
|
71
|
Wang Y, Li H, Shi Y, Wang S, Xu Y, Li H
and Liu D: miR-143-3p impacts on pulmonary inflammatory factors and
cell apoptosis in mice with mycoplasmal pneumonia by regulating
TLR4/MyD88/NF-κB pathway. Biosci Rep.
40(BSR20193419)2020.PubMed/NCBI View Article : Google Scholar
|
|
72
|
Jiang B, Yuan C, Han J, Shen M, Zhou X and
Zhou L: miR-143-3p inhibits the differentiation of osteoclast
induced by synovial fibroblast and monocyte coculture in
adjuvant-induced arthritic rats. Biomed Res Int.
2021(5565973)2021.PubMed/NCBI View Article : Google Scholar
|
|
73
|
Tang J, Pan H, Wang W, Qi C, Gu C, Shang A
and Zhu J: MiR-495-3p and miR-143-3p co-target CDK1 to inhibit the
development of cervical cancer. Clin Transl Oncol. 23:2323–2334.
2021.PubMed/NCBI View Article : Google Scholar
|
|
74
|
Zhang G, Liu Z, Zhong J and Lin L:
Circ-ACAP2 facilitates the progression of colorectal cancer through
mediating miR-143-3p/FZD4 axis. Eur J Clin Invest.
51(e13607)2021.PubMed/NCBI View Article : Google Scholar
|
|
75
|
Long Z, Gong F, Li Y, Fan Z and Li J:
Circ_0000285 regulates proliferation, migration, invasion and
apoptosis of osteosarcoma by miR-409-3p/IGFBP3 axis. Cancer Cell
Int. 20(481)2020.PubMed/NCBI View Article : Google Scholar
|
|
76
|
Zielinska HA, Daly CS, Alghamdi A, Bahl A,
Sohail M, White P, Dean SR, Holly JMP and Perks CM: Interaction
between GRP78 and IGFBP-3 affects tumourigenesis and prognosis in
breast cancer patients. Cancers (Basel). 12(3821)2020.PubMed/NCBI View Article : Google Scholar
|
|
77
|
Cai Q, Dozmorov M and Oh Y:
IGFBP-3/IGFBP-3 receptor system as an anti-tumor and
anti-metastatic signaling in cancer. Cells. 9(1261)2020.PubMed/NCBI View Article : Google Scholar
|
|
78
|
Kerr A and Baxter RC: Noncoding RNA
actions through IGFs and IGF binding proteins in cancer. Oncogene.
41:3385–3393. 2022.PubMed/NCBI View Article : Google Scholar
|
|
79
|
Li CL, Liu B, Wang ZY, Xie F, Qiao W,
Cheng J, Kuang JY, Wang Y, Zhang MX and Liu DS: Salvianolic acid B
improves myocardial function in diabetic cardiomyopathy by
suppressing IGFBP3. J Mol Cell Cardiol. 139:98–112. 2020.PubMed/NCBI View Article : Google Scholar
|
|
80
|
Tao A, Wang X and Li C: Effect of lycopene
on oral squamous cell carcinoma cell growth by inhibiting IGF1
pathway. Cancer Manag Res. 13:723–732. 2021.PubMed/NCBI View Article : Google Scholar
|
|
81
|
Xie F, Li Y, Wang M, Huang C, Tao D, Zheng
F, Zhang H, Zeng F, Xiao X and Jiang G: Circular RNA BCRC-3
suppresses bladder cancer proliferation through miR-182-5p/p27
axis. Mol Cancer. 17(144)2018.PubMed/NCBI View Article : Google Scholar
|
|
82
|
Ding C, Ding X, Zheng J, Wang B, Li Y,
Xiang H, Dou M, Qiao Y, Tian P and Xue W: miR-182-5p and
miR-378a-3p regulate ferroptosis in I/R-induced renal injury. Cell
Death Dis. 11(929)2020.PubMed/NCBI View Article : Google Scholar
|
|
83
|
Barbu MG, Thompson DC, Suciu N, Voinea SC,
Cretoiu D and Predescu DV: The roles of MicroRNAs in male
infertility. Int J Mol Sci. 22(2910)2021.PubMed/NCBI View Article : Google Scholar
|
|
84
|
Lian J, Zhang X, Tian H, Liang N, Wang Y,
Liang C, Li X and Sun F: Altered microRNA expression in patients
with non-obstructive azoospermia. Reprod Biol Endocrinol.
7(13)2009.PubMed/NCBI View Article : Google Scholar
|
|
85
|
Redman CW and Staff AC: Preeclampsia,
biomarkers, syncytiotrophoblast stress, and placental capacity. Am
J Obstet Gynecol. 213 (4 Suppl):S9.e1, S9–S11. 2015.PubMed/NCBI View Article : Google Scholar
|
|
86
|
Sheriff FR, Lopez A, Lupo PJ, Seth A,
Jorgez C and Agopian AJ: Maternal hypertension and hypospadias in
offspring: A systematic review and meta-analysis. Birth Defects
Res. 111:9–15. 2019.PubMed/NCBI View Article : Google Scholar
|
|
87
|
Gunel T, Zeybek YG, Akcakaya P, Kalelioglu
I, Benian A, Ermis H and Aydınlı K: Serum microRNA expression in
pregnancies with preeclampsia. Genet Mol Res. 10:4034–4040.
2011.PubMed/NCBI View Article : Google Scholar
|
|
88
|
Jaszczuk I, Koczkodaj D, Kondracka A,
Kwasniewska A, Winkler I and Filip A: The role of miRNA-210 in
pre-eclampsia development. Ann Med. 54:1350–1356. 2022.PubMed/NCBI View Article : Google Scholar
|
|
89
|
Ackerman D and Gems D: Insulin/IGF-1 and
hypoxia signaling act in concert to regulate iron homeostasis in
Caenorhabditis elegans. PLoS Genet.
8(e1002498)2012.PubMed/NCBI View Article : Google Scholar
|