Introduction
Hiatal hernias (HHs) are a relatively common
condition in the general population that occur due to elevated
intra-abdominal pressure, leading to the protrusion of the stomach
and other abdominal viscera into the mediastinum (1). The diagnosis of gastroesophageal reflux
disease (GERD) appears to be associated with symptomatic instances
of HHs, with regurgitation and retrosternal burning being typical
signs of the condition (2,3). Less common symptoms include dysphagia,
chest discomfort, or epigastric pain, along with chronic
iron-deficiency anemia (4,5).
Apart from their primary gastrointestinal impacts,
HHs have been linked to respiratory symptoms. Various studies with
patient-reported outcomes reinforce the widely held belief that
surgical intervention positively affects respiratory function in
patients with HHs, adding to the evidence supporting the intricate
association between HHs and respiratory symptoms (6-8).
However, respiratory issues in these patients have been
historically overlooked. Previous studies have demonstrated a
significant association between idiopathic pulmonary fibrosis and
GERD due to the presence of HH, resulting in an increased risk of
respiratory-related mortality, particularly among patients with HH
and idiopathic pulmonary fibrosis (9,10).
Furthermore, as of early 2023, the incidence of
COVID-19 in Lebanon stood at 0.94%, highlighting its ongoing impact
on respiratory health worldwide (11). Given the overlap between conditions
such as COVID-19 and other respiratory illnesses, it is essential
to factor in its potential contribution to respiratory symptoms
observed in the general population during this period.
Nevertheless, the connection between HHs and other
pulmonary conditions, such as asthma, remains unclear. The present
study thus aimed to estimate the prevalence of asthma among
patients with radiologically confirmed HH and compare to data in
the general population. Additionally, the present study sought to
assess the severity of asthma in diagnosed patients using a
validated score and determine the frequency of non-specific
respiratory symptoms related to asthma in otherwise asthma-free
patients.
Patients and methods
Study design
The present study was a cross-sectional descriptive
epidemiological study with a retrospective data collection scheme,
conducted using a computerized database sourced from the Radiology
Department of Hotel Dieu de France of Beirut, Lebanon spanning from
January, 2020 to May, 2023.
Computed tomography scans, conducted using a
64-slice scanner, were independently analyzed by two radiologists
working in tandem. Through a blinded evaluation, the radiologists
unanimously agreed on the diagnosis of HH. It is crucial to note
that the scans used for HH detection encompassed thoracic,
abdominal, thoraco-abdominal and thoraco-abdomino-pelvic scans.
Selection of patients
The process of selecting patients for the present
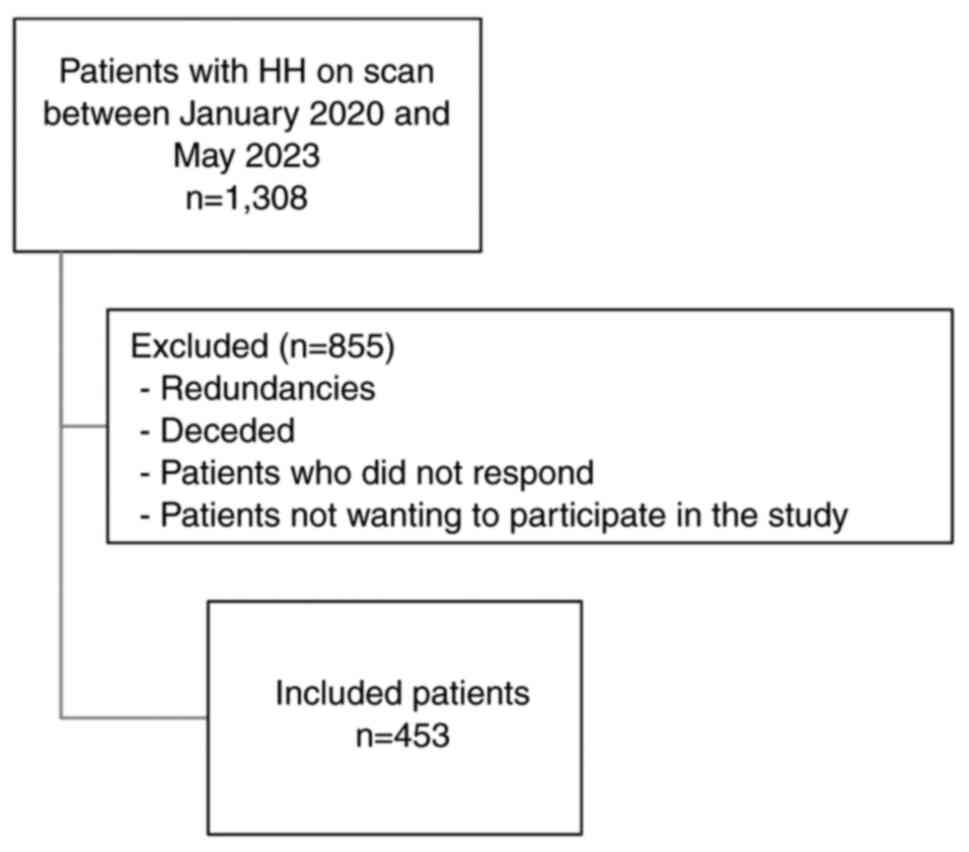
study is outlined in Fig. 1. From an
initial group of 1,308 patients diagnosed with HH between January,
2020 and May, 2023, a meticulous selection procedure was undertaken
to ensure a well-represented study sample. Patient identifiers were
retrieved from the hospital system, and consecutive patients were
contacted by phone and asked to engage in the study. Notably, 456
patient names were redundant. Additionally, 65 patients passed away
during the study period. The eligibility criteria included a
clinically and radiologically confirmed diagnosis of HH and a
comprehensive respiratory evaluation with a detailed history of
asthma symptoms. Patients who did not undergo complete diagnostic
workups for both HH and asthma, or those with a recent respiratory
infection or who did not fully respond to the questionnaire, were
excluded from the study. Among the remaining cohort, 334 patients
did not respond to phone calls or declined participation.
Ultimately, 453 patients were retained for the analysis.
Data collection
Data were retrospectively collected from existing
medical records and entered onto a database hosted on Microsoft
Office Excel. Patients were also administered a structured
questionnaire and were assisted in completing the questionnaire,
which remained in English. The interview process, based on patient
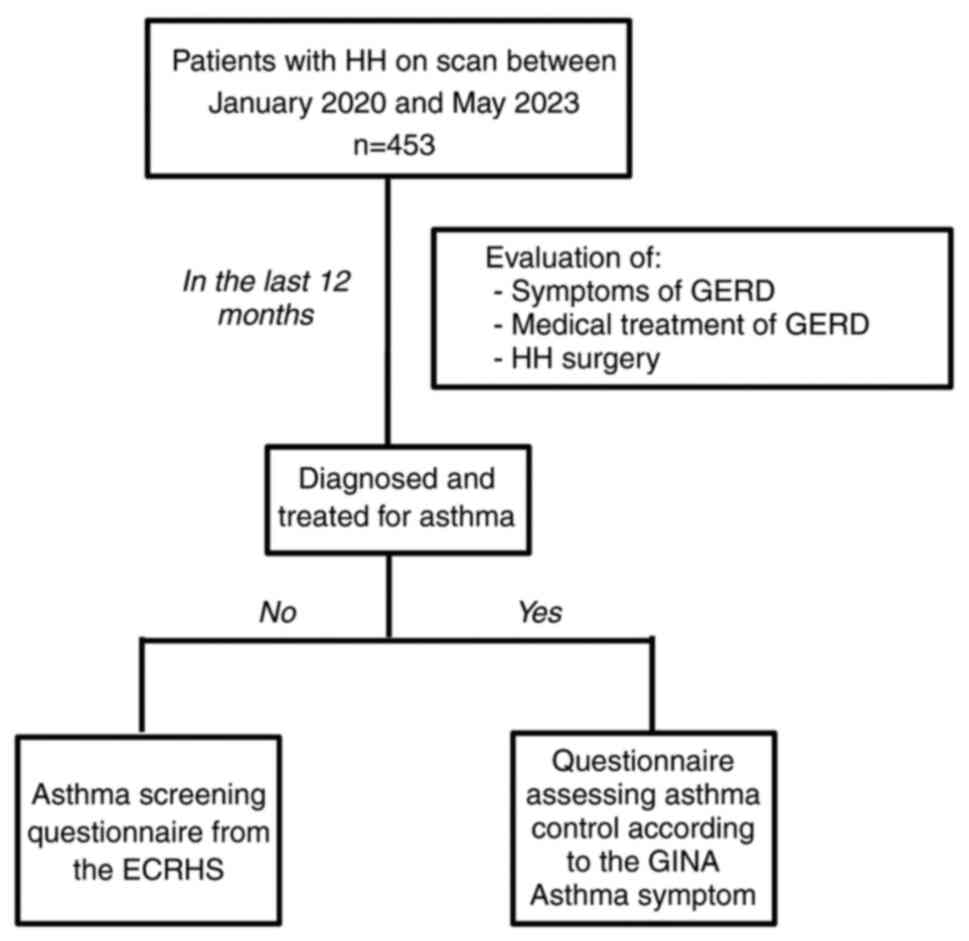
responses, is summarized in Fig.
2.
The initial segment included inquiries about GERD
symptoms, medication use for GERD and a surgical history related to
HH (please see ‘eTable I’, Data
S1). Screening questions for asthma-associated respiratory
symptoms were exclusively addressed to asthma-free patients. These
patients were invited to complete a questionnaire adapted from the
European Community Respiratory Health Survey (ECRHS), a widely
employed tool in epidemiological investigations to quantify
respiratory symptoms and screen for asthma (please see ‘eTable II’,
Data S1).
Patients diagnosed with and treated for asthma by a
pulmonologist were requested to complete the GINA questionnaire
assessing the severity of asthma and comprehensively evaluating
asthma control in adults, adolescents and children (please see
‘eTable II’, Data S1).
Objectives
The main objective of the present study was to
estimate the prevalence of asthma in patients with radiologically
detected HH, and compare it with prevalence data reported in the
literature for the general population. Additionally, among patients
diagnosed with asthma, disease severity was assessed using a
validated score, and among asthma-free patients, the prevalence of
non-specific respiratory symptoms related to asthma were
determined. The present study also compared the prevalence of
asthma among patients with symptomatic HH (reflux symptoms) and
asymptomatic HH (no reflux symptoms), and also based on sex and on
the use of HH therapy.
Ethics approval
The present study obtained ethics approval from
Hotel Dieu de France's review board [Tfem-2023-8]. The present
study received ethics approval in 2022, and patient information was
collected retrospectively for the period between 2020 and 2023. The
ethics approval allowed for the use of existing patient records up
until the final data collection date. Each patient contacted for
participation in the study received comprehensive information about
the research objectives. Patients were fully aware that their
participation in the study was entirely voluntary and that they had
full authority to refuse participation. Their confidentiality was
strictly protected throughout the study. Patients who agreed to
participate provided verbal consent.
Statistical analysis
In the descriptive approach, counts and percentages
were used to estimate the prevalence of asthma among patients with
radiologically detected HH. Additionally, frequencies of different
symptoms, severity scores, as well as other relevant parameters
were calculated. For prevalence comparisons between subgroups, the
Chi-squared test was used to assess statistical significance. A
P-value <0.05 was considered to indicate a statistically
significant difference. All statistical analyses were performed
using Microsoft Office Excel 2023.
Results
A total of 453 patients were included in the
analysis. The demographic characteristics of the patients are
summarized in Table I. In this
comprehensive survey, the frequency of HH was determined across a
variety of computed tomography scans, including thoracic,
thoraco-abdomino-pelvic and abdominal scans. Over the period
spanning from January, 2020 to May, 2023, 1,308 patients out of
17,374 scans examined (7.53%) had radiologically confirmed HH.
 | Table IDemographic profile of the study
population. |
Table I
Demographic profile of the study
population.
| Demographic
characteristics | Total (n=453) |
|---|
| Age, years (median;
Q1-Q3) | 67 (63-83) |
| Sex, n (%) | |
|
Males | 173 (38.19%) |
|
Females | 280 (61.81%) |
HH and GERD
Initially, the presence of GERD-related symptoms was
examined, including retrosternal burning, unexplained cough and the
sensation of a lump in the throat. In fact, 279 participants
reported experiencing GERD symptoms, accounting for 61.59% of a
total of 453 patients. Conversely, 174 participants reported no
GERD symptoms, representing 38.41% of the surveyed population.
The use of medications, such as proton pump
inhibitors (PPIs) and other reflux treatments for HH was reported
and categorized as medications as part of polypharmacy or
medications specifically used for symptom management. Among the
participating patients, 167 (36.87%) reported taking medications
for reasons, such as gastric protection, often as part of a broader
polypharmacy regimen, even in the absence of GERD-related symptoms.
Additionally, 119 (26.27%) patients were using medications to treat
HH-specific symptoms. Another 167 (36.87%) patients were not taking
any medications for HH.
Among the 453 patients retained for the analysis,
there were 16 instances (3.53%) of surgical intervention for HH
repair, notably Nissen fundoplication. The remainder of the
patients, [437 (96.47%)], had not undergone any HH-related surgical
intervention for HH.
Concomitant diagnosis of HH and
asthma
Among the study population of 453, 67 patients
(14.79%) reported a diagnosis of asthma and ongoing management by
pulmonologists.
History of asthma among patients with HH. Of
note, two perspectives were considered when assessing the severity
of asthma in the 67 patients. Firstly, the frequency of specific
components contributing to asthma symptom control was reported.
Specifically, 29 patients (43.28%) reported experiencing daytime
asthma symptoms more than twice a week, and another 29 patients
reported nocturnal awakenings due to asthma. Half of the patients
[34 (50.75%)] used short-acting β-agonist inhalers to relieve their
symptoms more than twice a week, and 39 patients (58.21%) reported
some form of activity limitation attributed to asthma.
Secondly, the GINA scoring system was used to assess
overall asthma control (Table II),
with modified descriptors for each score. In this framework, 16
patients (23.88%) had a score of 0, indicating ‘well-controlled’
asthma, 23 (34.33%) had a score of 1 or 2, indicating ‘partially
controlled’ symptoms and 28 (41.79%) had a score of 3 or 4,
indicating ‘uncontrolled’ asthma symptoms.
 | Table IIControl of asthma according to GINA
asthma symptom control. |
Table II
Control of asthma according to GINA
asthma symptom control.
| Asthma control | Score | No. of patients | Total no. of
patients | Percentage |
|---|
| Controlled
asthma | Score 0 | 16 | 16 | 23.88 |
| Partially controlled
asthma | Score 1 | 10 | 23 | 34.33 |
| | Score 2 | 13 | | |
| Uncontrolled
asthma | Score 3 | 17 | 28 | 41.79 |
| | Score 4 | 11 | | |
Prevalence of other respiratory symptoms among
patients with HH. Non-specific respiratory symptoms reported by
the asthma-free study participants are presented in Table III. The proportion of patients
reporting at least one respiratory symptom over the previous 12
months was 35.23% (136 out of 386 participants). The average number
of symptoms was 0.6 symptom per subject. No patient reported
experiencing all five proposed symptoms during the same period.
Finally, within this population of asthma-free patients, the most
frequently reported symptom was waking up with a sensation of
tightness or shortness of breath (22.54%). Furthermore, 26.68% of
this patient population reported nasal allergies, including hay
fever.
 | Table IIIPercentage of asthma-related
respiratory symptoms according to ECRHS. |
Table III
Percentage of asthma-related
respiratory symptoms according to ECRHS.
| Symptom | No. of
patients | Percentage |
|---|
| Asthma attacks | 7 | 1.81 |
| Chest wheezing | 52 | 13.47 |
| Shortness of breath
with wheezing | 22 | 5.7 |
| Waking up with a
feeling of tightness or shortness of breath | 87 | 22.54 |
| Waking up due to a
coughing fit | 54 | 13.99 |
| Symptoms and
score | | |
|
Score
0/5 | 250 | 64.77 |
|
Score
1/5 | 82 | 21.24 |
|
Score
2/5 | 30 | 7.77 |
|
Score
3/5 | 16 | 4.15 |
|
Score
4/5 | 8 | 2.07 |
|
Score
5/5 | 0 | 0.00 |
| Average no. per
subject | 0.8 | |
Prevalence of asthma in different
population subgroups
Subsequently, within the population of patients with
HH, the prevalence of asthma was compared based on sex, the
presence GERD symptoms, and the use of HH medications (Table IV).
 | Table IVAssociation of GERD symptoms, medical
treatment and sex with the prevalence of asthma. |
Table IV
Association of GERD symptoms, medical
treatment and sex with the prevalence of asthma.
| | Known to have
asthma | |
|---|
| Variables | Yes (%) | No (%) | P-value |
|---|
| Symptoms of GERD, n
(%) | | | 0.1 |
|
Present | 47 (16.85) | 232 (83.15) | |
|
Absent | 20 (11.49) | 154 (88.51) | |
| Medical treatment
of GERD, n (%) | | | 0.03 |
|
Present | 50 (17.48) | 236 (82.52) | |
|
Absent | 17 (10.18) | 150 (89.82) | |
| Sex, n (%) | | | 0.07 |
|
Male | 19 (10.98) | 154 (89.02) | |
|
Female | 48 (17.14) | 232 (82.86) | |
Based on sex. Patients with HH and a
diagnosis of asthma included 19 males (10.98%) and 48 females
(17.14%); the P-value for the difference between males and female
was 0.07, suggesting the absence of a statistically significant
association between sex and the prevalence of asthma among patients
with HH.
Based on GERD symptoms. Patients were
stratified into two subgroups, based on the presence and absence of
GERD symptoms (such as chest burns and cough). Asthma was present
among 47 patients with GERD symptoms (16.85%) and among 20 patients
with no GERD symptoms (11.49%); the P-value for the difference
between those with GERT symptoms and those without was 0.1,
indicating the absence of a statistically significant association
between the presence of GERD symptoms and a diagnosis of
asthma.
Based on the use of HH medications. The
prevalence of asthma was compared between patient subgroups based
on the use of HH medications. Among the patients with HH taking
medications (a total of 286 patients), 50 (17.48%) had asthma,
while 236 were asthma-free. Among patients reporting no medication
use (a total of 167 patients), a lower proportion of patients had
asthma [17 (10.18%)], and 150 were asthma-free. The P-value for
this difference was 0.03, suggesting a significant association
between the use of medications for GERD and the prevalence of
asthma.
Discussion
To the best of our knowledge, the present study is
first to examine the prevalence of asthma within a patient
population with radiologically confirmed HH. Innovatively, the
present study also aimed to assess disease severity in patients
with a known diagnosis of asthma, and to conduct early screening
for respiratory symptoms in patients with HH and no known
respiratory disease. The present study provides a comprehensive
perspective on the prevalence, severity and early screening of
asthma, and other respiratory conditions in patients with
radiologically confirmed HH.
The clinical relevance of HH is well-established,
given its implication in GERD, its association with interstitial
lung diseases and micro-aspirations (12,13), and
its tendency to decrease quality of life (14). However, little is known about the
prevalence of HH and its association with asthma. This is, at least
in part, due to the fact that asymptomatic individuals are rarely
subjected to standard HH tests, such as endoscopy, manometry, or
esophago-gastro-duodenal transit.
The present study reported a 7.53% prevalence of
radiologically detected HH, including the diaphragmatic hiatus, in
computed tomography scans analyzed for 17,374 patients, over the
period from January, 2020 to May, 2023. This finding aligns with
the results of a study conducted by Rahman et al (15), which reported a similar prevalence of
8.8%.
The average age of the patients with HH was 67 years
(interquartile range, 63-83), a result consistent with a
meta-analysis including data from seven studies (16). That meta-analysis revealed a
significant association with an age >50 years and the occurrence
of HH. This association may be attributed to the decreased
elasticity of the phrenoesophageal ligament, responsible for
anchoring the esophagus to the diaphragm and potentially
contributing to increased susceptibility to the development of
HH.
Furthermore, 280 (61.81%) patients were female, a
distribution consistent with the results of another study (17), suggesting a potential female
predilection among patients with HH. In the present our study
population, 3.53% of the patients with HH had undergone surgical
intervention for HH repair, a percentage higher than reported in
comparable studies (18). This may
be attributed to factors, such as an increased awareness of
surgical interventions for HH, evolving surgical techniques, or
higher severity of hernias prompting surgical management in this
particular cohort.
Collectively, these findings underscore the critical
role of HH as a structural contributor to GERD (19,20).
In the present study, it was observed that 67 out of
the 453 patients with HH had asthma (14.79%), previously diagnosed
and managed clinically. Notably, this prevalence exceeds the global
prevalence of asthma in the general population, which was estimated
at 3.57% in 2017(21), as well as
the reported prevalence of physician-diagnosed asthma in Lebanon,
reported at 6.7% (22). The elevated
rates of asthma in patients with HH underscores a potentially
higher prevalence of asthma among patients with HH, compared to
global and national averages. This finding is consistent with that
of another study where HH was more frequently observed in patients
with asthma rather than asthma-free subjects (62 vs. 34%, P=0.02)
(23). This observation is warranted
to prompt medical attention and asthma screening in this patient
subset.
Several explanations have been proposed to elucidate
the association between asthma and HH-induced GERD. Pulmonary
hyperinflation is common among patients with asthma. The descent of
the diaphragm during pulmonary hyperinflation and increased
respiratory effort results in a high-pressure gradient between the
abdomen and thorax. This can lead to the herniation of the lower
esophageal sphincter into the thorax, impairing its barrier
function. Consequently, this may allow increased gastric reflux in
asthmatics with hyperinflation (24).
Additionally, certain asthma medications attempting
to reduce hyperinflation may worsen acid reflux. β-agonists have
been shown to reduce the tone of the lower esophageal sphincter in
a dose-dependent manner (25). This
raises the possibility of a vicious cycle where asthma symptoms
caused by GERD lead to the increased use of bronchodilators,
exacerbating GERD symptoms.
Other pathways may shed light on the potential
mechanisms by which GERD may cause or exacerbate airway obstruction
in patients with asthma. Firstly, repetitive GERD-related acid
micro-aspiration into the larynx and upper airways can exacerbate
respiratory conditions, including asthma and chronic obstructive
pulmonary disease, according to a previous histological lung
analysis in a rat model of surgically-induced GERD (26). Secondly, reflux episodes can trigger
an esophago-bronchial vagal reflex and increased bronchial
reactivity, potentially leading to bronchoconstriction. A previous
study involving intra-esophageal acid infusions demonstrated a
decrease in peak expiratory flow even without signs of
micro-aspiration, implying vagally-mediated reflex involvement
(27). Of note, these
broncho-constrictive reactions were not dependent on esophageal
mucosal inflammation or micro-aspiration. These molecular findings
clarify the complex link between asthma and GERD induced by HH.
Using the GINA assessment of asthma control, the
present study revealed distinct trends in the severity of asthma
among the study participants. Specifically, 23.88% had
‘well-controlled’ asthma symptoms, 34.33% had ‘partially
controlled’ asthma, and 41.79% had ‘uncontrolled’ asthma symptoms.
This distribution is in contrast with the results of the PRISMA
study (28), a large cross-sectional
study involving 2,853 adults with asthma, where up to 64.4% of
patients had controlled asthma, 15.8% had partially controlled
asthma and 19.8% had uncontrolled asthma. Similarly, another recent
study conducted in the general population (29), reported that uncontrolled asthma was
present in 26% and partially controlled asthma in 22.9% of
patients.
In the present study, it was reported that patients
with HH appeared to have a higher prevalence of ‘uncontrolled’ and
‘partially controlled’ asthma. This may be due to the potential
impact of HH on respiratory function, leading to an increased
asthma severity, or underlying common mechanisms contributing to
both conditions.
In the present study, the frequency of respiratory
symptoms (outside of an asthma diagnosis) was reported using a
previously published questionnaire that evaluated the status of
occupational asthma in a cohort of workers (30). The findings obtained herein indicated
notable differences between patients diagnosed with HH and those
with untreated asthma-like symptoms described in the worker cohort
in the previous study (30). Chest
wheezing (13.74 vs. 26.20%) and waking up due to coughing (13.99
vs. 51.50%) were less common in the population of patients with HH
compared with the worker population. However, the patients in the
present study reported a higher prevalence of symptoms, such as
waking up due to shortness of breath (22.54 vs. 11.20%) and asthma
attacks (1.81 vs. 0.6%). These differences might be due to inherent
bias in the study sample selection. The overestimation of
respiratory symptoms in the worker population may be attributed to
variations in age and occupational exposure. Specifically, the
worker population was younger than the average age of 67 years
reported in the present study and environmental factors specific to
the workplace could play a role in exacerbating respiratory
symptoms.
The evaluation of GERD symptoms and asthma among
patients with HH indicated a higher rate of asthma among patients
with GERD symptoms (16.85%) compared to those without (11.49%);
however, this difference did not reach statistical significance
(P-value=0.1). In a previous study on >100,000 patients,
patients with GERD were 1.51-fold (1.43-1.59) more likely to suffer
from asthma than patients without GERD (31).
Patients undergoing treatment for GERD with PPIs or
other anti-reflux medications had a higher prevalence of asthma
(17.48%) than those not receiving anti-acid treatment (10.18%),
with a statistically significant P-value of 0.03. This aligns with
the findings of a broader research (32), where the overall incidence of asthma
was 1.58-fold higher in patients treated with PPIs than those not
receiving PPIs.
While the present study did not detect a significant
sex predilection for asthma presentation in patients with HH,
previous studies have suggested that in adulthood, females exhibit
increased an prevalence and severity of asthma (33-35).
In order to manage their GERD symptoms, patients
with HH are frequently advised to make certain lifestyle changes,
such as losing weight, changing their diet and sleeping at an
angle. Controlling asthma may be indirectly affected by these
lifestyle modifications. For example, losing weight can help
asthmatic outcomes by enhancing lung function and decreasing
systemic inflammation. However, the stress of dealing with several
chronic illnesses (HH, asthma and GERD) may cause patients to
adhere to these lifestyle treatments less than optimally,
particularly if they prioritize one ailment over the others.
Finally, it is important to recognize the
psychological toll that having both asthma and HH can have. The
symptoms of both conditions, fatigue, chest discomfort and
shortness of breath, can overlap and result in severe anxiety,
which may lead to a psychosomatic amplification of symptoms and
worsen asthma severity, as well as the perceived impact of HH on
daily life. Including mental health services in the treatment plan
for these patients may help lessen this burden and enhance symptom
control.
In summary, although there are clear physiological
connections between HH and asthma, patients with both conditions
require a comprehensive approach to care that takes into account
not only pharmacological interventions, but also lifestyle,
psychosocial and even neurogenic aspects. The knowledge gained from
this research opens the door to more complex, interdisciplinary
approaches to treatment, guaranteeing that patients get
all-encompassing assistance for both their digestive and
respiratory systems.
As regards, limitations and bias, firstly, the
present study relied on a retrospective design, which may have
introduced biases related to data collection. A limitation of the
present study is the variability in patient data collection over
time, as the health status of patients from earlier years, such as
2020, may have evolved by the final analysis in 2023. Disease
progression in conditions such as HH and asthma could mean that
more recent data could better reflect current health trends.
Additionally, in the absence of a control group (patients without
HH evaluated for asthma or other respiratory conditions), a direct
association between the prevalence of asthma and the presence of HH
cannot be established. Comparisons had to be primarily made with
data from the literature. In addition, the present study did not
stratify the results based on potential confounders, such as
comorbidities, age and smoking status; which limits the
generalizability of the results.
In conclusion, the present study uniquely identifies
a significantly higher prevalence of asthma (14.79%) among patients
with HH compared to global asthma prevalence rates. Notably, 41.79%
of asthmatic patients demonstrated an inadequate control of their
condition, indicating that asthma severity in this population may
be underrecognized and undertreated. The findings from the present
study suggest that HH may play an underappreciated role in
respiratory health, warranting increased clinical vigilance and a
proactive approach to diagnosing and managing respiratory
comorbidities in this population.
In clinical practice, while the presence of HH
should be considered when evaluating patients with
difficult-to-control asthma, further evidence is required to
support their routine identification as a key factor in asthma
management. Longitudinal studies with more robust data and extended
follow-up are necessary to confirm the potential progression of
asthma in patients with HH and to evaluate the impact of specific
interventions on respiratory outcomes. Additionally, more detailed
research into the underlying mechanisms linking HH and asthma may
shed light on potential pathways for targeted therapies and refined
treatment approaches for these patients.
Supplementary Material
Questionnaires and permissions
Acknowledgements
The authors would like to express their sincere
gratitude to the following individuals for their substantial
contributions to our research: To Dr Karl Semaan (Dana-Farber
Cancer Institute, Boston, MA, USA) for his dedicated assistance
with proofreading the manuscript, ensuring accuracy and clarity;
and to Dr Bassem Haber (Department of Pulmonary and Critical Care
Medicine, Hotel Dieu de France Hospital, Beirut, Lebanon) for
providing critical feedback and valuable suggestions during the
drafting process, which significantly enhanced the quality of the
final manuscript. Their expertise and support were instrumental in
the successful completion of this work. The abstract of this
article was presented at the ERS Congress in Vienna, Austria, from
September 7 to 11, 2024.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
MAK, HE, MR and ZAB conceived and designed the
study. MR was involved in reviewing the literature. KH, MF and EC
assisted with data collection. MAK performed the statistical
analysis and data interpretation and wrote the manuscript. KH and
MF confirm the authenticity of all the raw data. All authors have
read the manuscript, critically revised it for intellectual
content, and approved the final version.
Ethics approval and consent to
participate
The present study was conducted in accordance with
the Declaration of Helsinki. Ethical approval was obtained from the
Institutional Review Board (IRB) of Hotel Dieu de France, Beirut,
Lebanon with the reference number [Tfem-2023-8]. Verbal informed
consent was obtained from all individual participants included in
the study.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Authors' information
The ORCID IDs of the authors are as follows: Michel
Abou Khalil: 0009-0007-2038-7678; Zeina Aoun Bacha:
0000-0001-9723-1267; Hind Eid: 0000-0002-9306-4040; Moussa Riachy:
0000-0003-2146-6945.
References
|
1
|
Oleynikov D and Jolley JM: Paraesophageal
hernia. Surg Clin North Am. 95:555–565. 2015.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Argyrou A, Legaki E, Koutserimpas C,
Gazouli M, Papaconstantinou I, Gkiokas G and Karamanolis G: Risk
factors for gastroesophageal reflux disease and analysis of genetic
contributors. World J Clin Cases. 6:176–182. 2018.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Sugimoto M, Uotani T, Ichikawa H, Andoh A
and Furuta T: Gastroesophageal Reflux disease in time covering
eradication for all patients infected with Helicobacter pylori in
Japan. Digestion. 93:24–31. 2016.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Patoulias D, Kalogirou M, Feidantsis T,
Kallergis I and Patoulias I: Paraesophageal hernia as a cause of
chronic asymptomatic anemia in a 6 years old boy; case report and
review of the literature. Acta Medica (Hradec Kralove). 60:76–81.
2017.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Stein J, Connor S, Virgin G, Ong DEH and
Pereyra L: Anemia and iron deficiency in gastrointestinal and liver
conditions. World J Gastroenterol. 22:7908–1925. 2016.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Kamarajah SK, Boyle C, Navidi M and
Phillips AW: Critical appraisal of the impact of surgical repair of
type II-IV paraoesophageal hernia (POH) on pulmonary improvement: A
systematic review and meta-analysis. Surgeon. 18:365–374.
2020.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Dallemagne B, Kohnen L, Perretta S, Weerts
J, Markiewicz S and Jehaes C: Laparoscopic repair of paraesophageal
hernia. Long-term follow-up reveals good clinical outcome despite
high radiological recurrence rate. Ann Surg. 253:291–296.
2011.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Hazebroek EJ, Gananadha S, Koak Y, Berry
H, Leibman S and Smith GS: Laparoscopic paraesophageal hernia
repair: Quality of life outcomes in the elderly. Dis Esophagus.
21:737–741. 2008.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Tossier C, Dupin C, Plantier L, Leger J,
Flament T, Favelle O, Lecomte T, Diot P and Marchand-Adam S: Hiatal
hernia on thoracic computed tomography in pulmonary fibrosis. Eur
Respir J. 48:833–842. 2016.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Noth I, Zangan SM, Soares RV, Forsythe A,
Demchuk C, Takahashi SM, Patel SB, Strek ME, Krishnan JA, Patti MG
and Macmahon H: Prevalence of hiatal hernia by blinded
multidetector CT in patients with idiopathic pulmonary fibrosis.
Eur Respir J. 39:344–351. 2012.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Du J, Lang HM, Ma Y, Chen AW, Qin YY,
Zhang XP and Huang CQ: Global trends in COVID-19 incidence and case
fatality rates (2019-2023): A retrospective analysis. Front Public
Health. 12(1355097)2024.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Mays EE, Dubois JJ and Hamilton GB:
Pulmonary fibrosis associated with tracheobronchial aspiration. A
study of the frequency of hiatal hernia and gastroesophageal reflux
in interstitial pulmonary fibrosis of obscure etiology. Chest.
69:512–515. 1976.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Raghu G and Meyer KC: Silent
gastro-oesophageal reflux and microaspiration in IPF: Mounting
evidence for anti-reflux therapy? Eur Respir J. 39:242–245.
2012.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Perdikis G, Hinder RA, Filipi CJ, Walenz
T, McBride PJ, Smith SL, Katada N and Klingler PJ: Laparoscopic
paraesophageal hernia repair. Arch Surg. 132:586–591.
1997.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Rahman U, Till B, Thosani DS, Ruane B,
Muse E, Barta J, Grenda T, Evans NR III and Okusanya OT: Prevalence
and management of hiatal hernia found on imaging during lung cancer
screening. Foregut. 3:166–171. 2023.
|
|
16
|
Menon S and Trudgill N: Risk factors in
the aetiology of hiatus hernia: a meta-analysis. Eur J
Gastroenterol Hepatol. 23:133–138. 2011.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Kim J, Hiura GT, Oelsner EC, Yin X, Barr
RG, Smith BM and Prince MR: Hiatal hernia prevalence and natural
history on non-contrast CT in the Multi-Ethnic Study of
Atherosclerosis (MESA). BMJ Open Gastroenterol.
8(e000565)2021.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Dunn CP, Patel TA, Bildzukewicz NA,
Henning JR and Lipham JC: Which hiatal hernia's need to be fixed?
Large, small or none? Ann Laparosc Endosc Surg. 5(29)2020.
|
|
19
|
Hyun JJ and Bak YT: Clinical significance
of hiatal hernia. Gut Liver. 5:267–277. 2011.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Kahrilas PJ: The role of hiatus hernia in
GERD. Yale J Biol Med. 72:101–111. 1999.PubMed/NCBI
|
|
21
|
Mattiuzzi C and Lippi G: Worldwide asthma
epidemiology: Insights from the global health data exchange
database. Int Forum Allergy Rhinol. 10:75–80. 2020.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Akiki Z, Saadeh D, Farah R, Hallit S,
Sacre H, Hosseini H and Salameh P: Asthma prevalence and associated
factors among lebanese adults: The first national survey. BMC Pulm
Med. 21(162)2021.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Carmona-Sánchez R, Valdovinos-Díaz MA,
Facha MT, Aguilar L, Cachafeiro M, Solana S, Carrillo G, Chapela R,
Mejía M, Pérez-Chavira R and Salas J: Hiatal hernia in asthmatic
patients: Prevalence and its association with gastroesophageal
reflux. Rev Invest Clin. 51:215–220. 1999.PubMed/NCBI(In Spanish).
|
|
24
|
Zerbib F, Guisset O, Lamouliatte H,
Quinton A, Galmiche JP and Tunon-De-Lara JM: Effects of bronchial
obstruction on lower esophageal sphincter motility and
gastroesophageal reflux in patients with asthma. Am J Respir Crit
Care Med. 166:1206–1211. 2002.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Crowell MD, Zayat EN, Lacy BE,
Schettler-Duncan A and Liu MC: The effects of an inhaled
beta(2)-adrenergic agonist on lower esophageal function: A
dose-response study. Chest. 120:1184–1189. 2001.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Oue K, Mukaisho KI, Higo T, Araki Y,
Nishikawa M, Hattori T, Yamamoto G and Sugihara H: Histological
examination of the relationship between respiratory disorders and
repetitive microaspiration using a rat gastro-duodenal contents
reflux model. Exp Anim. 60:141–150. 2011.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Amarasiri DL, Pathmeswaran A, de Silva HJ
and Ranasinha CD: Response of the airways and autonomic nervous
system to acid perfusion of the esophagus in patients with asthma:
A laboratory study. BMC Pulm Med. 13(33)2013.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Allegra L, Cremonesi G, Girbino G,
Ingrassia E, Marsico S, Nicolini G and Terzano C: PRISMA
(PRospectIve Study on asthMA control) Study Group. Real-life
prospective study on asthma control in Italy: Cross-sectional phase
results. Respir Med. 106:205–214. 2012.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Alghamdi M, Aljaafri ZA, Alhadlaq KH,
Alamro SA, Alfaryan SM, Al Swaidan O and Mohamud M: Association
between asthmatic patients' asthma control test score and the
number of exacerbations per year in king abdulaziz medical city,
Riyadh. Cureus. 14(e24001)2022.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Demange V, Penven E, Paris C and Wild P:
Identification of asthma during the visit to the occupational
health service. Occupational Health References. 159:33–42.
2019.
|
|
31
|
el-Serag HB and Sonnenberg A: Comorbid
occurrence of laryngeal or pulmonary disease with esophagitis in
United States military veterans. Gastroenterology. 113:755–760.
1997.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Wang YT, Tsai MC, Wang YH and Wei JCC:
Association between proton pump inhibitors and asthma: A
population-based cohort study. Front Pharmacol.
11(607)2020.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Zhang GQ, Özuygur Ermis SS, Rådinger M,
Bossios A, Kankaanranta H and Nwaru B: Sex disparities in asthma
development and clinical outcomes: Implications for treatment
strategies. J Asthma Allergy. 15:231–247. 2022.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Dodge RR and Burrows B: The prevalence and
incidence of asthma and asthma-like symptoms in a general
population sample. Am Rev Respir Dis. 122:567–575. 1980.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Harju T, Keistinen T, Tuuponen T and
Kivelä SL: Hospital admissions of asthmatics by age and sex.
Allergy. 51:693–696. 1996.PubMed/NCBI
|