Introduction
Since 1957, when Simpson classified the extent of
resection of meningiomas into five subdivisions, various efforts
have been made to achieve a more extensive surgical excision. The
post-operative recurrence rates of patients with meningiomas were
associated with the extent of resection, and when the patients
survived for 6 months following surgery with Simpson grades I, II,
III and IV, the recurrence rates were 9, 16, 29 and 39%,
respectively (1-4).
However, the latest monitoring devices, surgical equipment such as
the Isocool® bipolar forceps or the cavitronic
ultrasonic surgical aspirators (CUSA) device, and diagnostic
procedures such as magnetic resonance imaging (MRI) are currently
commonly used, markedly improving the extent of resection of
meningiomas, and thus reducing the recurrence rate. However, the
necessity of post-operative radiation therapy for World Health
Organization (WHO) grade I meningiomas based on the Simpson grade
system still remains uncertain. According to certain reports, the
use of the proliferation tumor marker MIB-1 may be useful (5-10).
However, the association between the location of the meningioma and
recurrence warrants further and more in-depth investigations.
The present study investigated the role of the
Simpson grade system, meningioma location and grade in the risk of
recurrence, and aimed to assess the proliferative index in a series
of surgically removed meningiomas using immunohistochemical methods
with immunohistochemical marker (MIB-1) labeling indices (LI)
associating this index with clinical, radiological and histological
factors.
Patients and methods
Study protocol and patients
Between January, 2008 and January, 2018, the present
study retrospectively evaluated all patients undergoing craniotomy
for resection of a histopathologically confirmed meningioma from
the General University Hospital of Ioannina (Ioannina, Greece). A
total of 103 patients were derived into two groups as follows:
Group A (91 patients) without recurrence and group B (12 patients)
that had detected a meningioma recurrence. When the patient
underwent multiple surgeries, only the data from the first surgery
were included. Patients with neurofibromatosis, acoustic neurinomas
and radiation treatment prior to surgery were excluded. In
addition, patients with any other intracranial tumor history or
recurrent meningioma whose primary surgery was performed at another
institute were not included. The present study received
institutional ethical approval from the General University Hospital
of Ioannina (reference no. 9769/24-6-2019). The present study was
performed in line with the Declaration of Helsinki (1995; as
revised in Edinburgh 2000). Written informed consent was obtained
from all the included patients.
Study outcomes
The study end-points comprised neurological
improvement as the main outcome on the quality of life of patients.
The follow-up period for the patients was 6 to 123 months.
Immunohistochemistry
Immunohistochemistry was performed according to
standardized methods on paraffin-embedded sections of meningioma
specimens. The thickness of the sections used was 4 µm.
Deparaffinized tissue sections were treated with 10% hydrogen
peroxide (H2O2) in methanol at room
temperature for 4 min. Antigen retrieval was performed by autoclave
for 10 min at 120˚C. The sections were incubated in 8% skim
milk-Tris-buffered saline at 37˚C for 40 min to prevent
non-specific reactions and subsequently at 4˚C overnight with the
following primary antibodies: Mouse anti-cytokeratin (AE1/AE3; cat.
no. ab961; Abcam.), mouse anti-vimentin (cat. no. sc-6260; Santa
Cruz biotechnologies, Inc.), rabbit anti-claudin-1 (polyclonal;
cat. no. SAB4503546; MerckMillipore), mouse anti-E-cadherin (cat.
no. ab287970; Abcam), mouse anti-β-catenin (cat. no. 14-2567-82;
Thermo Fisher Scientific, Inc.), mouse anti-N-cadherin (cat. no.
ab98952; Abcam), and mouse anti-Ki-67 (cat. no. MBS9700363;
MyBioSource). The secondary antibodies used were anti-mouse or
anti-rabbit Envision horseradish peroxidase-labelled polymer
(9003-99-0; Merck Millipore) which were then applied at 37˚C for 40
min. Finally, the reactions were visualized with 0.05%
3-3'-diaminobenzidine and 0.03% hydrogen peroxide in
Tris-hydrochloric acid buffer, followed by a counterstain with
Mayer's hematoxylin at a temperature of 4˚C for ~3 sec. The
sections were viewed under an Olympus BX60 fluorescent microscope
with appropriate filters (Olympus Corporation), and those
exhibiting 90% nuclei with signals were evaluated, with 100 to 200
intact nonoverlapping nuclei scored for the number of fluorescent
signals.
Statistical analysis
Statistical analyses were performed using the
Statistical Package for the Social Sciences (SPSS 11; SPSS, Inc.).
Fisher's exact test was used to compare the groups, and continuous
data were compared using the Mann-Whitney U test. Receiver
operating characteristic (ROC) analysis was used to reveal the
factors that are related to first recurrence and affect the
outcomes of patients following meningioma surgery. A P-value
<0.05 was considered to indicate a statistically significant
difference.
Results
After applying the exclusion criteria, 103 patients
(30 males and 73 females) were included in the study. The mean and
the median ages of the patients were 62.6 and the 65.5 years,
respectively, (range, 22-83 years). A summary of the patient data
is presented in Table I. Following a
mean follow-up period of 67.3±33 months (range, 6 to 123 months),
there were 12 cases (11.6%) of tumor recurrence. There was no
significant difference between meningioma recurrence risk and age,
sex, or tumor location. When comparing the risk of recurrence
between Simpson grade V, WHO grade III, histology and the
recurrence interval, the difference was statistically significant
(P<0.05; Table I).
 | Table IBaseline demographic characteristics
of the patients. |
Table I
Baseline demographic characteristics
of the patients.
| Parameters | All patients, n=103
(100%) | Group A, n=91
(88.3%) | Group B, n=12
(11.6%) | P-value |
|---|
| Age, mean ± SD
(years) | 62.6±12.6 | 62.3±12.3 | 65.0±14.7 | 0.318 |
| Sex, n (%) | | | | 0.738 |
|
Male | 30 (29.1) | 27 (26.2) | 3 (2.9) | |
|
Female | 73 (70.9) | 64 (62.1) | 9 (8.7) | |
| Anticoagulant, n
(%) | | | | 0.442 |
|
Yes | 45 (43.6) | 41 (39.8) | 4 (3.8) | |
|
No | 58 (56.3) | 50 (48.5) | 8 (7.7) | |
| Diabetes, n (%) | | | | 0.281 |
|
Yes | 31(30) | 29 (28.1) | 2 (1.9) | |
|
No | 72 (69.9) | 62 (60.1) | 10 (9.7) | |
| Hypertension, n
(%) | | | | 0.346 |
|
Yes | 29 (28.1) | 27 (26.2) | 2 (1.9) | |
|
No | 74 (71.8) | 64 (62.1) | 10 (9.7) | |
| History of seizures,
n (%) | | | | 0.513 |
|
Yes | 25 (24.2) | 23 (22.3) | 2 (1.9) | |
|
No | 78 (75.7) | 68 (66.0) | 10 (9.7) | |
| WHO grade | | | | |
|
I, n
(%) | 80 (76.6) | 75 (72.8) | 5 (4.8) | 0.002a |
|
II, n
(%) | 13 (12.6) | 11 (10.6) | 2 (1.9) | 0.653a |
|
III, n
(%) | 10 (9.7) | 5 (4.8) | 5 (4.8) | 0.001a |
| Location | | | | 0.223 |
|
Convexity, n
(%) | 70 (67.9) | 64 (62.1) | 6 (5.8) | |
|
Cerebellum,
n (%) | 4 (3.8) | 3 (2.9) | 1 (0.9) | |
|
Parasagittal,
n (%) | 21 (20.3) | 16 (15.5) | 5 (4.8) | |
|
Sella
turcica, n (%) | 6 (5.8) | 6 (5.8) | 0 (0) | |
|
Multiple, n
(%) | 2 (1.9) | 2 (1.9) | 0 (0) | |
| Histology | | | | 0.001 |
|
Anaplastic
or atypical, n (%) | 12 (11.6) | 4 (3.8) | 8 (7.7) | |
|
Other, n
(%) | 91 (88.3) | 77 (74.7) | 14 (13.5) | |
| MIB-1 LI, n
(%) | | | | 0001 |
|
>3 | 28 (27.1) | 18 (17.4) | 10 (9.7) | |
|
<3 | 75 (72.8) | 73 (70.8) | 2 (1.9) | |
| Simpson grade | | | | |
|
I, n
(%) | 69 (66.9) | 68(66) | 1 (0.9) | 0.001a |
|
II, n
(%) | 21 (20.3) | 16 (15.5) | 5 (4.8) | 0.052a |
|
III, n
(%) | 5 (4.8) | 3 (2.9) | 2 (1.9) | 0.043a |
|
IV, n
(%) | 1 (0.9) | 1 (0.9) | 0 (0) | 0.715a |
|
V, n
(%) | 8 (7.7) | 4 (3.8) | 4 (3.8) | 0.001a |
| Recurrence
interval, mean ± SD (years) | 0.8±2.7 | 0 | 7.3±4.1 | 0.001 |
Subsequently, univariate analysis for neurological
improvement revealed that there was a statistically significant
difference in the following patient parameters: WHO grade III,
histology (anaplastic or atypical), MIB-1 LI >3, Simpson grade V
and the recurrence interval between the participants who were
operated on for meningiomas (P<0.05, Table II). Multivariate analysis (Table III) revealed that among the
aforementioned parameters, the recurrence interval and Simpson
grade V were independent factors associated with neurological
improvement during follow-up with P<0.05 and P=0.049,
respectively, and the combination of the WHO grade III, histology
(anaplastic or atypical), MIB-1 LI >3, and Simpson grade I
parameters can predict the meningioma recurrence.
 | Table IIUnivariate analysis for neurological
improvement. |
Table II
Univariate analysis for neurological
improvement.
| Parameters | No improvement,
n=17 (16.5%) | With improvement,
n=86 (83.4%) | P-value |
|---|
| Age, mean ± SD
(years) | 66.7±13 | 61.8±12 | 0.093 |
| Sex, n (%) | | | 0.231 |
|
Male | 7 (6.7) | 23 (22.3) | |
|
Female | 10 (9.7) | 63 (61.1) | |
| Anticoagulant, n
(%) | | | 0.400 |
|
Yes | 9 (8.7) | 36 (34.9) | |
|
No | 8 (7.7) | 50 (48.5) | |
| Diabetes, n
(%) | | | 0.946 |
|
Yes | 5 (4.8) | 26 (25.2) | |
|
No | 12 (11.6) | 60 (58.2) | |
| Hypertension, n
(%) | | | 0.100 |
|
Yes | 2 (1.9) | 27 (26.2) | |
|
No | 15 (14.5) | 59 (57.2) | |
| History of
seizures, n (%) | | | 0.938 |
|
Yes | 4 (3.8) | 21 (20.3) | |
|
No | 13 (12.6) | 65 (63.1) | |
| WHO grade | | | |
|
I, n
(%) | 10 (9.7) | 69 (66.9) | 0.056a |
|
II, n
(%) | 3 (2.9) | 10 (9.7) | 0.495a |
|
III, n
(%) | 4 (3.8) | 6 (5.8) | 0.035a |
| Location | | | 0.621 |
|
Convexity, n
(%) | 11 (10.6) | 59 (57.2) | |
|
Cerebellum,
n (%) | 1 (0.9) | 3 (2.9) | |
|
Parasagittal,
n (%) | 5 (4.8) | 16 (15.5) | |
|
Sella
turcica, n (%) | 0 (0) | 6 (5.8) | |
|
Multiple, n
(%) | 0 (0) | 2 (1.9) | |
| Histology | | | 0.005 |
|
Anaplastic
or atypical, n (%) | 8 (7.7) | 14 (13.5) | |
|
Other, n
(%) | 9 (8.7) | 72 (69.9) | |
| MIB-1 LI, n
(%) | | | 0.006 |
|
>3 | 10 (9.7) | 18 (17.4) | |
|
<3 | 7 (6.7) | 68 (66.0) | |
| Simpson grade | | | |
|
I, n
(%) | 8 (7.7) | 61 (59.2) | 0.056a |
|
II, n
(%) | 4 (3.8) | 17 (16.5) | 0.725a |
|
III, n
(%) | 1 (0.9) | 4 (3.8) | 0.829a |
|
IV, n
(%) | 0 (0) | 1 (0.9) | 0.655a |
|
V, n
(%) | 4 (3.8) | 4 (3.8) | 0.008a |
| Recurrence
interval, mean ± SD (years) | 3.9±4.0 | 0.8±2.7 | 0.001 |
 | Table IIIMultivariate analysis and ROC
analysis for neurological improvement. |
Table III
Multivariate analysis and ROC
analysis for neurological improvement.
| A, Multivariate
analysis |
|---|
| | 95% CI for Exp
(B) |
|---|
| Parameter | P-value | Exp (B) | Lower | Upper |
|---|
| WHO grade, III | 0.351 | 0.108 | -0.151 | 0.421 |
| Histology,
anaplastic or atypical | 0.249 | -0.132 | -0.325 | 0.085 |
| MIB-1 LI >3 | 0.396 | -0.082 | -0.229 | 0.091 |
| Simpson grade,
V | 0.049 | -0.173 | -0.478 | 0.001 |
| Recurrence
interval | 0.001 | -0.437 | -0.088 | -0.031 |
| B, ROC
analysis |
| Parameter | P-value | Area | Std. Error | CI (95%)
lower-upper |
| Recurrence
interval | 0.001 | 0.781 | 0.076 | 0.633-0.930 |
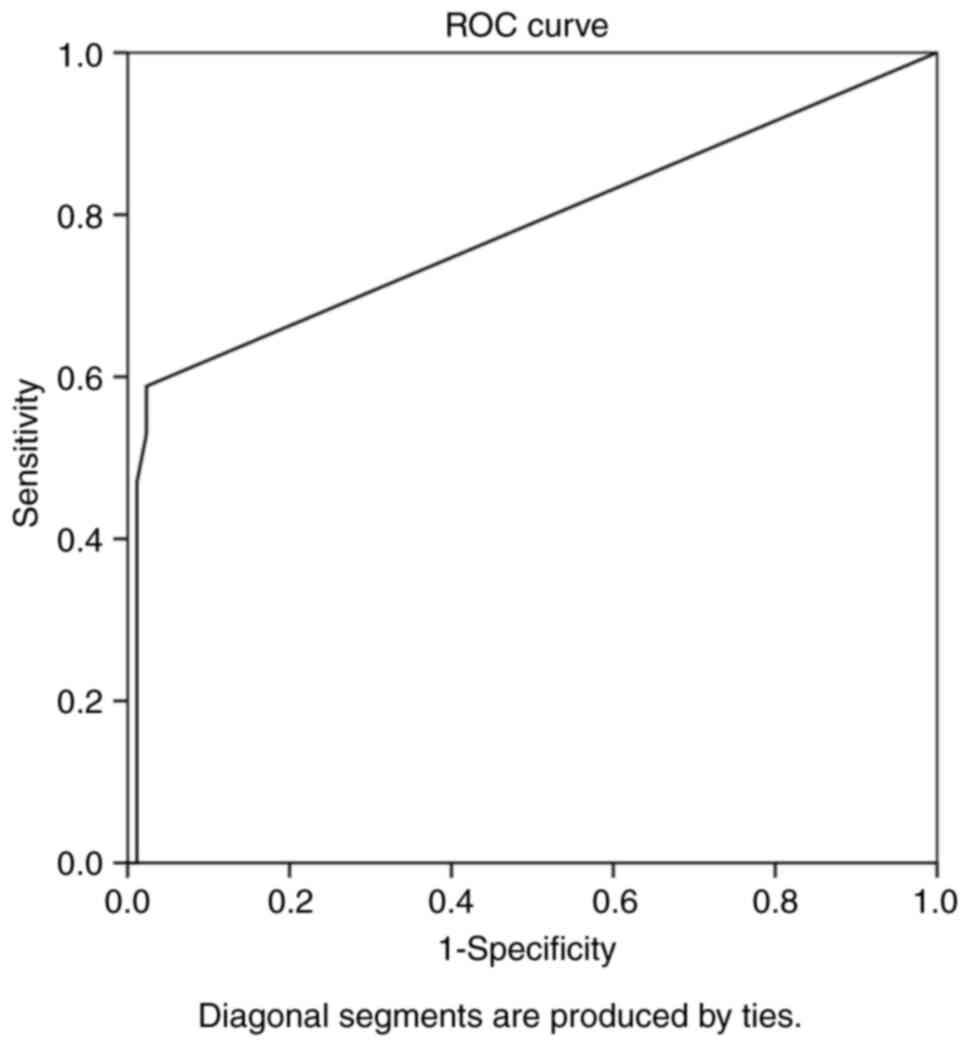
Overall, ROC analysis demonstrated that the
recurrence interval exhibited the optimal performance to predict
meningioma reappearance, as evaluated by an area under the curve
standard error [AUC(SE)] of [0.781 (0.076) and (P=0.001)] and
[0.633 (0.930)] (Table III and
Fig. 1). In addition, ROC analysis
demonstrated that, among the variables, an interval from surgical
removal at 5.5 years with 89% sensitivity and 98% specificity
exhibited a better dispersion to predict tumor recurrence, as
evaluated by an area under the curve standard error [AUC(SE)] of
[0.781 (0.076)] and (P=0.001)] Table
III and Fig. 1.
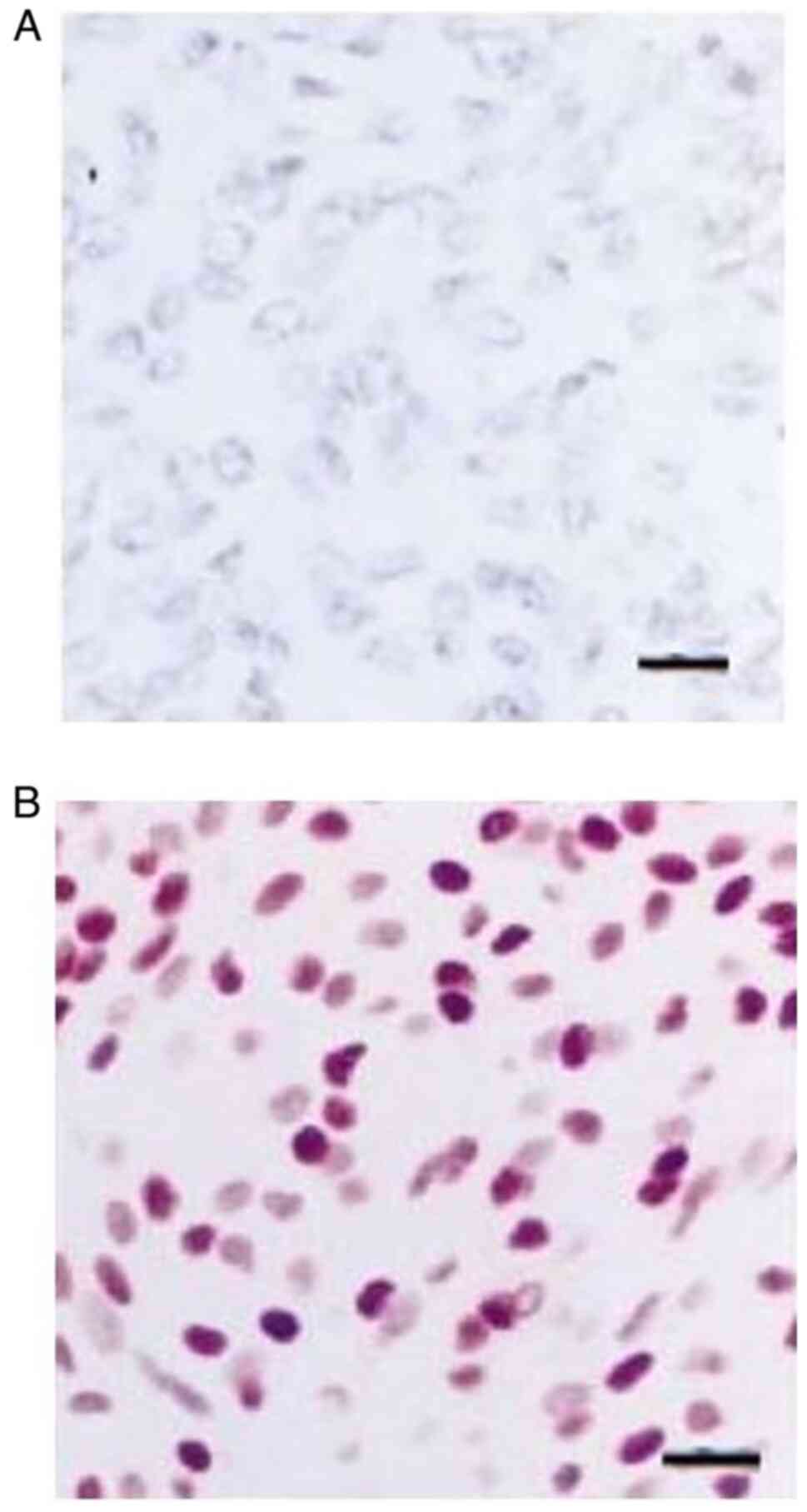
Immunohistochemical analysis revealed partially positive staining
for epithelial membrane antigen for non-anaplastic meningiomas, but
a lack of expression following staining for epithelial membrane
antigen in an anaplastic meningioma (Fig. 2).
Discussion
The results of the present study suggested that
simple decompression with or without biopsy and surgical resection
of meningiomas (Simpson grade V) was one of the main factors in
predicting the risk of meningioma recurrence. Overall, WHO grade
III, histology (anaplastic or atypical), and MIB-1 LI >3
parameters were not independent factors in predicting the
recurrence of meningiomas (Fig. 2).
This cohort proposes that the role of a high MIB-1 index (>3%)
as a key factor associated with meningioma recurrence is limited
compared with the literature (20-24)
and only the combination with WHO grade III histology (anaplastic
or atypical) may increase the risk of tumor recurrence. In
addition, instead of the most effective and widely accepted
treatment among neurosurgeries worldwide, namely the Simpson grade
I resection for reducing tumor recurrence (11-14),
the present study suggests that particularly for surgical
resection, according to Simpson grade I, II, III and IV, the
recurrence rate is the same, but markedly changes when the Simpson
grade is V (only decompression with or without biopsy). This means
that even a subtotal tumor resection (Simpson grade IV),
independent of the tumor location, could not affect the recurrence
rate. If the histological type is not WHO III or
anaplastic/atypical, simple tumor removal is sufficient in the
majority of cases. Of note, the present study proposed that if the
recurrence occurs, it is more likely to occur in an interval after
5.5 years of surgical intervention.
For a number of years, the gross total surgical
resection (GTR) of meningiomas with the affected dura and
underlying bone (Simpson grade I) was the most effective and widely
accepted treatment among neurosurgeries worldwide, reducing tumor
recurrence (11-14).
On the other hand, there are new reports demonstrating that simply
removing the entire tumor, even if small areas are left close to
critical structures, achieves the same result compared with the
more aggressive resection of the dura and underlying bone (2). This is the most effective treatment
mainly for the optic nerve sheath meningiomas, where fractionated
radiotherapy complements the surgery (15). In the present study on 103 cases
undergoing surgery, 12 cases (11.6%) of recurrent meningiomas were
elicited; 2 of these cases (1.9%) were located at the convexity,
despite the en block tumor removal. This is the reason why
the surgical plan for meningiomas must be revised based on the
collective results of the meningiomas location, Simpson grade
scale, and MIB-1 index in order to have a better understanding of
the different factors that may play a role in tumor recurrence. For
example, as regards the location of meningiomas, Zhang et al
(16) reported that petroclival
meningiomas, due to deep location and for being adjacent to
neurovascular structures are generally considered to be associated
with a high rate of recurrence.
Post-operative radiation therapy, pre-operative
endovascular embolization, intraoperative monitoring, the
widespread use of new surgical equipment such as the CUSA,
microscope, ISOCOOL bipolar device and the benefits of the MRI on
the definition of recurrence have changed the importance of the
Simpson grade system in the modern era. The goal of meningiomas
surgery is currently to eliminate, to the greatest extent possible,
tumors without efforts to achieve a higher Simpson grade score by
removing the dura or the bone. This is more important in skull-base
meningiomas (2). On the other hand,
particularly for convexity meningiomas, the Simpson grade I
resection appears to be the main target during surgery, reducing
the tumor recurrence rate (3). In
addition to the same meningioma subtype, other research has
reported that despite the entire tumor removal, the pial
participation and/or vascular attachments play a crucial role in
recurrence (4). When analyzing the
association between Simpson grade categories of meningiomas and
recurrence, it was established that there were statistically
significant differences among Simpson grade I, II, III and IV vs.
grade V in the groups, with no statistically significant findings
between the Simpson grade I, II, III and IV groups. This indicates
that, particularly for surgical resection, according to Simpson
grade I, II, III and IV, the recurrence rate is the same but
markedly changes when the Simpson grade becomes V.
It is important to note that currently, the ability
to correctly discriminate between higher-grade meningiomas and thus
to rule them out of any studies for benign meningiomas, compared to
the past 20-30 years, is more valuable (17-21).
The present study evaluated 103 cases undergoing surgery for
intracranial meningiomas of WHO I, II and grade III. After 6-123
months of follow-up, 12 cases (11.6%) with tumor recurrence were
found. When analyzing the association between the WHO grade
categories of meningiomas and recurrence, it was established that
there were statistically significant differences among WHO I vs. II
and III and WHO I via III groups, with no statistically significant
findings between the WHO I and II groups. This means that,
particularly for the histological types WHO I and II, the
recurrence rate is the same, but markedly changes when the grade of
proliferation becomes III.
It has been well recognized through several studies
that a high MIB-1 index (>3%) is associated with the recurrence
of meningioma (21-25).
In this cohort, the role of MIB-1 as an important factor associated
with meningioma recurrence is limited. The present study found a
trend towards a significant association between MIB-1 and the
recurrence of meningioma. In the present study, the MIB-1 LI >3
parameter was not an independent factor to predict the recurrence
of meningioma; only the combination with WHO grade III histology
(anaplastic or atypical) may increase the risk of tumor
recurrence.
The present study has certain limitations which
should be mentioned. One limitation was that in clinical practice,
the rate of recurrence varies according to the location of the
meningioma; meningiomas located at the skull base, particularly
those in the foramen magnum, petroclival region, anterior skull
base and sphenoid ridge, are more prone to recurrence, as it is
difficult to achieve Simpson grade I resection for meningiomas
located in these areas; thus, due to the small number of cases, the
present study was unable to reflect this point. In addition, the
results demonstrated that the Simpson grade was associated with the
risk of recurrence; however, due to the small number of excisions
of Simpson grades ≥3, there may have been a significant deviation
in the results of recurrence. In addition, the follow-up period of
6-123 months, may have been too short for the recurrence of
meningioma, as meningiomas often occur following a long period of
time (>10 years) post-surgery. Finally, the small number of 12
cases of recurrence, may have caused bias in the results and thus
to the excluded factors, such as MIB-1 LI, Simpson grade, WHO
grade, which are recognized to affect the post-operative recurrence
of meningiomas.
In conclusion, while for the histological types WHO
I and II, the recurrence rate does not differ, when the grade of
proliferation becomes III, the behavior of the meningioma markedly
changes. The GTR with all affected dura and the underlying bone
(Simpson grade I) had a significant sorter risk for recurrence, but
with the same recurrence rate compared with Simpson grade II, III
or IV resection. This may demonstrate that if the histological type
is not WHO III or anaplastic/atypical, the simple removal of the
tumor is sufficient in most cases. However, the risk of recurrence
markedly changes when the Simpson grade of proliferation becomes V.
Notably, the present study suggests that if recurrence occurs, it
is more likely to occur at an interval of 5.5 years following
surgical intervention.
The role of MIB-1 as a key factor associated with
meningioma recurrence is limited, which indicates that the risk of
tumor recurrence is low.
The present study suggests that the surgical plan
for meningiomas needs to be revised on the basis of the combined
influence of the meningiomas histological type, Simpson grade
scale, MIB-1 LI value, and eventually, its location in order to
achieve improved outcomes with aggressive surgical and
post-surgical treatment, often recommending radiation therapy. In
addition, further multi-center collaborative studies are required
in order to obtain a more in-depth understanding of the different
factors that may play a role in the recurrence of meningiomas.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
SV and GF conceptualized the study. SV, GA, GF, ΝΤ,
EA, AAM, VEG and DAS made a substantial contribution to data
interpretation and analysis and wrote and prepared the draft of the
manuscript. SV and GF analyzed the data and provided critical
revisions. SV and GF confirm the authenticity of all the raw data.
All authors contributed to manuscript revision and have read and
approved the final version of the manuscript.
Ethics approval and consent to
participate
The present study received institutional ethical
approval from the General University Hospital of Ioannina
(reference no. 9769/24-6-2019). The present study was performed in
line with the Declaration of Helsinki (1995; as revised in
Edinburgh 2000). Written informed consent was obtained from all the
included patients.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Oya S, Kawai K, Nakatomi H and Saito N:
Significance of Simpson grading system in modern meningioma
surgery: Integration of the grade with MIB-1 labeling index as a
key to predict the recurrence of WHO grade I meningiomas. J
Neurosurg. 117:121–128. 2012.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Sughrue ME, Kane AJ, Shangari G, Rutkowski
MJ, McDermott MW, Berger MS and Parsa AT: The relevance of Simpson
Grade I and II resection in modern neurosurgical treatment of World
Health Organization Grade I meningiomas. J Neurosurg.
113:1029–1035. 2010.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Hasseleid BF, Meling TR, Rønning P, Scheie
D and Helseth E: Surgery for convexity meningioma: Simpson grade I
resection as the goal: Clinical article. J Neurosurg. 117:999–1006.
2012.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Alvernia JE, Dang ND and Sindou MP:
Convexity meningiomas: Study of recurrence factors with special
emphasis on the cleavage plane in a series of 100 consecutive
patients. J Neurosurg. 115:491–498. 2011.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Aguiar PH, Tsanaclis AM, Tella OI Jr and
Plese JP: Proliferation rate of intracranial meningiomas as defined
by the monoclonal antibody MIB-1: Correlation with peritumoural
oedema and other clinicoradiological and histological
characteristics. Neurosurg Rev. 26:221–228. 2003.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Amatya VJ, Takeshima Y, Sugiyama K, Kurisu
K, Nishisaka T, Fukuhara T and Inai K: Immunohistochemical study of
Ki-67 (MIB-1), p53 protein, p21WAF1, and p27KIP1 expression in
benign, atypical, and anaplastic meningiomas. Hum Pathol.
32:970–975. 2001.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Kasuya H, Kubo O, Tanaka M, Amano K, Kato
K and Hori T: Clinical and radiological features related to the
growth potential of meningioma. Neurosurg Rev. 29:293–296.
2006.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Kim YJ, Ketter R, Steudel WI and Feiden W:
Prognostic significance of the mitotic index using the mitosis
marker anti-phosphohistone H3 in meningiomas. Am J Clin Pathol.
128:118–125. 2007.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Colli BO, Carlotti CG Jr, Assirati JA Jr,
Dos Santos MB, Neder L and Dos Santos AC: Parasagittal meningiomas:
Follow-up review. Surg Neurol. 66 (Suppl 3):S20–S27.
2006.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Durand A, Labrousse F, Jouvet A, Bauchet
L, Kalamaridès M, Menei P, Deruty R, Moreau JJ, Fèvre-Montange M
and Guyotat J: WHO grade II and III meningiomas: A study of
prognostic factors. J Neurooncol. 95:367–375. 2009.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Black PM, Morokoff AP and Zauberman J:
Surgery for extra-axial tumors of the cerebral convexity and
midline. Neurosurgery. 62 (Suppl 3):S1115–S1121. 2008.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Celenk F, Erkilic S, Durucu C, Baysal E
and Kanlikama M: Late metastasis of an intracranial meningioma to
the hard palate. J Craniofac Surg. 23:1912–1914. 2012.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Wang YC, Chuang CC, Wei KC, Hsu YH, Hsu
PW, Lee ST, Wu CT, Tseng CK, Wang CC, Chen YL, et al: Skull base
atypical meningioma: Long term surgical outcome and prognostic
factors. Clin Neurol Neurosurg. 128:112–116. 2015.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Ivan ME, Cheng JS, Kaur G, Sughrue ME,
Clark A, Kane AJ, Aranda D, McDermott M, Barani IJ and Parsa AT:
Association of morbidity with extent of resection and cavernous
sinus invasion in sphenoid wing meningiomas. J Neurol Surg B Skull
Base. 73:76–83. 2012.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Bloch O, Sun M, Kaur G, Barani IJ and
Parsa AT: Fractionated radiotherapy for optic nerve sheath
meningiomas. J Clin Neurosci. 19:1210–1215. 2012.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Zhang L, Wang Q, Zhu J and Luo C: A
modified anterior petrosectomy approach for resection of
petroclival meningioma; with management of complications. World
Neurosurg. 187(101)2024.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Bozkurt SU, Ayan E, Bolukbasi F, Elmaci I,
Pamir N and Sav A: Immunohistochemical expression of SPARC is
correlated with recurrence, survival and malignant potential in
meningiomas. APMIS. 117:651–659. 2009.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Krejchi D, Caldemeyer KS, Vakili ST and
Pritz MB: Neurosarcoidosis resembling meningioma: MRI
characteristics and pathologic correlation. J Neuroimaging.
8:177–179. 1998.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Liu Y, Liu M, Li F, Wu C and Zhu S:
Malignant meningiomas: A retrospective study of 22 cases. Bull
Cancer. 94:E27–E31. 2007.PubMed/NCBI
|
|
20
|
Nakane Y, Natsume A, Wakabayashi T, Oi S,
Ito M, Inao S, Saito K and Yoshida J: Malignant
transformation-related genes in meningiomas: Allelic loss on 1p36
and methylation status of p73 and RASSF1A. J Neurosurg.
107:398–404. 2007.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Rosenberg LA, Prayson RA, Lee J, Reddy C,
Chao ST, Barnett GH, Vogelbaum MA and Suh JH: Long-term experience
with World Health Organization grade III (malignant) meningiomas at
a single institution. Int J Radiat Oncol Biol Phys. 74:427–432.
2009.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Tao Y, Liang G, Li Z, Wang Y, Wu A, Wang
H, Lu Y, Liu Z and Hu G: Clinical features and immunohistochemical
expression levels of androgen, estrogen, progesterone and Ki-67
receptors in relationship with gross-total resected meningiomas
relapse. Br J Neurosurg. 26:700–704. 2012.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Jensen R and Lee J: Predicting outcomes of
patients with intracranial meningiomas using molecular markers of
hypoxia, vascularity, and proliferation. Neurosurgery. 71:146–156.
2012.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Mukherjee S, Ghosh SN, Chatterjee U and
Chatterjee S: Detection of progesterone receptor and the
correlation with Ki-67 labeling index in meningiomas. Neurol India.
59:817–822. 2011.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Jiang L, Wang T, Bao Y, Qian J, Wu XJ, Hu
GH and Lu YC: A study of UbcH10 expression and its association with
recurrence of meningiomas. J Surg Oncol. 106:327–331.
2012.PubMed/NCBI View Article : Google Scholar
|