Introduction
Since 1889, it has been hypothesized that tumor
cells with a special affinity for the surrounding environment were
necessary for the growth and spread of cancer (1), and an uneven distribution of breast
cancer metastases was observed in different organs and tissues. It
was believed that the tumor cells, as ‘seeds’, would only grow if
they were in the matching fabric, the ‘soil’. A contemporary
definition of the ‘seed and soil’ hypothesis is based on two
principles. Firstly, neoplasms are heterogeneous and composed of
cells with different biological properties. Secondly, multiple
interactions between tumor cells and host factors cause tumor
growth and dissemination (2).
Carcinoma-associated fibroblasts (CAFs) are found in
a major proportion of solid tumors and their metastases. These
cells, as a heterogeneous group of fibroblasts in many cases, may
be activated and have myofibroblastic differentiation (3–5).
Certain research has suggested that CAFs play an important
pathophysiological role in tumor progression and angiogenesis in
vitro and in vivo; thus they are of great significance
in the development of targeted treatment strategies (6–10).
It was believed that CAFs derived from
organ-specific local permanent fibroblasts were mediated by
cytokines, such as transforming growth factor (TGF)-1 and
platelet-derived growth factor (PDGF) (3,4,11).
In addition, other potential origins were the transdifferentiation
from blood or bone marrow stem cells, and transdifferentiation of
mesenchymal epithelial cells was also experimentally described
(12,13). In pancreatic tumor tissue of mice,
bone marrow was a source of myofibroblasts for 25% of tumor tissues
(14).
Colorectal cancer is one of the main causes of
cancer mortality in the world (15,16).
The poor prognosis of the disease is caused by the presence of
metastases, which occurs mostly in the liver (17). It has been postulated that the
micro-architecture and the cellular components of the liver form a
favorable environment for the adhesion of tumor cells and the
formation of metastases (2).
However, little is yet known about the oncogenic mechanisms of
CAFs. This study aimed to explore the microenvironmental factors
involved in the tumor progression directed by CAFs in liver
metastases.
Materials and methods
Tissue samples and cell culture
The tissue samples were obtained from 20 patients
with liver metastases of colorectal cancer. The tissue was obtained
from partial hepatic resections. All patients in the study signed
an informed consent form. The study protocol was approved by the
local research ethics committee of the Second Affiliated Hospital
of Zhengzhou University. The tissue samples were mechanically
crushed with sterile forceps for 60 min at 37°C in 30 ml of medium
199 (Invitrogen, Carlsbad, CA, USA), and incubated in 1 mg/ml
collagenase type IV (Sigma, Steinheim, Germany). Thereafter, the
digested tissue pieces were placed in 30 ml Dulbecco’s modified
Eagle’s medium (DMEM) with 10% fetal calf serum (FCS, Invitrogen)
and 1% antibiotic/antimycotic solution (Invitrogen), consisting of
penicillin, streptomycin and amphotericin B. Subsequently, samples
were transferred to 145 cm2 culture plates (Nunclon™)
and cultured in a 5% CO2-containing humidified
atmosphere at 37°C. The first medium change occurred once the cells
adhered, after approximately 48 to 72 h.
Immunohistochemical staining
To characterize the tumor-free liver tissue
phenotype, the CAFs in human metastatic tissue and tumor-free liver
fibroblasts were stained with the following primary antibodies
(monoclonal mouse anti-human antibodies) in PBS: α-SMA (1/50, clone
1A4, IgG2a; Dako, Glostrup, Denmark), vimentin (1/50, clone V9,
Dako), Thy-1 (1/25, clone 5E10, BD Biosciences, Bedford, MA, USA),
desmin (1/50, clone D33, Dako), CD45 (1/50, clone T29/33, Dako),
cytokeratin 7 (1/50 clone OV-TL 12/30, Dako), cytokeratin 19
(1/100, RCK108 clone, Dako), NCAM (1/25, clone T1, Dako), ICAM-1
(1/50, clone 6.5B5, Dako), laminin (1/25, clone 4C7, Dako) and
glial fibrillary acidic protein (GFAP, 1/25, clone 6F2, Dako). The
staining against CD45 was carried out according to the protocol of
DakoCytomation EnVision and System-HRP (Dako Co., Carpinteria, CA,
USA).
RNA isolation
In order to extract the total RNA from tissue
samples, the RNA Midi kit (Qiagen, Hilden, Germany) was used. The
sample was crushed by a disintegrator (MicroDismembrator, Braun
Instrumente, Melsungen, Germany), and lysed in 3.8 ml RLT buffer
(Qiagen). The lysed sample was centrifuged for 5 min at 4,000 rpm.
Ethanol (70%; 3.8 ml) was added into the supernatant, then the
supernatant was planned and mixed for 1 min. To fix the RNA in the
membranes of the kit columns, several wash steps were followed
according to the manufacturer’s instructions. In order to dissolve
the RNA from the membrane, the filter was transferred to a new 15
ml collection tube and washed twice with 250 ml of RNase-free water
(Sigma). In order to determine the concentration, an aliquot was
produced at a dilution of 1/50 in RNase-free water from the kit,
and the absorbance was measured at 260 and 280 nm photometrically
(Hach Lange DR 2800 VIS photometer, Germany).
Northern blotting
The transfer of nucleic acids to a nylon membrane
was initially from a gel. Based on the concentration gradient of
SSC solutions, the transport of RNA was performed by the gel onto
the membrane. A total of 20 h after the transfer was canceled using
a UV cross linker (Hoefer™, Amersham Biosciences UK Ltd., UK), the
RNA could be linked to the nylon membrane. For subsequent
assignment of the bands sizes, the signals of the 18S and 28S rRNA
subunits were marked under ultraviolet light. The membrane was
either used immediately or sealed and stored in 20X SSC at 4°C.
Hybridization
Total RNA of the liver samples was transferred to
the inward-facing RNA-side of the nylon membrane in hybridization
tubes filled with 10 ml EasyHyb hybridization buffer (Roche, USA).
The RNA with membrane-covered points was prehybridized for 1 h at
50°C in a rotary hybridization oven (Amersham-Pharmacia, UK). The
nonspecific binding of DIG antibody was used to prevent the
blocking of the membrane. A hybridization probe was heated for 10
min at 68°C in a water bath, then stored at −20°C. After the expiry
of prehybridization, buffer was discarded, and the membrane was
hybridized at 50°C under constant rotation overnight. Following
hybridization, the probe was frozen at −20°C again. Thereafter, the
membrane was washed twice for 15 min at 20°C in wash buffer (2X SSC
+ 0.1% SDS) and twice for 15 min at 68°C in wash buffer (0.5X SSC +
0.1% SDS).
ELISA
For measurement of PAI-1 concentration in the
culture supernatant, the human PAI ELISA kit was used. Firstly, 100
μl anti-mouse HRP-conjugated PAI-1 antibodies were placed into each
well. The microplate was coated with another monoclonal anti-PAI-1
antibody. The 100 μl standard sample was introduced directly into
the appropriate wells, after which the immune reaction started. The
PAI-1-Ag was bound to a defined epitope on the solid phase-bound
monoclonal anti-PAI-1 antibodies and bound to another
HRP-conjugated anti-PAI-1 monoclonal antibody defined epitope.
Following gentle mixing, the plate was incubated at room
temperature for 1 h and then washed with 300 μl wash solution (at
least 20-fold concentrated). After the unbound proteins were
washed, 200 μl of peroxidase substrate
3,3′,5,5′-tetramethylbenzidine (TMB)/H2O2 was
added for 5 min at room temperature, and the reaction was stopped
with 50 μl 0.45 M sulfuric acid. Thus the formation of a dye was
induced, and the intensity was directly proportional to the
concentration of the human PAI-1 in the sample. The color
stabilization lasted 10 min. The absorbance was read at 450 nm and
the blank value was subtracted. A microplate reader (Dynatech MR
5000) and the software Microwin 3.0 (Microtek) were applied for
measurement and evaluation.
Statistical analysis
The result of each experiment was shown as the mean
± standard deviation (SD) where applicable. Statistically
significant differences in each assay were determined by SPSS
version 18.0 statistical software package (SPSS Inc., Chicago, IL,
USA). Differences in each group were tested for significance using
the ANOVA analysis of variance. P<0.05 was considered to
indicate a statistically significant difference.
Results
Morphological and histological
characterization of CAFs
Fibroblastic cell populations were classified
according to their immunohistochemical marker profile. We observed
the cell populations in histological sections of colorectal cancer
liver metastases (at least 3 cm). The cells present within the
metastatic stroma showed differentiation, as occurred in stationary
fibroblasts in continuous portal vessels of the liver tissue.
Therefore, fibroblasts could be identified by co-expression of
vimentin, Thy-1 and α-SMA, while perisinusoidal hepatic stellate
cells (HSCs) were localized in the normal liver. Due to the
staining in the transition area between the liver tissue and
metastases, the phenomenon of fibroblastic cells in the liver tumor
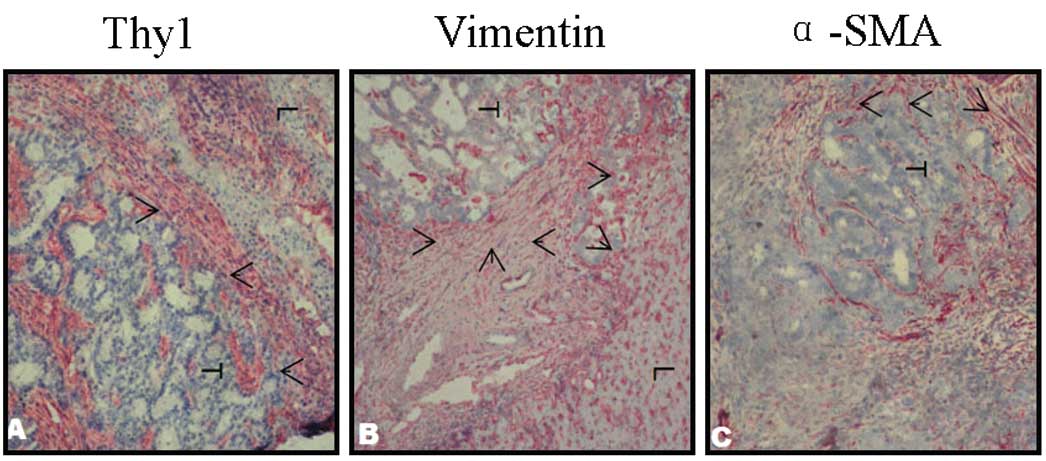
could be observed, as shown in Fig.
1A-C, and α-SMA, vimentin and Thy-1 were positive in tumor
cells.
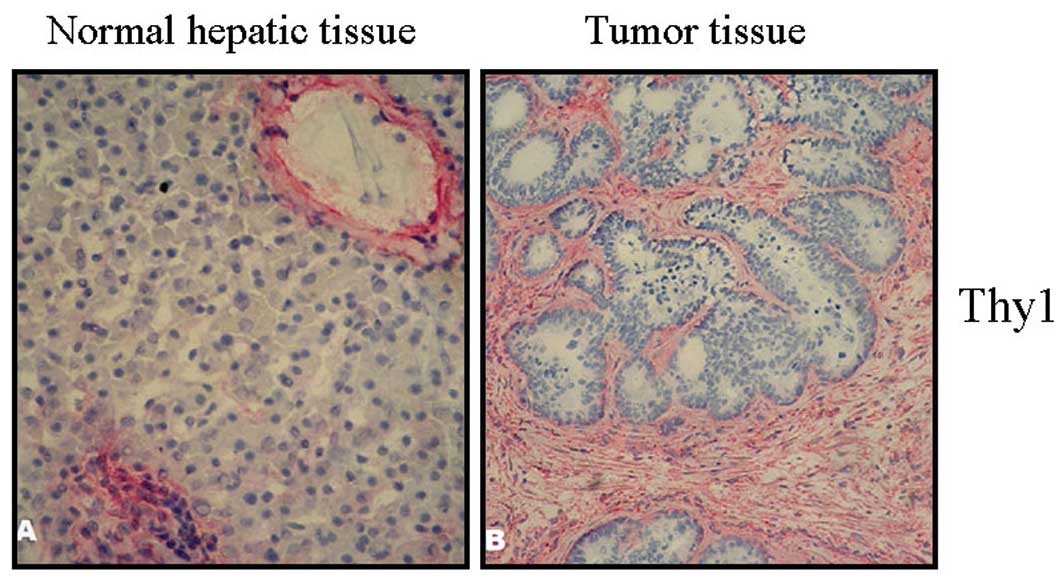
Clear staining of the marker Thy-1 is shown in
Fig. 2. The sections showed marked
staining in the septa. Thy-1 positive fibroblasts were distributed
throughout the stroma and were intensified in the border region
between the tumor and liver tissue (Fig. 2B). In healthy tissue, predominantly
the hepatic portal area, but also the vascular wall and bile duct
structures were stained (Fig.
2A).
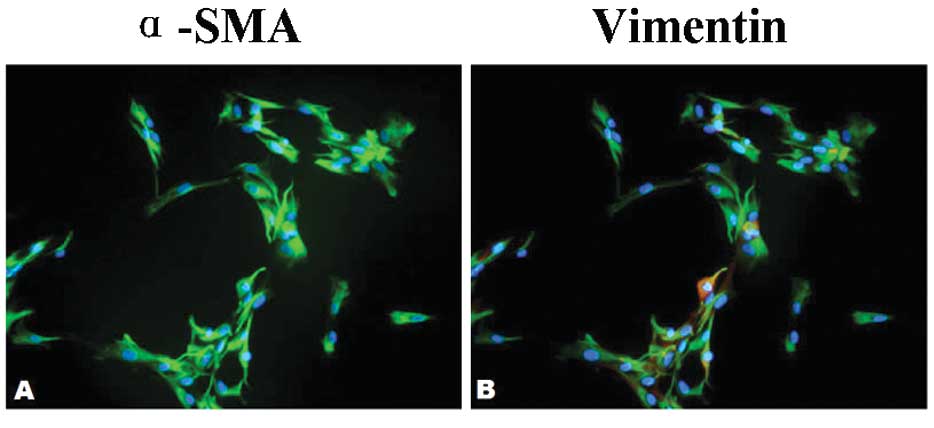
Using a double immunofluorescence stain against
α-SMA and vimentin, the proportion of α-SMA-positive cells was
quantified. While all the CAFs were positive for vimentin, the
percentage of α-SMA-positive cells was between 50 and 90% (Fig. 3A-B). The majority of the CAFs
therefore had a vimentin+/α-SMA+/Thy-1+ fibroblast phenotype. This
expression profile matched that of the CAFs in situ. Thus it
was concluded that the majority of cultured CAFs was representative
of the population found in fibroblastic tumors in situ.
Effect of different growth factors on the
expression of α-SMA
CAFs are the targets for both cellular growth
factors and pro-angiogenic factors. Therefore, CAFs are likely to
play a key role in hepatic invasion and metastasis in colorectal
carcinoma, as well as endothelial cells. To determine the effect of
growth factors on the properties of CAFs, the established cell
lines were incubated with increasing concentrations of TGF-1, PDGF,
tumor necrosis factor (TNF) and epidermal growth factor (EGF).
Subsequently, mRNA was isolated from the cells and analyzed by
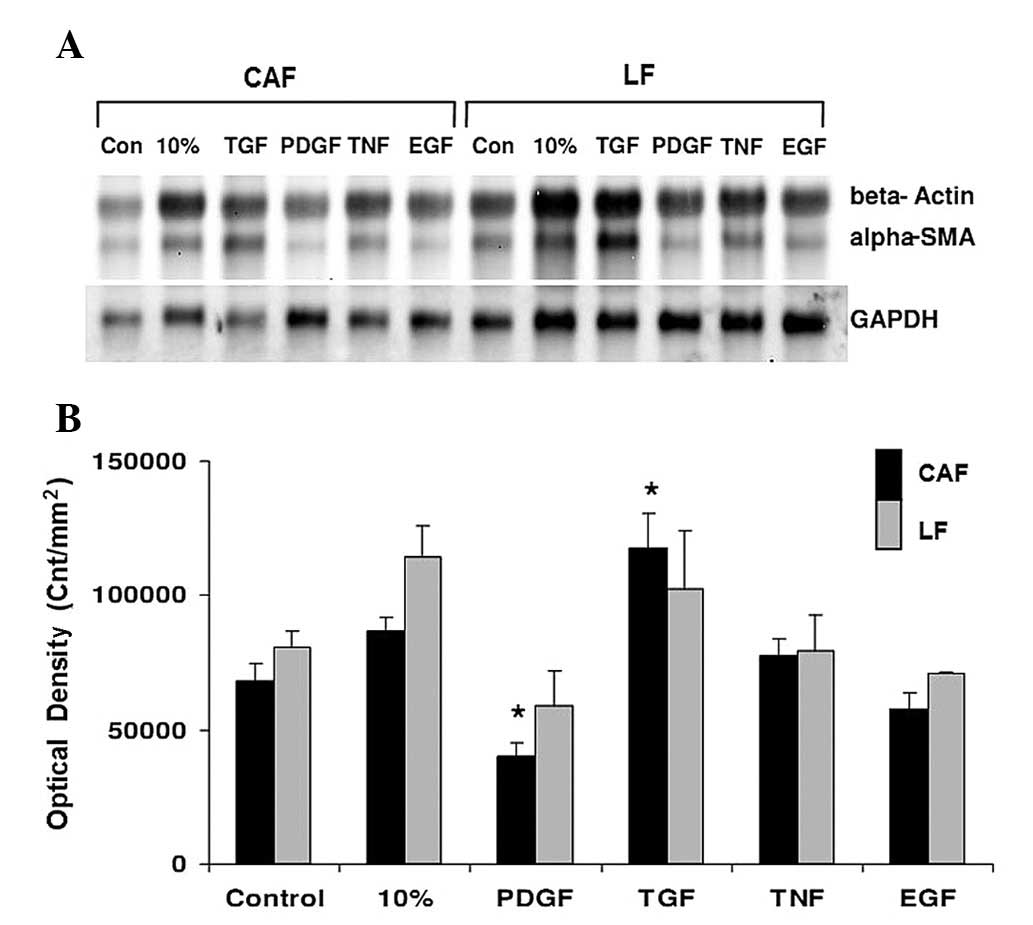
northern blotting. As shown in Fig.
4A-B, α-SMA mRNA expression increased significantly after CAFs
were treated with the growth factor TGF-1 (P<0.05), while EGF
and TNF led to no increase in α-SMA mRNA expression, and PDGF
caused a significant suppression of α-SMA expression
(P<0.05).
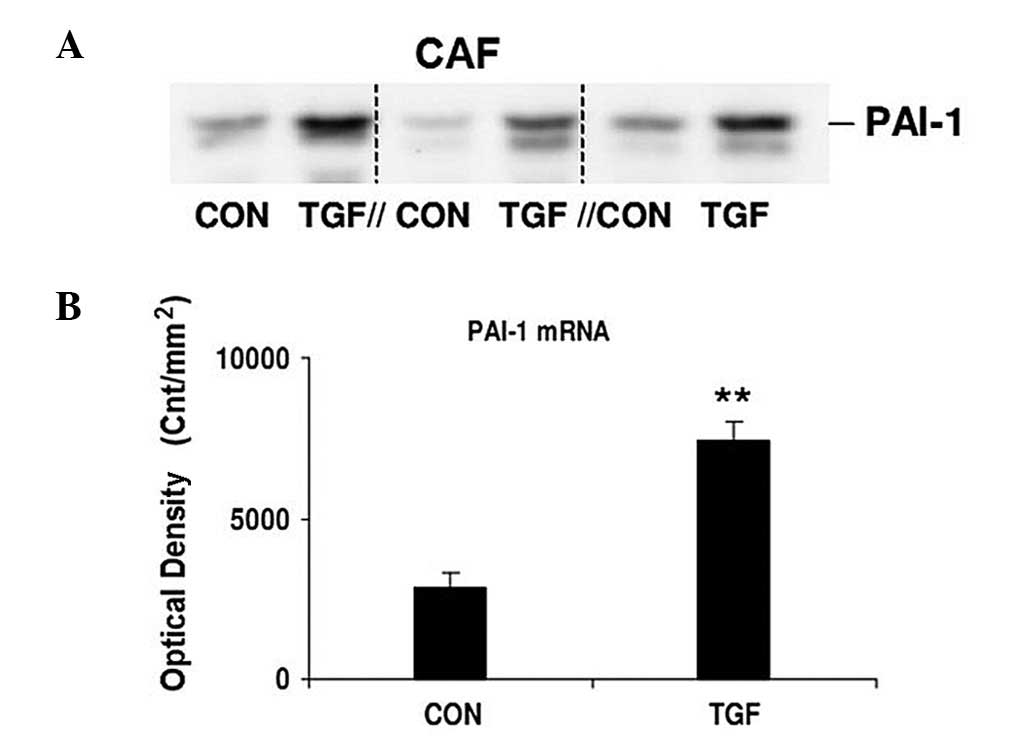
TGF-1 promotes the expression of
PAI-1
Compared with CAFs without stimulation, the PAI-1
mRNA expression significantly increased in those stimulated by
TGF-1 (5 ng/ml), as shown in Fig. 5A
and B. The PAI-1 mRNA expression was significantly lower in
unstimulated CAFs (P<0.01).
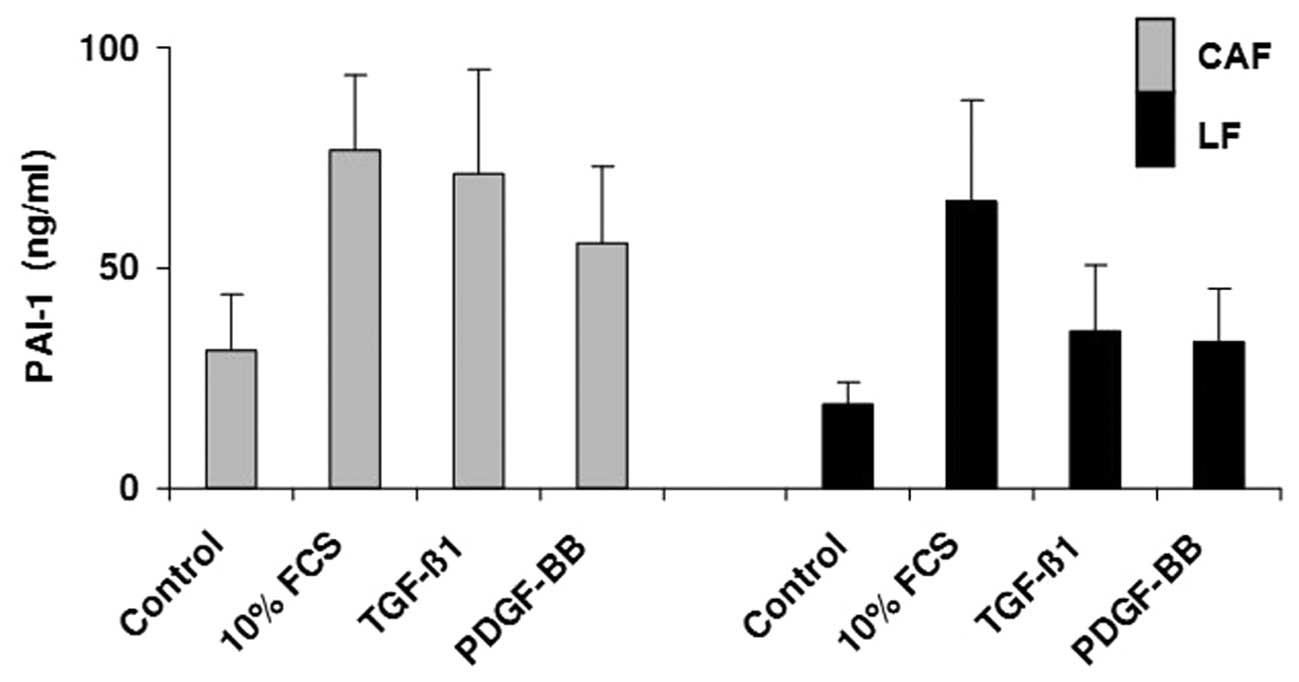
Secretion of PAI-1
The PAI-1 expression in CAFs from colorectal liver
metastases and liver fibroblasts from healthy liver tissue was also
analyzed by ELISA, as shown in Fig.
6. The strongest PAI-1 expression was 93.77 ng/ml in CAFs
cultured with 10% FCS (control, 53.9 ng/ml), while it was 79.26
ng/ml in liver fibroblasts cultured with 10% FCS (control, 24.49
ng/ml). The influence of different growth factors on cells was
strong. The average PAI-1 expression in the fibroblasts from the
healthy liver tissues increased to 43.445 ng/ml under TGF-1 culture
with 39.01 ng/ml for PDGF, while the average PAI-1 expression in
CAFs increased to 75.45 ng/ml under TGF-1 culture, with 59.53 ng/ml
for PDGF. Overall, the PAI-1 production increase in normal tissue
was less than that in the metastatic CAFs.
Discussion
CAFs are involved in tissue invasion and
neo-angiogenesis of tumors in a number of ways. They primarily
affect the reorganization of tissue architecture and the production
of the tumor stroma (18). They
also affect endothelial cell recruitment and vascular infiltration
of tumor tissue over pro-angiogenic growth factors (19). In addition, they form the
structural basis for tumors (18).
Moreover, CAFs are capable of directly affecting the growth of
tumor cells (20). Our study
demonstrated positivity for vimentin, α-SMA and Thy-1 expression
and myofibroblastic differentiation of CAFs as well as the
phenomenon of recruitment between liver tumor and healthy liver
tissue.
In previous studies, it was assumed that CAFs were
transdifferentiated from HSCs (21). Liver fibroblasts could be
identified by co-expression of vimentin, Thy-1 and α-SMA, while
perisinusoidal HSCs were localized in the normal liver. Our
findings suggested that the cultured CAFs and HFs were
representative of the respective cell types.
It was clear that the behavior of CAFs of colorectal
liver metastases was similar to liver fibroblasts. In fact, it
cannot be excluded that the functional similarities of CAFs and
liver fibroblasts, and differences between the two cell types
decreased continuously during the cultivation. The same change of
α-SMA expression after growth factor stimulation also showed that
CAFs and liver fibroblasts were similar.
More than 95% of the CAFs and the liver fibroblasts
in situ and in vitro were positive for the marker
Thy-1, a cell surface glycoprotein, which was found in
heterogeneous fibroblast cell isolation from different tissues. The
transdifferentiation of fibroblasts into myofibroblasts was
attributed to TGF-1 stimulation (22,23).
Thy-1 had been considered as a potential differentiation marker
between HSCs and portal fibroblasts. However, it was not determined
whether activated or transdifferentiated HSCs expressed Thy-1.
The ELISA results showed that PAI-1 expression was
very high in CAFs with lower levels in liver fibroblasts. The
increases in the metastatic tissue were only slight, possibly
reflecting the mixed cell composition. Therefore, CAFs produced a
significant proportion of PAI-1.
Furthermore, the potential role of CAFs was
investigated for the invasion of tumor cells, which was not carried
out specifically in liver metastases and liver tumors. Following
stimulation by growth factors, including PDGF, EGF, TNF and TGF-1,
CAFs and liver fibroblasts expressed more PAI-1. The PAI-1
expression was significantly increased by TGF-1. Since the PAI-1
expression in unstimulated cells was much lower, CAFs were
stimulated in humans by growth factors (24). Whether these growth factors were
secreted by the tumor cells themselves or by other cells involved
in tumor growth is currently being studied and pharmacological
approaches require exploration (25).
The plasminogen activator (PA) system played a key
role in tumor progression, possibly by increasing extracellular
matrix degeneration and tumor cell migration. Consequently,
urokinase-type plasminogen (u-PA) was discovered as an inhibitor of
tumor growth and angiogenesis. Paradoxically, the high
concentration of PAI-1 is associated with a poor survival prognosis
(26). Through its function as an
anti-protease, PAI-1 thus led to proangiogenesis in general to a
more aggressive tumor process, and thus to a poorer prognosis
(27–29). Furthermore, it was found that tumor
cells could be protected from apoptosis by PAI-1 (30).
In conclusion, our findings suggest that CAFs may
play an important role in migration, matrix degradation, invasion
and thus angiogenesis of tumors. Furthermore, TGF-1 may promote the
activation of PAI-1 transcription in CAFs.
References
|
1
|
Welch DR, Steeg PS and Rinker-Schaefer CW:
Molecular biology of breast cancer metastasis. Genetic regulation
of human breast carcinoma metastasis. Breast Cancer Res. 2:408–416.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Duda DG, Duyverman AM, Kohno M, et al:
Malignant cells facilitate lung metastasis by bringing their own
soil. Proc Natl Acad Sci USA. 107:21677–21682. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bhowmick NA, Neilson EG and Moses HL:
Stromal fibroblasts in cancer initiation and progression. Nature.
43:332–337. 2004. View Article : Google Scholar
|
|
4
|
Polanska UM, Acar A and Orimo A:
Experimental generation of carcinoma-associated fibroblasts (CAFs)
from human mammary fibroblasts. J Vis Exp. 56:e32012011.PubMed/NCBI
|
|
5
|
Mazzocca A, Dituri F, Lupo L, et al:
Tumor-secreted lysophostatidic acid accelerates hepatocellular
carcinoma progression by promoting differentiation of peritumoral
fibroblasts in myofibroblasts. Hepatology. 54:920–930. 2011.
View Article : Google Scholar
|
|
6
|
Fujiita M, Hayashi I, Yamashina S, et al:
Angiotensin type 1a receptor signaling-dependent induction of
vascular endothelial growth factor in stroma is relevant to
tumor-associated angiogenesis and tumor growth. Carcinogenesis.
26:271–219. 2005. View Article : Google Scholar
|
|
7
|
Fabris VT, Sahores A, Vanzulli SI, et al:
Inoculated mammary carcinoma-associated fibroblasts: contribution
to hormone independent tumor growth. BMC Cancer. 10:2932010.
View Article : Google Scholar
|
|
8
|
Ishikawa S, Takenaka K, Yanagihara K, et
al: Matrix metalloproteinase-2 status in stromal fibroblasts, not
in tumor cells, is a significant prognostic factor in
non-small-cell lung cancer. Clin Cancer Res. 10:6579–6585. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Orimo A, Gupta PB, Sgroi DC, et al:
Stromal fibroblasts present in invasive human breast carcinomas
promote tumor growth and angiogenesis through elevated SDF-1/CXCL12
secretion. Cell. 121:335–348. 2005. View Article : Google Scholar
|
|
10
|
Orimo A and Weinberg RA: Stromal
fibroblasts in cancer: a novel tumor-promoting cell type. Cell
Cycle. 5:1597–1601. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Rosenthal E, McCrory A, Talbert M, et al:
Elevated expression of TGF-beta1 in head and neck cancer-associated
fibroblasts. Mol Carcinog. 40:116–121. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
LaRue A, Masuya M and Ebihara Y:
Hematopoietic origins of fibroblasts: I. in vivo studies of
fibroblasts associated with solid tumors. Exp Hematol. 34:208–218.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Liu C, Chen Z, Chen Z, et al: Multiple
tumor types may originate from bone marrow-derived cells.
Neoplasia. 8:716–724. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Direkze N, Hodivala-Dilke K, Jeffery R, et
al: Bone marrow contribution to tumor-associated myofibroblasts and
fibroblasts. Cancer Res. 64:8492–8495. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Jemal A, Bray F, Center MM, et al: Global
cancer statistics. CA Cancer J Clin. 61:69–90. 2011. View Article : Google Scholar
|
|
16
|
Rougier P and Mitry E: Epidemiology,
treatment and chemoprevention in colorectal cancer. Ann Oncol.
14:ii3–5. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Tajima Y, Ishibashi K, Ishiguro T, et al:
Analysis of hepatic lymph node metastasis in liver metastases from
colorectal cancer. Gan To Kagaku Ryoho. 38:2228–2231. 2011.(In
Japanese).
|
|
18
|
Olaso E, Salado C, Egilegor E, et al:
Proangiogenic role of tumor-activated hepatic stellate cells in
experimental melanoma metastasis. Hepatology. 37:674–685. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Claffey KP, Abrams K, Shih SC, et al:
Fibroblasts growth factor 2 activation of stromal cell vascular
endothelial growth factor expression and angiogenesis. Lab Invest.
81:61–75. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lee HO, Mullins SR, Franco-Barraza J, et
al: FAP-overexpressing fibroblasts produce an extracellular matrix
that enhances invasive velocity and directionality of pancreatic
cancer cells. BMC Cancer. 11:2452011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Gulubova MV: Ito cell morphology,
alpha-smooth muscle actin and collagen type IV expression in the
liver of patients with gastric and colorectal tumors. Histochem J.
32:151–164. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Koumas L, Smith TJ, Feldon S, et al: Thy-1
expression in human fibroblast subsets defines myofibroblastic or
lipofibroblastic phenotypes. Am J Pathol. 163:1291–1300. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Barker TH, Grenett HE, MacEwen MW, et al:
Thy-1 regulates fibroblast focal adhesions, cytoskeletal
organization and migration through modulation of p190 RhoGAP and
Rho GTPase activity. Exp Cell Res. 295:4884–4896. 2004.
|
|
24
|
Wakahara K, Kobayashi H, Yagyu T, et al:
Transforming growth factor-beta1- dependent activation of Smad2/3
and up-regulation of PAI-1 expression is negatively regulated by
Src in SKOV-3 human ovarian cancer cells. J Cell Biochem.
93:437–453. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Hirashima Y, Kobayashi H, Suzuki M, et al:
Transforming growth factor-beta1 produced by ovarian cancer cell
line HRA stimulates attachement and invasion through an
up-regulation of plasminogen activator inhibitor type-1 in human
peritoneal mesothelial cells. J Biol Chem. 278:26793–26802. 2003.
View Article : Google Scholar
|
|
26
|
Halamkova J, Kiss I, Pavlovsky Z, et al:
Clinical significance of the plasminogen activator system in
relation to grade of tumor and treatment response in colorectal
carcinoma patients. Neoplasma. 58:377–385. 2011. View Article : Google Scholar
|
|
27
|
Zubac DP, Wentzel-Larsen T, Seidal T, et
al: Type 1 plasminogen activator inhibitor (PAI-1) in clear cell
renal cell carcinoma (CCRCC) and its impact on angiogenesis,
progression and patient survival after radical nephrectomy. BMC
Urol. 10:202010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Grebenchtchikov N, Maguire TM, Riisbro R,
et al: Measurement of plasminogen activator system components in
plasma and tumor tissue extracts obtained from patients with breast
cancer: an EORTC Receptor and Biomarker Group. Oncol Rep.
14:235–239. 2005.PubMed/NCBI
|
|
29
|
Nishioka N, Matsuoka T, Yashiro M, et al:
Linoleic acid enhances angiogenesis through suppression of
angiostatin induced by plasminogen activator inhibitor 1. Br J
Cancer. 105:1750–1758. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Romer MU, Due AK, Larsen JK, et al:
Indication of a role of plasminogen activator inhibitor type I in
protecting murine fibrosarcoma cells against apoptosis. Thromb
haemost. 94:859–866. 2005.PubMed/NCBI
|