Introduction
The herb Liriope platyphylla (LP) has been
used to treat asthma and inflammation of the bronchi and lungs in
oriental medicine (1). LP is found
throughout the temperate climate area of the northern hemisphere
and is a perennial seed-reproducing plant. In Korea, the leaves of
LP remain green all year round and the plant grows in low mountain
regions, at altitudes of less than 500 m above sea level (2). A number of previous studies have
reported the preventative effects of LP root extracts on obesity,
diabetes, inflammation and neurodegenerative disease (3–7).
Among these effects, LP has also exhibited therapeutic potential in
human subjects suffering from Alzheimer’s disease (AD). In
particular, the steroidal saponin, spicatoside A, isolated from LP
induces neuritic outgrowth similar to nerve growth factor (NGF) and
activates extracellular signal-regulated kinase 1/2 (ERK1/2) and
phosphatidylinositol 3-kinase (PI3-kinase)/Akt in PC12 cells
(7). In two types of neuronal
cells, B35 and C6, 10% water extracts of LP induced an increase in
NGF secretion, PC12 cell differentiation and NGF mRNA expression
(8).
Steaming medicinal plants is a process that is
commonly used to increase the levels or effectiveness of functional
components and cause the chemical transformation of certain
constituents (9). The ginseng
plant is steamed and its derivatives are administered orally as
adaptogens, aphrodisiacs and nourishing stimulants, as well as to
treat type II diabetes and male sexual dysfunction (10–12).
Korean ginseng is found in two forms: Korean white ginseng (KWG,
Panax ginseng C.A. Meyer), which is air-dried, and Korean red
ginseng (KRG, Ginseng Radix rubra), which is steamed
(9). When ginseng is steamed, a
number of components, including ginsenosides, acidic
polysaccharides and phenolics, are chemically transformed into
different molecules. New compounds, including
non-saponinpolyaceylene, maltol and amino acids, are also formed
(13,14). Recently, red LP (RLP) was produced
by the steaming process and its effects on the insulin secretion
ability and insulin receptor signaling pathway were examined. The
maximum insulin secretion was observed in the INS cells treated
with LP extract steamed for 3 h (3-SLP) and with two repeated steps
(3 h steaming and 24 h air-dried) carried out 9 times (9-SALP)
(15). Despite these primary
results, no studies have been performed on how RLP affects the NGF
secretion ability and NGF receptor signaling pathway to improve
neuronal cell functionality in the treatment of neurodegenerative
disease.
Therefore, this study examined the effects of the LP
steaming time and frequency on the NGF secretion ability and NGF
receptor signaling pathways in order to develop a novel therapeutic
drug. These results provide a scientific basis for determining the
optimal conditions for the LP steaming process when applied to
neuronal relative diseases.
Materials and methods
Preparation of LP and ginseng sample
The roots of LP were collected from plantations in
the Miryang area (Korea) and dried in a hot-air drying machine
(JSR, Seoul, Korea) at 60°C. To prepare five extracts at different
steaming times (0-, 3-, 9-, 15- and 24-SLP), 200 g of dry roots
were steamed at 99°C for 0, 3, 9, 15 and 24 h, respectively, and
air-dried for 24 h at 70°C. These steamed roots were reduced to a
powder using an electric blender. The water extracts were purified
for 2 h at 100°C using circulating extraction equipment (IKA
Labortechnik, Staufen, Germany) after adding 200 ml of distilled
water. In addition, a solution of the extracts was concentrated to
dry pellets in a rotary evaporator (EYELA, Tokyo, Japan) and stored
at −80°C until further use. To prepare six extracts with different
steaming frequencies (0-, 1-, 3-, 5-, 7- and 9-SALP), the two-step
process (200 g of dry roots were steamed at 99°C for 3 h and
air-dried at 70°C for 24 h) was carried out for a different number
of repetitions (0, 1, 3, 5, 7 and 9 times, respectively). The roots
obtained using these processes were treated with the same
procedures to prepare dry pellets (15).
Two types of Korean ginseng (KWG and KRG) were
purchased from Cheng-Kwan-Jang in the Korea Ginseng Corp. (Daejon,
Korea). These roots were treated with the same procedures described
above to prepare dry pellets.
Cell culture
The B35 neuronal cell line, which secretes NGF, and
PC12 pheochromocytoma cell line were obtained from the the Korean
Cell Line Bank (Seoul, Korea). The B35 cell line was maintained for
24–36 h in Dulbecco’s Modified Eagle’s Medium (DMEM, Hyclone,
Logan, UT, USA) containing 10% fetal bovine serum (FBS, Hyclone),
100 IU/ml of penicillin and 100 μg/ml of streptomycin. The PC12
cells were cultured in RPMI-1640 (Hyclone) supplemented with 10%
FBS, 100 IU/ml of penicillin and 100 μg/ml of streptomycin. The
cells were maintained in a humidified incubator at 37°C and 5%
CO2.
MTT assay
The B35 cells were seeded at a density of
4×104 cells/200 μl in 96-well plates and grown for 24 h
in a 37°C incubator. When the cells reached 70–80% confluence, they
were exposed to distilled water (vehicle), various types of LP
extracts (50 μg/ml) or two ginseng extracts (50 μg/ml) dissolved in
dH2O for a further 24 h. Cell proliferation was
determined using the tetrazolium compound, MTT
(3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide;
Sigma-Aldrich, St. Louis, MO, USA). After discarding the
supernatants from the vehicle- and extract-treated wells, 200 μl of
fresh DMEM and 50 μl of an MTT solution (2 mg/ml in
phosphate-buffered saline; PBS) were added to each well. The cells
were then incubated in a 37°C incubator. The reduction of MTT to
insoluble purple formazan dye crystals by the viable cells was
evaluated in a 220 μl sample recovered after 4 h. The formazan
precipitate was dissolved in DMSO and the absorbance in the wells
was read directly at 540 nm using a Soft Max Pro5 spectrophotometer
(Molecular Devices, Sunnyvale, CA, USA). The data were analyzed in
terms of the cell number versus absorbance, allowing the changes in
cell proliferation to be quantified.
ELISA for NGF detection
The levels of NGF in the culture supernatant
isolated from the B35 cells, which had been cultured and treated
under the same conditions as the MTT assay, were detected using an
ultra-sensitive assay and reagents in the NGF ELISA kit (Chemicon
International Inc., Temecula, CA, USA). Briefly, the sample and
standards were incubated overnight on antibody-coated plates in a
plate shaker at 100–150 rpm at 2–8°C. The wells were then washed
four times with a wash buffer, after which the anti-mouse NGF
monoclonal antibody (100 μl) was added to each of the wells. The
plates were then incubated in a shaker for 2 h at room temperature.
The peroxidase-conjugated donkey anti-mouse IgG polyclonal antibody
(100 μl) was then added to each well and the cells were incubated
at room temperature for 2 h. After washing, 100 μl of TMB/E
substrate was added to each well and the cells were incubated at
room temperature for 15 min. The reaction was quenched by the
addition of 100 μl of a stop solution. The plates were analyzed by
evaluating the absorbance at 450 nm using the same
spectrophotometer as for the MTT assay.
Neuritic outgrowth assay
To confirm the NGF ability on cell differentiation,
conditional medium (CM) was collected from B35 cells treated with
LP extracts (50 μg/ml), which had been processed under a range of
conditions for 24 h, and added to the undifferentiated PC12 cells.
After 24 h, the cell morphology was observed by optical microscopy
and the dendrite length of the PC12 cells was measured using a
Leica Application Suite (Leica Microsystems, Heerbrugg,
Switzerland).
Western blot analyses
Following treatment of the PC12 cells with various
types of CM for 24 h, the cells were harvested from 100 mm-diameter
culture dishes and collected in a PRO-PREP protein extraction
solution (INtRON Biotechnology, Seongnam, Korea) containing 1.0 mM
phenylmethylsulfonyl fluoride (PMSF), 1.0 mM ethylenediamine
tetraacetic acid (EDTA), 1 μM pepstatin A, 1 μM leupeptin and 1 μM
aprotinin. The resulting mixture was centrifuged for 10 min at
13,000 rpm at 4°C. The homogenized proteins were separated for 2 h
at 100 V by 10% sodium dodecyl sulfate-polyacrylamide gel
electrophoresis and transferred to nitrocellulose membranes over 2
h at 40 V. The membranes were then incubated with the following
primary antibodies to determine the levels of each protein:
anti-p75NTR antibody (Cell Signaling Technology, Beverley, MA,
USA), anti-RhoA antibody (Cell Signaling Technology), anti-TrkA
antibody (Cell Signaling Technology), anti-p-TrkA antibody (Cell
Signaling Technology), anti-Akt antibody (Cell Signaling
Technology), anti-p-Akt antibody (Cell Signaling Technology),
anti-ERK antibody (Santa Cruz Biotechnology, Santa Cruz, CA, USA),
anti-p-ERK antibody (Santa Cruz Biotechnology) and anti-β-actin
(Sigma-Aldrich). Each membrane was washed with a buffer (137 mM
NaCl, 2.7 mM KCl, 10 mM NaHPO4 and 0.05% Tween-20) and
incubated with a 1:1,000 dilution of horseradish peroxidase
(HRP)-conjugated goat anti-rabbit IgG antibody at room temperature
for 2 h. The membrane blots were developed using an Enhanced
Chemiluminescence Reagent Plus kit (Amersham Life Science,
Piscataway, NJ, USA).
ELISA for extracellular calcium
detection
The extracellular calcium concentration in the
culture supernatant isolated from the B35 cells, which had been
cultured and treated under the same conditions used in the MTT
assay, was detected using the ultra-sensitive assay procedure and
reagents in a Calcium colorimetric Assay kit (BioVision Research
Products, Milpitas, CA, USA). Briefly, the sample and standards (50
μl) were placed in an individual well and 90 μl of the Chromogenic
reagent and 60 μl of the Calcium Assay buffer were added. The
plates were then incubated in a shaker at 100–150 rpm for 2 h at
room temperature under light-protection conditions. Following
incubation, the plates were analyzed by evaluating the absorbance
at 575 nm using an ELISA reader (VERSA max, micro-reader, Molecular
Devices).
Detection of intracellular calcium
concentration using fura-2
The concentration of intracellular calcium was
determined by the Grynkiewicz method using fura-2/AM (16). Briefly, the prepared B35 cells were
incubated with 0.5 μM fura-2/AM (F1221, Invitrogen, Carlsbad, CA,
USA) at 37°C for 40 min in fresh serum-free RPMI-1640 medium with
continuous stirring. A total of 2×106 cells were
aliquoted for each assay in Ca-free Locke’s solution [154 mM NaCl,
5.6 mM KCl, 1.2 mM MgCl2, 5 mM HEPES (pH 7.3), 10 mM
glucose and 0.2 mM EGTA]. The fluorescence changes at the dual
excitation wavelengths of 340 and 380 nm and the emission
wavelength of 500 nm were measured and the calibrated fluorescence
ratio was translated into calcium concentration.
Statistical analysis
Tests for the significance between the various types
of LP- and vehicle-treated groups in B35 cells were carried out
using the one-way ANOVA test of variance (SPSS for Windows, Release
10.10, Standard Version, Chicago, IL, USA). Values were reported as
the mean ± SD. P<0.05 was considered to indicate a statistically
significant result.
Results
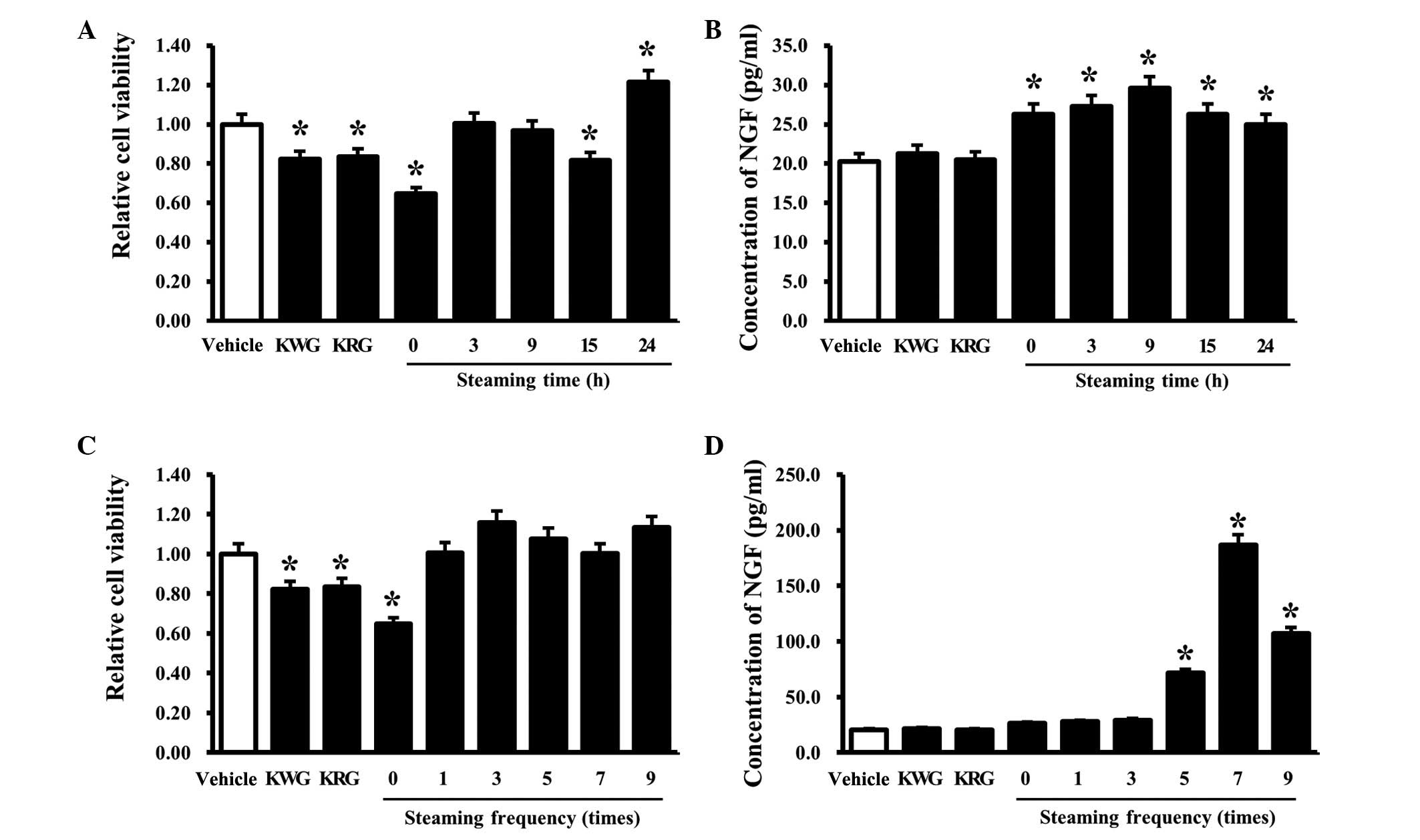
Effects of RLP treated for different
steaming times on the viability and NGF secretion ability of B35
cells
The effects of the steaming time on the viability
and NGF secretion of B35 cells was evaluated by determining the
cell viability and NGF concentration in B35 cells treated with five
types of LPs manufactured for different steaming times (0, 3, 9, 15
and 24 h). In the case of the ginseng treatment group, the cell
viability was slightly lower in the KRG- and KWG-treated cells than
the cells treated with the vehicle alone. Among the LP-treated
cells, an increase in cell viability was detected only in the
24-SLP-treated cells, whereas a decrease in cell viability was
detected in the 0- and 15-SLP-treated groups (Fig. 1A). Results of the NGF ELISA
analysis showed that the B35 cells treated with RLP had slightly
higher NGF concentrations than the group of cells treated with the
vehicle alone. By contrast, the increase in the NGF concentration
ratio was greater in the 9-SLP-treated B35 cells than in the other
groups. Moreover, the cells treated with KWG and KRG maintained the
levels observed in the vehicle-treated cells (Fig. 1B). These results suggest that a
steaming time for 9 h should be considered optimal for increasing
the NGF secretion ability of B35 cells. This is consistent with the
results of a previous study, which found that 9-SLP induced a
maximum increase in insulin secretion (15), although the induction ratio was
similar to that observed with 0-SLP to 24-SLP.
Effects of RLP manufactured using
different steaming frequencies on the viability and NGF secretion
ability of B35 cells
The cell viability and NGF concentration of B35
cells treated with the six types of LPs manufactured under
different steaming frequencies (0, 1, 3, 5, 7 and 9 times) were
evaluated to determine the effects of the LP steaming frequency on
the NGF secretion ability. The cells treated with KWG and KRG
showed slightly lower levels of viability than the vehicle-treated
cells. Among the LP-treated cells, the lowest viability was
observed in the B35 cells treated with 0-SALP. The B35 cells
treated with other forms of SALP showed similar or slightly higher
viability than the vehicle-treated group (Fig. 1C). However, the NGF concentrations
were significantly higher in the B35 cells treated with 7-SALP,
whereas a similar level was maintained for the vehicle-treated
cells in the cells treated with 0-, 1 and 3-SALP. The 5- and
9-SALP-treated cells maintained the intermediate level of NGF
concentrations (Fig. 1D). These
results show that 7-SALP is able to induce a maximum increase in
NGF secretion ability in B35 cells.
Effect of RLP manufactured for different
steaming times and frequencies on the differentiation of PC12
cells
NGF secreted from neuronal cells induced a cell
differentiation through the NGF receptors TrkA and
p75NTR(17,18). To determine whether NGF secreted
from B35 cells induced undifferentiated PC12 cells, the length of
the PC12 cells was observed following treatment with CM collected
from the B35 cells treated with the five types of LPs manufactured
for different steaming times (0, 3, 9, 15 and 24 h) and steaming
frequencies (0, 1, 3, 5, 7 and 9 times). For the different steaming
times, the length of the PC12 cells was increased significantly in
the supernatants treated with all types of SALP as well as the
supernatants treated with ginseng compared with the supernatant
treated with the vehicle. By contrast, the ratio of the increase
differed according to the group examined (Fig. 2A and B). In the case of different
steaming frequencies, the greatest length of PC12 cells was
detected in the PC12 cells treated with the 7-SALP supernatant,
followed by the PC12 cells treated with 9-, 5- and 1-SALP (Fig. 2A and B). Therefore, the NGF
secreted from B35 cells following treatment with the LPs
manufactured under the different conditions was able to induce the
differentiation of PC12 cells.
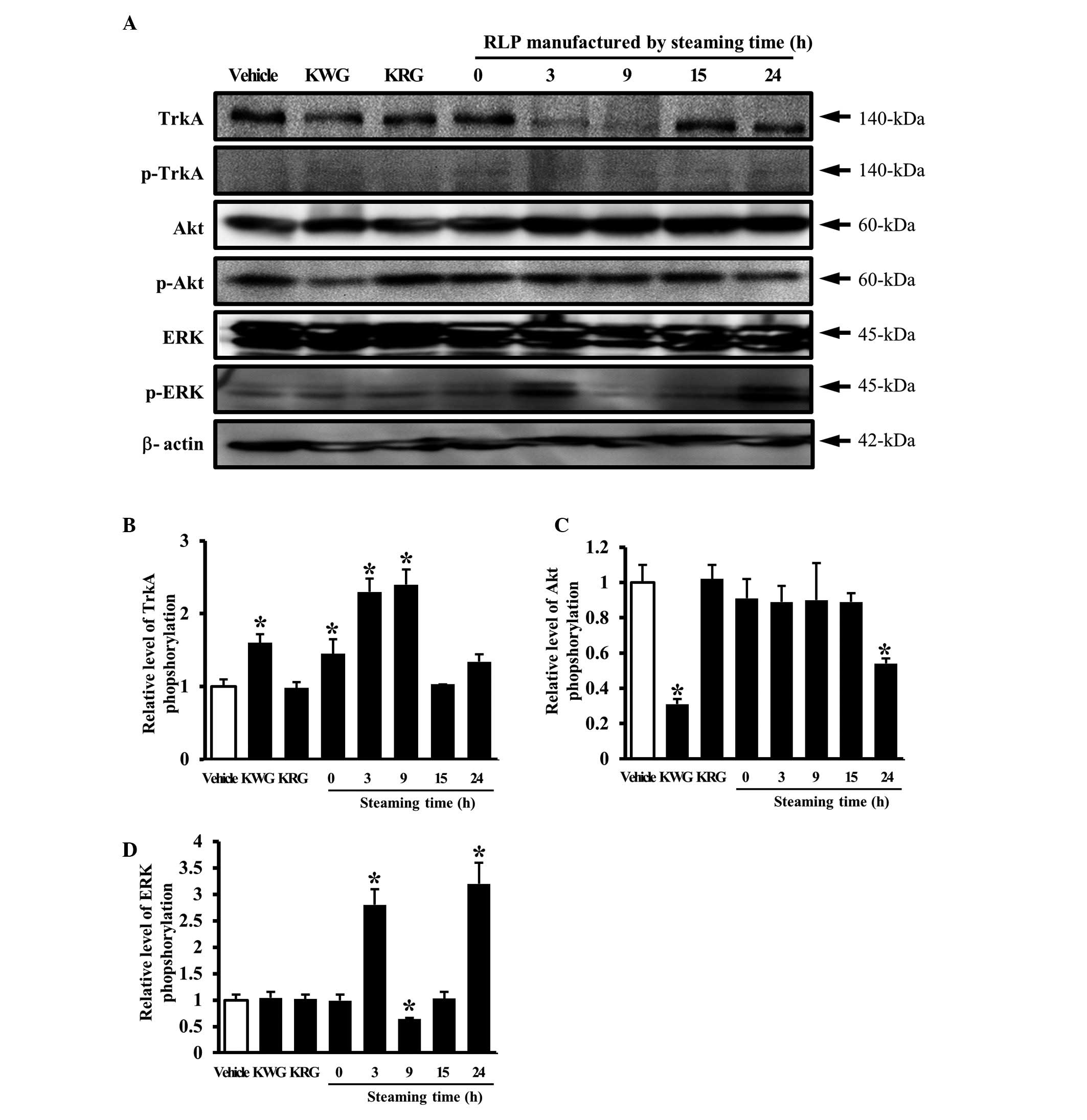
Effect of RLP manufactured for different
steaming times and frequencies on the NGF receptor TrkA signaling
pathway
The secreted NGF transduced the signal into the
cytosol by binding the two types of NGF receptor located on the
cell membrane. Of the two types of receptors, the high affinity
receptor, TrkA, is capable of inducing neuritic outgrowth via the
Akt and ERK signaling pathways (19). Therefore, this study examined the
effects of several types of CM collected from B35 cells on the NGF
receptor TrkA signaling pathway. In an analysis of the steaming
time effects, the phosphorylation of TrkA was increased by the
downregulation of unphosphorylated TrkA in the group treated with
3- and 9-SLP CM, whereas the level of TrkA phosphorylation was
maintained in the group treated with 15- and 24-SLP CM (Fig. 3A and B). The alteration of ERK or
Akt protein phosphorylation was then detected in the cells treated
with several types of CM to determine which protein was involved in
the signaling pathway activated by TrkA activation. A significant
change in phosphorylation was detected only in the ERK protein. In
particular, the level of ERK phosphorylation was higher in the
cells treated with 3- and 24-SLP CM than the cells treated with the
vehicle CM (Fig. 3A and D). By
contrast, the level of Akt phosphorylation was maintained in the
cells treated with most of the LP CMs, with the exception of 24-SLP
CM (Fig. 3A and C).
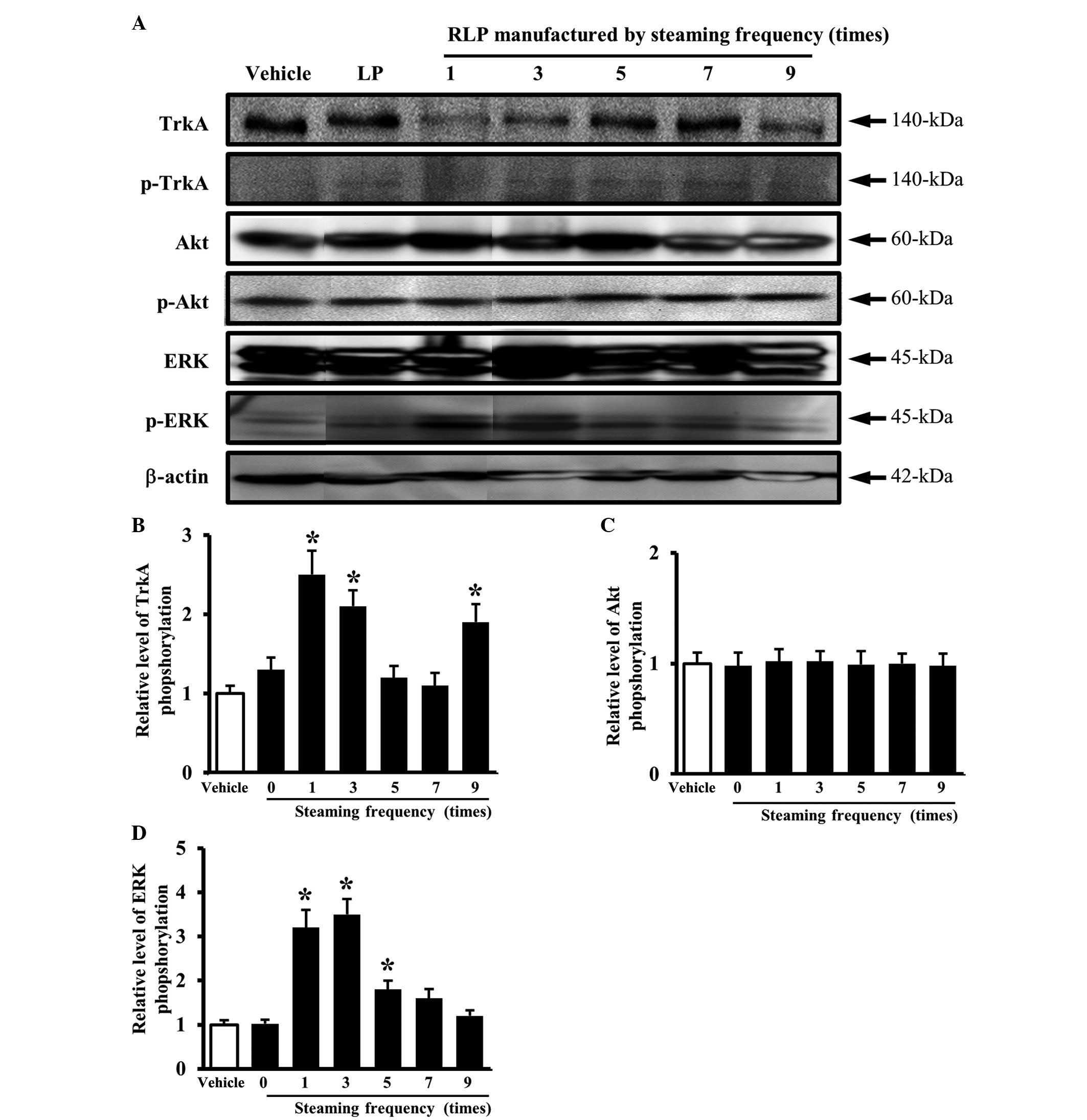
Similar results were obtained in an analysis of the
steaming frequency. The level of TrkA phosphorylation was higher in
the cells treated with 1-, 3- and 9-SALP CM due to the
downregulation of the unphosphorylated TrkA protein compared with
the vehicle CM (Fig. 4A and B). In
the downstream TrkA signaling pathway, the pattern of ERK
phosphorylation was similar to that of TrkA phosphorylation. The
cells treated with 1- and 3-SALP CM showed a higher level of ERK
phosphorylation than the cells treated with the vehicle CM
(Fig. 4A and D), whereas, the
level of Akt phosphorylation did not show any significant changes
(Fig. 4A and C). These results
suggest that the steaming time and frequency are able to induce the
ability of LP to activate the NGF receptor TrkA signaling pathway.
In particular, the LP steamed for 3 h and for 3 different times was
found to be the most appropriate formulation for activating the NGF
receptor TrkA signaling pathway.
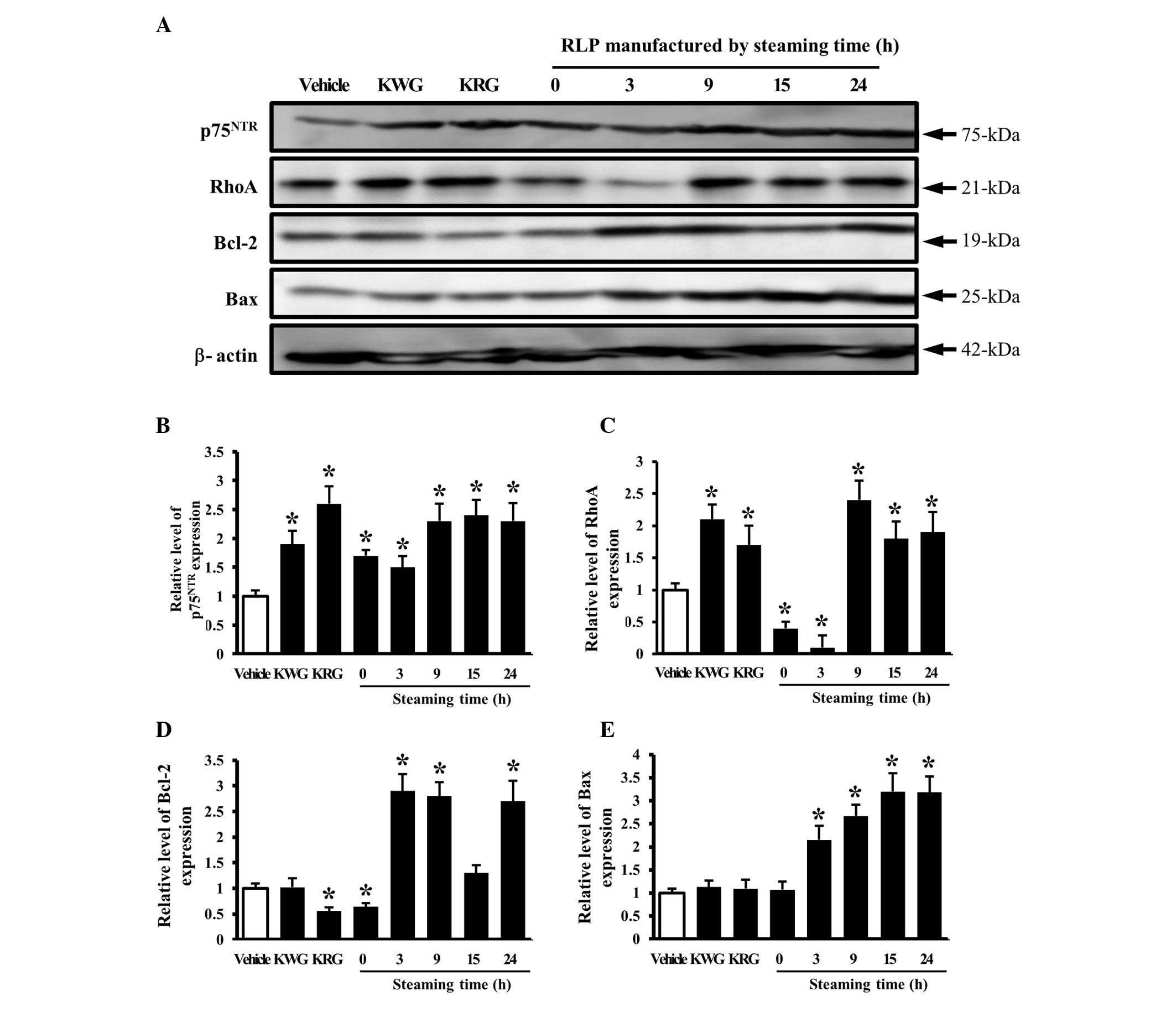
Effect of RLP manufactured for different
steaming times and frequencies on the NGF receptor
p75NTR signaling pathway
The effects of LP manufactured for different
steaming times and frequencies on the signaling pathway of the NGF
receptor p75NTR as a low affinity receptor for NGF were
examined. The level of p75NTR expression was higher in
most groups treated with different CM than in the group treated
with the vehicle CM. In particular, the maximum level was detected
in the groups treated with 9-, 15- and 24-SLP CM (Fig. 5A and B). In addition, the
expression of the p75NTR downstream component, RhoA, was
significantly higher in the cells treated with 9-, 15- and 24-SLP
CM, whereas the level was lower in the cells treated with 0- and
3-SLP CM (Fig. 5A and B). These
patterns of protein expression were also observed with the
apoptosis-related proteins, Bcl-2 and Bax. The levels of Bcl-2 and
Bax expression were higher in the cells treated with 3-to 24-SLP CM
than in the cells treated with the vehicle or 0-SLP CM (Fig. 5A, D and E).
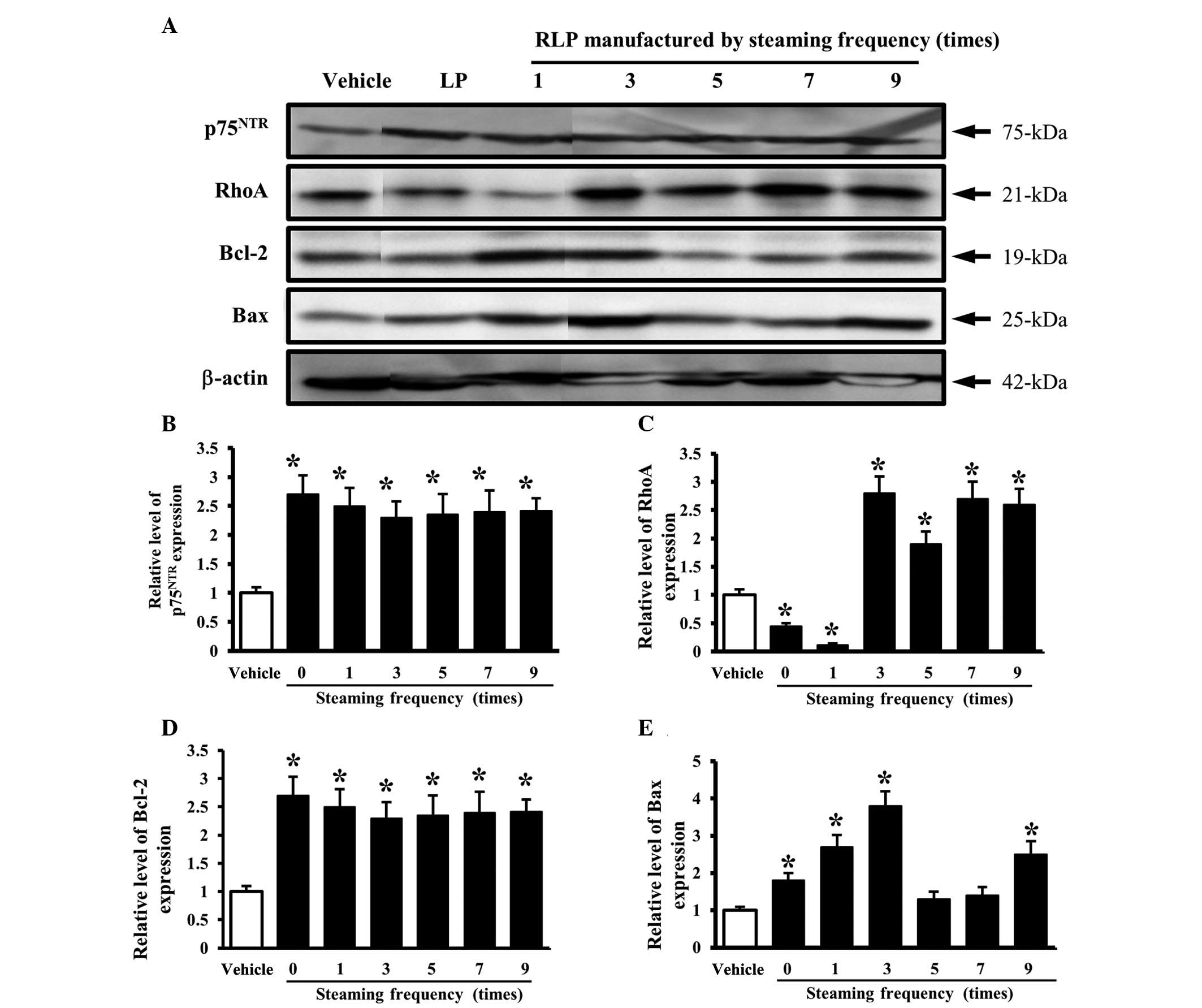
An analysis of the effects of the steaming frequency
revealed a constant level of p75NTR expression but a
higher level than the cells treated with the vehicle CM (Fig. 6A and B). However, the level of RhoA
expression was higher only in the cells treated with 3-, 5-, 7- and
9-SALP CM than in the vehicle-treated cells, but was lower in the
cells treated with 0- and 1-SALP CM (Fig. 6A and C). Furthermore, the final
components of the NGF receptor p75NTR downstream
pathway, Bcl-2 and Bax, showed a similar pattern of protein
expression. The significant increase in expression was commonly
detected in the cells treated with 1- and 3-SALP CM. In the other
groups, the levels of Bcl-2 and Bax were decreased or unchanged
according to the steaming frequency (Fig. 6A, D and E). These results show that
the steaming time and frequency of LP are capable of inducing
significant changes in the NGF receptor p75NTR signaling
pathway. In particular, 9-SLP and 3-SALP were considered to be the
most appropriate conditions to induce neuronal differentiation.
Effect of RLP on the regulation of the
calcium concentration
The physical function of neuronal cells is regulated
by the entry of extracellular calcium through the voltage- or
receptor-gated channels as well as the release of calcium from the
endoplasmic reticulum (20). The
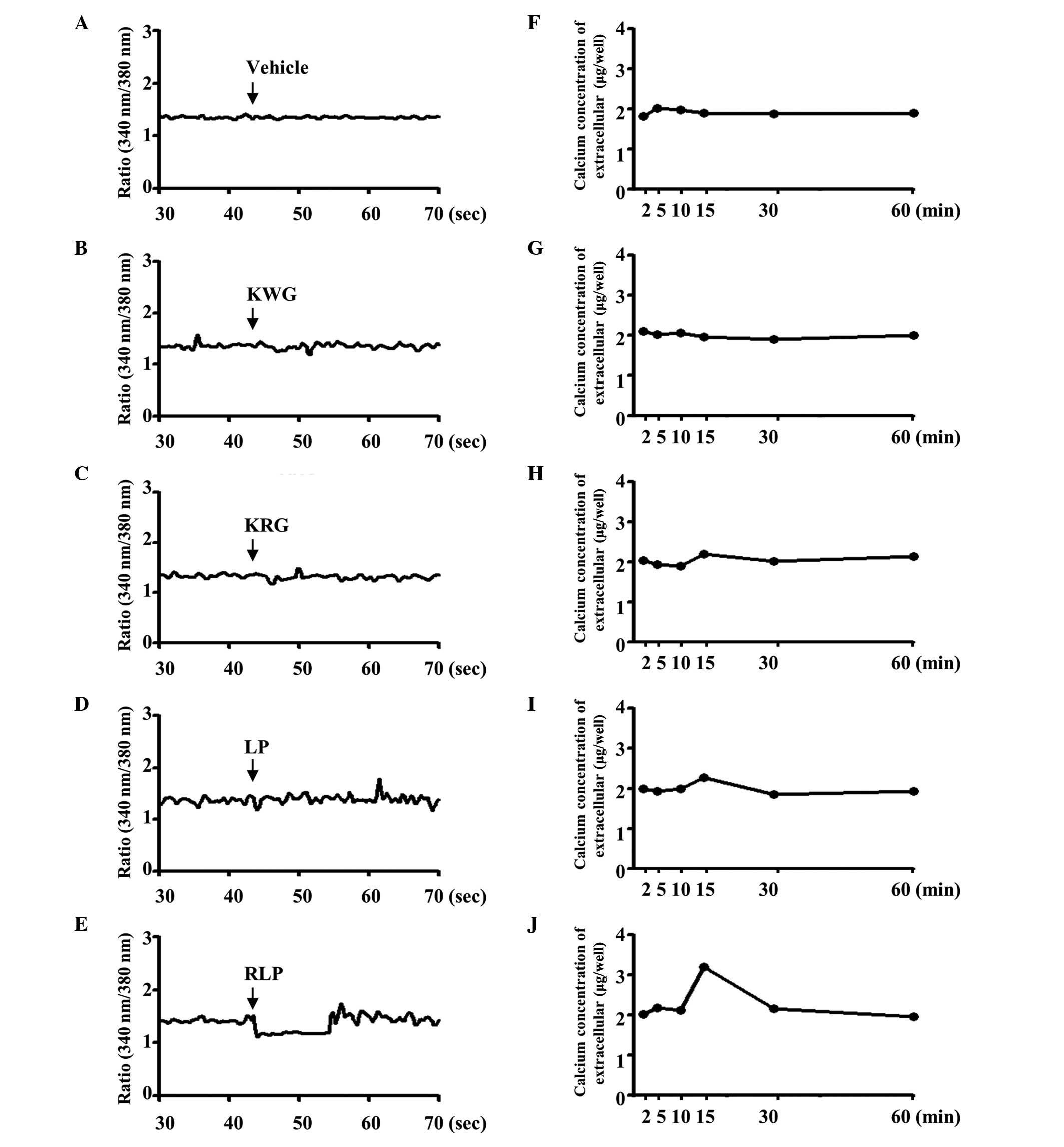
calcium level was measured in the supernatant of B35 cells treated
with various extracts to determine the effect of RLP (7-SALP) on
the regulation of the extracellular calcium concentration. In the
control group, a significant change in the extracellular calcium
concentration was not detected in the KWG-, KRG- and
vehicle-treated cells (Fig. 7F, G and
H), whereas, in the LP-treated group, the calcium level was
increased by 2% at 20 min after the treatment (Fig. 7I). Following the RLP treatment, the
extracellular calcium concentration increased markedly (~2-fold) at
the same point (Fig. 7J).
Furthermore, the alternation of intracellular calcium was similar
to the patterns of extracellular calcium concentration when the B35
cells were treated with the compounds KWG, KRG, LP and RLP. The
KWG-, KRG- and vehicle-treated cells did not exhibit a significant
change in the intracellular calcium concentration (Fig. 7A–C). The intracellular calcium
concentration was markedly decreased in RLP-treated cells, although
a slight decrease was detected in LP-treated cells (Fig. 7D and E). Therefore, RLP may induce
an increase in the extracellular calcium concentration and a
decrease in the intracellular calcium concentration.
Discussion
Neurotrophic factors are proteins that are produced
and released by astrocytes in the brain and periphery. They support
the survival of neuronal cells and also contribute to the growth
and differentiation of neurons (21). Among the neurotrophic factors, NGF
is a member of the neurotrophic factors family and plays a role in
the growth, maintenance and survival of nerve cells. In particular,
NGF is critical for axonal growth and the survival of sympathetic
and sensory neurons (22,23). NGF has powerful beneficial effects
on the damaged or dying neurons which are observed in
neurodegenerative disorders, including Alzheimer’s disease and
dementia, in that elevating the levels of the appropriate
neurotrophic factor may aid the restoration of the health and
vitality of injured neurons. However, NGF is not used for medical
applications since this molecule cannot penetrate the blood-brain
barrier owing to its large molecular weight and susceptibility
(24). Therefore, a number of
studies have focused on the identification of novel agents that
increase the level of NGF secretion or enhance the activity of NGF.
This study examined the effects of the steaming time and frequency
of manufactured RLP on the NGF secretion ability and the NGF
receptor signaling pathway to develop a novel agent increasing NGF
secretion. As shown in Fig. 1,
7-SALP under various steaming frequencies fully induced NGF
secretion without damaging the cell viability.
Several studies have reported a correlation between
LP and neuritic outgrowth. C6 and astrocyte cells were incubated
with the butanol fraction of LP for 24 h and the resulting media
were then added to PC12 cells, which exhibited neuritic outgrowth
as a result. Neuritic outgrowth was increased significantly in a
dose-dependent manner and blocked using an NGF antibody and an
inhibitor of protein kinase (6).
The results from the present study were similar to the results from
the butanol fraction. In this study, the maximum length of PC12
cells was observed in the cells treated with 7-SALP CM, although
most conditions for the steaming process induced the neuritic
outgrowth of PC12 cells. Spicatoside A, a steroid saponin isolated
from LP, induced the neuritic outgrowth of PC12 cells similar to
NGF (50 ng/ml) (7). Nevertheless,
this study focused not on mimicking the effect of NGF, but on the
NGF secretion ability.
Generally, the steaming process induced a chemical
transformation or the formation of novel compounds according to the
conditions. The steamed ginseng was manufactured by steaming raw
ginseng at 98–100° for 2–3 h, although the steaming process was
repeated under several conditions (9). The present study examined the optimal
conditions for the LP steaming process on the NGF secretion
ability. Of the four steaming times, the maximum NGF secretion was
observed in the cells treated with LP, which had been steamed for 9
h (Fig. 2B), whereas, there was
little difference between the maximum and minimum levels of
secretion. Furthermore, this study determined the optimal frequency
of the steaming process at a 3-h steaming time. The highest NGF
concentrations were detected in the cells treated with LP steamed 7
times (Fig. 2D). Therefore, the
increase in NGF secretion induced by a treatment with steamed LP
might be induced by the component changes caused by the steaming
process.
There is ethnopharmacological evidence suggesting
that KWG and KRG have regulatory effects on NGF secretion. However,
most studies have focused on KWG and only one study has examined
the effect of KRG, which is manufactured by a steaming process
(25–27). In a study using KRG, administration
of estradiol valerate was found to induce a significant increase in
NGF protein and NGF mRNA in the ovaries of polycystic ovary-induced
rats. These levels were decreased by administering a KRG extract
(28). In this study, the steaming
process induced a different effect from the NGF secretion ability
observed under the condition of the LP treatment. NGF secretion was
markedly induced by the 9-SLP and 7-SALP treatments.
Thus far, there is no evidence that the RLP-CM is
able to regulate the signaling pathway of the NGF receptor,
although one study reported that spicatoside A activated ERK 1/2
and PI3-kinase/Akt via TrkA sequentially to induce the neuritic
process (7). In the present study,
LP CM activated TrkA phosphorylation and then their signal
stimulated neuritic outgrowth via the ERK pathway. These results
showed a similar pattern under the two conditions regardless of the
manufacturing methods.
In conclusion, the steam-processed LP induced
profound NGF secretion compared with the unsteamed LP under in
vitro analysis. Changes in the NGF receptor signaling pathway
were observed in the PC12 cells treated with steam-processed LP.
The steam-processed LP may therefore have applications as a
therapeutic drug in the treatment of neurodegenerative
diseases.
Acknowledgements
This study was supported by grants to Professor Dae
Youn Hwang from the Korea Institute of Planning Evaluation for
Technology of Food, Agriculture, Forestry and Fisheries
(110119-3).
References
|
1
|
Lee YC, Lee JC, Seo YB and Kook YB:
Liriopis tuber inhibit OVA-induced airway inflammation and
bronchial hyper responsiveness in murine model of asthma. J
Ethnopharmacol. 101:144–152. 2005. View Article : Google Scholar
|
|
2
|
Huh MK, Huh HW, Choi JS and Lee BK:
Genetic diversity and population structure of Liriope
platyphylla (Liliaceae) in Korea. J Life Sci. 17:328–333. 2007.
View Article : Google Scholar
|
|
3
|
Choi SB, Wha JD and Park S: The insulin
sensitizing effect of homoisoflavone-enriched fraction in
Liriope platyphylla Wang et Tang via PI3-kinase pathway.
Life Sci. 75:2653–2664. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Jeong S, Chae K, Jung YS, Rho YH, Lee J,
Ha J, Yoon KH, Kim GC, Oh KS, Shin SS and Yoon M: The Korean
traditional medicine Gyeongshingangjeehwan inhibits obesity through
the regulation of leptin and PPAR alpha action in OLETF rats. J
Ethnopharmacol. 119:245–251. 2008. View Article : Google Scholar
|
|
5
|
Kim SW, Chang IM and Oh KB: Inhibition of
the bacterial surface protein anchoring transpeptidase sortase by
medicinal plants. Biosci Biotechnol Biochem. 66:2751–2754. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hur J, Lee P, Kim J, Kim AJ, Kim H and Kim
SY: Induction of nerve growth factor by butanol fraction of
Liriope platyphylla in C6 and primary astrocyte cells. Biol
Pharm Bull. 27:1257–1260. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hur J, Lee P, Moon E, Kang I, Kim SH, Oh
MS and Kim SY: Neurite outgrowth induced by spicatoside A, a
steroidal saponin, via the tyrosine kinase A receptor pathway. Eur
J Pharmacol. 620:9–15. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Choi SI, Park JH, Her YK, Lee YK, Kim JE,
Nam SH, Goo JS, Jang MJ, Lee HS, Son HJ, et al: Effects of water
extract of Liriope platyphylla on the mRNA expression and
protein secretion of nerve growth factors. Korean J Medicinal Crop
Sci. 18:291–297. 2010.(In Korean).
|
|
9
|
Kim K and Kim HY: Korean red ginseng
stimulates insulin release from isolated rat pancreatic islets. J
Ethnopharmacol. 120:190–195. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lu JM, Yao Q and Chen C: Ginseng
compounds: an update on their molecular mechanisms and medical
applications. Curr Vasc Pharmacol. 7:293–302. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ng TB: Pharmacological activity of sanchi
ginseng (Panax notoginseng). J Pharm Pharmacol.
58:1007–1019. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kiefer D and Pantuso T: Panax ginseng. Am
Fam Physician. 68:1539–1542. 2003.PubMed/NCBI
|
|
13
|
Baek NI, Kim DS, Lee YH, Park JD, Lee CB
and Kim SI: Ginsenoside Rh4, a genuine dammarane glycoside from
Korean red ginseng. Planta Medica. 62:86–87. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yun TK, Lee YS, Kwon KH and Choi KJ:
Saponin contents and anticarcinogenic effects of ginseng depending
on types and ages in mice. Zhongguo Yao Li Xue Bao. 17:293–298.
1996.PubMed/NCBI
|
|
15
|
Choi SI, Lee HR, Goo JS, Kim JE, Nam SH,
Hwang IS, Lee YJ, Prak SH, Lee HS, Lee JS, et al: Effects of
steaming time and frequency for manufactured red Liriope
platyphylla on the insulin secretion ability and insulin
receptor signaling pathway. Lab Anim Res. 27:117–126. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Grynkiewicz G, Poenie M and Tsien RY: A
new generation of Ca2+ indicators with greatly improved
fluorescence properties. J Bio Chem. 260:3440–3450. 1985.
|
|
17
|
Perini G, Della-Bianca V, Politi V, Della
Valle G, Dal-Pra I, Rossi F and Armato U: Role of p75 neurotrophin
receptor in the neurotoxicity by β-amyloid peptides and synergistic
effect of inflammatory cytokines. J Exp Med. 195:907–918. 2002.
|
|
18
|
Russo C, Dolcini V, Salis S, Venezia V,
Zambrano N, Russo T and Schettini G: Signal transduction through
tyrosine-phosphorylated C-terminal fragments of amyloid precursor
protein via an enhanced interaction with Shc/Grb2 adaptor proteins
in reactive astrocytes of Alzheimer’s disease brain. J Biol Chem.
277:35282–35288. 2002.
|
|
19
|
Tsui-Pierchala BA and Ginty BB:
Characterization of an NGF-P-TrkA retrograde-signaling complex and
age-dependent regulation of TrkA phosphorylation in sympathetic
neurons. J Neurosci. 19:8207–8218. 1999.PubMed/NCBI
|
|
20
|
Berridge MJ: Neuronal calcium signaling
review. Neuron. 21:13–26. 1998. View Article : Google Scholar
|
|
21
|
Barres BA and Barde Y: Neuronal and glial
cell biology. Curr Opin Neurobiol. 10:642–648. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Levi-Montalcini R: The nerve growth
factor: thirty-five years later. EMBO J. 6:1145–1154.
1987.PubMed/NCBI
|
|
23
|
Fiore M, Chaldakov GN and Aloe L: Nerve
growth factor as a signaling molecule for nerve cells and also for
the neuroendocrine-immune systems. Rev Neurosci. 20:133–145.
2009.PubMed/NCBI
|
|
24
|
Diaz Brinton R and Yamazaki RS: Advances
and challenges in the prevention and treatment of Alzheimer’s
disease. Pharm Res. 15:386–398. 1998.
|
|
25
|
Liang W, Ge S, Yang L, Yang M, Ye Z, Yan
M, Du J and Luo Z: Ginsenosides Rb1 and Rg1 promote proliferation
and expression of neurotrophic factors in primary Schwann cell
cultures. Brain Res. 1357:19–25. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Yabe T, Tuchida H, Kiyohara H, Takeda T
and Yamada H: Induction of NGF synthesis in astrocytes by
onjisaponins of Polygala tenuifolia, constituents of kampo
(Japanese herbal) medicine, Ninjin-yoei-to. Phytomedicine.
10:106–114. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Pak SC, Kim SE, Oh DM, Shim KM, Jeong MJ,
Lim SC, Nah SY, Park SH, Kang SS, Moon CJ, et al: Effect of Korean
red ginseng extract in a steroid-induced polycystic ovary murine
model. Arch Pharm Res. 32:347–352. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Pak SC, Lim SC, Nah SY, Lee J, Hill JA and
Bae CS: Role of Korea red ginseng total saponins in rat infertility
induced by polycystic ovaries. Fertil Steril. 84:1139–1143. 2005.
View Article : Google Scholar : PubMed/NCBI
|