Introduction
Bone marrow stromal stem cells or mesenchymal stem
cells (MSCs) have been under intense scrutiny for many years
(1–3). In addition to the multilineage
potential of MSCs in vitro and in vivo(3–6),
further interest in the clinical application of MSCs has been
raised in recent years by the observation that MSCs are capable of
exerting a profound immunosuppressive ability (7–9).
Injections of MSCs have been reported to successfully prolong the
survival of mismatched skin grafts in animals (10) and have also been proven to be of
therapeutic use in graft-versus-host disease (GVHD) treatment in
clinical trials (11). On the
basis of these findings, some researchers employed MSCs to treat
experimental autoimmune encephalomyelitis (EAE) and successfully
alleviated this T-cell-mediated autoimmune disease in an animal
model (12). Furthermore, our
group recently demonstrated that MSCs could exert profound
suppressive effects on type II collagen-reactive T cells from
rheumatoid arthritis (RA) patients (13). Improved knowledge of these
immunological properties of MSCs creates a potential
cell-immunotherapeutic approach for arthritic diseases, and MSCs
have also been gaining attention from rheumatologists (14).
Although the potential of MSCs in therapy is
encouraging, the low frequency of MSCs in bone marrow necessitates
their in vitro expansion prior to clinical use. In recent
years, long-term culture and sequential passages in vitro by
adherence to plastic has been used as the most popular strategy for
MSC isolation, purification and expansion (3,4).
However, potential difficulties are the subtle changes these cells
undergo as they are expanded in culture. A previous study has
proven that the telomere length of MSCs shortens after each
division cycle (15), which leads
to a gradual senescence of MSCs. Some researchers believe that MSCs
enter senescence almost undetectably from the moment of culture
in vitro(16). Despite the
premature aging of stem cells that has been reported, which may
have implications for other cell lineages and may provide the basis
for immune-mediated tissue damage and the breakdown of
self-tolerance (17), our
understanding of the effects of in vitro amplification on
the immunological properties of MSCs, which is critical to future
cell-therapeutic applications, remains in its infancy.
With this in mind, in the present study we
characterized the changes of morphology, immunophenotype, growth
rate and the biological characteristics of MSCs during expansion
in vitro; we further evaluated the effects of MSCs separated
from different passages (designated as P1, P7 and P13,
respectively) on the proliferation, activation and cytokine
production of allogenic T cells.
Materials and methods
Isolation and long-term culture of human
MSCs
Bone marrow aspirates (10 ml) were obtained from the
iliac crests of 5 healthy donors (3 males and 2 females, aged 20–30
years). The procedure was approved by the Ethics Committee at
Xijing Hospital, and the donors provided informed consent. The
widely used method of MSC culture, which was firstly adopted 10
years ago (3) and was used in a
recent published study (18), was
also adopted in the current study. Briefly, mononuclear cells were
isolated from Percoll-separated bone marrow and resuspended in
medium consisting of Dulbecco’s modified Eagle’s medium-low glucose
(DMEM-LG; Gibco-BRL, Carlsbad, CA, USA), supplemented with 10%
fetal bovine serum (FBS; Hyclone, Logan, UT, USA). Mononuclear
cells were plated at 2×107 cells/cm2 in 75
cm2 flasks (Corning, Acton, MA, USA) for primary
culture, and the culture was maintained at 37°C in a humidified
atmosphere containing 5% CO2. Cells were fed by
completely replacing the medium every 3 days. When fibroblast-like
cells at the base of the flask reached more than 90% confluence,
the cells were trypsinized in trypsin-EDTA (Gibco-BRL, Denmark),
and reseeded at 5×103 cells/cm2 in 75
cm2 flasks. On reaching confluence, all cultures were
passaged sequentially until the cells reached their maximal life
span, as evidenced by growth arrest where the cells failed to
become confluent within 4 weeks of culturing (19). After every 6th passage, some of the
expanded cells were separated for studying. The adherent cells
derived from the 5 donors were all identified as MSCs according to
the method described in our previous report (13).
Characterization of morphology of
long-term cultured MSCs
To study the morphological characteristics of
long-term cultured MSCs, cell culture flasks were observed at every
medium re-feeding interval to detect any abnormalities in cell
morphology and medium. When any variation was observed, the changes
were recorded.
Quantification of MSC growth in long-term
culture
To determine the number of cumulative population
doublings (PD), MSCs were trypsinized, counted and reseeded at a
density of 5×103/cm2 in 75 cm2
flasks during sequential passages following primary culture. Cell
growth was monitored by determining the number of PDs using the
following formula: Log N/log 2, where N is the cell number of the
confluent monolayer divided by the initial number of cells
seeded.
Assessment of senescence-associated
β-galactosidase staining in long-term culture
Senescence-associated β-galactosidase (SA β-gal)
staining was performed as described previously (20). MSCs separated from different
passages (P1, P7 and P13, respectively) were seeded on slides
(5×104 cells/cm2) and cultured to 90%
confluence. They were washed in phosphate-buffered saline (PBS),
fixed for 3–5 min at room temperature in 2% formaldehyde/0.2%
glutaraldehyde (or 3% formaldehyde), and incubated overnight at
37°C (without CO2) with fresh SA β-gal staining solution
(1 mg/ml 5-bromo-4-chloro-3-indolyl β-D-galactoside, 40 mM citric
acid/sodium phosphate, pH 6.0, 5 mM potassium ferrocyanide, 5 mM
potassium ferricyanide, 150 mM NaCl and 2 mM MgCl2).
Staining was evident in 2–4 h and maximal in 12–16 h.
Determination of the immunophenotype of
long-term cultured MSCs
Multiple surface markers were determined on MSCs at
different stages during the long-term culture (P1, P7 and P13,
respectively). The monoclonal antibodies used were anti-CD44
fluorescein isothiocyanate (FITC), anti-CD90 FITC, anti-CD105 FITC,
anti-CD106 FITC, anti-HLA-ABC FITC, anti-HLA-DR FITC, anti-CD29
phycoerythrin (PE), anti-CD34 PE, anti-CD80 PE, anti-CD86 PE,
anti-CD14 peridinin chlorophyll protein (Percp) and anti-CD45 Percp
(all from Pharmingen, San Diego, CA, USA). Relevant isotope control
antibodies were also used. Flow cytometry was performed on a
FACScan (Becton Dickinson, ,Franklin Lakes, NJ, USA), and data were
analyzed using Cellquest software.
Isolation of allogenic T cells
Heparinized peripheral blood (PB) was collected from
5 healthy donors (3 males and 2 females, aged 19–31 years) under
sterile conditions with the approval of the Ethics Committee at
Xijing Hospital and diluted 1:1 with DMEM-LG. Informed consent was
obtained from all 5 donors prior to the study. Mononuclear cells in
PB (PBMCs) were isolated by density gradient centrifugation on
Ficoll-Hypaque (1.077 g/ml). Cell viability was 95% by trypan blue
exclusion. PBMCs were then separated immunomagnetically into T
cells and non-T cells using anti-CD3 microbeads (Miltenyi Biotec,
Auburn, CA, USA). Non-T cells were used as antigen-presenting cells
(APCs).
Proliferation assay
Non-T cells and MSCs (from P1, P7 and P13,
respectively) were all irradiated (30 Gy) prior to being cultured
with T lymphocytes. Each culture was performed in triplicate at
1×105 cells/well for T cells in 96-well round-bottomed
microtiter plates (Corning) in a total volume of 0.2 ml DMEM-LG
supplemented with 10% FBS. Non-T cells, acting as APCs, were mixed
with T-cells at a ratio of 1:1. MSCs separated from different
passages were then added to the plates at a ratio of 1:1 to T cells
with the stimulation of phytohemagglutinin (PHA; 20 ng/ml; Sigma,
St. Louis, MO, USA). T cells cultured only with non-T cells in the
presence of PHA served as controls. The plates were incubated in a
humidified atmosphere of 5% CO2 at 37°C for 3 days.
Twelve hours prior to the end of culture, 1 μCi 3H-thymidine (NEN
Life Science Products, Boston, MA, USA) was added to each well.
Cells were harvested onto glass-fiber filter paper, dried and the
incorporated 3H-thymidine was analyzed using a liquid scintillation
counter. Data were expressed as median counts per minute (cpm) of
triplicate samples. The inhibition capacity was calculated using
the following formula: [1-(proliferation of PHA-stimulated T cells
in the presence of MSCs) (cpm)/(proliferation of T cells stimulated
with PHA alone) (cpm)] ×100%.
Activation assay
T cell activation assays were performed in 24-well
round-bottomed plates (Corning) in a total volume of 1 ml DMEM-LG
in triplicate. T cells were mixed at a 1:1 ratio with MSCs (from
P1, P7 and P13, respectively) at a density of
1×106/well. Activation markers were stained on day 1
(for CD3/CD69, Pharmingen) and day 3 (for CD3/CD25, Pharmingen).
The cells were then analyzed by flow cytometry.
Cytokine quantification
After 3 days of co-culture (ratios of MSCs to T
cells, 1:1), with or without PHA stimulation, fresh supernatants
were collected. Quantitative analyses of IL-10, IFN-γ and TNF-α
production were performed by enzyme-linked immunosorbent assay
(ELISA) using commercially available kits (R&D Systems,
Minneapolis, MN, USA). Supernatants of MSCs (from P1, P7 and P13,
respectively) and T cells that were cultured alone served as
controls. The detection limits were 15 pg/ml for IL-10, 4 pg/ml for
IFN-γ and 7 pg/ml for TNF-α, respectively.
Statistics
Results were expressed as the means ± SD of the
mean. Differences between experimental conditions were analyzed by
t-test (paired when possible). P<0.05 was considered to indicate
a statistically significant difference.
Results
Morphological characterization of MSCs in
long-term culture
MSCs were successfully isolated and expanded from
all the 5 donors. After approximately 10 days of primary
incubation, marrow-derived cells that adhered to the flasks
gradually formed a confluent heterogeneous stromal cell layer and
appeared microscopically to be a relatively homogeneous population
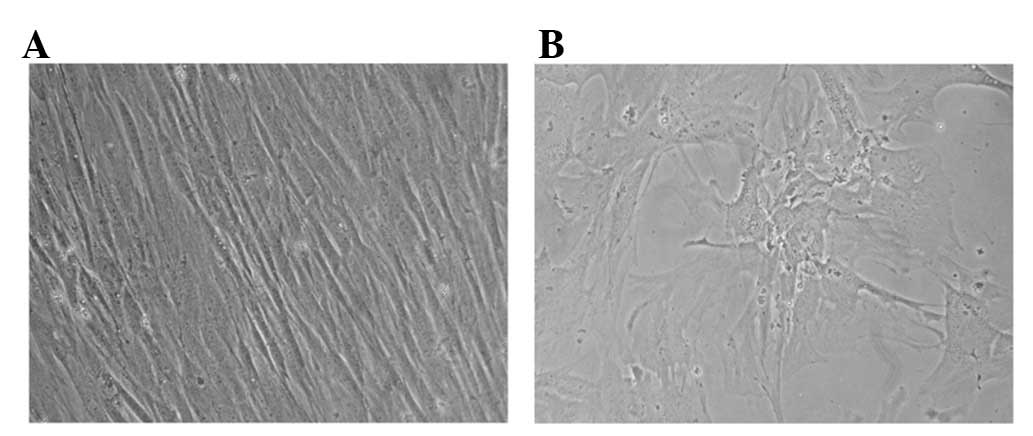
of fibroblast-like cells after the first passage (P1,Fig. 1A).
However, MSCs increased in size and shape with a
long period of conventional expansion in vitro, and showed
abnormalities typical of the Hayflick model of cellular aging.
Moreover, increasing numbers of non-attached floating MSCs were
also consistently observed in prolonged passage. On average,
granules were gradually noted in the cytoplasm of MSCs after P7,
and debris formation was observed in medium of culture after
approximately P10 of primary culture (data not shown). Particularly
in later stages (around P13 in some cultures), MSCs lost their
fibroblast-like morphology and began to be vacuolated, and finally
detached from the base of the flasks (Fig. 1B).
Growth kinetics of MSCs in long-term
culture
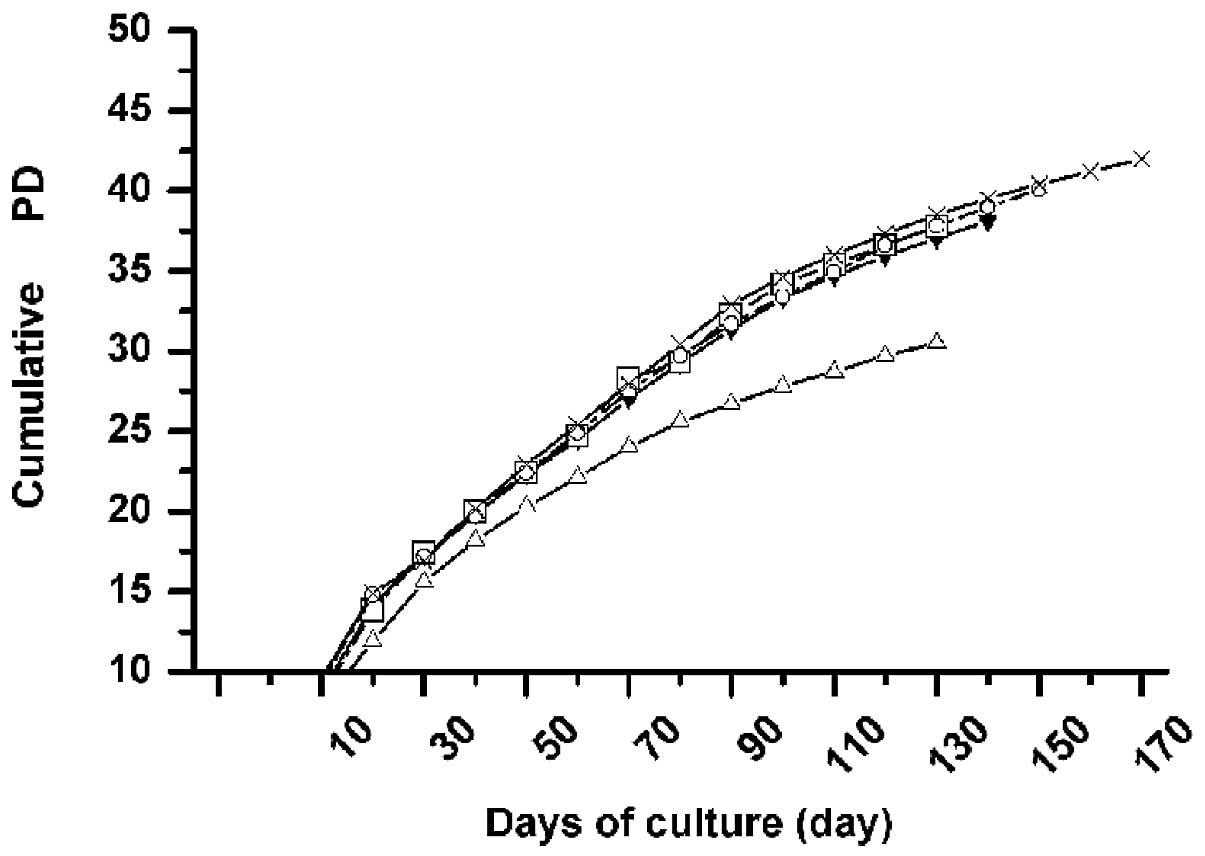
Starting from the primary passage, we analyzed the
kinetics of growth of MSCs from all 5 donors, with respect to the
passage number in multiple donors. In all donors tested, MSCs were
expanded over at least 13 passages (range from P13 to P17, mean
P14.4), with similar growth kinetics. The average time of culture
was approximately 142 days (range, 130–170 days) until reaching
their maximal life span, and the average number of cumulative PDs
was approximately 37.7 (range, 30–42) in these days. The curve
relationship between cumulative PD and the duration of culture
demonstrates a relatively linear decreasing PD rate with the
progression of time. Furthermore, an appreciable decrease in the
number of PD was observed in the late days of culture (>100 days
in culture; Fig. 2), indicating
that the proliferative potential of MSCs decreased with long-term
culture in vitro.
Cellular senescence markers expressed on
MSCs in long-term culture
An increase in the number of cells staining positive
for SA β-gal was observed in the long-term in vitro culture.
As shown in Fig. 3, early-passage
MSCs (P1) only contained 2±0.4% positive cells, while the
proportion of positive cells significantly increased to 16±3% in P7
(16±3 vs. 2±0.4%, P<0.001). When MSCs were passaged to P10, the
cells displaying detectable SA β-gal activity reached up to 35±2%
(35±2 vs. 2±0.4%, P<0.001; 35±2 vs. 16±3%, P<0.001),
demonstrating that MSCs would be senescent during the long-term
expansion in vitro. MSCs from all 5 donors showed similar
results in the long-term culture.
Immunophenotype of MSCs in long-term
culture
We employed multiple monoclonal antibodies to detect
the immunophenotype of MSCs from P1, P7 and P13 to investigate the
effects of long-term culture on the immunophenotype of MSCs. As
Fig. 4 shows, MSCs from P1 were
uniformly positive for the expression of CD29, CD44, CD90, CD105,
CD106 and HLA-ABC, but negative for the expression of CD14, CD34,
CD45, CD80, CD86 and HLA-DR. These immunophenotypes expressed on
MSCs from P7 and P13 were similar to that of MSCs from P1 (data not
shown), indicating that long-term culture exerts fewer effects on
the immunophenotype of MSCs.
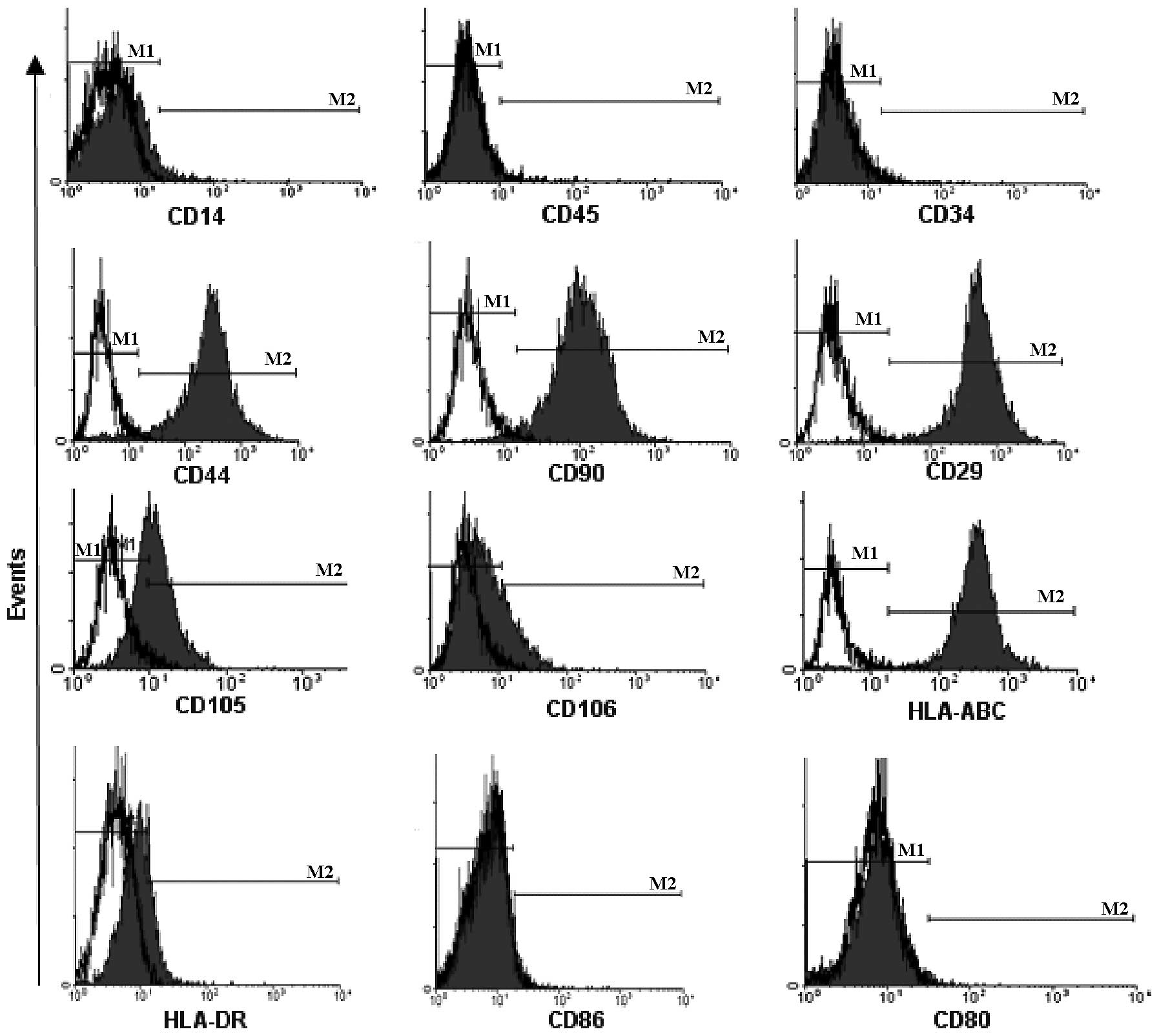 | Figure 4Immunophenotype of MSCs in long-term
culture. MSCs were uniformly positive for the expression of CD29,
CD44, CD90, CD105, CD106 and HLA-ABC, but negative for the
expression of CD14, CD34, CD45, CD80, CD86 and HLA-DR. Data showed
a representative histogram of immunophenotype of MSCs from passage
1 (P1). Black open histogram, blank control; black solid histogram,
expression of immunophenotype. MSCs, mesenchymal stem cells. |
Effects of MSCs in long-term culture on
the proliferation of T cells
Compared to the control, T cells alone in culture,
no significant proliferation of T cells was observed against
allogeneic MSCs whether they were from early passages (P1) or late
passages (P13) (data not shown), demonstrating that MSCs in the
long-term culture still retained their low immunogenicity and were
not recognized by antigen-specific T cells.
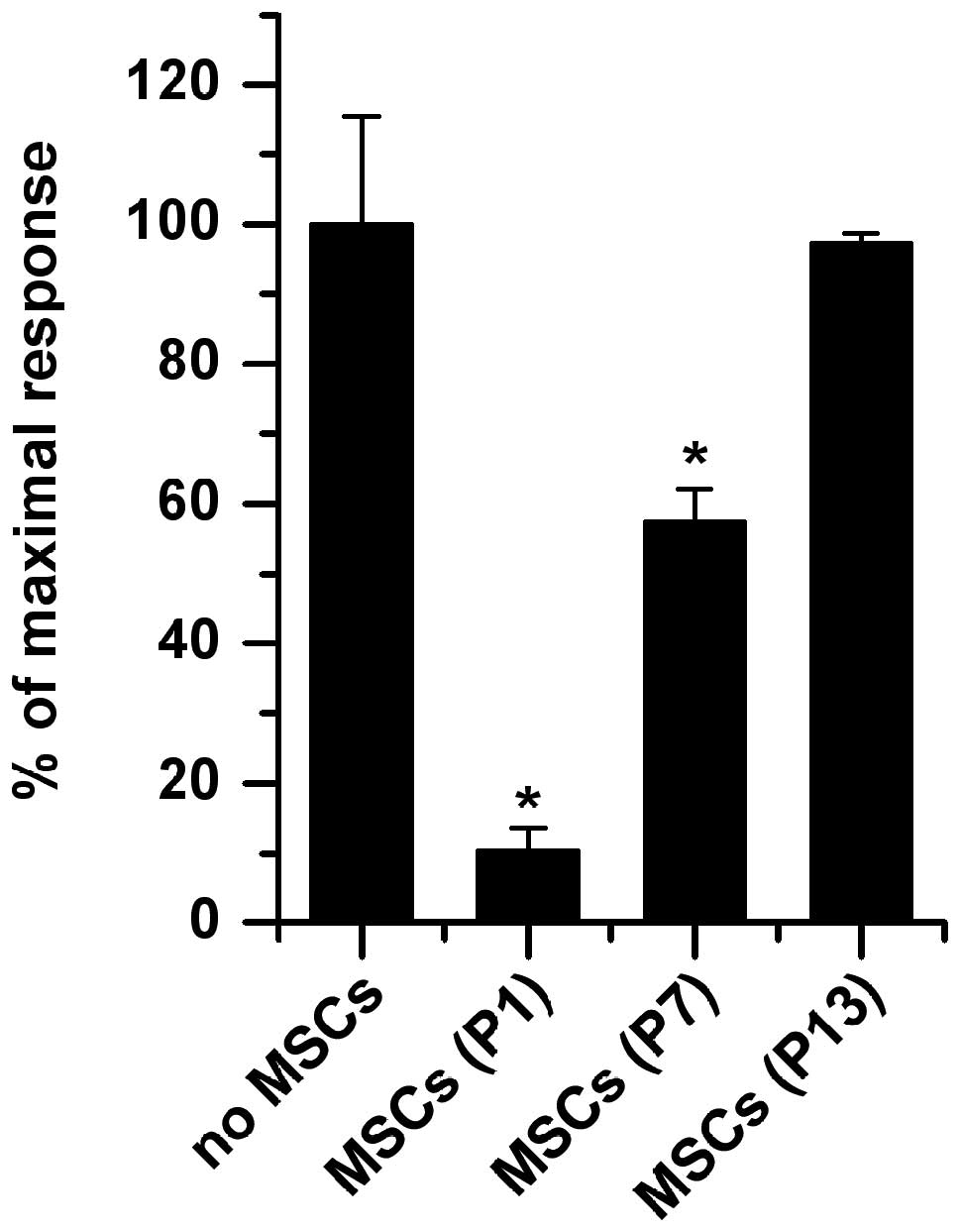
However, the inhibitory capacity of MSCs on T-cell
proliferation decreased in long-term culture. As Fig. 5 shows, in the presence of MSCs from
P1, PHA-stimulated T-cell proliferation was significantly inhibited
(89.7±3.5% in the presence of MSCs, P<0.001) compared to the no
MSCs group with the stimulation of PHA, which is in accordance with
our previous study (13). However,
the inhibitory capacity of MSCs decreased with their successive
expansion in vitro. As for MSCs from P7, they could also
significantly inhibit T-cell proliferation compared to the no MSCs
group (42.6±4.2% in the presence of MSCs, P<0.001); however,
they exerted only approximately half of the inhibitory effects
compared to that of MSCs from P1 (42.6±4.2 vs. 89.7%±3.5%,
P<0.001). When passaged to P13, the inhibitory capacity of MSCs
almost disappeared (2.7±1.5%, P=0.148) compared with the no MSCs
group. Although MSCs from the 5 donors possessed different
inhibitory capacities at the same passage of T-cell proliferation,
their tendency for decreased inhibitory capacity with long-term
expansion was consistent.
Effects of MSCs in long-term culture on
the activation of T cells
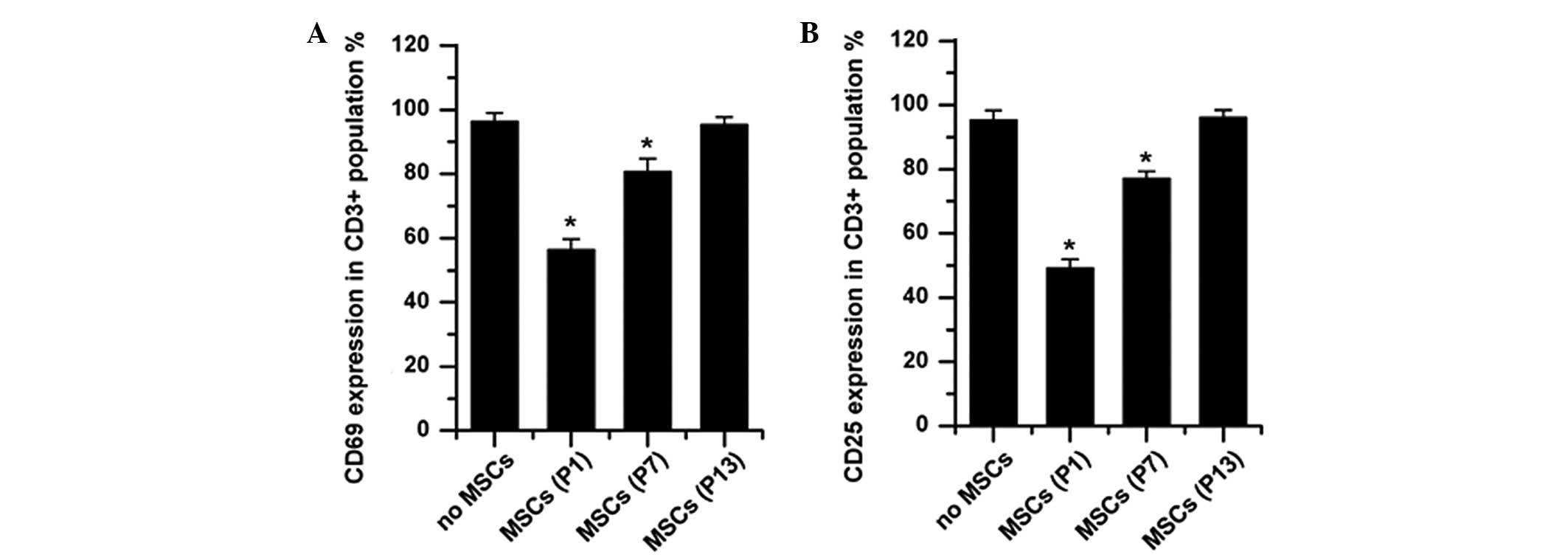
Allogenic MSCs from any passage did not elicit the
upregulation of CD69 and CD25 on T cells, indicating that long-term
culture did not impair the low immunogenicity of MSCs (data not
shown); while MSCs significantly inhibited PHA-induced upregulation
of CD69 and CD25, which is consistent with previous reports
(21–23). Although MSCs from P1 significantly
downregulated the percentages of CD69 (56.4±3.3% in the presence of
MSCs vs. 96.3±2.7% in the absence of MSCs, P<0.001) and CD25
(49.2±2.8% in the presence of MSCs vs. 95.6±3.0% in the absence of
MSCs, P<0.001) expressed on PHA-stimulated CD3+ T cells, the
percentages of CD69 and CD25 increased up to 80.7±4.0% (P=0.005 vs.
no MSCs control) and 77.2±2.2% (P=0.001 vs. no MSCs control),
respectively, when MSCs from P7 were added to the co-culture. The
addition of MSCs from P13 did not exert any suppressive influences
on the expression of activation antigens on the PHA-stimulated T
cells (for both CD25 and CD69, P>0.05 vs. no MSCs control;
Fig. 6). All these data further
suggested that the inhibitory effects of MSCs on T-cell activation
decreased during long-term culture in vitro.
Effects of MSCs in long-term culture on
cytokine production by T cells
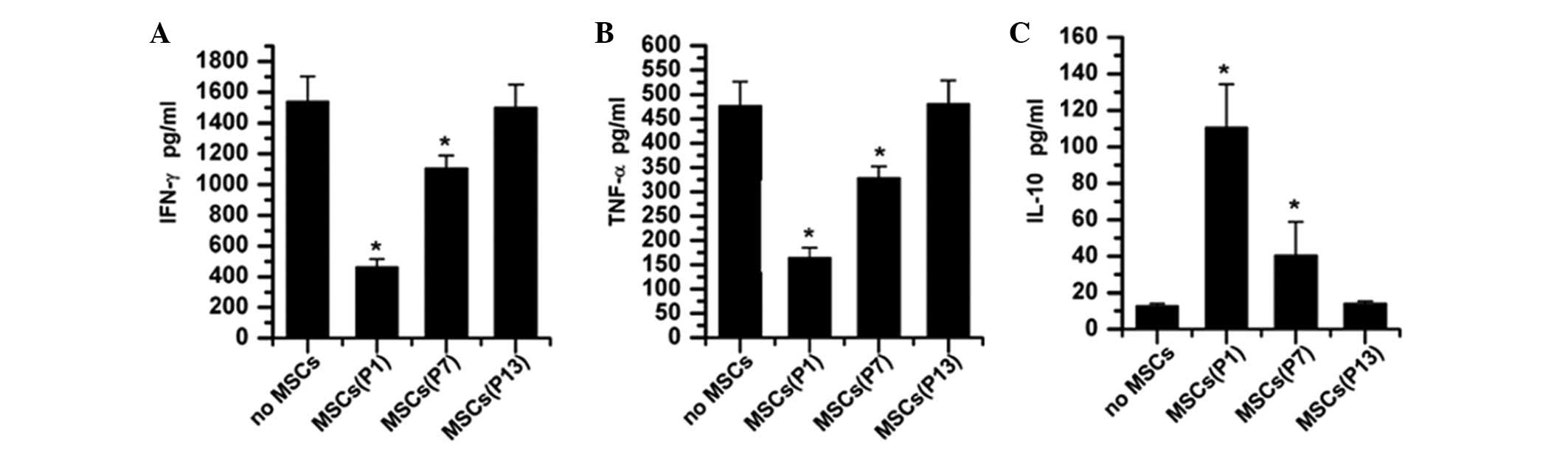
Cytokines IL-10, IFN-γ and TNF-α were not detected
in the supernatants of MSCs whether they were from P1, P7 or P13,
with or without the stimulation of PHA. Allogeneic MSCs from any
passage did not stimulate T cells to produce the pro-inflammatory
cytokines IFN-γ and TNF-α (data not shown), which further confirmed
that MSCs in long-term culture still retain their immune-tolerance
properties. With the stimulation of PHA, T cells secreted
considerable amounts of the pro-inflammatory cytokines IFN-γ
(1540.89±162.44 pg/ml) and TNF-α (476.34±49.52 pg/ml) in the
absence of MSCs, which were inhibited by allogenic MSCs in a
passage-dependent fashion (Fig. 7A and
B). However, PHA-stimulated T cells secreted significantly
lower doses of IFN-γ (463.28±52.22 pg/ml vs. 1540.89±162.44 pg/ml,
P<0.001) and TNF-α (164.57±20.75 pg/ml, P<0.001) in the
presence of MSCs from P1. Although still significantly inhibited by
MSCs from P7, the amount of IFN-γ and TNF-α secreted by
PHA-stimulated T cells increased to 1104.46±85.37 pg/ml (P=0.015
vs. no MSC group) and 328.17±24.36 pg/ml (P=0.01 vs. no MSC group),
respectively. When cultured to late passages (such as P13), MSCs
almost lost their suppressive effects on the production of IFN-γ
and TNF-α inPHA-stimulated T cells (Fig. 7A and B).
Whether stimulated by PHA or not, T cells secreted
similarly low levels of the anti-inflammatory cytokine IL-10
(12.76±1.38 vs. 11.43±1.31 pg/ml, P=0.289). When MSCs from
different passages (P1, P7 and P13) were added to the culture with
the stimulation of PHA, the level of IL-10 in the supernatant was
found to be significantly elevated (110.64±23.72 pg/ml for MSCs
from P1 and 40.57±18.30 pg/ml for MSCs from P7, respectively)
compared to no-MSC controls (P<0.001, Fig. 7C), while the level was almost
unchanged in the presence of MSCs from P13 (14.05±1.42 pg/ml,
P=0.332). In addition, MSCs from P1 showed a significantly stronger
capacity to elevate the level of IL-10 than that of MSCs from P7
(110.64±23.72 vs. 40.57±18.30 pg/ml, P=0.015). All these data
suggested that long-term culture deprived MSCs of the capability of
elevating the anti-inflammatory cytokine IL-10, which, in turn,
decreased their inhibitory capacity.
Discussion
In the present study, we confirmed that long-term
in vitro expansion leads to the aging of MSCs. In addition,
we further demonstrated that this type of senescence impaired the
immunosuppressive properties of MSCs.
MSCs are present in a variety of tissues, and are
most prevalent in the bone marrow compartment. They were first
described in 1968 by Friedenstein et al(24), and have attracted much attention
due to their multipotential properties with regard to
differentiation, and their possible use for cell and gene therapy
(25,26). Recently, the potential of MSCs to
serve as a potent immunotherapy has also been explored and
confirmed by several studies (10–12).
Despite the great interest in MSCs, however,
systemic immunotherapy using MSCs would require a greater abundance
of these cells than tissue engineering can provide and there
remains no well-defined protocol for isolation and expansion of
MSCs in culture. Most experiments, including recently published
studies, have been conducted using MSCs isolated primarily from
bone marrow aspirates by their tight adherence to plastic dishes,
as described by Friedenstein et al(27) 35 years ago, which means that
cellular immunotherapy would require a longer-term culture for MSC
expansion. Although stem cells have the ability to continuously
proliferate and differentiate (develop) into various other types of
cells/tissues, several studies still demonstrated that long-term
expansion impaired the telomere length and activity of telomerase,
in turn leading to senescence of MSCs and damaging their
multilineage potential (28–31).
However, the effects of long-term in vitro amplification on
the immunological properties of MSCs remain unknown.
To better investigate the effects of this widely
used method for MSC culture on immunological properties, we
isolated and cultured MSCs in common medium, as most previous
experiments did in vitro. During the successive passages, we
observed changes of MSCs including morphology, immunophenotype and
growth rate at different time points. In addition, SA β-gal was
also detected on MSCs in the long-term culture. SA β-gal has been
used as an important marker for aged cells as it may be related to
increased lysosomal activities and altered cytosolic pH during
aging and has been demonstrated to increase with aging of
fibroblasts both in vitro and in vivo(19). As a result, we found there were no
MSCs that could be passaged permanently in routine culture, and the
maximal life span of the 5 donor-derived MSCs was only
approximately 170 days. Although the immunophenotype of MSCs did
not change very much during the expansion, MSCs altered from a
fibroblast-like cell to a flat cell with some granules in the
cytoplasm, and the latter morphology was usually shown by senescent
cells. The PD rate, usually used to judge the growth activities of
the cells, was also shown to be decreased with the passage of MSCs,
while SA β-gal expression on MSCs was observed to increase. All
these data were consistent with the previous studies and suggested
that the long-term in vitro culture could induce the aging
of MSCs.
In the following experiments, MSCs derived from
different passages were evaluated to observe their effects on the
allogenic T-cell responses. As the results show, although MSCs from
P1 significantly suppressed the PHA-stimulated T-cell
proliferation, activation marker (such as CD25 and CD69)
expression, and even the secretion of pro-inflammatory cytokines
IFN-γ and TNF-α, their inhibitory abilities were decreased with
successive passages. MSCs from P10 almost lost their inhibitory
capacity. In addition, the ability of MSCs to elevate the secretion
of anti-inflammatory IL-10 by T cells was also lost in the
long-term culture. Though the immunosuppressive capacity may be
impaired, the low immunogenicity of MSCs was not significantly
altered in long-term culture.
Currently, MSCs are favored by many researchers as
they may be a potential candidate for treating certain refractory
T-cell-mediated diseases, such as GVHD, RA and EAE, based on their
profound suppressive capacities on allogenic T cells without being
rejected by the host. In addition to their damaged multilineage
potential as previous reported, MSCs were further confirmed to lose
their immunosuppressive ability during long-term culture in
vitro in our study. Thus, an important challenge in
immunotherapy is to improve the replicative capacity of MSCs. In
the past 10 years, several methods have been developed for MSC
isolation/expansion in vitro, such as using flow cytometry
or mononuclear cell gravity sedimentation for isolation (32,33),
and using magnetic nanoparticles or basement membrane extracellular
matrix, together with culture medium supplementation with different
growth factors for expansion (34,35).
Although these methods improved MSC yield and reduced expansion
time compared to the standard accepted protocols, the senescence of
MSCs in the culture has not been completely eliminated. Forced
expression of telomerase in MSCs by transferring the telomerase
reverse transcriptase gene (usually using a viral vector) has been
used recently and has proven to markedly increase their
proliferative life span (36,37).
However, it remains unknown whether these modified cells are
capable of maintaining their immunological properties and whether
these cells have a risk of transformation into tumors in
recipients.
We demonstrated that long-term culture in
vitro leads to the aging of MSCs, which impaired the
immunosuppressive properties of MSCs. Our conclusion further
suggested that finding a better expansion method for MSCs remains a
necessary step prior to extensive clinical use of MSCs in
immunotherapy.
Acknowledgements
We wish to thank Wang Yan-Hong and Fan Chun-Mei for
their excellent technical assistance. This study was supported by a
grant from the Major State Basic Research Development Program of
China (2009CB521705).
References
|
1
|
Caplan AI: Mesenchymal stem cells. J
Orthop Res. 9:641–650. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Deans RJ and Moseley AB: Mesenchymal stem
cells: biology and potential clinical uses. Exp Hematol.
28:875–884. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Pittenger MF, Mackay AM, Beck SC, et al:
Multilineage potential of adult human mesenchymal stem cells.
Science. 284:143–147. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ferrari G, Cusella-De Angelis G, Coletta
M, et al: Muscle regeneration by bone marrow-derived myogenic
progenitors. Science. 279:1528–1530. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Liechty KW, MacKenzie TC, Shaaban AF, et
al: Human mesenchymal stem cells engraft and demonstrate
site-specific differentiation after in utero transplantation in
sheep. Nat Med. 6:1282–1286. 2000. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Pereira RF, O’Hara MD, Laptev AV, et al:
Marrow stromal cells as a source of progenitor cells for
nonhematopoietic tissues in transgenic mice with a phenotype of
osteogenesis imperfecta. Proc Natl Acad Sci USA. 95:1142–1147.
1998.PubMed/NCBI
|
|
7
|
Di Nicola M, Carlo-Stella C, Magni M, et
al: Human bone marrow stromal cells suppress T-lymphocyte
proliferation induced by cellular or nonspecific mitogenic stimuli.
Blood. 99:3838–3843. 2002.
|
|
8
|
Krampera M, Glennie S, Dyson J, et al:
Bone marrow mesenchymal stem cells inhibit the response of naive
and memory antigen-specific T cells to their cognate peptide.
Blood. 101:3722–3729. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Aggarwal S and Pittenger MF: Human
mesenchymal stem cells modulate allogeneic immune cell responses.
Blood. 105:1815–1822. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Bartholomew A, Sturgeon C, Siatskas M, et
al: Mesenchymal stem cells suppress lymphocyte proliferation in
vitro and prolong skin graft survival in vivo. Exp Hematol.
30:42–48. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Le Blanc K, Rasmusson I, Sundberg B, et
al: Treatment of severe acute graft-versus-host disease with third
party haploidentical mesenchymal stem cells. Lancet. 363:1439–1441.
2004.PubMed/NCBI
|
|
12
|
Zappia E, Casazza S, Pedemonte E, et al:
Mesenchymal stem cells ameliorate experimental autoimmune
encephalomyelitis inducing T-cell anergy. Blood. 106:1755–1761.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zheng ZH, Li XY, Ding J, Jia JF and Zhu P:
Allogeneic mesenchymal stem cell and mesenchymal stem
cell-differentiated chondrocyte suppress the responses of type II
collagen-reactive T cells in rheumatoid arthritis. Rheumatology
(Oxford). 47:22–30. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Chen FH and Tuan RS: Mesenchymal stem
cells in arthritic diseases. Arthritis Res Ther. 10:2232008.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Baxter MA, Wynn RF, Jowitt SN, Wraith JE,
Fairbairn LJ and Bellantuono I: Study of telomere length reveals
rapid aging of human marrow stromal cells following in vitro
expansion. Stem Cells. 22:675–682. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Bonab MM, Alimoghaddam K, Talebian F,
Ghaffari SH, Ghavamzadeh A and Nikbin B: Aging of mesenchymal stem
cell in vitro. BMC Cell Biol. 71:42006.
|
|
17
|
Weyand CM and Goronzy JJ: Stem cell aging
and autoimmunity in rheumatoid arthritis. Trends Mol Med.
10:426–433. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Morimoto D, Kuroda S, Kizawa T, et al:
Equivalent osteoblastic differentiation function of human
mesenchymal stem cells from rheumatoid arthritis in comparison with
osteoarthritis. Rheumatology (Oxford). 48:643–649. 2009. View Article : Google Scholar
|
|
19
|
Kassem M, Ankersen L, Eriksen EF, Clark BF
and Rattan SI: Demonstration of cellular aging and senescence in
serially passaged long-term cultures of human trabecular
osteoblasts. Osteoporos Int. 7:514–524. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Dimri GP, Lee X, Basile G, et al: A
biomarker that identifies senescent human cells in culture and in
aging skin in vivo. Proc Natl Acad Sci USA. 92:9363–9367. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Groh ME, Maitra B, Szekely E and Koc ON:
Human mesenchymal stem cells require monocyte-mediated activation
to suppress alloreactive T cells. Exp Hematol. 33:928–934. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Le Blanc K, Rasmusson I, Gotherstrom C, et
al: Mesenchymal stem cells inhibit the expression of CD25
(interleukin-2 receptor) and CD38 on phytohaemagglutinin-activated
lymphocytes. Scand J Immunol. 60:307–315. 2004.PubMed/NCBI
|
|
23
|
Zhu P, Li XY, Wang HK, et al: Oral
administration of type-II collagen peptide 250–270 suppresses
specific cellular and humoral immune response in collagen-induced
arthritis. Clin Immunol. 122:75–84. 2007.
|
|
24
|
Fridenshtein A, Petrakova KV, Kuralesova
AI and Frolova GI: Precursor cells for osteogenic and hemopoietic
tissues. Analysis of heterotopic transplants of bone marrow.
Tsitologiia. 10:557–567. 1968.PubMed/NCBI
|
|
25
|
Lu L, Zhao C, Liu Y, et al: Therapeutic
benefit of TH-engineered mesenchymal stem cells for Parkinson’s
disease. Brain Res. 15:46–51. 2005.PubMed/NCBI
|
|
26
|
Studeny M, Marini FC, Dembinski JL, et al:
Mesenchymal stem cells: potential precursors for tumor stroma and
targeted-delivery vehicles for anticancer agents. J Natl Cancer
Inst. 96:1593–1603. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Friedenstein AJ, Deriglasova UF, Kulagina
NN, et al: Precursors for fibroblasts in different populations of
hematopoietic cells as detected by the in vitro colony assay
method. Exp Hematol. 2:83–92. 1974.PubMed/NCBI
|
|
28
|
Dressler MR, Butler DL and Boivin GP:
Effects of age on the repair ability of mesenchymal stem cells in
rabbit tendon. J Orthop Res. 23:287–293. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Liu L, DiGirolamo CM, Navarro PA, Blasco
MA and Keefe DL: Telomerase deficiency impairs differentiation of
mesenchymal stem cells. Exp Cell Res. 294:1–8. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Stolzing A and Scutt A: Age-related
impairment of mesenchymal progenitor cell function. Aging Cell.
5:213–224. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zheng H, Martin JA, Duwayri Y, Falcon G
and Buckwalter JA: Impact of aging on rat bone marrow-derived stem
cell chondrogenesis. J Gerontol A Biol Sci Med Sci. 62:136–148.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Carrancio S, Lopez-Holgado N,
Sanchez-Guijo FM, et al: Optimization of mesenchymal stem cell
expansion procedures by cell separation and culture conditions
modification. Exp Hematol. 36:1014–1021. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zohar R, Sodek J and McCulloch CA:
Characterization of stromal progenitor cells enriched by flow
cytometry. Blood. 90:3471–3481. 1997.PubMed/NCBI
|
|
34
|
Matsubara T, Tsutsumi S, Pan H, et al: A
new technique to expand human mesenchymal stem cells using basement
membrane extracellular matrix. Biochem Biophys Res Commun.
313:503–508. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Schallmoser K, Rohde E, Reinisch A, et al:
Rapid large-scale expansion of functional mesenchymal stem cells
from unmanipulated bone marrow without animal serum. Tissue
engineering. 14:185–196. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Hung SC, Yang DM, Chang CF, et al:
Immortalization without neoplastic transformation of human
mesenchymal stem cells by transduction with HPV16 E6/E7 genes. Int
J Cancer. 110:313–319. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Shi S, Gronthos S, Chen S, et al: Bone
formation by human postnatal bone marrow stromal stem cells is
enhanced by telomerase expression. Nat Biotechnol. 20:587–591.
2002. View Article : Google Scholar : PubMed/NCBI
|