Introduction
Ovarian cancer is the second most common
gynecological malignancy and affects more than 200,000 women
worldwide each year. The symptoms of ovarian cancer are
non-specific and therefore two-thirds of cases are not diagnosed
until the later stages (1).
Aggressive surgical reduction and new chemotherapeutic agents have
improved the prognosis of advanced ovarian cancer (2,3);
however, the 5-year survival rate remains low in more than half of
diagnosed women (4–8). Therefore, it is critical to
investigate the biological behavior of ovarian cancer cells and
identify new prognostic factors and therapeutic targets.
RAB25 belongs to the Rab family of small GTPases,
which regulates various aspects of membrane recycling and
trafficking to the plasma membrane (9). Unlike the other members of the
ubiquitously expressed RAB11 sub-family, RAB25 expression is
restricted to epithelial tissue (10) and is correlated with several
epithelial cancers. Overexpression of RAB25 has been reported in
liver (10) and bladder cancer
(11) and is also associated with
the aggressive behavior of ovarian and breast cancers (12,13).
RAB25 has been implicated in the promotion of cell proliferation,
evasion of apoptosis and acceleration of in vivo tumor
growth in ovarian cancer (12,14,15).
Autophagy refers to the process of autodigestion in
which the cell’s own components are degraded and recycled by the
lysosomal machinery (16).
Autophagy is induced by starvation, hypoxia and high temperature.
Through the partial digestion of cell components, autophagy
provides nutrients that are necessary to maintain cell viability
and prolonged survival (17).
However, autophagy may result in the destruction of vital
organelles, leading to cell death (18). Previous studies have suggested a
correlation between the decline in autophagic activity and
tumorigenesis (19,20), while chemotherapeutic agents
stimulate autophagic cell death (21,22).
The role of RAB25 in ovarian cancer cells was
evaluated in vitro. The present study reveals that knockdown
of RAB25 by siRNA promoted autophagy through activation of the
ERK1/2 signaling pathway. It indicated that knockdown of RAB25
resulted in the inhibition of cell proliferation and the induction
of apoptosis. These results support the tumorigenic role of RAB25
in ovarian cancer cells.
Materials and methods
Cell culture and treatment
HEY and ES-2 human ovarian cancer cell lines
(donated by the University of Texas, M. D. Anderson Cancer Center,
Houston, TX, USA) were grown in RPMI-1640 medium (Gibco, Grand
Island, NY, USA) supplemented with 100 IU/ml penicillin, 100 μg/ml
streptomycin and 10% FBS (Hyclone, Logan, UT, USA) in a humidified
atmosphere of 5% CO2 at 37°C. These cells were
sub-cultured by adding 0.05% trypsin-0.01% EDTA (Gibco) when the
cells reached 80% confluence. For experiments involving the
pharmacological inhibitor, the cells were serum-starved for 12 h
and then treated with U0126 (Sigma Aldrich, St. Louis, MO, USA) at
a concentration of 10 μM for 24 h. Cells treated with DMSO (Sigma
Aldrich) served as the control.
siRNA transfection
The RAB25 ON-TARGET plus SMART pool siRNA (siRab)
and siGLO non-targeting siCONTROL siRNA (siNon) were purchased from
Dharmacon (Lafayette, CO, USA). Cells were transfected with siRab
or siNon using DharmaFCET 1 reagent (Dharmacon) according to the
manufacturer’s instructions. Briefly, the siRNA and transfection
reagent were diluted in serum-free Opti-MEM and mixed. Following
incubation at room temperature for 20 min, the mixture was added to
the cells at a final siRNA concentration of 50 nM. Following
incubation for 6 h, FBS was added to achieve a final concentration
of 10% and the cells were incubated for 24 h prior to subsequent
treatment. Cells treated with DharmaFECT 1 reagent served as the
control.
RNA extraction and quantitative real-time
PCR
Total RNA was extracted from HEY and ES-2 cells with
TRIzol reagent (Invitrogen, Carlsbad, CA, USA) according to the
manufacturer’s instructions. Reverse transcription was performed
using the Oligo (dT) 18 primer according to the RevertAid
First-Strand cDNA Synthesis kit protocol (Fermentas, Vilnius,
Lithuania). Quantitative PCR was performed with the SYBR Green
Premix Ex Taq kit (Takara Bio, Inc., Dalian, China), which
consisted of 2 μl cDNA template, 10 μl SYBR-Green Real-time PCR
Master mix and 0.2 μM forward and reverse primers in a final volume
of 20 μl. The primer sequences were as follows: RAB25 sense,
5′-GCCCTGGACTCTACCAAT GTTGA-3′; RAB25 antisense,
5′-GCTGTTCTGTCTCTGCTT GGACAC-3′; GAPDH sense, 5′-GCACCGTCA
AGGCTGAGA AC-3′; and GAPDH antisense, 5′-TGGTGAAGACGCCAG TGGA-3′.
The reactions were carried out with an ABI PRISM 7000 Sequence
Detection System (Applied Biosystems, Foster City, CA, USA) for 40
cycles (95°C for 5 sec, 60°C for 31 sec) after an initial 10 sec of
incubation at 95°C. The fold change in the expression of each gene
was calculated using the -ΔΔCt method and GAPDH was used as the
internal control.
Western blot analysis
Cultured cells were washed with PBS and then lysed
with RIPA solution (Shanghai Bocai Bio. Technology Co., Ltd.,
Shanghai, China). The lysate was cleared by centrifugation at
12,000 rpm for 30 min and the protein was then quantified using the
bicinchoninic acid (BCA) (Shanghai Bocai Bio. Technology Co., Ltd.)
method according to the manufacturer’s instructions. Proteins (30
μg/lane) were resolved by SDS-PAGE and transferred onto a PVDF
membrane (Millipore, Billerica, MA, USA). Following blocking with
5% BSA-TBST for 1 h at room temperature, the membrane was incubated
with the primary antibodies overnight at 4°C. The membrane was then
incubated with HRP-conjugated secondary antibodies (Kangchen
Bioengineering Corp., Shanghai, China; 1:5,000) or IRDye 700DX
conjugated affinity goat anti-mouse IgM (1:2,500) for 1 h at 37°C.
Protein bands were visualized by ECL (Perkin Elmer, Waltham, MA,
USA) or by Odyssey infrared imaging system (Li-Cor, Lincoln, NE,
USA), with GAPDH as a loading control. The mouse anti-GAPDH
monoclonal antibody (1:5,000) was purchased from Kangchen
Bioengineering Corporation; mouse anti-RAB25 monoclonal antibodies
(1:500) were purchased from Abcam Inc. (Cambridge, MA, USA); rabbit
anti-survivin polyclonal antibody (1:1,000) was purchased from
R&D Systems, Inc. (Minneapolis, MN, USA); rabbit anti-LC3B
(1:1000), rabbit anti-Beclin 1 (1:1000), mouse anti-cyclin D1
(1:2000), mouse anti-cyclin B1 (1:2000), rabbit
anti-cleaved-caspase-3 (1:1000) and rabbit anti-phospho-ERK1/2
(1:2000) antibodies were purchased from Cell Signaling Technology,
Inc. (Danvers, MA, USA).
Immunocytofluorescence
Cells were seeded on a cover glass and cultured in
Opti-MEM for 24 h. Adherent cells were fixed with 95% ethanol at
4°C for 20 min and then incubated with 0.5% Triton-X 100 (Sangon,
Shanghai, China) for 15 min. Cells were blocked by adding goat
serum blocking buffer (Mingrui, Shanghai, China) at room
temperature for 30 min and then incubated with rabbit anti-RAB25
(Cell Signaling Technology, Inc.) primary antibody (1:500) at 4°C
overnight. The following day, cells were washed three times with
PBS and incubated with Northern Lights anti-mouse IgG-NL557
(R&D systems Inc.; 1:200) at 37°C for 1 h in the dark.
Following mounting with VECTASHIELD mounting medium with DAPI
(Vector Laboratories, Burlingame, CA, USA), the cell images were
acquired using DP 7.0 software and an Olympus BX51 fluorescence
microscope.
Acridine orange staining assay
The formation of acidic vesicle organelles (AVOs) is
a characteristic of autophagic cell death (23). To stain AVOs in cells, acridine
orange (Sangon) was added into the medium at a final concentration
of 1 μg/ml. The cells were incubated at 37°C for 15 min and then
harvested with trypsin and immediately analyzed on a flow cytometer
(BD Biosciences, Franklin Lakes, NJ, USA). AVOs were quantified
using the Cy5-Phycoerythrin emission (PE) signal detector.
GFP-microtubule-associated protein 1
light chain 3 (LC3) transfection
LC3 is involved in microtubule assembly and
autophagy. Endogenous LC3 is processed into LC3-I and then
lipidated to LC3-II. LC3-II is associated with the autophagosome
membrane and thus regarded as a promising marker of autophagy.
Ovarian cancer cells were transfected with siRNA as described
previously on day 1 and then transfected with pEGFP-LC3 plasmid
(kindly provided by N. Mizushima and T. Yoshimori, National
Institute for Basic Biology, Okazaki, Japan) on day 2. pEGFP-LC3
was added at a final concentration of 1.6 μg/ml using Lipofectamine
2000 transfection reagent (Invitrogen) according to the
manufacturer’s instructions. Following incubation for 6 h, cells
were replenished with serum-free Opti-MEM for 12 h. The resultant
cells were fixed with 95% ethanol for microscopic analysis.
Sulphorhodamine B (SRB) assay
Cell proliferation was evaluated with SRB by
measuring cellular protein content. Cells in the exponential growth
phase were seeded in 96-well culture plates at a final
concentration of 5×103 cells per well. After 48 h, cells
were fixed with 100 μl of 30% iced trichloroacetic acid at 4°C for
1 h. Following washing and air-drying, 100 μl of 0.4% (w/v) SRB
solution in 1% acetic acid was added into each well and incubated
for 30 min at room temperature. Excess dye was removed by washing 5
times with 1% acetic acid and the plates were then allowed to air
dry. The optical density values of resuspended SRB in 10 mM Tris
buffer were read at 570 nm on a microplate spectrophotometer to
evaluate cell proliferation.
Cell cycle analysis
Cells were synchronized in G1-phase by serum
starvation for 12 h. The collected cells were then fixed in 70%
ethanol at 4°C overnight. The following day, cells were washed with
PBS and dyed with Propidium Iodide (PI) solution (Dingguo,
Shanghai, China) for 30 min at room temperature in the dark. PE
signals were detected by flow cytometry. Modfit 3.0 software
(Verity software Inc., Topsham, ME, USA) was used for cell cycle
analysis.
Apoptosis assay
Cells were harvested 48 h after siRNA transfection,
washed twice with PBS and resuspended in 1X binding buffer
(Invitrogen). Following incubation with Annexin V-FITC and PI
staining solution (Invitrogen) at room temperature for 15 min in
the dark, cells were analyzed immediately by flow cytometry. The
signal of Annexin V-FITC was detected using the FITC-signal
detector and PI with the PE signal detector. The Annexin
V-FITC-positive/PI-negative population represented early apoptotic
cells.
Statistical analysis
Data are expressed as the mean ± SD obtained from
three individual experiments. Statistical differences between the
control and treatment groups were determined by one-way ANOVA
followed by Dunnet’s test. P<0.05 was considered to indicate
statistically significant differences. Representative images from
western blotting and microscope analysis are shown.
Results
RAB25 siRNA downregulates the expression
of RAB25 and increases the expression of pERK1/2 in ovarian cancer
cells
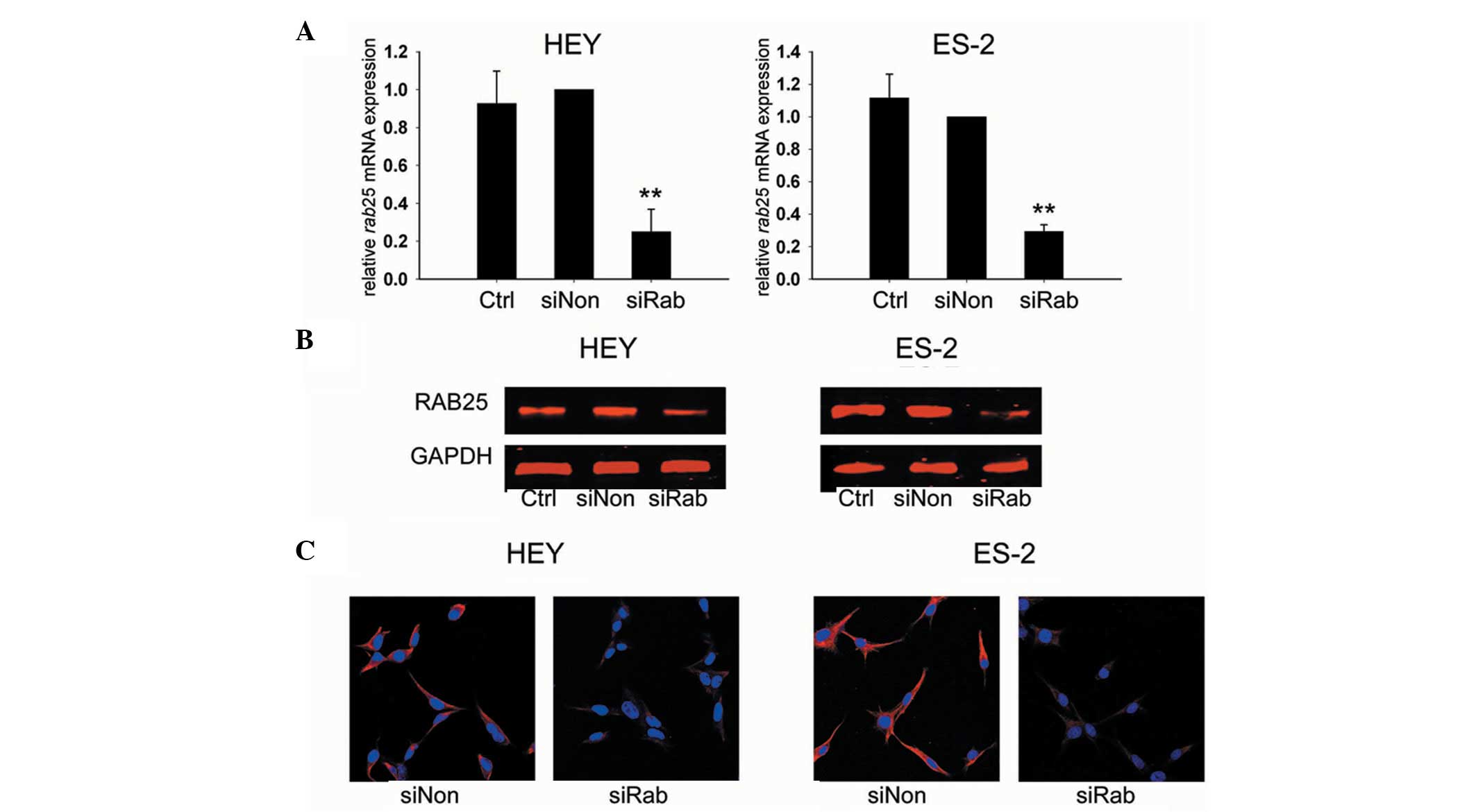
HEY and ES-2 ovarian cancer cells were assessed for
the knockdown efficiency of RAB25 siRNA 24 h after transfection.
Transfection with siRab resulted in a 75.1% reduction of RAB25 mRNA
levels in HEY cells (P<0.01) and a 70.7% reduction in ES-2 cells
(P<0.01) compared with siNon-treated cells (Fig. 1A). The RAB25 protein expression in
transfected cells was consistent with the transcription assay
results (Fig. 1B and C). These
data revealed that RAB25 was effectively downregulated by siRab in
the ovarian cancer cell lines used in this study.
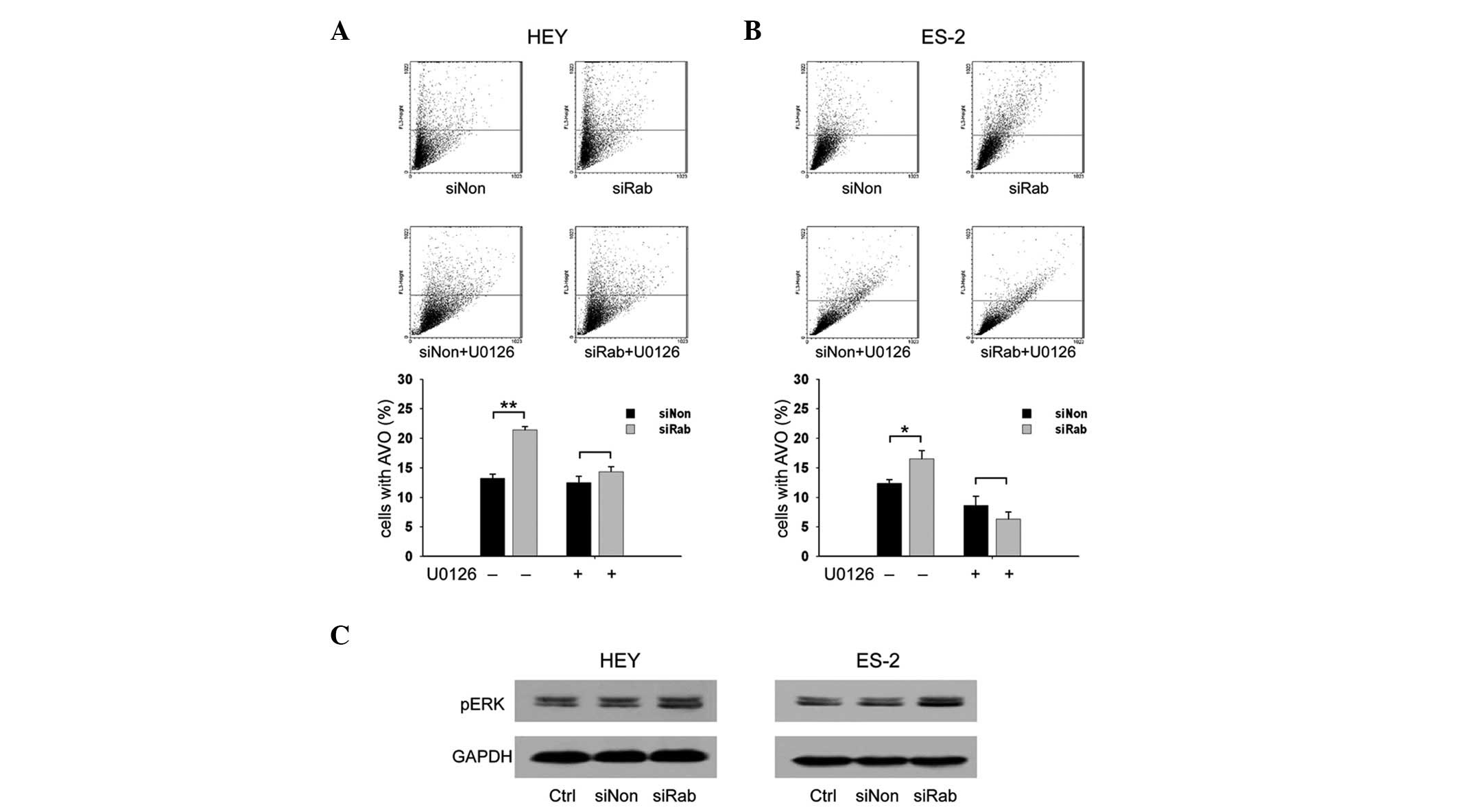
Knockdown of RAB25 increases AVOs through
the ERK1/2 signaling pathway in ovarian cancer cells
To investigate whether RAB25 is involved in
autophagy, the level of AVOs was examined in transfected ovarian
cancer cells. The percentages of cells with AVOs in
siRab-transfected cells were significantly higher than those in
siNon-transfected cells (21.4 vs. 13.2% in HEY cells and 16.5 vs.
12.4% in ES-2 cells, P<0.05; Fig.
2A). These data suggest that knockdown of RAB25 promotes
autophagy in ovarian cancer. The ERK1/2 signaling pathway plays a
role in regulating autophagy (24–27)
and therefore the effect of RAB25 siRNA on the ERK signaling
pathway in ovarian cancer cells was examined. Compared with siNon,
transfection with siRab increased the expression of phospho-ERK1/2
(Fig. 2B). The transfected cells
were then treated with U0126, a MEK 1 inhibitor, to test whether
the ERK1/2 signaling pathway is involved in RAB25 knockdown-induced
autophagy in ovarian cancer cells. Increased AVOs were noted in the
untreated siRab-transfected cells but not in the U0126-treated
cells (Fig. 2A), suggesting that
the effect of RAB25 on autophagy is mediated through the ERK1/2
signaling pathway.
Knockdown of RAB25 increased GFP-LC3
punctate fluorescence in ovarian cancer cells
The levels of GFP-LC3 punctate fluorescence was
examined in transfected ovarian cancer cells. RAB25 siRNA
significantly increased the percentage of cells with GFP-LC3
punctate fluorescence in HEY (Fig.
3A) and ES-2 cells (Fig. 3B)
compared with controls (44.4 vs. 73.8% in HEY and 39.8 vs. 71.7% in
ES-2 cells, P<0.05). It was also demonstrated that the
upregulation of GFP-LC3 punctate fluorescence induced by silencing
of RAB25 was reversed by U0126 in HEY and ES-2 cells.
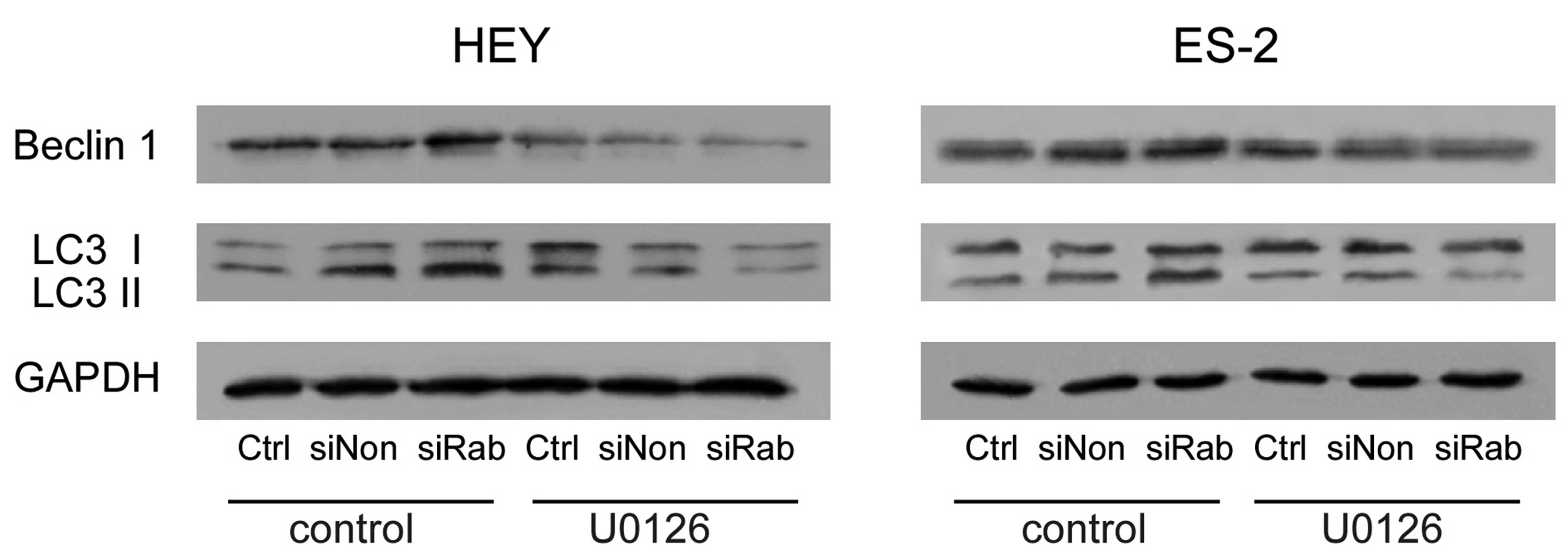
Knockdown of RAB25 upregulated autophagic
proteins in ovarian cancer cells
Beclin 1, an autophagy-related protein, is required
for the initiation of autophagy. Knockdown of RAB25 increased
Beclin 1 expression and conversion of LC3-I to LC3-II. These
changes were reversed by U0126 treatment (Fig. 4). These findings further confirm
that RAB25 regulates autophagy through the ERK1/2 signaling
pathway.
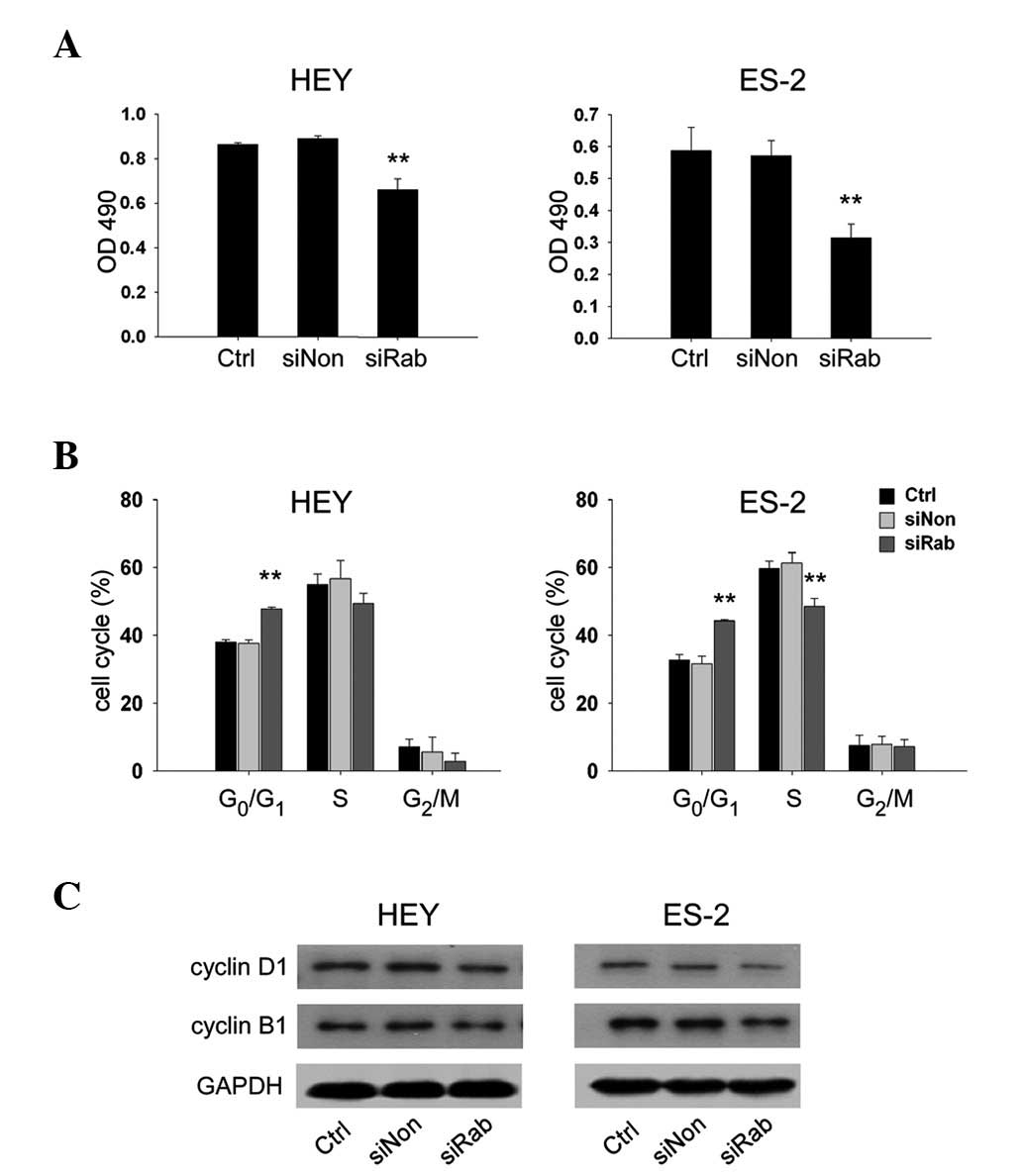
Knockdown of RAB25 inhibited cell growth
in ovarian cancer cells
To determine whether knockdown of RAB25 impacted
ovarian cancer cell growth, the proliferation of transfected HEY
and ES-2 cells was evaluated. Transfection with siRab reduced cell
growth by 25.9% in HEY cells (P<0.01) and 36.6% in ES-2 cells
(P<0.01) compared with siNon (Fig.
5A). These results were further confirmed by cell cycle
analysis, which revealed that siRab transfection significantly
increased the proportion of cells in G0/G1 phase (P<0.05) and
decreased the proportion of cells entering S phase (P<0.05)
compared with siNon (Fig. 5B).
Protein expression of cyclin D1, a marker of G1/S progression and
cyclin B1, a marker of G2/M progression, were analyzed by western
blotting. The data indicated that siRab-transfected ovarian cancer
cells exhibited lower expression of cyclin D1 and cyclin B1
compared with siNon (Fig. 5C).
Knockdown of RAB25 induced apoptosis in
ovarian cancer cells
Transfected ovarian cancer cells were stained with
Annexin V-FITC and PI, and the cells were then analyzed by flow
cytometry to explore whether knockdown of RAB25 impacted apoptosis.
Compared with siNon, siRab transfection increased the Annexin
V-FITC-positive/PI-negative population in HEY (5.8 vs. 3.3%,
P<0.01; Fig. 6A) and ES-2 cells
(8.3 vs. 5.8%, P<0.01; Fig.
6B). These results indicated that knockdown of RAB25 increases
apoptosis in ovarian cancer cells. Since survivin plays a critical
role in cell survival and apoptosis, the expression of survivin was
analyzed, which revealed a reduction following siRab transfection.
The level of cleaved caspase-3, a marker of apoptosis, was also
increased by siRab transfection (Fig.
6C).
Discussion
Cancer cell transformation involves alterations in
cell shape and behavior. Cancers of epithelial origin arise from a
loss of polarized epithelial monolayer and adoption of migratory
behaviors that lead to invasion (28). RAB25 regulates the
membrane-trafficking system that changes epithelial cell polarity
and aids the localization of integrin-recycling vesicles that
enhance invasive ability (29),
suggesting a role for RAB25 in tumorigenesis.
Overexpression of RAB25 has been reported in ovarian
cancer and is associated with decreased survival rates (12). Studies on ovarian cancer have
demonstrated that RAB25 facilitates cell proliferation and
anti-apoptotic effects (12,14,15).
In agreement with previous studies, these results indicated that
knockdown of RAB25 in ovarian cancer cells inhibited cell growth
and induced apoptosis. Studies on RAB25 expression in other types
of cancer, however, have shown mixed results. RAB25 has been
reported to be overexpressed in liver and bladder cancer, but
downregulated in colon cancers (28). RAB25 expression has been associated
with invasiveness in breast cancer, but loss of expression has also
been reported in some breast cancer tissue (30,31).
The role of RAB25 in tumorigenesis may be tissue-specific and
requires further elucidation.
Autophagy occurs after nutrient deprivation or
following chemotherapy in cancer cells (32,33).
The impact of autophagy on cancer progression is controversial and
whether autophagic activities in cells actually cause death or are
a survival mechanism remains unclear. However, excessive autophagy
damages the cell. In the present study, knockdown of RAB25 promoted
autophagy, suggesting that suppression of RAB25 was stressful to
ovarian cancer cells and induced programmed cell death mechanisms.
This result implies that RAB25 is a new candidate for targeted
cancer therapy.
Previous studies have demonstrated a correlation
between the MAPK/ERK pathway and autophagy (24–27).
Prolonged activation and cytoplasmic sequestration of ERK1/2 induce
autophagy (34–37). In this study, knockdown of RAB25
with siRNA activated the ERK1/2 pathway and promoted autophagy.
Blocking ERK1/2 concurrently inhibited autophagy, suggesting that
the ERK signaling pathway mediates the effects of RAB25 on
autophagy in ovarian cancer cells.
In conclusion, knockdown of RAB25 promoted
autophagy, inhibited cell proliferation and induced apoptosis in
ovarian cancer cells. These results support the tumorigenic role of
RAB25 in ovarian cancer cells.
Acknowledgements
This study was supported by the National Natural
Science Foundation of China (grant numbers 30872755 and 81020108027
for Y. Feng) and the Shanghai Leading Academic Discipline Project
(grant number B117 for Y. Feng). This research received no specific
grant from any funding agency in the public, commercial, or
not-for-profit sector.
References
|
1
|
Colombo N, Van Gorp T, Parma G, et al:
Ovarian cancer. Crit Rev Oncol Hematol. 60:159–179. 2006.
View Article : Google Scholar
|
|
2
|
Armstrong DK, Bundy B, Wenzel L, et al:
Intraperitoneal cisplatin and paclitaxel in ovarian cancer. New
Engl J Med. 354:34–43. 2006.
|
|
3
|
Bristow RE, Tomacruz RS, Armstrong DK, et
al: Survival effect of maximal cytoreductive surgery for advanced
ovarian carcinoma during the platinum era: A meta-analysis. J Clin
Oncol. 20:1248–1259. 2002.
|
|
4
|
Shih IeM and Davidson B: Pathogenesis of
ovarian cancer: clues from selected overexpressed genes. Future
Oncol. 5:1641–1657. 2009.PubMed/NCBI
|
|
5
|
Pisano C, Bruni GS, Facchini G, et al:
Treatment of recurrent epithelial ovarian cancer. Ther Clin Risk
Manag. 5:421–426. 2009.PubMed/NCBI
|
|
6
|
Nelson AE, Francis JE and Zorbas H:
Population screening and early detection of ovarian cancer in
asymptomatic women. Aust N Z J Obstet Gynecol. 49:448–450. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Marth C, Hiebl S, Oberaigner W, et al:
Influence of department volume on survival for ovarian cancer:
results from a prospective quality assurance program of the
Austrian Association for Gynecologic Oncology. Int J Gynecol
Cancer. 19:94–102. 2009.
|
|
8
|
Holschneider CH and Berek JS: Ovarian
cancer: Epidemiology, biology, and prognostic factors. Semin Surg
Oncol. 19:3–10. 2000.
|
|
9
|
Goldenring JR, Shen KR, Vaughan HD, et al:
Identification of a small GTP-binding protein, RAB25, expressed in
the gastrointestinal mucosa, kidney, and lung. J Biol Chem.
268:18419–18422. 1993.
|
|
10
|
He H, Dai F, Yu L, et al: Identification
and characterization of nine novel human small GTPases showing
variable expressions in liver cancer tissues. Gene Expr.
10:231–242. 2002.
|
|
11
|
Mor O, Nativ O, Stein A, et al: Molecular
analysis of transitional cell carcinoma using cDNA microarray.
Oncogene. 22:7702–7710. 2003.PubMed/NCBI
|
|
12
|
Cheng KW, Lahad JP, Kuo WL, et al: The
RAB25 small GTPase determines aggressiveness of ovarian and breast
cancers. Nat Med. 10:1251–1256. 2004.
|
|
13
|
Caswell PT, Spence HJ, Parsons M, et al:
Rab25 associates with alpha 5 beta 1 integrin to promote invasive
migration in 3D microenvironments. Dev Cell. 13:496–510. 2007.
|
|
14
|
Cheng KW, Lu YL and Mills GB: Assay of
Rab25 function in ovarian and breast cancers. Method Enzymol.
403:202–215. 2005.
|
|
15
|
Fan Y, Xin XY, Chen BL, et al: Knockdown
of Rab25 expression by RNAi inhibits growth of human epithelial
ovarian cancer cells in vitro and in vivo. Pathology. 38:561–567.
2006.
|
|
16
|
Tsujimoto Y and Shimizu S: Another way to
die: autophagic programmed cell death. Cell Death Differ.
12:1528–1534. 2005.
|
|
17
|
Lum JJ, Bauer DE, Kong M, et al: Growth
factor regulation of autophagy and cell survival in the absence of
apoptosis. Cell. 120:237–248. 2005.
|
|
18
|
Ogier-Denis E and Codogno P: Autophagy: a
barrier or an adaptive response to cancer. Biochim Biophys Acta.
1603:113–128. 2003.
|
|
19
|
Qu XP, Yu J, Bhagat G, et al: Promotion of
tumorigenesis by heterozygous disruption of the beclin 1 autophagy
gene. J Clin Invest. 112:1809–1820. 2003.
|
|
20
|
Mathew R, Karp CM, Beaudoin B, et al:
Autophagy suppresses tumorigenesis through elimination of p62.
Cell. 137:1062–1075. 2009.
|
|
21
|
Groth-Pedersen L, Ostenfeld MS,
Hoyer-Hansen M, et al: Vincristine induces dramatic lysosomal
changes and sensitizes cancer cells to lysosome-destabilizing
siramesine. Cancer Res. 67:2217–2225. 2007.
|
|
22
|
Ulasov IV, Sonabend AM, Nandi S, et al:
Combination of adenoviral virotherapy and temozolomide chemotherapy
eradicates malignant glioma through autophagic and apoptotic cell
death in vivo. Br J Cancer. 100:1154–1164. 2009.
|
|
23
|
Paglin S, Hollister T, Delohery T, et al:
A novel response of cancer cells to radiation involves autophagy
and formation of acidic vesicles. Cancer Res. 61:439–444. 2001.
|
|
24
|
Wu WK, Cho CH, Lee CW, et al:
Macroautophagy and ERK phosphorylation counteract the
antiproliferative effect of proteasome inhibitor in gastric cancer
cells. Autophagy. 6:228–238. 2010.PubMed/NCBI
|
|
25
|
Mujumdar N, Mackenzie TN, Dudeja V, et al:
Triptolide induces cell death in pancreatic cancer cells by
apoptotic and autophagic pathways. Gastroenterology. 139:598–608.
2010. View Article : Google Scholar
|
|
26
|
Wang J, Whiteman MW, Lian H, et al: A
non-canonical MEK/ERK signaling pathway regulates autophagy via
regulating Beclin 1. J Biol Chem. 284:21412–21424. 2009.
|
|
27
|
Subramaniam S and Unsicker K:
Extracellular signal-regulated kinase as an inducer of
non-apoptotic neuronal death. Neuroscience. 138:1055–1065.
2006.
|
|
28
|
Goldenring JR and Nam KT: Rab25 as a
tumour suppressor in colon carcinogenesis. Br J Cancer. 104:33–36.
2011.
|
|
29
|
Caswell PT, Spence HJ, Parsons M, et al:
Rab25 associates with alpha5beta1 integrin to promote invasive
migration in 3D microenvironments. Dev Cell. 13:496–510. 2007.
|
|
30
|
Cheng JM, Volk L, Janaki DK, et al: Tumor
suppressor function of Rab25 in triple-negative breast cancer. Int
J Cancer. 126:2799–2812. 2009.
|
|
31
|
Cheng JM, Ding M, Aribi A, et al: Loss of
RAB25 expression in breast cancer. Int J Cancer. 118:2957–2964.
2006.
|
|
32
|
Chatterjee SJ and Pandey S:
Chemo-resistant melanoma sensitized by tamoxifen to low dose
curcumin treatment through induction of apoptosis and autophagy.
Cancer Biol Ther. 11:216–228. 2011.
|
|
33
|
Schnekenburger M, Grandjenette C, Ghelfi
J, et al: Sustained exposure to the DNA demethylating agent,
2′-deoxy-5-azacytidine, leads to apoptotic cell death in chronic
myeloid leukemia by promoting differentiation, senescence, and
autophagy. Biochem Pharmacol. 81:364–378. 2011.
|
|
34
|
Cagnol S and Chambard JC: ERK and cell
death: mechanisms of ERK-induced cell death-apoptosis, autophagy
and senescence. FEBS J. 277:2–21. 2010.
|
|
35
|
Mebratu Y and Tesfaigzi Y: How ERK1/2
activation controls cell proliferation and cell death: Is
subcellular localization the answer? Cell Cycle. 8:1168–1175.
2009.
|
|
36
|
Glading A, Koziol JA, Krueger J, et al:
PEA-15 inhibits tumor cell invasion by binding to extracellular
signal-regulated kinase 1/2. Cancer Res. 67:1536–1544. 2007.
|
|
37
|
Mebratu YA, Dickey BF, Evans C, et al: The
BH3-only protein Bik/Blk/Nbk inhibits nuclear translocation of
activated ERK1/2 to mediate IFNgamma-induced cell death. J Cell
Biol. 183:429–439. 2008.
|