Introduction
Bladder cancer, the fourth most common cancer in
males and ninth most common in females, is a significant health
problem. Despite the numerous advances made in the past few
decades, the prognosis of patients with bladder cancer remains
poor. Superficial bladder cancers have a high rate of tumor
recurrence and 10–30% of cases are likely to progress to invasive
cancer (1). In addition, the
results of treating patients who have advanced or metastatic
bladder cancer are unsatisfactory in the majority of cases. Despite
localized therapy with cystectomy and/or radical radiotherapy, the
5-year survival rate of patients with muscle-invasive transitional
cell carcinoma is approximately 50% (2). Therefore, efforts are ongoing to
explore novel mechanism-based targets and strategies for the
management of bladder cancer.
Green tea, derived from the plant Camellia
sinensis, is one of the most common beverages consumed
worldwide, particularly in China. Epigallocatechin-3-gallate (EGCG)
accounts for 50–80% of the catechins in green tea (3). Studies conducted on cell-culture
systems and animal models as well as human epidemiological studies
have shown that the polyphenols that are present in green tea may
afford protection against a variety of cancer types, including
bladder cancer (4,5). However, the molecular mechanism
underlying the invasion and migration effects of EGCG is not yet
fully understood in bladder cancer.
Metastasis is one of the major causes of mortality
in bladder cancer patients. Tumor invasion and metastasis are
regarded as multistep phenomena that involve cell proliferation,
proteolytic degradation of the basement membrane and extracellular
matrix (ECM), altered cell adhesion, cell migration through the
basement membranes to reach the circulatory system and the
remigration and growth of tumors at metastatic sites (6). Components of the ECM are fundamental
in the process of tumor invasion. Matrix metalloproteinases (MMPs)
degrade the ECM, allowing urinary bladder transitional-cell
carcinoma cells to spread and diffusely infiltrate the bladder
parenchyma (7,8). MMP-9 is postulated to play a critical
role in tumor invasion. It has been reported that the expression of
MMP-9 is regulated by nuclear factor-κB (NF-κB) since the MMP-9
promoter region contains DNA binding sites for NF-κB.
The present study revealed that in human bladder
carcinoma T24 cells, EGCG (a) inhibits the adhesion activities,
cellular motility and invasional ability, (b) downregulates MMP-9
at the protein and mRNA levels and (c) inhibits NF-κB
activation.
Materials and methods
Reagents
EGCG was obtained from Sigma (St. Louis, MO, USA).
Primary antibodies to MMP-9, NF-κB and β-actin and secondary
antibodies were purchased from Santa-Cruz Biotechnology, Inc.
(Santa Cruz, CA, USA). The bicinchoninic acid protein assay kit was
obtained from Pierce Biotechnology (Rockford, IL, USA).
Cell culture
The human bladder cancer cell line T24 was obtained
from the Shanghai Institute of Cell Biology, Chinese Academy of
Sciences, Shanghai, China. The cells were cultured in RPMI-1640
medium supplemented with 10% heat-inactivated fetal bovine serum,
penicillin (100 U/ml) and streptomycin (100 mg/l) at 37°C in a
humidified atmosphere containing 5% CO2. EGCG was
dissolved in PBS (pH 7.4) and used for the treatment of cells. The
study was approved by the ethics committee of the First Affiliated
Hospital, Zhejiang University School of Medicine, Hangzhou,
China
Cell adhesion assay
For the cell-matrix adhesion assay (9,10),
96-well plates were incubated with 100 μl fibronectin (10 μg/ml)
for 60 min and blocked with 1% bovine serum albumin at 37°C for 30
min. The cells (5×105 cells/ml) of 200 μl were added to
each well and incubated in EGCG (10–80 μg/ml) or vehicle alone for
90 min at 37°C. After washing three times with PBS to remove
nonadherent cells, 20 μl MTT (5 mg/ml) was added to each well and
incubated at 37°C for 4 h. The plates were spun and the purple
precipitates of formazan were dissolved in 150 μl dimethyl
sulfoxide. Absorbance was measured at 490 nm using an ELISA plate
reader. The adhesion of cells treated with the vehicle was
established as 100%.
Cell migration assay
T24 motility was assessed using a wound closure
assay (11). Approximately
1×105 T24 cells were cultured in 6-well plates. When the
cells had grown to full confluence, a wound was induced on the
monolayer cells by scraping a gap using a micropipette tip and
removing any cell debris with PBS. The cells were then incubated in
EGCG (10–80 μg/ml) or vehicle alone for 24 h. Images were captured
under ×100 magnification using phase-contrast microscopy (Olympus
IX70, Olympus, Tokyo, Japan) immediately or at 6, 12 and 24 h after
wound incision. Image-Pro Plus 5.0 software (Media Cybernetics,
Bethesda, MD, USA) was used to quantify the non-recovered wound
area over the period of the experiment. The percentage of
non-recovered wound area was calculated by dividing the
non-recovered area by the initial wound area at zero time.
Cell invasion assay
The cell invasion assay was performed as previously
described (12,13). Briefly, T24 cells were plated at
5×106 cells/ml in the upper compartment of a 8 μm-pore
size Transwell migration chamber (Cat. no. 3422; Corning Inc.,
Corning, NY, USA) and cultured in medium containing EGCG (10–80
μg/ml) or vehicle alone for 24 h. Cells were incubated for 24 h and
those that did not migrate through the pores were removed by
scraping the upper surface of the membrane with a cotton swab.
Cells that had migrated to the lower surface of the membrane were
fixed and stained. The cells that invaded through the insert were
counted in 5 randomly selected microscopic fields (x400) per
filter. The invasion of cells treated with the vehicle was
established as 100%.
Total RNA extraction and mRNA detection
by reverse transcription-polymerase chain reaction (RT-PCR)
Total cellular RNA was extracted using the TRIzol
reagent (Invitrogen, Carlsbad, CA, USA) following the
manufacturer’s instructions. RNA (3 μg) was reverse transcribed
using oligo(dT) primers and M-MLV Reverse Transcriptase (Promega,
Madison, WI, USA). The resulting cDNA was amplified by PCR using
gene-specific primers. The PCR primers for MMP-9 were
5′-CACTGTCCACCCCTCAGAGC-3′ (sense) and 5′-GCCACTTGTCGGCGATAAGG-3′
(antisense) and for GAPDH were 5′-ATGGCACCG TCAAGGCTGAG-3′ (sense)
and 5′-GCAGTGATGGCATGGACTGT-3′ (antisense). PCR amplification
consisted of an initial denaturation step (95°C for 3 min), 32
cycles of denaturation (94°C for 45 sec), annealing (57°C for 45
sec) and extension (72°C for 60 sec) followed by a final incubation
at 72°C for 10 min. The PCR products were analyzed on 1.5% agarose
gel. The gel was photographed and then quantitatively measured by
scanning densitometry.
Western blot analysis
Western blot analysis was used to analyze the
expression of various proteins as described previously (14). Briefly, cells were harvested at 24
h following EGCG treatment, washed with lysis buffer (10 mmol/l
Tris-HCl, 0.25 mol/l sucrose, 5 mmol/l EDTA, 50 mmol/l NaCl, 30
mmol/l sodium pyrophosphate, 50 mmol/l NaF, 1 mmol/l
Na3VO4, 1 mmol/l PMSF, pH 7.5). Protein
concentration in the resulting lysate was determined using the
bicinchoninic acid protein assay. Appropriate amounts of protein
(20–30 μg) were resolved by electrophoresis in 10–15% tris-glycine
polyacrylamide gels and transferred to nitrocellulose membranes.
Membranes were blocked and then incubated overnight with the
appropriate primary antibody at dilutions specified by the
manufacturer. The membranes were then washed and incubated with the
corresponding horseradish peroxidase-conjugated secondary antibody
at 1:1,000 dilution in TBST. Bound secondary antibody was detected
using an enhanced chemiluminescence (ECL) system (Pierce
Biotechnology Inc.). To determine NF-κB cellular localization,
nuclear and cytoplasmic proteins were isolated from cells using a
cell fractionation kit (Keygen, Nanjing, China). NF-κB expression
in the nuclear and cytoplasmic compartments was determined by
immunoblot analysis.
Statistical analysis
All the experiments were performed in triplicate and
performed a minimum of three times. All values are expressed as the
mean ± SD. Statistical significance was compared between various
treatment groups and controls using the one-way analysis of
variance (ANOVA). Data were considered to be statistically
different when P<0.05.
Results
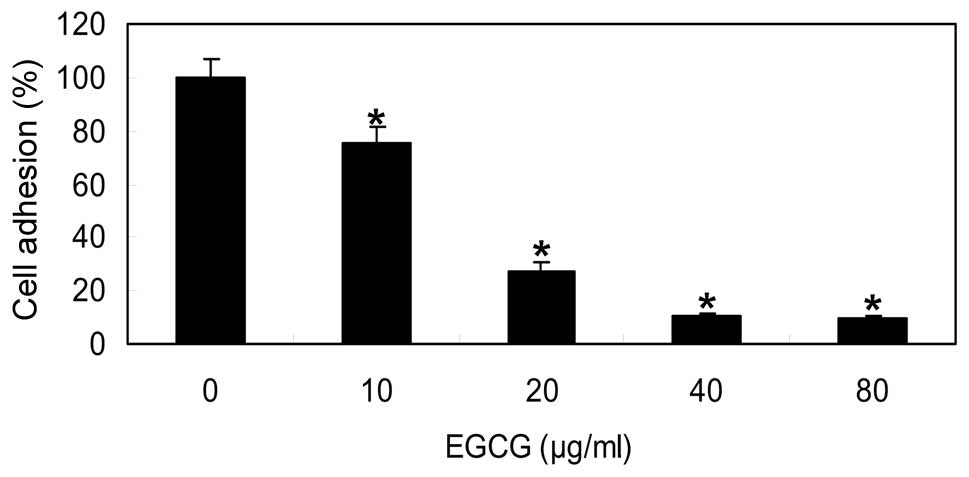
EGCG inhibits T24 cell adhesion to
fibronectin
Adhesive interactions between tumor cells and ECM
proteins, including fibronectin, are deeply involved in a tumor
growth, invasion and metastasis. Cell migration is intimately
associated with cell adhesive properties. Thus the effects of EGCG
on T24 cell adhesion were assessed. When the adhesion of T24 cells
to wells coated with fibronectin in the presence of EGCG in
solution was examined, it was revealed that EGCG inhibited cell
adhesion to fibronectin dose-dependently (Fig. 1).
EGCG inhibits T24 cell migration
The ability of tumor cells to migrate has been
associated with their metastatic potential and thus, the migration
of EGCG-treated cells was investigated by the wound scratch assay.
This assay is commonly used for testing the effects of pro- and
anti-migratory agents on cultured cells. The migration of the cells
into the wound area was assessed at time 0 and following the
indicated hours. As shown in Fig.
2A, EGCG significantly inhibited the migration of T24 cells
into the wounded area. In the wound scratch tests, the larger
non-recovered wound area may reveal that the anti-metastatic effect
was more notable in these areas. Results demonstrated that at
higher dosages of EGCG, the T24 cells spreading along the wound
edges became slower than those treated with vehicle alone. The
anti-metastatic effect of EGCG on T24 cells was thus confirmed to
be dose-dependent (Fig. 2B).
EGCG inhibits T24 cell invasion
The in vitro cell invasion assay was based on
the Boyden chamber assay. The Matrigel matrix served as a
reconstituted basement membrane and the number of cells that
migrated through the matrix was counted. The relevance of this
assay for other invasion assays and for in vivo malignancy
has been documented extensively. The invasion inhibition was
determined following exposure to EGCG, as shown in Fig. 3. EGCG dose-dependently inhibited
the invasion of T24 cells; at 80 μg/ml EGCG inhibited the invasion
of T24 cells by 90% (P<0.01). The results show that EGCG
inhibited the invasion of highly metastatic human bladder carcinoma
T24 cells in vitro.
EGCG downregulates MMP-9 at the protein
and mRNA levels in the T24 cell line
The effect of EGCG on the expression of MMP-9 was
examined in order to study the possible anti-metastatic mechanisms
of EGCG. An RT-PCR analysis was conducted to investigate the effect
of EGCG on MMP-9 mRNA expression in T24 cells. As shown in Fig. 4A and B, electrophoresis scanning
quantitative analysis indicated that EGCG dose-dependently
suppressed the MMP-9 mRNA in T24 cells. Western blot analysis was
used to evaluate the protein expression of MMP-9 and the result was
consistent with the changes of mRNA expression (Fig. 4C and D).
EGCG downregulates NF-κB in the T24 cell
line
NF-κB family members are pleiotropic transcription
factors that control the expression of numerous genes involved in a
variety of cellular responses, including those significant for cell
invasion and metastasis, such as MMP-9. To further elucidate the
effect of EGCG on NF-κB activity, western blotting was applied to
investigate the change of NF-κB nuclear translocation in the T24
cells treated with various concentrations of EGCG. As shown in
Fig. 4E, EGCG significantly
inhibited NF-κB activation in T24 cells in a dose-dependent manner.
This result suggests that NF-κB is likely to be involved in the
inhibitory effect of EGCG on MMP-9 expression.
Discussion
In this study, the anti-metastatic potential of EGCG
against bladder cancer and its mechanism of action was evaluated.
Consistent with earlier observations, EGCG inhibited the invasion
and metastatic potential of bladder cancer cells by the
inactivation of NF-κB and then the inhibition of the expression of
MMP-9.
Metastasis is a series of sequential steps,
including cell proliferation, angiogenesis, cell adhesion,
migration and invasion into the surrounding tissue. In the majority
of cases, mortality from bladder cancer results from metastatic
disease. Therefore, the inhibition of metastasis is one of the most
significant issues in bladder cancer research. Numerous studies
have reported that EGCG exerts its anticancer effects in various
experimental systems. Using the T24 human bladder cancer cell line,
previous studies (14) have
demonstrated that EGCG treatment caused the dose- and
time-dependent inhibition of cellular proliferation and viability
and induced apoptosis. In addition to having chemopreventive
activity, EGCG has been shown to inhibit tumor invasion and
angiogenesis, which are essential for the growth and metastasis of
all solid tumors. Therefore, in this study, the effect of EGCG on
the invasive ability of human bladder cancer T24 cells was
investigated. It was revealed that EGCG efficiently and
dose-dependently inhibited the adhesion, migration and invasion of
T24 cells.
The process of metastasis is promoted by expressing
and secreting various proteolytic enzymes that degrade the majority
of ECM components. MMPs are a family of zinc- and calcium-dependent
endopeptidases, consisting of four subclasses based on substrate,
including collagenases, gelatinases, stromelysins and
membrane-associated MMPs. MMPs degrade ECM allowing urinary bladder
transitional-cell carcinoma cells to spread and diffusely
infiltrate the bladder parenchyma. To date, more than 20 human MMPs
have been identified (15). MMP-9
is a key enzyme in tumor invasion for degrading type IV collagen,
which is the major structural protein component in the ECM and
basement membrane. Numerous studies have shown that MMP-9 is
associated with invasiveness and bladder cancer progression.
Therefore, in the current study, the effect of EGCG against MMP-9
expression was investigated. EGCG efficiently dose-dependently
downregulated MMP-9 at the protein and mRNA levels in human bladder
cancer T24 cells. The inhibitory effect of EGCG on MMP-9 may be
partly responsible for its anti-metastatic potential.
It has been reported that the expression of MMP-9 is
regulated by NF-κB since the MMP-9 promoter region contains the DNA
binding sites of NF-κB. NF-κB is typically a heterodimer consisting
of p65 and p50 proteins. It is kept in an inactive form in the
cytoplasm by inhibitory proteins called inhibitors of κB (I κB). In
response to an activation signal, NF-κB is released from I κB and
translocates from the cytoplasm to the nucleus. Following the
translocation, in the nucleus, NF-κB binds to the promoter region
of MMP-9, leading to gene expression. To further elucidate the
effect of EGCG on NF-κB expression, western blotting was applied to
investigate the change of NF-κB nuclear translocation in the T24
cells treated with various concentrations of EGCG. As shown in
Fig. 4E, EGCG downregulated the
expression of p65 protein in the nucleus, suggesting that p65
nuclear translocation was prevented by EGCG. This result suggests
that NF-κB is involved in the inhibitory effect of EGCG on MMP-9
expression.
The phosphatidylinositide-3 kinase (PI3K)/Akt
signaling pathway is significant in regulating the expression of
MMPs by transcriptional factors, including NF-κB (16–18).
Metastasis is also regulated by the PI3K/Akt pathway, which has
been implicated in a number of cellular functions, including cell
survival, adhesion and metastasis. Accumulating evidence indicates
a role for the PI3K pathway in the invasion of bladder cancer
(19–21). Previous studies have shown that
EGCG treatment resulted in a significant dose-dependent inhibition
of constitutively elevated levels of phosphorylated PDK1 and
phosphorylated (active) Akt (at Ser473 and Thr308) in T24 cells
(14). The decrease in the
expression of MMP-9 by EGCG was attributed to the inactivation of
the PI3K/Akt signaling pathways and NF-κB activity. This
suppressive effect may contribute to the inhibition of invasion of
T24 cells by EGCG.
In conclusion, the data from our study indicate that
EGCG efficiently and dose-dependently inhibited the adhesion,
migration and invasion of human bladder cancer T24 cells. This may
be due to EGCG inhibition of PI3K/Akt, followed by the inactivation
of NF-κB and then the inhibition of the expression of MMP-9 and
ultimately suppressed the invasion and metastasis. The results of
this study provide evidence that EGCG may be a potential
therapeutic candidate against tumor invasion. Further studies on
more detailed mechanisms and functions of EGCG are required.
Acknowledgements
This study was supported by grants from the National
Natural Science Foundation of China (grant no. 30900552) and the
Administration of Traditional Chinese Medicine of Zhejiang, China
(grant no. 2009CA057).
References
|
1
|
Ashughyan VR, Marihart S and Djavan B:
Chemopreventive trials in urologic cancer. Rev Urol. 8:8–13.
2006.
|
|
2
|
Pectasides D, Pectasides M and Nikolaou M:
Adjuvant and neoadjuvant chemotherapy in muscle invasive bladder
cancer: literature review. Eur Urol. 48:60–67; discussion 67–68.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Khan N, Afaq F, Saleem M, Ahmad N and
Mukhtar H: Targeting multiple signaling pathways by green tea
polyphenol (−)-epigallo-catechin-3-gallate. Cancer Res.
66:2500–2505. 2006.
|
|
4
|
Yang CS, Maliakal P and Meng X: Inhibition
of carcinogenesis by tea. Annu Rev Pharmacol Toxicol. 42:25–54.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kemberling JK, Hampton JA, Keck RW, Gomez
MA and Selman SH: Inhibition of bladder tumor growth by the green
tea derivative epigallocatechin-3-gallate. J Urol. 170:773–776.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Liotta LA and Stetler-Stevenson WG: Tumor
invasion and metastasis: an imbalance of positive and negative
regulation. Cancer Res. 51(Suppl 18): 5054s–5059s. 1991.PubMed/NCBI
|
|
7
|
Choi YD, Cho NH, Ahn HS, Cho KS, Cho SY
and Yang WJ: Matrix metalloproteinase expression in the recurrence
of superficial low grade bladder transitional cell carcinoma. J
Urol. 177:1174–1178. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Di Carlo A, Terracciano D, Mariano A and
Macchia V: Urinary gelatinase activities (matrix metalloproteinases
2 and 9) in human bladder tumors. Oncol Rep. 15:1321–1326.
2006.PubMed/NCBI
|
|
9
|
Liu J, Zhang X, Yang F, Li T, Wei D and
Ren Y: Antimetastatic effect of a lipophilic ascorbic acid
derivative with antioxidation through inhibition of tumor invasion.
Cancer Chemother Pharmacol. 57:584–590. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Suzuki Y and Isemura M: Inhibitory effect
of epigallocatechin gallate on adhesion of murine melanoma cells to
laminin. Cancer Lett. 173:15–20. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hochman E, Castiel A, Jacob-Hirsch J,
Amariglio N and Izraeli S: Molecular pathways regulating
pro-migratory effects of Hedgehog signaling. J Biol Chem.
281:33860–33870. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Garbisa S, Biggin S, Cavallarin N, Sartor
L, Benelli R and Albini A: Tumor invasion: molecular shears blunted
by green tea. Nat Med. 5:12161999. View
Article : Google Scholar : PubMed/NCBI
|
|
13
|
Meng Q, Qi M, Chen DZ, et al: Suppression
of breast cancer invasion and migration by indole-3-carbinol:
associated with up-regulation of BRCA1 and E-cadherin/catenin
complexes. J Mol Med (Berl). 78:155–165. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Qin J, Xie LP, Zheng XY, et al: A
component of green tea, (−)-epigallocatechin-3-gallate, promotes
apoptosis in T24 human bladder cancer cells via modulation of the
PI3K/Akt pathway and Bcl-2 family proteins. Biochem Biophys Res
Commun. 354:852–857. 2007.
|
|
15
|
Overall CM and López-Otin C: Strategies
for MMP inhibition in cancer: innovations for the post-trial era.
Nat Rev Cancer. 2:657–672. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chakraborti S, Mandal M, Das S, Mandal A
and Chakraborti T: Regulation of matrix metalloproteinases: an
overview. Mol Cell Biochem. 253:269–285. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kim D, Kim S, Koh H, et al: Akt/PKB
promotes cancer cell invasion via increased motility and
metalloproteinase production. FASEB J. 15:1953–1962. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Rangaswami H, Bulbule A and Kundu GC:
Nuclear factor-inducing kinase plays a crucial role in
osteopontin-induced MAPK/IκBα kinase-dependent nuclear factor
κB-mediated promatrix metalloproteinase-9 activation. J Biol Chem.
279:38921–38935. 2004.PubMed/NCBI
|
|
19
|
Chiang GJ, Billmeyer BR, Canes D, et al:
The src-family kinase inhibitor PP2 suppresses the in vitro
invasive phenotype of bladder carcinoma cells via modulation of
Akt. BJU Int. 96:416–422. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Rieger-Christ KM, Lee P, Zagha R, et al:
Novel expression of N-cadherin elicits in vitro bladder cell
invasion via the Akt signaling pathway. Oncogene. 23:4745–4753.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wu X, Obata T, Khan Q, Highshaw RA, De
Vere White R and Sweeney C: The phosphatidylinositol-3 kinase
pathway regulates bladder cancer cell invasion. BJU Int.
93:143–150. 2004. View Article : Google Scholar : PubMed/NCBI
|