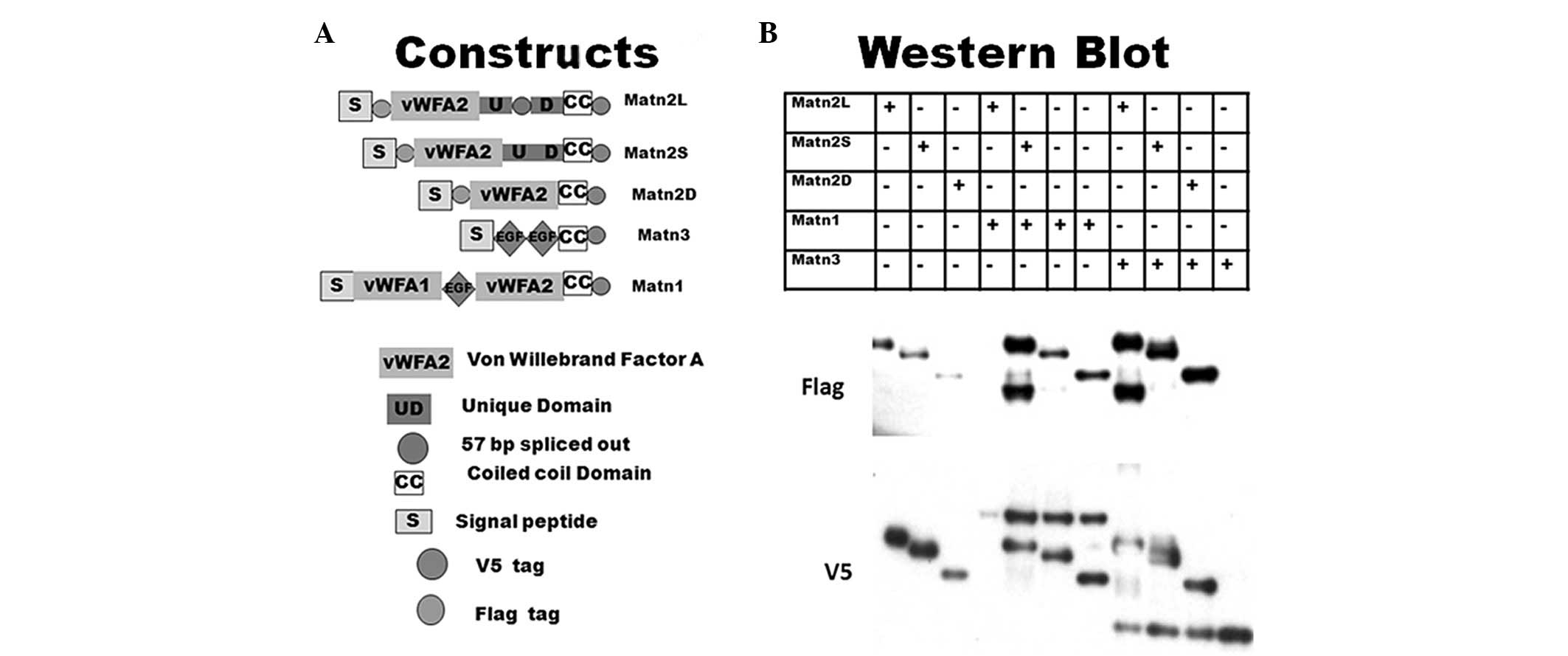
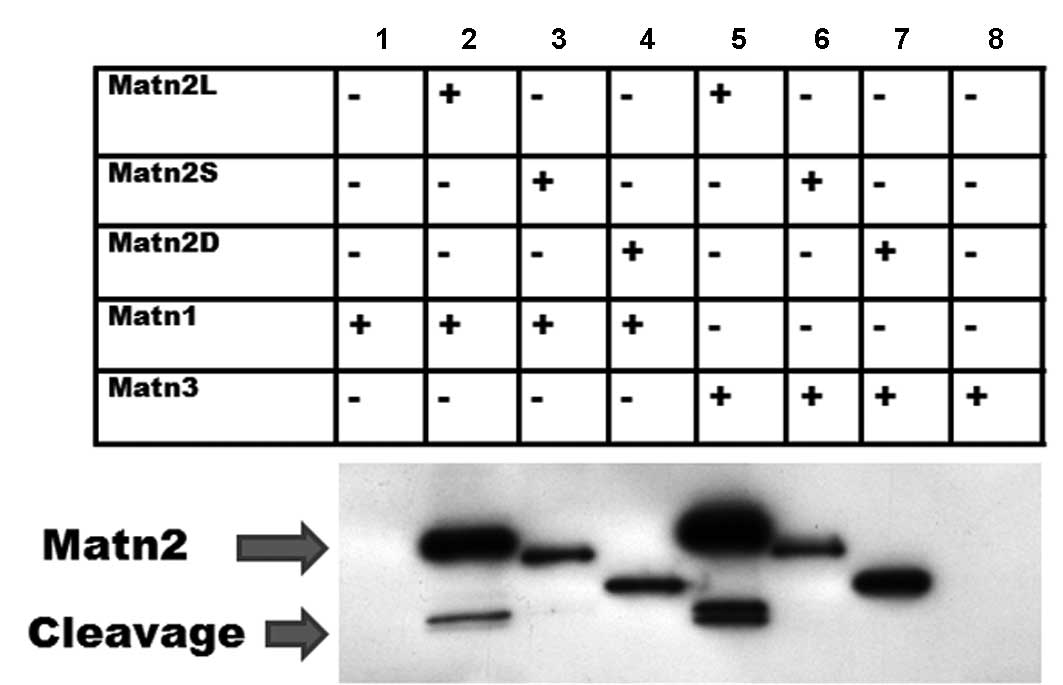
|
1
|
Klatt AR, Becker AA, Neacsu CD, Paulsson M
and Wagener R: The matrilins: modulators of extracellular matrix
assembly. Int J Biochem Cell Biol. 43:320–330. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Deak F, Piecha D, Barchrati C, Paulsson M
and Kiss I: Primary structure and expression of matrilin-2, the
closest relative of cartilage matrix protein within the von
Willebrand factor type A-like module superfamily. J Biol Chem.
272:9268–9274. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Piecha D, Muatoglu S, Morgelin M, Hauser
N, Studer D, Kiss I, Paulsson M and Deak F: Matrilin-2, a large,
oliomeric protein, is expressed by a great variety of cells and
forms fibrillar networks. J Biol Chem. 274:13353–13361. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Whittaker CA and Hynes RO: Distribution
and evolution of von Willebrand/integrin A domains: widely
dispersed domains with roles in cell adhesion and elsewhere. Mol
Biol Cell. 13:3369–3387. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Luo J and Wan Y: Tightly regulated
distribution of family members of proteins is related to social
property in the open body system. Int J Mol Med. 17:411–418.
2006.PubMed/NCBI
|
|
6
|
Wouters MA, Riquoutsos I, Chu CK, Feng LL,
Sparrow DB and Dunwoodie SL: Evolution of distinct EGF domains with
specific functions. Protein Sci. 14:1091–1103. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Rao Z, Handford P, Mayhew M, Knott V,
Brownlee GG and Stuart D: The structure of Ca (+2)-binding
epidermal growth factor-like domain: its role in protein-protein
interactions. Cell. 82:131–141. 1995.
|
|
8
|
Pan OH and Beck K: The C-terminal domain
of matrilin-2 assembles into a three-stranded alpha-helical coiled
coil. J Biol Chem. 273:14205–14209. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Paulsson M, Piecha D, Segat D, Smyth N and
Wagener R: The matrilins: a growing family of A-domain-containing
protein. Biochem Soc Trans. 27:824–826. 1999.PubMed/NCBI
|
|
10
|
Frank S, Schulthess T, Landwehr R, Lustig
A, Mini T, Jeno P, Engel J and Kammerer RA: Characterization of the
matrilin coiled-coil domains reveals seven novel isoforms. J Biol
Chem. 277:19071–19079. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Piecha D, Wiberg C, Morgelin M, Reinhardt
DP and Deak F: Matrilin-2 interacts with itself and with other
extracellular matrix proteins. Biochem J. 367:715–721. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Muratoglu S, Krysan K, Balazs M, Sheng H,
Zakany R, Modis L, Kiss I and Deak F: Primary structure of human
matrilin-2, chromosome location of the MATN2 gene and conservation
of an AT-AC intron in matrilin genes. Cytogenet Cell Genet.
90:323–327. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhang Y and Chen Q: Changes of matrilin
forms during endochondral ossification. Molecular basis of
oligomeric assembly. J Biol Chem. 275:32628–32634. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Chen Q, Zhang Y, Johnson DM and Goetinck
PF: Assembly of a novel cartilage matrix protein filamentous
network: molecular basis of differential requirement of von
Willebrand factor A domains. Mol Biol Cell. 10:2149–21462. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Mates L, Nicolae C, Morgelin M, Deak F,
Kiss I and Aszodi A: Mice lacking the extracellular matrix adaptor
protein matrilin-2 develop without obvious abnormalities. Matrix
Biol. 23:195–204. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Szabo E, Korpos E, Batmunkh E, Lotz G,
Holczbauer A, Kovalszky H, Deak F, Kiss I, Schaff Z and Kiss A:
Expression of matrilin-2 in liver cirrhosis and hepatocellular
carcinoma. Pathol Oncol Res. 14:15–22. 2008. View Article : Google Scholar
|
|
17
|
Ichikawa T, Suenaga Y, Koda T, Ozaki T and
Nakagawara A: ΔNp63/BMP-7-dependent expression of matrilin-2 is
involved in keratinocyte migration in response to wounding. Biochem
Biophys Res Comm. 369:994–1000. 2008.
|
|
18
|
Malin D, Sonnenberg-Riethmacher E, Guseva
D, Wagener R and Aszodi A: The extracellular-matrix protein
matrilin 2 participates in peripheral nerve regeneration. J Cell
Sci. 122:995–1004. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Goetsch SC, Hawke TJ, Gallarde TD,
Richardson JA and Garry DJ: Transcriptional profiling and
regulation of the extracellular matrix during muscle regeneration.
Physiol Genomics. 14:261–271. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sharma MK, Watson MA, Lyman M, Perry A,
Aldape KD, Deak F and Gutmann DH: Matrilin-2 expression
distinguishes clinically relevant subset of pilocytic astrocytoma.
Neurol. 66:127–130. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhang Y, Wang Z, Luo J, Kanbe K and Chen
Q: Multiple functions of the von Willebrand factor A domain in
matrilins: secretion, assembly, and proteolysis. J Orthop Surg Res.
3:212008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ehlen HW, Sengle G, Klatt AR, Talke A,
Muller S and Paulsson M: Proteolytic processing causes extensive
heterogeneity of tissue matrilin forms. J Biol Chem.
284:21545–21556. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
O’Reilly MS, Holmgren L, Chen C and
Folkman J: Angiostatin induces and sustains dormancy of human
primary tumors in mice. Nat Med. 2:689–692. 1996.PubMed/NCBI
|
|
24
|
Bergers G, Javaherian K, Lo KM, Folkman J
and Hanahan D: Effects of angiogenesis inhibitors on multistage
carcinogenesis in mice. Science. 284:808–812. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Krieg T and LeRoy EC: Diseases of the
extracellular matrix. J Mol Med. 76:224–225. 1998. View Article : Google Scholar
|