Introduction
Ionizing radiation (IR) induces a variety of
cellular responses at clinically relevant doses. MCF-7 cells were
arrested at the G1 and G2 phases of the cell cycle and MDA-MB231
cells were arrested at the G2 phase in response to IR (1,2). It
has been demonstrated that X-rays at extremely low doses, between
1–5 cGy, stimulate cell proliferation, whereas at doses >1 Gy,
IR is lethal to cells (3).
Topoisomerase IIα (Topo IIα), a nuclear enzyme involved in a number
of cellular processes, is necessary for eukaryotic survival. Topo
IIα is significant in the replication and repair of DNA and takes
part in chromosome condensation and segregation during mitosis
(4). Previous studies have
demonstrated that DNA Topo IIα expression levels correlate with
chromatid radiosensitivity (5).
Topo IIα has also been shown to play a significant role in male
mouse meiosis and its activity is required at the
metaphase-anaphase transition of the two meiotic divisions for
proper chromosome disjunction (6,7). The
expression of Topo IIα changes periodically during cell growth and
therefore, Topo IIα is thought to be a marker of cell proliferation
(8–10). Topo IIα expression is affected by
numerous environmental factors, including heat-shock and IR. It has
been reported that, if the activity of Topo IIα is restrained or
the distribution of Topo IIα in cells changes as the result of
genetic mutations, the aneuploid ratio of the cells increases
(11,12).
Grp75, a member of the HSP70 family, is involved in
multiple functions that are required to maintain cell metabolism,
including the stress response, cell proliferation and
differentiation (13). Previous
studies have demonstrated that Grp75 levels appear to correlate
with mitochondrial activity and biogenesis (14) and that Grp75 expression is induced
by cerebral ischemia (15),
glucose deprivation (16) and low
doses of IR (17). The gene
expression of Grp75 in HT29 and MCF-7 cells was upregulated
following exposure to IR (17,18).
To investigate the potential link between the expression of Grp75
and Topo IIα and how they affect cell proliferation in response to
low dose IR, the IR-induced proliferation and Topo IIα expression
levels in regular and Grp75-overexpressing PC12 cells were
analyzed.
Materials and methods
Cell culture
The rat adrenal pheochromocytoma cell line PC12 used
in this study was obtained from the Chinese Academy of Sciences,
Beijing, China. The cells were maintained in Dulbecco’s modified
Eagle’s medium (Gibco, Gaithersburg, MD, USA) supplemented with 10%
fetal bovine serum (FBS; Gibco), penicillin (100 U/ml; Gibco) and
streptomycin (100 mg/ml; Gibco), in a humidified atmosphere of 5%
CO2 and 95% air at 37°C.
Cell transfection
The coding sequence of the Grp75 gene (NM_001100658)
was cloned and inserted into pcDNA3 vectors. The plasmids of the
pcDNA3-Grp75 and pcDNA3 vectors were separately transfected into
PC12 cells using Lipofectamine 2000 (Invitrogen, Carlsbad, CA,
USA), following the manufacturer’s instructions. Transfected cells
stably expressing Grp75 were selected using 800 μg/ml of G418 after
48 h. Control cells were transfected with pcDNA3 alone. Grp75
expression in transfected PC12 cells was determined by western blot
analysis.
IR treatment
Cells were exposed to various doses of IR or the
same dose of IR at a regular intervals (Gammatron-3; dose rate,
0.0244 Gy/min) at room temperature. To measure radiation
sensitivity, the cells were seeded on four 100-mm culture dishes at
a density of 5×105 cells per dish and 24 h later they
were irradiated with various doses of IR, ranging from 1 to 5 Gy or
with 3 Gy of γ-rays 1–4 times at regular intervals of 24 h. The
cells were washed twice with ice-cold phosphate-buffered saline
(PBS) 24 h after IR treatment and harvested for western blotting.
Experiments were repeated at least three times.
Cell proliferation assay
Cells were seeded in 96-well tissue culture plates
at a density of 5×103 cells per well. The following day,
cells were treated with IR at the indicated doses and were
incubated for 24 h. Cell proliferation was determined using an MTT
assay. Fresh medium containing 0.5 mg/ml MTT (Amresco, Solon, OH,
USA) was added to each well. Following incubation at 37°C for 4 h,
the MTT solution was removed and replaced with approximately 150 μl
of dimethylsulfoxide. The cells were then incubated for another 10
minutes at 37°C with gentle agitation. The absorbance was measured
using a microplate reader (Thermo Scientific, Anaheim, CA, USA) at
a wavelength of 492 nm. Experiments were performed in quadruplicate
and repeated at least three times.
Western blot analysis
The cells were washed and lysed in buffer containing
50 mM Tris-HCl (pH 8.0), 150 mM NaCl, 1% NP-40, 0.5% Doc, 0.1%
sodium dodecyl sulfate, 1 μg/ml of aprotinin and 100 μg/ml of
phenylmethylsulfonyl fluoride supplemented with phosphatase
inhibitor (Sigma, St. Louis, MO, USA) cocktails. Cell lysates were
separated by 10% SDS-polyacrylamide gel electrophoresis and
transferred to 0.45-μm polyvinylidene fluoride membranes (Amersham
Pharmacia Biotech, Amersham, UK) in a Bio-Rad Trans-Blotting
apparatus (100 V, 90 min). The membranes were blocked for at least
1 h in 5% (W/V) non-fat milk in TBS-T buffer (20 mM Tris-HCl, pH
7.6, 137 mM NaCl, and 0.05% Tween-20; pH 7.0). The membranes were
incubated with primary antibodies in blocking buffer at 4°C
overnight. After being washed with 0.2% TBS-T for 20 min, the
membranes were incubated with horseradish peroxidase conjugated
secondary antibody for 45 min at room temperature and were
visualized with the enhanced chemiluminescent reagents (Pierce
Biotechnology Inc., Rockford, IL, USA). Antibodies specific for
Grp75 and GAPDH were obtained from Cell Signaling Technology
(Danvers, MA, USA) and Topo IIα was purchased from BioWorld
(Visalia, CA, USA).
Statistical analysis
The data are expressed as the mean ± SEM from at
least three replicates of the experiment. The comparison of groups
was performed using a Student’s t-test or ANOVA. P<0.05 was
considered to indicate statistically significant differences.
Results
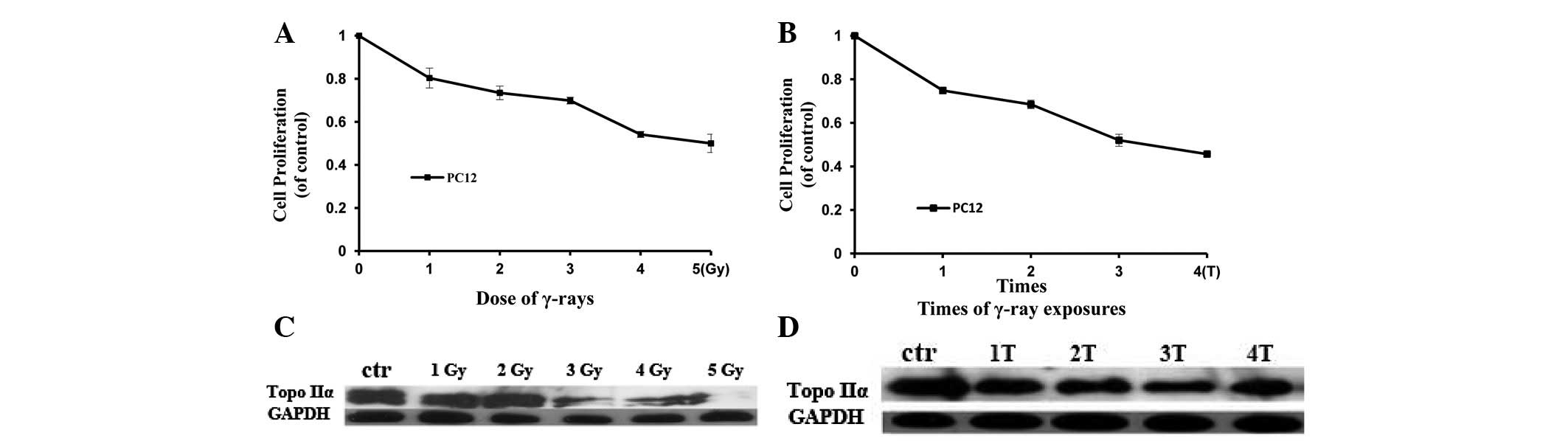
Inhibitory effect of IR on PC12 cell
proliferation
To determine whether the inhibitory effect of IR on
cell proliferation acted in a dose-dependent manner or whether it
correlated with treatment frequency, PC12 cells were either treated
with IR for various doses from 1–5 Gy or repeatedly treated with IR
(3 Gy of γ-rays) from 1–4 times at regular intervals of 24 h. Cell
proliferation was analyzed by an MTT assay 24 h after treatment. As
shown in Fig. 1A and B, the cells
demonstrated a gradual decrease in proliferative ability when
compared with control cells with increasing doses or frequency of
IR treatment. These results suggest that IR exposure not only
inhibits the proliferation of PC12 cells but such inhibition is
dependent on either dose or time of treatment.
Previous studies have demonstrated that Topo IIα
plays a crucial role in DNA replication and therefore is
significant in cell proliferation (19). To explore a potential link between
the inhibition of cell proliferation induced by IR and Topo IIα
expression, the level of Topo IIα protein was analyzed in PC12
cells. These cells were either treated with IR at various doses
from 1 to 5 Gy or repeatedly treated with IR (3 Gy) from 1 to 4
times at regular intervals of 24 h, and the level of Topo IIα
protein was measured 24 h after treatment by anti-Topo IIα western
blot analysis. As shown in Fig. 1C and
D, Topo IIα protein was downregulated by IR treatment.
Inhibition of Topo IIα expression was observed at 1 Gy and
following the first treatment of IR, Topo IIα protein level
decreased as the dose of IR and frequency of IR treatment
increased. In IR dose-dependent or frequency of treatment-dependent
experiments, the observed decreased cell proliferation correlated
with the reduced expression level of Topo IIα protein. These data
suggest that IR inhibits PC12 cell proliferation by repressing the
expression of Topo IIα protein.
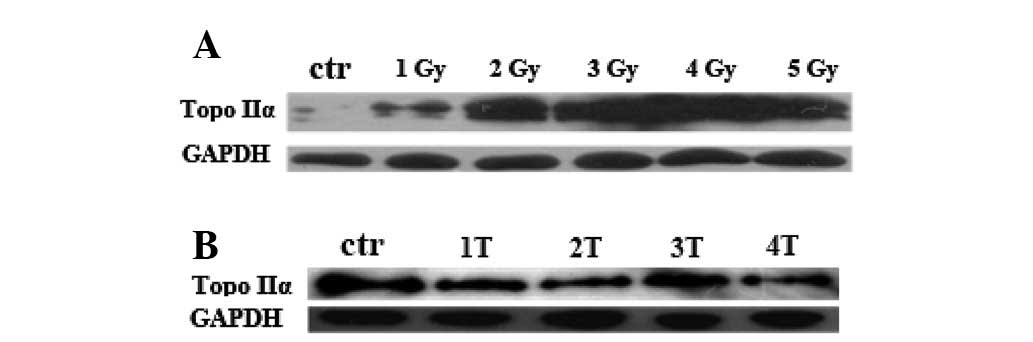
The expression of Grp75 was induced by IR
in PC12 cells
Previous studies have reported that the expression
of Grp75 is inducible in response to IR treatment (17,18).
However, there is no evidence concerning whether the induction of
the Grp75 gene depends on the amount of IR or the frequency of IR.
In this study, the regulation of Grp75 expression in PC12 cells by
various doses or frequencies of IR treatment was examined. The
level of endogenous Grp75 protein was determined by western blot
analysis 24 h after treatment. As shown in Fig. 2, increasing doses/frequency of IR
treatment gradually led to the upregulation of Grp75 protein. These
data demonstrate that the expression of Grp75 in PC12 cells is
induced by IR in a dose- and frequency-dependent manner.
Grp75 overexpression protects PC12 cells
against IR-induced injury
The expression of Grp75 has been shown to be
adaptively increased in response to IR (Fig. 2). This study investigated whether
the overexpression of Grp75 protects the cells from IR-induced
injury. PC12 cells were transfected with a pcDNA3.1-Grp75 vector to
enhance the expression of Grp75 protein and determine the
expression of Grp75 using western blotting. As shown in Fig. 3A, enhancement of Grp75 expression
in positive clone2 was evident compared with control cells
transfected with an empty vector. The effect of IR on the
proliferation of PC12 cells overexpressing Grp75 was then examined.
As shown in Fig. 3B and C, PC12
cells overexpressing Grp75 demonstrated a moderate decrease in cell
proliferation upon IR treatment when compared with controls. These
results indicate that Grp75 overexpression reduced IR injury in
PC12 cells and thus overexpressing Grp75 protected PC12 cells
against IR injury.
The level of Topo IIα protein in
Grp75-overexpressing PC12 cells treated with IR was examined
(Fig. 3A and B). Western blot
analysis demonstrated a dose-dependent expression of Topo IIα
protein in response to increasing IR, whereas the level of Grp75
protein was almost constant during treatments 1–4 (Fig. 4A and B). These data suggest that
the protective role of Grp75 against IR-induced cell proliferation
inhibition acts through either enhancing or stabilizing the
expression of Topo IIα protein, which plays a crucial role in cell
proliferation.
Discussion
IR, which causes DNA damage and inhibits cell
proliferation, has become a predominant therapeutic tool to treat
tumors clinically. Consistent with previous studies, cell
proliferation was inhibited after PC12 cells were exposed to IR.
Notably, a dose/time-dependent inhibition of cell proliferation in
IR-treated PC12 cells was demonstrated. It has been shown that Topo
IIα is significantly involved in chromosome condensation during DNA
replication and repair to confer cell survival and multiplication
(20). Previous studies have
reported that the repression of Topo IIα mRNA is induced by IR in
25 cell lines (21). However, the
action of Topo IIα protein in response to various doses/frequencies
of IR treatment has not been previously recognized. In keeping with
previous studies, the expression of Topo IIα was downregulated in
PC12 cells which were treated with IR (Fig. 1C and D). Significantly, IR
inhibited the expression of Topo IIα protein in a dose- or
frequency of treatment-dependent manner which was correlated with
the observed inhibition of cell proliferation under the same
treatment conditions. The data suggests that IR-induced Topo IIα
protein repression may be responsible for IR-induced cell
proliferation inhibition.
Grp75 is a member of the heat-shock protein family
that is not heat-inducible and is mainly located at multiple
subcellular sites, including in mitochondria. Grp75 has been
implicated in various biological processes of human cells (13) and has been shown to respond to
numerous forms of stress, including ischemia (15), glucose deprivation (16), oxidative injury (22) and drug resistance (23). In addition, data from previous
studies have shown that the treatment of IR led to the upregulation
of the Grp75 protein (17,18). In this study, the expression of
Grp75 induced by IR was highlighted and a dose/time-dependent
induction of Grp75 expression in response to IR treatment was
identified (Fig. 2A and B). The
level of Topo IIα protein in cells treated by IR and whether the
overexpression of Grp75 could affect cell proliferation was then
evaluated.
Grp75 overexpression markedly attenuated IR-induced
cell injury as demonstrated by higher levels of cell proliferation
and hence increased expression of Topo IIα (Fig. 3A and B). These results suggest that
the stable expression of Grp75 desensitizes the cells to IR injury.
Thus, it is reasonable to speculate that changes in Grp75 reflect a
protective response in cells exposed to IR and maintaining the
endogenous expression of Topo IIα might be one of the mechanisms by
which Grp75 reduces cell injury induced by IR.
The expression of Grp75 gradually increased as the
dose of IR increased, whereas the level of Topo IIα decreased.
Although the expression of Grp75 protein is enhanced in response to
increasing doses of IR treatment, the level of Grp75 protein
remains too low to restore the expression of Topo IIα protein.
In conclusion, the current study reveals a
protective effect of Grp75 against IR-induced cell injury which may
be associated with the increased expression of Topo IIα, a marker
of cell proliferation. This study focused on a cellular model of IR
injury and further in vivo studies are required to confirm
the protective effects of Grp75 against radiation damage. This is
likely to aid in developing novel strategies to improve
radiotherapy based on the manipulation of Grp75.
Acknowledgements
We thank Lichong Yan for critical reading of the
manuscript and valuable suggestions. This study was supported by
the National Natural Science Foundation of China (81000978).
References
|
1
|
Khan QA and Dipple A: Diverse chemical
carcinogens fail to induce G(1) arrest in MCF-7 cells.
Carcinogenesis. 21:1611–1618. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Yi T, Li H, Wang X and Wu Z: Enhancement
radiosensitization of breast cancer cells by deguelin. Cancer
Biother Radiopharm. 23:355–362. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Suzuki K, Kodama S and Watanabe M:
Extremely low-dose ionizing radiation causes activation of
mitogen-activated protein kinase pathway and enhances proliferation
of normal human diploid cells. Cancer Res. 61:5396–5401. 2001.
|
|
4
|
Adachi Y, Luke M and Laemmli UK:
Chromosome assembly in vitro: topoisomerase II is required
for condensation. Cell. 64:137–148. 1991.
|
|
5
|
Terry SY, Riches AC and Bryant PE: A role
for topoisomerase IIα in the formation of radiation-induced
chromatid breaks. Br J Cancer. 99:670–674. 2008.
|
|
6
|
Mailhes JB, Marchetti F, Young D and
London SN: Numerical and structural chromosome aberrations induced
by etoposide (VP16) during oocyte maturation of mice: transmission
to one-cell zygotes and damage to dictyate oocytes. Mutagenesis.
11:357–361. 1996. View Article : Google Scholar
|
|
7
|
Kallio M and Lähdetie J: Fragmentation of
centromeric DNA and prevention of homologous chromosome separation
in male mouse meiosis in vivo by the topoisomerase II
inhibitor etoposide. Mutagenesis. 11:435–443. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Turley H, Comley M, Houlbrook S, et al:
The distribution and expression of the two isoforms of DNA
topoisomerase II in normal and neoplastic human tissues. Br J
Cancer. 75:1340–1346. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Heck MM and Earnshaw WC: Topoisomerase II:
A specific marker for cell proliferation. J Cell Biol.
103:2569–2581. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hsiang YH, Wu HY and Liu LF:
Proliferation-dependent regulation of DNA topoisomerase II in
cultured human cells. Cancer Res. 48:3230–3235. 1988.PubMed/NCBI
|
|
11
|
Grue P, Grasser A, Sehested M, et al:
Essential mitotic functions of DNA topoisomerase IIα are not
adopted by topoisomerase IIβ in human H69 cells. J Biol Chem.
273:33660–33666. 1998.
|
|
12
|
Yu Q, Mirski SE, Sparks KE and Cole SP:
Two COOH-terminal truncated cytoplasmic forms of topoisomerase IIα
in a VP-16-selected lung cancer cell line result from partial gene
deletion and alternative splicing. Biochemistry. 36:5868–5877.
1997.PubMed/NCBI
|
|
13
|
Wadhwa R, Taira K and Kaul SC: An Hsp70
family chaperone, mortalin/mthsp70/PBP74/Grp75: what, when, and
where? Cell Stress Chaperones. 7:309–316. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ornatsky OI, Connor MK and Hood DA:
Expression of stress proteins and mitochondrial chaperonins in
chronically stimulated skeletal muscle. Biochem J. 311(Pt 1):
119–123. 1995.PubMed/NCBI
|
|
15
|
Massa SM, Longo FM, Zuo J, Wang S, Chen J
and Sharp FR: Cloning of rat grp75, an hsp70-family member, and its
expression in normal and ischemic brain. J Neurosci Res.
40:807–819. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Liu Y, Liu W, Song XD and Zuo J: Effect of
GRP75/mthsp70/PBP74/mortalin overexpression on intracellular ATP
level, mitochondrial membrane potential and ROS accumulation
following glucose deprivation in PC12 cells. Mol Cell Biochem.
268:45–51. 2005. View Article : Google Scholar
|
|
17
|
Sadekova S, Lehnert S and Chow TY:
Induction of PBP74/mortalin/Grp75, a member of the hsp70 family, by
low doses of ionizing radiation: a possible role in induced
radioresistance. Int J Radiat Biol. 72:653–660. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Carette J, Lehnert S and Chow TY:
Implication of PBP74/mortalin/GRP75 in the radio-adaptive response.
Int J Radiat Biol. 78:183–190. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Burden DA and Osheroff N: Mechanism of
action of eukaryotic topoisomerase II and drugs targeted to the
enzyme. Biochim Biophys Acta. 1400:139–154. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Uemura T, Ohkura H, Adachi Y, Morino K,
Shiozaki K and Yanagida M: DNA topoisomerase II is required for
condensation and separation of mitotic chromosomes in S.
pombe. Cell. 50:917–925. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Goldwasser F, Bae I, Pommier Y and Fornace
AJ Jr: Evidence of a reduced DNA topoisomerase II mRNA expression
after ionizing radiation. Anticancer Res. 19:3167–3171.
1999.PubMed/NCBI
|
|
22
|
Rezzani R, Buffoli B, Rodella L,
Stacchiotti A and Bianchi R: Protective role of melatonin in
cyclosporine A-induced oxidative stress in rat liver. Int
Immunopharmacol. 5:1397–1405. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Shi S, He Z, Cai J and Qiu J: RNA
Interference targeting GRP75 decreases cisplatin resistance in
human lung adenocarcinoma cell. Zhongguo Fei Ai Za Zhi. 14:305–310.
2011.(In Chinese).
|