Introduction
Cervical carcinoma is the second most common type of
cancer among women worldwide. More than 370,000 women are diagnosed
annually with an overall survival rate of 40% (1). Of note, the high rate of mortality
has remained relatively static. Although the preventive uterine
cervical carcinoma (UCC) vaccine has come onto the market, it only
targets human papillomavirus (HPV) which is not the sole cause of
UCC. Therefore, the identification of new biological markers may be
useful for predicting patient outcome.
Annexins are a group of at least 13 structurally
related proteins that share a core domain containing four repeats
(eight for Annexin VI) of approximately 70 conserved amino acids
(2). Each of these repeats
contains Ca2+ and phospholipid-binding sites. The
N-terminus, specific for each annexin, contains phosphorylation
sites for protein kinase C (PKC) as well as for other kinases.
Annexins have been associated with a wide variety of functions,
including membrane organization, exocytosis, endocytosis, ion
channel regulation, ion channel activity and
membrane-to-cytoskeleton linkage. In contrast with the others,
annexin A5 (ANXA5), which is more often considered as a major
intracellular Ca2+-binding protein, has a very short
N-terminus which is not phosphorylated. It is known for inducing
Ca2+ influx by forming Ca2+ channels
(3) and for inhibiting the
activities of enzymes linked to Ca2+ activation,
including phospholipase A2 and PKC (4). ANXA5 has thus been suggested to
mediate Ca2+ signaling, cell cycle regulation, signal
transduction, membrane trafficking and organization (5). It also binds to negatively charged
phosphatidylserine (PS) with high affinity in the presence of
Ca2+ ions. It has been proposed that the binding of
ANXA5 to PS accounts for its anticoagulant (6), anti-apoptotic (7) and anti-inflammatory (8) effects.
In the present study, using reverse
transcription-polymerase chain reaction (RT-PCR), western blot
analysis and immunohistochemistry, we found that the ANXA5
expression levels in uterine cervical squamous cell carcinomas
(UCSCCs) were significantly higher than those in normal cervical
tissues. We also found that ANXA5 levels in the blood serum of
patients with UCSCC were much higher than those of the control
group with a higher positive ratio than that of squamous cell
carcinoma antigen (SCCAg). The levels of ANXA5 and SCCAg were
determined to have a positive correlation by relativity analysis.
The results from our study demonstrate that ANXA5 is significantly
overexpressed in human UCSCC and that it is closely associated with
histological differentiation, which may have implications for the
diagnosis and prognosis of UCSCC.
Materials and methods
Tissue samples
Surgical tissue specimens from 60 patients with
UCSCC who consecutively underwent resection of their tumors at the
Affiliated Hospital of Chengde Medical College, Chengde, China,
were obtained for this prospective study, following institutional
review board guidelines. Informed consent was obtained from each
patient. None of them had received radiochemotherapy prior to
surgical intervention. A total of 25 clinically normal cervical
tissues were also collected. All tissue samples were stored at
−80°C until analysis. A portion of the surgical tissue specimen was
fixed in 4% buffered formaldehyde for use in immunohistochemical
staining and the other portion was prepared for use in RT-PCR and
western blot assays.
Among the UCSCCs with stage I-II disease, 18 (30%)
were well differentiated, 26 (43.33%) moderately differentiated and
16 (26.66%) poorly differentiated. The mean age of the patients was
57.3 (median). The stage of disease was determined following the
surgical resection of the tumor according to the current
tumor-node-metastasis staging (TNM) system of the International
Union Against Cancer. The histological grade was determined
according to the degree of differentiation of the tumor (Broders’
classification).
Apparatus and reagents
The ANXA5 mouse monoclonal antibody was purchased
from Sigma (St. Louis, MO, USA); the TRIzol reagent, enzyme-linked
immunosorbent assay (ELISA) kit and protein assay kit were
purchased from Invitrogen Life Technologies (Carlsbad, CA, USA);
and the gel image analysis system and electrophoresis apparatus
were purchased from Bio-Rad (Hercules, CA, USA). The digital image
analysis system was obtained from Nikon (Tokyo, Japan) and the
ultraviolet analyzer was from Beckman Coulter (Miami, FL, USA).
Immunohistochemical staining
The formalin-fixed, paraffin-embedded tissues were
cut into 5-μm sections. The sections were deparaffinized with
standard xylene and hydrated through decreasing concentrations of
ethanol. Endogenous peroxidase activity was blocked by incubation
with 3% (wt/vol) H2O2 for 10 min and then
antigen retrieval was achieved by microwave treatment. The tissue
samples were stained via an immunohistochemical method according to
conventional staining procedures. Negative controls were run
synchronously. For the negative control, phosphate buffer was
substituted for the primary antibody. After blocking with serum,
ANXA5 mouse anti-human monoclonal antibodies were applied at a
dilution of 1:50 and the samples were incubated overnight at 4°C.
The samples were rinsed three times with PBS (pH 7.2) for 5 min
each, incubated with biotin-labeled goat anti-mouse IgG for 30 min
at room temperature, rinsed again and then incubated with
horseradish peroxidase (HRP)-conjugated streptavidin for 30 min at
37°C. Finally, the sections were rinsed and stained with a DAB
system kit. The semi-quantitative assay was conducted under a high
power lens (x400) with integration of the staining intensity and
the staining distribution. The ANXA5 immunohistochemical staining
score was assessed using MiVnt image analysis software.
Western blot analysis
The tissues were homogenized in a single detergent
lysis buffer (50 mM Tris, pH 8.0; 150 mM NaCl; 1% Triton X-100; and
0.5% each of protease and phosphatase inhibitor cocktails) and then
centrifuged at 12,000 × g for 20 min at 4°C. The supernatants were
transferred into new tubes, assayed for protein content using a
protein assay kit, aliquoted at a concentration of 5 μg/20 μl in
lysis buffer and stored at −80°C. Alternatively, they were used on
the same day. The samples were mixed with loading buffer (100 mM
Tris, pH 6.8; 200 mM DTT; 4% SDS; 20% glycerol; and 0.2%
bromophenol blue) at a 1:1 dilution, boiled for 5 min, quickly
chilled on ice and then separated on 10% SDS-polyacrylamide
Tris-glycine gels. The proteins were then transferred onto
polyvinylidene difluoride membranes and treated with 5% non-fat dry
milk in 1X phosphate-buffered saline-Tween (PBST) (1.46 mM
NaH2PO4H2O; 8.05 mM
Na2HPO4; and 144.72 mM NaCl; 5% Tween-20) for
2 h. The membranes were reacted with mouse anti-ANXA5 monoclonal
antibodies at 1:1,000 dilution overnight at 4°C and then reacted
with HRP-conjugated goat anti-mouse antibodies for 1 h at room
temperature. The bound antibodies were detected by
chemiluminescence according to the manufacturer’s instructions and
quantified using a digital image analysis system. All experiments
were performed three times in triplicate.
RNA extraction and cDNA synthesis
Total RNA was prepared using the TRIzol reagent
according to the manufacturer’s instructions. RNA was treated with
reverse transcriptase in the presence of 50 μM primers in 20 μl RT
buffer (1X Superscript II RT buffer, 10 mM DTT and 0.025 mM dNTP)at
25°C for 5 min, followed by 50°C for 50 min. Reverse transcriptase
was inactivated at 70°C for 15 min.
RT-PCR
Total RNA was isolated from the cervical cancer or
normal tissues with the TRIzol reagent kit according to the
manufacturer’s instructions. RNA (2 μg) was reverse-transcribed
with 10,000 units of reverse transcriptase and 0.5 μg/μl oligo-(dT)
primer. PCR amplification of the cDNA aliquots was performed with
the following sense and antisense primers: ANXA5 forward, 5′-ATG
GCA CAG GTT CTC AGA GGC ACTG-3′ and reverse, 5′-TTA GTC ATC TTC TCC
ACA GAG CAGC-3′. PCR was performed in 50 μl of 10 mM Tris-HCl (pH
8.3), 25 mM MgCl2, 10 mM dNTP, 100 units Taq DNA
polymerase and 0.1 μM of each primer. The reactions were initiated
at 94°C for 5 min and amplified for 30 cycles of 30 sec at 94°C, 30
sec at 58°C and 30 sec at 72°C. Final extensions were performed for
7 min at 72°C to complete the polymerization. The PCR products were
resolved on a 1% agarose gel and analyzed using a digital image
analysis system. All experiments were performed at least three
times, with similar results.
ELISA
Blood samples were immersed in ice and transported
immediately to a biosafety level-II laboratory for processing.
Plasma was separated by centrifugation (2,000 × g for 10 min) at
4°C. The serum was extracted and stored in 300 μl aliquots at −70°C
until analysis. The wells of 96-well Polysorb ELISA plates were
coated with 50 ng ANXA5 or SCCAg in bicarbonate buffer (0.1 M, pH
9.6). Serum samples were thawed at room temperature and 50 ml
patient serum or standard (0; 0.025; 0.050; 0.075; 0.1; 0.3; 1 and
2.5 mg/ml) were incubated with biotinylated primary antibody
(dilution: 1/1000 in PBS; 0.05% Tween-20; and 1% BSA) overnight at
4°C. The plates were washed and then incubated with blocking buffer
(PBS buffer pH 7.4, 3% BSA) for 1 h at room temperature. After
washing, the sample/antibody mixtures were added and the plates
were incubated for 4 h at room temperature. The plates were washed
again and incubated with streptavidin-HRP (dilution: 1/5000 in PBS
pH 7.4; 0.05% Tween-20; and 1% BSA) for 1 h at room temperature.
After washing, the plates were incubated with the HRP substrate
3,3′,5,5′-tetramethylbenzidine (TMB). The reaction was stopped with
1 N HCl and the plates were then read at 450 nm in a microplate
reader. The optical density at 570 nm (background) was subtracted.
The sample concentrations were determined using a standard curve
(fit type: four parameter logistic).
Statistical analysis
The western blot and RT-PCR bands were analyzed
using Bandscan 5.0 software. The levels of ANXA5 and SCCAg in the
cervical cancer patients and normal women were analyzed using a
t-test which was also employed to analyze the expression of ANXA5
in the cervical cancer and normal tissues. For the comparison of
ANXA5 expression levels between the different groups, the one-way
analysis of variance test was used. The correlation between the
serum concentrations of ANXA5 and SCCAg was analyzed using the
Pearson correlation co-efficient. All data are expressed as the
means ± standard deviation. P<0.05 was assumed to indicate a
statistically significant difference.
Results
Expression of ANXA5 protein increased in
UCSCCs
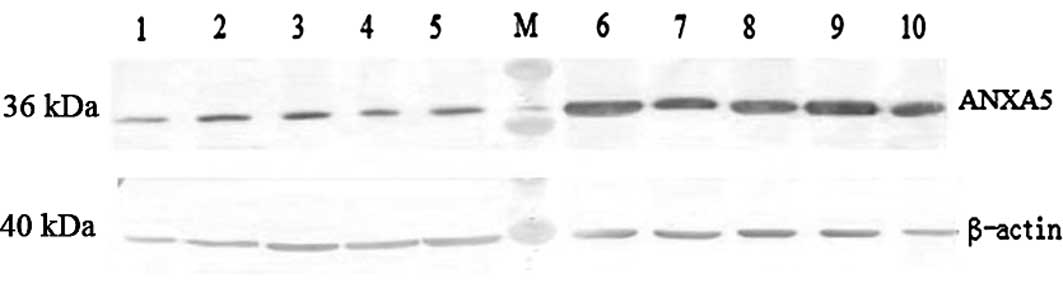
As shown in Fig. 1,
ANXA5 protein expression in the 25 normal cervical tissues and the
tumor tissues from 60 patients with primary UCSCC was analyzed
using a commercially available mouse monoclonal antibody against
ANXA5. The expression of ANXA5 was normalized using β-actin
protein. High expression levels of ANXA5 were detected in 48 of the
UCSCC samples whereas only a weak expression of ANXA5 was observed
in the 25 normal uterine cervical tissues. The positive rate of
ANXA5 expression was 80%. The data obtained from each group
revealed that the expression of ANXA5 protein in the cervical
cancer tissues (0.4699±0.018) was significantly increased compared
with that in the normal cervical tissues (0.2023±0.022; P<
0.05).
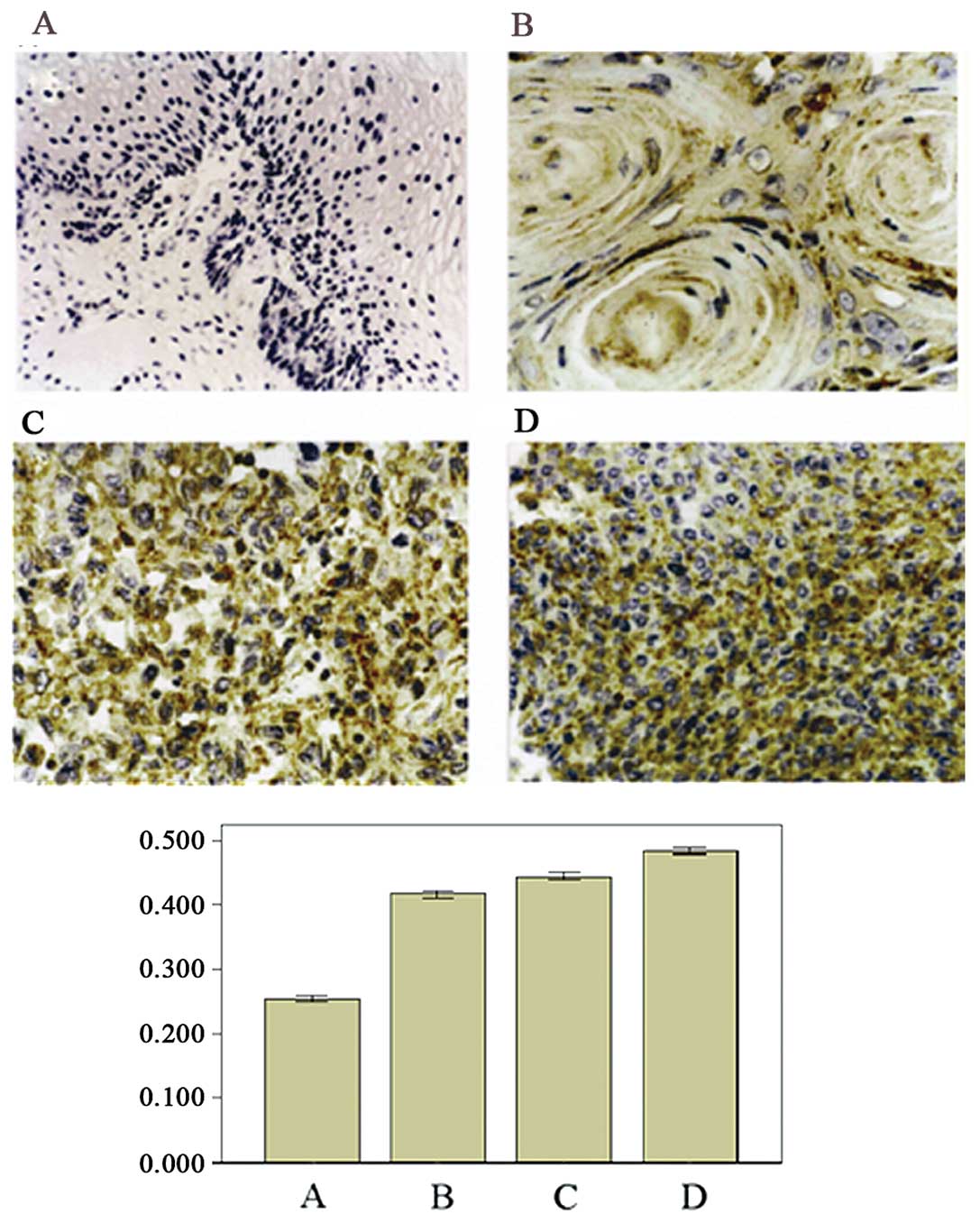
To confirm the results from the western blot
analysis and to investigate the presence of ANXA5 protein
expression in human UCC tissues, ANXA5 expression was further
examined by immunohistochemical staining. Compared with the normal
epithelia, the UCSCC specimens had a markedly increased ANXA5
expression. The ANXA5 staining was preferentially
cytoplasm-localized, although nuclear and membrane staining were
also noted in the superficial layers. ANXA5 expression was also
detected in the normal uterine cervical tissues with a weak
positive signal toward the basal cells and the most external layers
of the epithelia (Fig. 2).
ANXA5 expression correlates with
histological differentiation grade in UCSCC
From the immunohistochemical staining of the UCSCC
tissues, we found that ANXA5 expression correlated with the
histological differentiation grade of UCSCC. The images of the
immunohistochemical staining showed that from well differentiated
to moderately and poorly differentiated carcinoma, the number of
positive cells increased and the staining of the positive cells
became heavier (Fig. 3). The
positive cell ratio in the poorly differentiated group was the
highest (78.2±9.21%), while that in the moderately differentiated
group (51.86±7.62%) was the second highest and that in the well
differentiated group was the lowest (39.57±4.33%). The difference
between any two of the groups was significant (P<0.05).
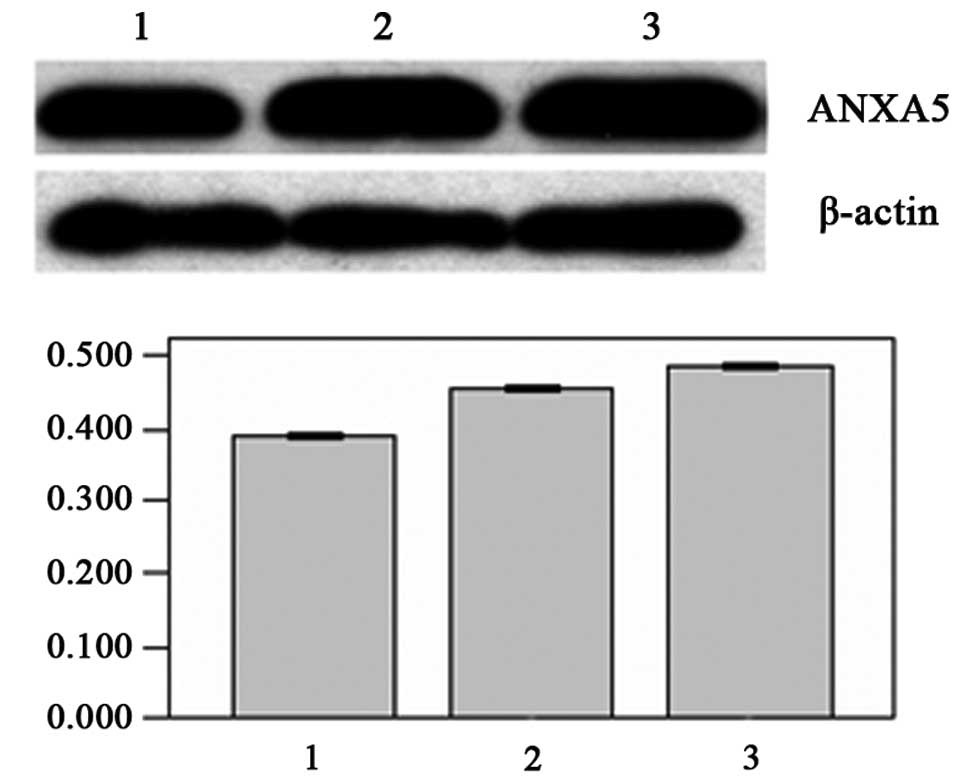
From the results of the western blot and RT-PCR
analyses, we also found that as the cells went from being well
differentiated to poorly differentiated, the expression levels of
the ANXA5 gene increased. The expression levels of the target gene
mRNA were highest in the poorly differentiated group
(2.9729±0.1056), which were 1.504-fold higher than the relative
expression levels of the normal group (1.9764±0.0899). The second
highest levels were in the moderately differentiated group
(2.8900±0.0552) and the lowest were in the well differentiated
group (2.2857±0.1213; P<0.05; Fig.
4). Moreover, the expression level of the ANXA5 protein in the
well differentiated group was the lowest (0.3929±0.0108), while the
expression levels of the target protein in the moderately and
poorly differentiated groups (0.4622±0.1939 and 0.4859±0.1567,
respectively) were significantly increased (P<0.05; Fig. 5).
ANXA5 and SCCAg serum concentration
levels increase in UCSCC patients
The serum levels of soluble ANXA5 and SCCAg were
measured in the untreated UCSCC patients and the normal group using
an ELISA kit. Each assay was calibrated using a standard curve
according to the manufacturer’s instructions. Each sample was
measured in duplicate. The level of ANXA5 in the serum of the UCSCC
patients was 52.77±11.54 μg/ml while that in normal women was
42.10±11.21 μg/ml, which was a significant difference (P<0.05).
The concentration of SCCAg was 17.90±7.85 μg/ml in the serum of the
UCSCC patients and 12.87±2.35 μg/ml in normal women, which was also
a significant difference (P<0.05). The positive ratio of ANXA5
in the UCSCC patients was 83.33% while that of SCCAg was 54% which
indicates that the specificity of ANXA5 was much higher than that
of SCCAg.
Association of pre-treatment serum ANXA5
and SCCAg levels
SCCAg is a frequently used biomarker for squamous
cell carcinoma. To assess the correlation between ANXA5 and SCCAg,
we performed Pearson correlation co-efficient analysis. SCCAg was
taken as the independent variable and ANXA5 as the dependent
variable. The linear regression equation was built as Ŷ=6.901+2.52X
and the result demonstrated that in the UCSCC group, ANXA5 and
SCCAg had a significant positive correlation (r=0.764,
P=0.010).
Discussion
Much of the complex fundamental biology of UCSCC
remains poorly understood. As with other epithelial neoplasms,
UCSCC appears to evolve through a multistep process involving
biomolecular changes, ensuing premalignant lesions and consequent
invasive cancer (9). Thus,
epithelial carcinogenesis has been divided into three phases,
specifically initiation, promotion and progression, that involve
genetic alteration, dysregulated epithelial differentiation,
abnormal proliferation and altered regulatory effects associated
with the abnormal expression of cellular factors that regulate
growth and development. The identification of molecular alterations
associated with these events may yield insights into the mechanisms
of initiation and progression of neoplasia and provide new tools
for diagnosis, treatment and prevention
It has previously been reported that annexin A1 is
able to induce differentiation in the U937 human histiocytic
lymphoma cell line (10) and
increasing numbers of annexin family members have been found to be
involved in tumorigenesis. Based on structural characteristics,
annexins have a number of physiological functions and have been
confirmed to participate in numerous physiological processes. Thus,
researchers have shown a keen interest in studying the role of
annexins in the pathogenesis of cancer. However, it appears that
the expression of different annexins is variable in various cancers
and that annexins play different roles in the course of genesis of
different cancers, which makes it difficult to associate their
physiological functions with their behaviors in certain specific
physiological processes. For example, in many cancers, including
colorectal (11), gastric
carcinoma (12), hepatic (13), lung (14) and pancreatic carcinoma (15), there is a sharp upregulation of
several members of the annexin family at the mRNA and protein
levels. However, studies have also demonstrated that in prostate
cancer the levels of annexin A7 and A2 mRNA and protein are reduced
(16). Although numerous
hypotheses have been proposed to explain how annexins function in
the genesis of different cancers, few of these hypotheses have been
confirmed directly.
As a member of the annexin family, the association
between tumors and ANXA5 is unclear. However, ANXA5 is highly
expressed in human pituitary adenoma and growth hormone-secreting
carcinoma (17) and is regarded to
be one of the biomarkers in cutaneous squamous cell carcinoma due
to its upregulated expression (18). In addition, ANXA5 is able to
regulate the invasive capacity of oral carcinoma cells (19). As regards UCC, over a decade ago,
Karube et al (20) reported
that the production of ANXA5 was suppressed in carcinoma of the
uterine cervix, whereas our study found that the expression of
ANXA5 was increased in UCSCC at the protein and mRNA levels. This
is in accordance with a study by Bae et al (21), who reported that the expression of
ANXA5 was upregulated in human ICC using a 2D method. Furthermore,
we detected that ANXA5 expression increased as the dysplastic
epithelium became less well differentiated, which indicates that
ANXA5 is associated with the differentiation of human carcinoma
cells, as has been identified in human colon adenocarcinoma cells
(22). Xue et al (23) reported that the expression of ANXA5
is associated with higher tumor stage and poor prognosis in
colorectal adenocarcinomas.
At the time of diagnosis, the disease stage is the
most important prognostic factor in the treatment of UCSCC and the
identification and early treatment of small cancers correlate with
excellent survival statistics. The ability to identify clinically
important therapeutic targets or biomarkers for the early detection
of cancer will ultimately rely on the consistency with which the
protein of interest changes with respect to the population norm.
Proteins with the best chances of clinical utility are those
proteins whose expression patterns vary consistently, not only
between different patients but also within the patient-matched
sets. This consistency is likely to reflect the most important
candidates for additional investigation in large validation
studies. The qualitative evaluation of biological features is
important for predicting the clinical course of a disease (24). Due to the histological diagnosis of
UCSCC being based, until now, on the traditional examination of
hematoxylin and eosin-stained specimens, supplementary techniques
that use specific, disease-relevant markers may enable a more
objective assessment of human uterine cervix pathology. Our
findings clearly identify ANXA5 as an effective differentiation
marker, providing the first demonstration of the potential utility
of ANXA5 immunostaining for the histopathological grading of UCSCC
and for the detection of epithelial dysplasia.
Previous studies have shown that biomarkers,
including Ki-67, p53, p16ink4 and SCCAg, have some specificity for
the diagnosis of UCC. However, each of them has certain
limitations. To further determine the potential of ANXA5 as a
diagnostic biomarker for UCSCC, we examined the levels of ANXA5 and
SCCAg in the serum of the patients. The ANXA5 positive ratio was
much higher than that of SCCAg and the two indexes had a positive
correlation, indicating that ANXA5 could not only be used as a
UCSCC biomarker by detecting serum levels but that it could also be
detected with SCCAg to increase the positive diagnosis ratio.
Our results provide evidence for the increased
expression of ANXA5 protein in UCSCC development. The high
frequency of ANXA5 upregulation in UCSCC tumors (48 out of 60
cases) suggests that ANXA5 is fundamentally important in human
tumorigenesis. Further experiments are required to establish the
pathogenic role of ANXA5 in UCSCC.
References
|
1
|
Greenlee RT, Hill-Harmon MB, Murray T and
Thun M: Cancer statistics. CA Cancer J Clin. 51:15–36. 2001.
|
|
2
|
Mortimer JC, Laohavisit A, Macpherson N,
Webb A, Brownlee C, Battey NH and Davies JM: Annexins:
multifunctional components of growth and adaptation. J Exp Bot.
59:533–544. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Camors E, Monceau V and Charlemagne D:
Annexins and Ca2+ handling in the heart. Cardiovasc Res.
65:793–802. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Russo-Marie F: Annexin V and phospholipid
metabolism. Clin Chem Lab Med. 37:287–291. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Gerke V and Moss SE: Annexins: from
structure to function. Physiol Rev. 82:331–371. 2002.PubMed/NCBI
|
|
6
|
Rand JH, Wu XX, Quinn AS and Taatjes DJ:
The annexin A5- mediated pathogenic mechanism in the
antiphospholipid syndrome: role in pregnancy losses and thrombosis.
Lupus. 19:460–469. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kenis H, van Genderen H, Deckers NM, Lux
PA, Hofstra L, Narula J and Reutelingsperger CP: Annexin A5
inhibits engulfment through internalization of PS-expressing cell
membrane patches. Exp Cell Res. 312:719–726. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kim DM, Noh HB, Park DS, Ryu SH, Koo JS
and Shim YB: Immunosensors for detection of Annexin II and MUC5AC
for early diagnosis of lung cancer. Biosens Bioelectron.
25:456–462. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Califano J, van der Riet P, Westra W, et
al: Genetic progression model for head and neck cancer:
implications for field cancerization. Cancer Res. 56:2488–2492.
1996.PubMed/NCBI
|
|
10
|
Hattori T, Hoffman T and Hirata F:
Differentiation of a histiocytic lymphoma cell line by lipomodulin,
a phospholipase inhibitory protein. Biochem Biophys Res Commun.
111:551–559. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Duncan R, Carpenter B, Main LC, Telfer C
and Murray GI: Characterisation and protein expression profiling of
annexins in colorectal cancer. Br J Cancer. 98:426–433. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kim JK, Kim PJ, Jung KH, et al: Decreased
expression of annexin A10 in gastric cancer and its overexpression
in tumor cell growth suppression. Oncol Rep. 24:607–612.
2010.PubMed/NCBI
|
|
13
|
Zhao P, Zhang W, Tang J, et al: Annexin II
promotes invasion and migration of human hepatocellular carcinoma
cells in vitro via its interaction with HAb18G/CD147. Cancer Sci.
101:387–395. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Liu YF, Xiao ZQ, Li MX, et al:
Quantitative proteome analysis reveals annexin A3 as a novel
biomarker in lung adenocarcinoma. J Pathol. 217:54–64. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhang Z, Huang L, Zhao W and Rigas B:
Annexin 1 induced by anti-inflammatory drugs binds to NF-kappaB and
inhibits its activation: anticancer effects in vitro and in vivo.
Cancer Res. 70:2379–2388. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Torosyan Y, Dobi A, Glasman M, et al: Role
of multi-hnRNP nuclear complex in regulation of tumor suppressor
ANXA7 in prostate cancer cells. Oncogene. 29:2457–2466. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Mulla A, Christian HC, Solito E, Mendoza
N, Morris JF and Buckingham JC: Expression, subcellular
localization and phosphorylation status of annexins 1 and 5 in
human pituitary adenomas and a growth hormone-secreting carcinoma.
Clin Endocrinol (Oxford). 60:107–119. 2004. View Article : Google Scholar
|
|
18
|
Dooley TP, Reddy SP, Wilborn TW and Davis
RL: Biomarkers of human cutaneous squamous cell carcinoma from
tissues and cell lines identified by DNA microarrays and qRT-PCR.
Biochem Biophys Res Commun. 306:1026–1036. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wehder L, Arndt S, Murzik U, Bosserhoff
AK, Kob R, von Eggeling F and Melle C: Annexin A5 is involved in
migration and invasion of oral carcinoma. Cell Cycle. 8:1552–1558.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Karube A, Shidara Y, Hayasaka K, Maki M
and Tanaka T: Suppression of calphobidin I (CPB I) production in
carcinoma of uterine cervix and endometrium. Gynecol Oncol.
58:295–300. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Bae SM, Min HJ, Ding GH, et al: Protein
expression profile using two-dimensional gel analysis in squamous
cervical cancer patients. Cancer Res Treat. 38:99–107. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Guzmán-Aránguez A, Olmo N, Turnay J,
Lecona E, Pérez-Ramos P, López de Silanes I and Lizarbe MA:
Differentiation of human colon adenocarcinoma cells alters the
expression and intracellular localization of annexins A1, A2, and
A5. J Cell Bioche. 94:178–193. 2005.PubMed/NCBI
|
|
23
|
Xue G, Hao LQ, Ding FX, et al: Expression
of annexin a5 is associated with higher tumor stage and poor
prognosis in colorectal adenocarcinomas. J Clin Gastroenterol.
43:831–837. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lomnytska MI, Becker S, Hellman K, et al:
Diagnostic protein marker patterns in squamous cervical cancer.
Proteomics Clin Appl. 4:17–31. 2010. View Article : Google Scholar : PubMed/NCBI
|