Introduction
One of the most important steps that limits
transfection efficiency in non-viral gene delivery is the entry of
nucleic acids across various membrane barriers and eventually into
the nucleus where transcription occurs (1,2).
Therefore, studies have focused on increasing transfection
efficiencies by passing through the nuclear pore (3). Yet, despite significant
diversification of gene delivery strategies, efforts to expand the
chemicals amenable to DNA-mediated gene transfer continue to
stumble over a recurrent obstacle, the nuclear pore. Different
methods have been used to deliver plasmid DNA across nuclear
membranes.
An alternative approach to improve transfection
efficiency is to incorporate penetration enhancers in formulations
(4). New and effective
transfection enhancers (non-ionic surfactants, bile salts and
menthol) have been used to increase gene transfer efficiency
(5). Another encouraging method to
increase transfection efficiency is to disturb the cell cycle which
significantly affects transfection efficiency. Cells treated with
dimethyl sulfoxide (DMSO) disturb the hydrophobic function of the
nuclear pore (6,7). Brunner et al demonstrated that
the addition of DMSO affects the transfer of plasmid DNA by
influencing the expression of genes related to the cell cycle and
cell hydrophobic properties (8).
DMSO has been commonly used for a number of years,
not only for laboratory, but also for clinical purposes. In recent
studies, DMSO was used as an efficient penetration enhancer for
gene transfer expression (9–11).
Li et al(12) revealed that
the transfection of exogenous DNA incubated with DMSO was more
efficient than without any treatment.
It has been found that menthol acts as a penetration
enhancer by passing through the membrane (13,14).
Menthol has been extensively used in many aspects of pharmaceutical
preparation. However, to the best of our knowledge, no report has
described menthol as a penetration enhancer for the gene delivery
system (4).
The exact conditions of how the chemicals, DMSO and
menthol, successfully enhance gene transfer efficiency remain
unclear. Unless plasmids enter the nucleus, they cannot be
transcribed (15). Our study
presents a novel approach that allows the expression of plasmid DNA
in the Bcap-37 human breast cancer cell line after treatment with
DMSO and menthol for the purposes of transgene expression. We aimed
to explore the ability of DMSO to deliver genes into the nucleus.
We also examined the ability of menthol to enhance the permeation
of plasmid DNA. The aim of our study was to evaluate the safety and
efficacy of using DMSO and menthol as permeability enhancers in
gene delivery systems.
Materials and methods
Plasmid DNA and cell line
Plasmid DNA pAC-GFP-N1 was kindly provided by the
College of Life Sciences at the Nanjing Agricultural University
(Nanjing, China). Plasmid DNA pGN was kept in our laboratory.
Information regarding the vector map and basic description is shown
in Fig. 1. Plasmid DNA was
amplified using Escherichia coli DH5a and purified using the
E.Z.N.A.® Endo-Free Plasmid Mini kit I (Omega,
Norcross, GA, USA).
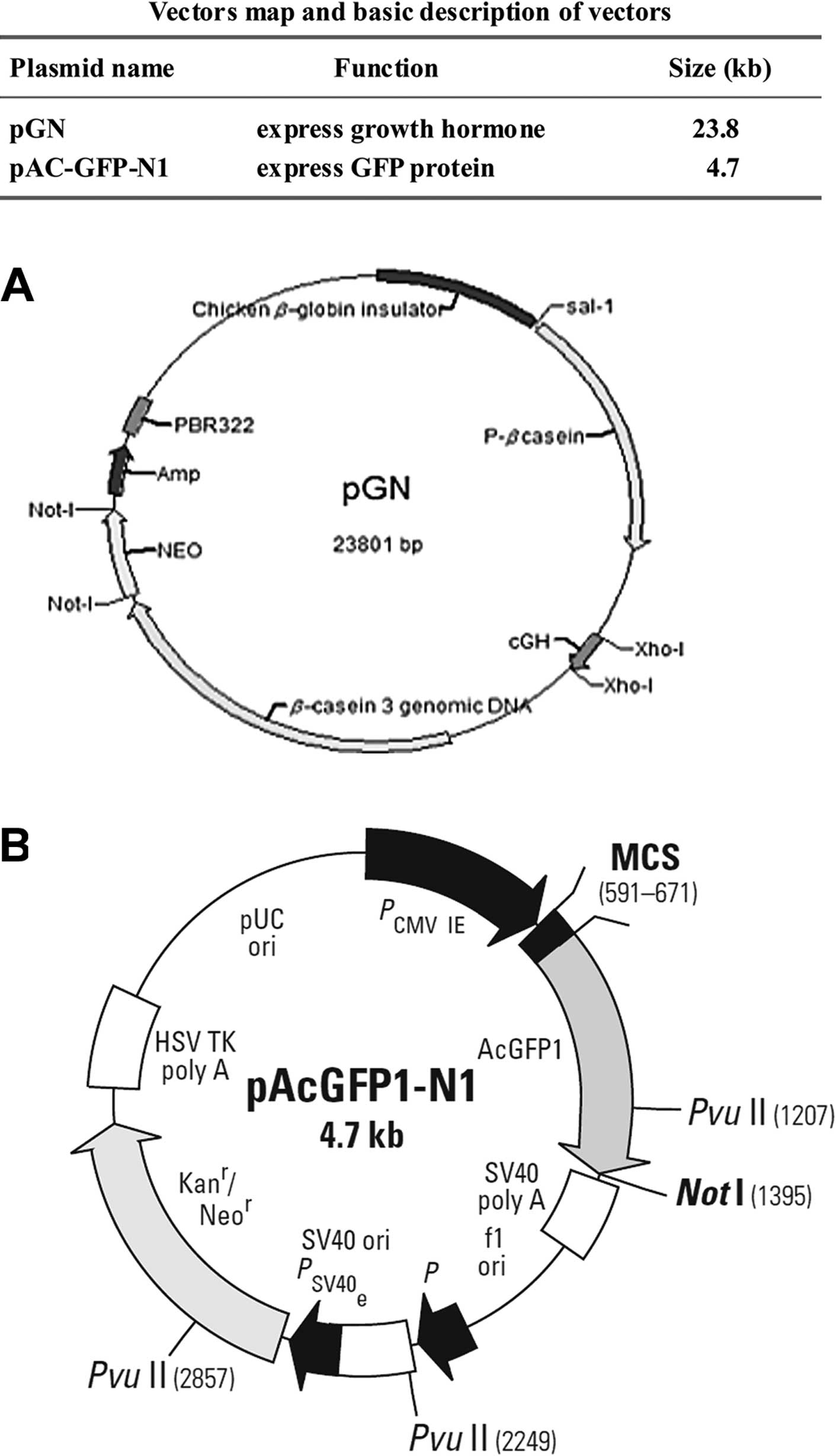 | Figure 1Vector map and basic description of
vectors. (A) The pGN plasmid was constructed by our laboratory for
the specific expression of growth hormone in the mammary gland to
increase milk production. The pGN plasmid was constructed under the
bone plasmid, pBC1, which was a 21.6-kb vector designed to
facilitate the expression of recombinant proteins in the milk of
transgenic animals. Successful expression of recombinant protein in
transgenic mice has generally been indicative of successful
expression in larger animals, such as goats or cows. The pGN
plasmid was a 23.8-kb vector which was difficult to be transfected
in producing transgenic animals. We searched for a transfection
enhancing agent and the pGN plasmid was used to evaluate the
transfection efficiency. (B) pAcGFP1-N1 encodes green fluorescent
protein (GFP) from Aequorea coerulescens (excitation
maximum, 475 nm; emission maximum, 505 nm). The coding sequence of
the AcGFP1 gene contains silent base changes, which correspond to
human codon-usage preferences. The MCS in pAcGFP1-N1 is between the
immediate early promoter of CMV (PCMV IE) and the AcGFP1 coding
sequences. Genes cloned into the multiple cloning site (MCS) will
be expressed as fusions to the N-terminus of AcGFP1 if they are in
the same reading frame as AcGFP1, and there are no intervening stop
codons. SV40 polyadenylation signals downstream of the AcGFP1 gene
direct proper processing of the 3′ end of the AcGFP1 mRNA. The
vector backbone also contains an SV40 origin for replication in
mammalian cells expressing the SV40 T antigen. A
neomycin-resistance cassette (Neo′), consisting of the SV40 early
promoter, the neomycin/kanamycin resistance gene of Tn5 and
polyadenylation signals from the herpes simplex virus thymidine
kinase (HSV TK) gene, allows stably transfected eukaryotic cells to
be selected using G418. A bacterial promoter upstream of the gene
expresses kanamycin resistance in Escherichia coli (E.
coli). The pAcGFP1-N1 backbone also provides a pUC origin of
replication for propagation in E. coli and an f1 origin for
single-stranded DNA production. |
The Bcap-37 human breast cancer cell line
(ER−, p53 mutated) was purchased from the Shanghai Cell
Collection, CAS (Shanghai, China). Bcap-37 cells were cultured in
DMEM (Gibco) medium, supplemented with 10% fetal bovine serum
(Gibco) at 37°C in a humidified atmosphere of 5% CO2.
Succinimidyl-[4-(psoralen-8-yloxy)]-butyrate (SPB)
was purchased from Pierce. Peptide derivative SPB-NLS was
synthesized by Sangon Biotech (Shanghai, China) with the following
sequences: SPB-PKKKRKV.
In vitro cytotoxicity assays (MTT
assays)
The cytotoxic effects of DMSO (J&K) and menthol
(J&K) on the Bcap-37 cell line were evaluated by MTT assays.
Approximately 5,000 Bcap-37 cells per population were plated in a
flat-bottomed 96-well plate and incubated at 37°C for 24 h before
the assays. Preliminary studies were performed to define the
optimal concentration of DMSO and menthol which was suitable for
testing. DMSO, ranging from 0.5 to 10% (v/v), was added into the
Bcap-37 cells to evaluate the toxicity. After incubation with DMSO
for 48 h at 37°C in complete medium, the medium was removed and the
cells were rinsed with PBS 3 times, then 75 μl complete medium and
25 μl MTT (2 mg/ml in PBS) were added for another 4 h. After 4 h,
the medium was aspirated and replaced with 100 μl DMSO to dissolve
formazan crystals. Absorbance was measured at 540 nm, with
untreated cells serving as the control. The same experiments were
carried out to define the safe working concentration of menthol.
The concentration of menthol that was added into the cells to
evaluate the toxicity ranged from 12.5 to 400 μM.
Quantitative RT-PCR studies of
transfection efficiency
Bcap-37 cells were incubated at 37°C for 48 h before
transfection. Transfection was performed with the pGN plasmid
(Lipofectamine® 2000 Reagent; Invitrogen™) following the
instructions of the manufacturer. The primers used for detecting
growth hormone (GH) mRNA expression are listed in Table I. The cells were divided into 12
groups (Tables II and III). Two groups were separately treated
with 12.5 μM menthol and 2% DMSO 48 h before transfection.
SPB-NLS/DNA complexes were prepared 30 min before transfection.
Another 2 groups were separately treated with 2% DMSO and 12.5 μM
menthol 2 h after transfection. DMEM medium was replaced with
complete medium 6 h after transfection. GH mRNA expression was
analyzed by quantitative RT-PCR 48 h after transfection. All
transfection experiments were performed in triplicate.
 | Table IQuantitative RT-PCR primers used in
detecting the pGN plasmid. |
Table I
Quantitative RT-PCR primers used in
detecting the pGN plasmid.
| Gene | Sense primer | Anti-sense
primer | Product length
(bp) |
|---|
| GH |
gagaagctgaaggacctgga |
tacgtctccgtcttgtgcag | 194 |
| Bcap-37 β-actin |
gatcattgctcctcctgagc |
tgtggacttgggagaggact | 385 |
 | Table IIRelative GH mRNA expression in the
DMSO-treated groups. |
Table II
Relative GH mRNA expression in the
DMSO-treated groups.
| Treatment | Blank | Control
(liposome) | Liposome/pGN |
Liposome/SPB-NLS/pGN |
|---|
| Untreated | 1.039±0.349 | 2.182±0.329 | 11.006±1.909 | 47.648±4.620 |
| Pre-DMSO
treatment | | | 97.087±11.091 | 100.521±10.032 |
| Post-DMSO
treatment | | | 344.605±50.708 | 155.090±12.675 |
 | Table IIIRelative GH mRNA expression in the
menthol-treated groups. |
Table III
Relative GH mRNA expression in the
menthol-treated groups.
| Treatment | Blank | Control
(liposome) | Liposome/pGN |
Liposome/SPB-NLS/pGN |
|---|
| Untreated | 1.031±0.31 | 3.132±1.676 | 9.749±1.608 | 70.677±18.145 |
| Pre-menthol
treatment | | | 277.110±23.371 |
2,256.667±178.046 |
| Post-menthol
treatment | | | 98.183±10.666 | 30.560±3.057 |
Fluorescence microscopy studies of
transfection efficiency
After detecting the mRNA expression levels, we
evaluated protein expression efficiency in the DMSO- and
menthol-treated groups. The pAC-GFP-N1 plasmid was used to evaluate
the transgene expression efficiency. Bcap-37 cells were seeded at
10,000/well in a flat-bottomed 12-well plate 24 h prior to the
experiments. Transfection was performed as mentioned above. After
transfection, the cells were incubated for 48 h at 37°C for green
fluorescent protein (GFP) expression. After incubation, the cells
were washed twice with phosphate-buffered saline (PBS). Images were
obtained by standard fluorescence microscopy (Olympus).
Flow cytometry analysis of transfection
efficiency
As the results of fluorescence microscopy showed
improved GFP expression efficiency in the DMSO- and menthol-treated
groups, we aimed to calculate the increased percentage of
transfected cells in the different groups. The capacity of flow
cytometry for the rapid, individual analysis of a large number of
cells make it ideally adapted for the study of transfection
efficiency. Transfection was performed as mentioned above with the
pAC-GFP-N1 plasmid. Cells were harvested and washed 3 times by PBS
48 h after transfection. Finally, the harvested cells were
suspended in 500 μl cold PBS and examined by a FACSCalibur flow
cytometer, which was equipped with an argon laser (488 nm).
Flow cytometry analysis of cell
cycle
Previous studies have suggested that DMSO and
menthol treatments significantly increase gene delivery efficiency
(14,16). In this study, in order to determine
the mechanism by which the chemicals, DMSO and menthol, improve
gene transfer efficiency, we examined the cell cycle changes in the
different groups by flow cytometry. Bcap-37 cells were treated with
DMSO and menthol 48 h before the assays. The cells were harvested
and washed 3 times with PBS. The cells were then treated with RNase
A (5 μg/ml) for 10 min at room temperature, followed by staining
with propidium iodide (PI; 5 μg/ml), a DNA-binding dye for 2 h at
room temperature. Subsequently, the cells were subjected to flow
cytometry to analyze the cell cycle changes.
Statistical analysis
Statistical analyses of transfection efficiency and
cell cycle changes were carried out using one-way analysis of
variance (ANOVA). A P-value <0.05 denoted statistically
significant differences. All data are expressed as the means ±
standard error of the mean.
Results
In vitro cell viability
One of the major requirements for gene delivery is
low cytotoxicity. In order to define the safe working concentration
of DMSO and menthol for the Bcap-37 cells, we performed MTT assays.
The viability of Bcap-37 cells in the absence or presence of
various concentrations of DMSO is shown in Fig. 2A. The results showed that cell
viability was surpassed by >90% when the concentration of DMSO
was <1% (v/v). However, cell viability only exceeded 80% when
the concentration of DMSO was <2% (v/v), and significantly
decreased at higher concentrations of DMSO [2.5 and 5% (v/v)]. The
minimum cell viability of Bcap-37 cells was 17.2 in 10% DMSO. As
shown in Fig. 2A, among 8 tested
concentrations of DMSO, 2% (v/v) DMSO was the optimum working
concentration.
We also detected the working concentration of
menthol. The viabilities in the absence or presence of various
concentrations of menthol are shown in Fig. 2B. We observed a strange phenomenon:
cell viability was surpassed by >80% when the concentration of
menthol was <50 μM. Once the concentration of menthol was up to
100 μM, cell viability sharply decreased to <10% (Fig. 2B). According to the literature,
cell proliferation speed significantly decreases when cells are
treated with high concentrations of menthol (17). According to the afore-mentioned
results, we selected 2% DMSO and 12.5 μM menthol for our
experiments.
Transfection efficiency experiments
DMSO and menthol enhance GH mRNA
expression in SPB-NLS-meditated transfection
We hypothesized that a correlation existed between
the ability of penetration enhancers to wreck membranes under
natural conditions and penetrating efficiency. To analyze the
transfection efficiency of DMSO and menthol treatments, we carried
out transient transfection with the pGN plasmid in the Bcap-37 cell
line.
The results showed that the expression of GH mRNA in
the pre-DMSO treatment group was 10-fold higher than that in the
liposome/pGN group. Compared to the liposome/pGN group, the
post-DMSO treatment group showed a significantly enhanced
expression of GH mRNA (up to 30-fold). However, the
pre-DMSO/SPB-NLS co-treatment group showed no difference in GH mRNA
expression levels compared to the pre-DMSO treatment group (both
groups showed a 10-fold increase compared to the liposome/pGN
group), while the post-DMSO/SPB-NLS co-treatment group showed even
lower levels in GH mRNA expression compared to the post-DMSO
treatment group (Table II).
Similar results were also observed in the
menthol-treated groups. Compared to the liposome/pGN group, the
expression of GH mRNA was greatly increased in the post-menthol
treatment group (10-fold), and significantly enhanced in the
pre-menthol treatment group (30-fold). However, there were 2
differences compared to the DMSO-treated groups. One was that the
pre-menthol/SPB-NLS co-treatment group had significantly increased
GH mRNA expression levels (up to 200-fold compared to the
liposome/pGN group). The other was that menthol treatment only
showed high transcriptional activities in the pre-menthol treatment
group and the pre-menthol/SPB-NLS co-treatment group (Table III).
Fluorescence microscopy of
transfection efficiency
After detecting GH mRNA expression, we aimed to
evaluate the function of DMSO and menthol in improving protein
expression efficiency by fluorescence microscopy. Protein
expression efficiency was evaluated using the pAC-GFP-N1 plasmid,
which expressed GFP as a reporter protein. Normal cells were used
as the blank group. Cells transfected with the pGN plasmid were
used as the negative control group. The results showed that there
was no GFP expression in the blank and negative groups, while only
small areas of fluorescence were observed in the
liposome/pAC-GFP-N1 group (Fig.
3A). Compared to the liposome/pAC-GFP-N1 group, the expression
of GFP in the DMSO- and menthol-treated groups was much more
efficient (Fig. 3B).
Flow cytometry analysis of
transfection efficiency
Our main goal was to develop a convenient and
efficient delivery system for functional gene transfer. As the
fluorescence microscopy results demonstrated a high GFP expression
efficiency in the DMSO- and menthol-treated groups, we aimed to
calculate the increased percentage of transfected cells in the
different groups by flow cytometry. The flow cytometry results
showed that the positive percentages of the blank, control
(liposome) and negative groups (liposome/pGN) were 0.91, 1.32 and
0.87%, respectively. Compared to the 8.55% positive percentage in
the liposome/pAC-GFP-N1 group, the positive percentage in the
pre-DMSO treatment group (9.58%) and the post-DMSO treatment group
(10.91%) was significantly increased. However, the positive
percentages in the pre-menthol treatment group (7.9%) and the
post-menthol treatment group (7.44%) were slightly lower compared
to the liposome/pAC-GFP-N1 group (8.55%). These results were
consistent with those obtained by fluorescence microscopy. Data
analysis also implied that the highest transfection efficiency was
observed in the SPB-NLS/post-DMSO co-treatment group (15.81%),
which was significantly higher than that in the SPB-NLS/pre-menthol
co treatment group (12.97%) (Fig.
4).
Cell cycle analysis
Although high efficiency was demonstrated in gene
transfer, the mechanism by which DMSO and menthol improve gene
delivery remained unclear. Cell cycle synchronization was confirmed
to have a crucial role in gene delivery. The flow cytometry results
showed that there were significant differences in the cell cycle
between the blank group (G1 phase, 61.41%; G2 phase, 7.01%; S
phase, 31.58%) and the liposome group (G1 phase, 56.79%; G2 phase,
4.29%; S phase, 38.93%). All 3 phases of the cell cycle were
affected by liposome treatment (Table
IV). Data analysis showed that the cell cycle phases were G1
57.48%, G2 8.21% and S 34.31% in the menthol-treated group, and G1
71.41%, G2 9.14% and S 19.45% in the DMSO-treated group, which were
significantly different from the blank group. DMSO and menthol
exposure resulted in an alteration in the percentage of nuclei,
mainly in the S and G1 phases. A significant increase in the
percentage of nuclei in the S phase and a significant decrease in
the percentage of nuclei in the G1 phase occurred following DMSO
treatment. Menthol treatment only led to a significant increase in
the percentage of nuclei in the G1 phase and a significant decrease
in the percentage of nuclei in the S phase. It should also be noted
that the significant changes in the percentage of nuclei in the G1
and S phases was observed at the working concentration of menthol
and DMSO, without altering the G2 phase. Previous studies using
synchronized cells showed that cells expressed between 50- and
3,000-fold more gene product when transfected in the G2 or G2-M
phase as compared to those transfected in the G1 phase (8,18).
 | Table IVCell cycle analysis of the different
treatments. |
Table IV
Cell cycle analysis of the different
treatments.
| Treatment | G1 phase (%) | S phase (%) | G2 phase (%) | Apoptosis |
|---|
| Blank | 61.41±0.42 | 31.58±0.89 | 7.01±0.47 | 1.11±0.29 |
| Liposome/pGN | 56.79±0.87 | 38.93±2.30 | 4.29±1.43 | 2.21±0.59 |
| DMSO | 71.41±1.33 | 19.45±1.14 | 9.14±0.24 | 1.40±0.10 |
| Menthol | 57.48±0.79 | 34.31±1.45 | 8.21±0.76 | 0.22±0.05 |
Discussion
Previously, methods of in vitro transfection
of different cells were limited to electric transfection (19), liposome-mediated(4) or retroviral infection (20). In this study, our main goal was to
develop a convenient and efficient delivery system for functional
gene delivery. Our results prove that the addition of DMSO and
menthol enhances liposome-mediated transfection efficiency in
Bcap-37 cells. Furthermore, we found that transfection efficiency
in the post-DMSO treatment group (21) had improved compared to that in the
pre-DMSO treatment group, while the efficiency of gene transfer in
the pre-menthol treatment group was extremely higher compared to
that in the post-menthol treatment group. In addition, we confirmed
that gene delivery efficiency in the DMSO/SPB-NLS and
menthol/SPB-NLS groups had greatly improved compared to that in the
DMSO- or menthol-treated groups. Although peptide derivative
SPB-NLS has been shown to be an effective transfection reagent
in vitro (data not shown), it has never been used in
conjunction with DMSO or menthol. DMSO and menthol have been shown
to improve the transfection efficiency of plasmid DNA by increasing
the permeability of cell membranes (22), the integrity of the nucleus
(23) or by affecting cell cycle
synchronization (24,25).
Quantitative evaluation of gene
transfer
Quantitative RT-PCR was conducted to evaluate the
transfection efficiency of the different treatments (DMSO and
menthol). The results revealed that the expression of GH mRNA in
the DMSO- and menthol-treated groups was significantly higher than
that in the liposome-mediated group. This result was consistent
with the study by Jain and Gewirtz (16). Our results clearly demonstrated
that the post-DMSO treatment group expressed much more GH mRNA than
the pre-DMSO treatment group, which suggests, as also shown by a
previous study, that DMSO affects the nuclear pore in gene transfer
(23). Taken together, our results
show that post-DMSO treatment is most effective in enhancing gene
delivery. Of note, the DMSO/SPB-NLS co-treatment was less efficient
in enhancing gene transfer compared to the post-DMSO treatment
group. Although the exact mechanism of the DMSO-mediated gene
delivery remains unknown, our results clearly demonstrate that the
addition of DMSO influences the transfection efficiency. We
speculated, as also shown in a previous study, that pre-DMSO
treatment may increase transfection efficiency by regulating the
gene controlled cell cycle and cell hydrophobic properties
(26), while post-DMSO treatment
may improve transfection efficiency by influencing the formation of
the nuclear pore.
Similar results were also found in the
menthol-treated groups. What was different from the DMSO-treated
groups was that pre-menthol treatment had more significant results
compared to post-menthol treatment concerning GH mRNA expression.
Another difference was that the menthol/SPB-NLS co-treatment group
achieved supreme transfer efficiency. As for the reason why
menthol/SPB-NLS co-treatment had such an impact on transfection
efficiency, it remains to be further investigated.
Quantitative estimation of gene
expression
After we investigated the function of DMSO and
menthol in improving GH mRNA expression, we evaluated protein (GFP)
expression efficiency in the DMSO- and menthol-treated groups by
fluorescence microscopy and flow cytometry. Flow cytometry
measurements made every accurate determination of fluorescence
intensities feasible. The results from both analyses showed that
DMSO and menthol treatments increased GFP expression efficiency.
DMSO and menthol treatments only slightly improved the expression
of GFP. These results are completely different from those obtained
by qRT-PCR. The reason behind this phenomenon may be the following:
the slight improvement in GFP expression in the DMSO- and
menthol-treated groups implied that DMSO and menthol treatments may
affect cell viability, which in turn limits gene expression.
We also found that the post-DMSO/SPB-NLS
co-treatment group achieved the highest GFP expression efficiency
(increased by 90%; flow cytometry). Similarly, the
pre-menthol/SPB-NLS co-treatment group also obtained a high
efficiency in GFP expression (increased by 52%; flow cytometry).
Notably, high-level transcription efficiency (DMSO- and
menthol-treated group) did not always mean high-level expression
efficiency. Among all treatments, the post-DMSO/SPB-NLS and the
pre-menthol/SPB-NLS co-treatment groups not only showed high
transcription levels (GH mRNA expression levels), but also
displayed high translation levels (GFP expression levels).
Methods involved in improving gene
transfer efficiency
Apart from some isolated reports (27,28),
the majority of studies have aimed at increasing DNA nuclear import
by adding NLS-containing proteins or using novel polymers. There
has been relatively little success transforming information
gathered into more effective methods for DNA delivery in
vitro. We aimed to search for versatile solutions to improve
transfection efficiency. DMSO and menthol seemed to be a suitable
way to solve this problem. Previous studies using DMSO have shown
that this amphiphilic molecule affects transfection by influencing
the formation of the nuclear pore (6). DMSO also alters the cell cycle to
accomodate gene transfer. Cell cycle synchronization has been
confirmed to be a crucial part in gene delivery (29,30).
Previous studies using synchronized cells have shown that cells
express more gene product when transfected in the G2 or G2-M phase
(8,18). DNA microinjected into the nucleus
produces robust gene expression, while cytoplasm injection results
in low expression levels. Thus, if plasmids were presented in the
cytoplasm, they could enter into the nucleus when the envelope was
disrupted (30). It was well known
that non-dividing or growth-arrested cells cannot be easily
transfected. By contrast, cells undergoing mitosis are much more
receptive to transfection (31).
The results from the present study demonstrate that DMSO stops the
cell cycle during mitosis, which explains the high gene delivery
efficiency in the DMSO-treated groups.
Menthol is a naturally-occurring cyclic terpene
alcohol of plant origin, which has been frequently used since
antiquity for medicinal purposes. However, as a penetration
enhancer, the ability of menthol in improving gene delivery
efficiency has not been reported. Menthol may be used to increase
the permeability of cell membranes or to affect the integrity of
the nucleus (23). As detected in
previous experiments, the mechanism by which menthol enhances
transmembrane permeation may include the possible reversible
disruption of the intercellular lipid domain (20,21).
Although high transfection efficiency has been
demonstrated, issues related to the effects of DMSO or menthol on
transfection efficiency and the mechanisms have not been fully
addressed. The present investigation aimed to generate versatile
solutions to further improve transfection efficiency. It would be
hopeful to provide impetus for searching for more versatile
solutions in transferring plasmid DNA.
In conclusion, DMSO can increase the transfection
efficiency by influencing the pore integrity of the nucleus or
regulating the gene controlled cell cycle and cell hydrophobic
properties. Additionally, it is likely that menthol facilitates the
gene transfer efficiency by increasing the permeability of cell
membranes. Our results clearly demonstrate that pre-menthol and
post-DMSO treatments both improve gene delivery efficiency.
The pre-menthol and post-DMSO treatments are
developing techniques. We stronlgy believe that only versatile
transfection techniques would aid in the development of a
controlled release gene transfer method, unlike many of the
conventional nucleic acid carriers that are purely liposome-based.
Hopefully, our research will lead to a safer alternative to viral
systems for gene delivery.
Acknowledgements
This study was supported by grants from the National
Animal Transgenic Breeding Grand Project (nos. 2008ZX08008-004 and
2011ZX08008-004).
References
|
1
|
Lechardeur D and Lukacs GL: Intracellular
barriers to non-viral gene transfer. Curr Gene Ther. 2:183–194.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Leong KW, Mao HQ, Truong-Le VL, Roy K,
Walsh SM and August JT: DNA-polycation nanospheres as non-viral
gene delivery vehicles. J Control Release. 53:183–193. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kamiya H, Tsuchiya H, Yamazaki J and
Harashima H: Intracellular trafficking and transgene expression of
viral and non-viral gene vectors. Adv Drug Deliv Rev. 52:153–164.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zhou M, Liu H, Xu X, et al: Identification
of nuclear localization signal that governs nuclear import of BRD7
and its essential roles in inhibiting cell cycle progression. J
Cell Biochem. 98:920–930. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Man N, Yu L, Zheng F, Li Y and Wen LP:
Efficient gene transfer to rat fetal osteoblastic cells by
synthetic peptide vector system. Protein Pept Lett. 16:368–372.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Notman R, den Otter WK, Noro MG, Briels WJ
and Anwar J: The permeability enhancing mechanism of DMSO in
ceramide bilayers simulated by molecular dynamics. Biophys J.
93:2056–2068. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ribbeck K and Gorlich D: The permeability
barrier of nuclear pore complexes appears to operate via
hydrophobic exclusion. Embo J. 21:2664–2671. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Brunner S, Sauer T, Carotta S, Cotten M,
Saltik M and Wagner E: Cell cycle dependence of gene transfer by
lipoplex, polyplex and recombinant adenovirus. Gene Ther.
7:401–407. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Guillard EC, Laugel C and Baillet-Guffroy
A: Molecular interactions of penetration enhancers within ceramides
organization: a FTIR approach. Eur J Pharm Sci. 36:192–199. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hewitt PG, Poblete N, Wester RC, Maibach
HI and Shainhouse JZ: In vitro cutaneous disposition of a topical
diclofenac lotion in human skin: effect of a multi-dose regimen.
Pharm Res. 15:988–992. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Mittal A, Sara UV, Ali A and Aqil M: The
effect of penetration enhancers on permeation kinetics of
nitrendipine in two different skin models. Biol Pharm Bull.
31:1766–1772. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Li L, Shen W, Min L, Dong H, Sun Y and Pan
Q: Human lactoferrin transgenic rabbits produced efficiently using
dimethylsulfoxide-sperm-mediated gene transfer. Reprod Fertil Dev.
18:689–695. 2006. View
Article : Google Scholar
|
|
13
|
Brain KR, Green DM, Dykes PJ, Marks R and
Bola TS: The role of menthol in skin penetration from topical
formulations of ibuprofen 5% in vivo. Skin Pharmacol Physiol.
19:17–21. 2006.PubMed/NCBI
|
|
14
|
Ho HO, Chen LC, Lin HM and Sheu MT:
Penetration enhancement by menthol combined with a solubilization
effect in a mixed solvent system. J Control Release. 51:301–311.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Bremner KH, Seymour LW, Logan A and Read
ML: Factors influencing the ability of nuclear localization
sequence peptides to enhance nonviral gene delivery. Bioconjug
Chem. 15:152–161. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Jain PT and Gewirtz DA: Enhancement of
liposomal gene delivery in human breast cancer cells by dimethyl
sulfoxide. Int J Mol Med. 1:609–611. 1998.PubMed/NCBI
|
|
17
|
Fiore M, Zanier R and Degrassi F:
Reversible G(1) arrest by dimethyl sulfoxide as a new method to
synchronize Chinese hamster cells. Mutagenesis. 17:419–424. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Escriou V, Carriere M, Bussone F, Wils P
and Scherman D: Critical assessment of the nuclear import of
plasmid during cationic lipid-mediated gene transfer. J Gene Med.
3:179–187. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
19
|
Mir LM: Nucleic acids
electrotransfer-based gene therapy (electrogenetherapy): past,
current, and future. Mol Biotechnol. 43:167–176. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ramezani A and Hawley RG: Strategies to
insulate lentiviral vector-expressed transgenes. Methods Mol Biol.
614:77–100. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Heckert RA, Elankumaran S, Oshop GL and
Vakharia VN: A novel transcutaneous plasmid-dimethylsulfoxide
delivery technique for avian nucleic acid immunization. Vet Immunol
Immunopathol. 89:67–81. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Sultana Y, Jain R, Aqil M and Ali A:
Review of ocular drug delivery. Curr Drug Deliv. 3:207–217. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wang H, Zhong CY, Wu JF, Huang YB and Liu
CB: Enhancement of TAT cell membrane penetration efficiency by
dimethyl sulphoxide. J Control Release. 143:64–70. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Campeau P, Chapdelaine P, Seigneurin-Venin
S, Massie B and Tremblay JP: Transfection of large plasmids in
primary human myoblasts. Gene Ther. 8:1387–1394. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Migita S, Hanagata N, Tsuya D, Yamazaki T,
Sugimoto Y and Ikoma T: Transfection efficiency for size-separated
cells synchronized in cell cycle by microfluidic device. Biomed
Microdevices. 13:725–729. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Srinivas S, Sironmani TA and Shanmugam G:
Dimethyl sulfoxide inhibits the expression of early growth-response
genes and arrests fibroblasts at quiescence. Exp Cell Res.
196:279–286. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Collas P and Alestrom P: Rapid targeting
of plasmid DNA to zebrafish embryo nuclei by the nuclear
localization signal of SV40 T antigen. Mol Mar Biol Biotechnol.
6:48–58. 1997.PubMed/NCBI
|
|
28
|
Arenal A, Pimentel R, Garcia C, Pimentel E
and Alestrom P: The SV40 T antigen nuclear localization sequence
enhances nuclear import of vector DNA in embryos of a crustacean
(Litopenaeus schmitti). Gene. 337:71–77. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Medina-Kauwe LK, Xie J and Hamm-Alvarez S:
Intracellular trafficking of nonviral vectors. Gene Ther.
12:1734–1751. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Xavier J, Singh S, Dean DA, Rao NM and
Gopal V: Designed multi-domain protein as a carrier of nucleic
acids into cells. J Control Release. 133:154–160. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Dean DA, Strong DD and Zimmer WE: Nuclear
entry of nonviral vectors. Gene Ther. 12:881–890. 2005. View Article : Google Scholar : PubMed/NCBI
|


















