Introduction
Bladder cancer is a common malignancy of the
urologic system. Approximately 75% of new patients have
non-muscle-invasive bladder cancer, and transurethral resection of
bladder tumor (TURBt) is the standard treatment for those patients
(1). However, following surgery,
60–70% of the cancers will recur, with 25% exhibiting a higher
stage or grade, which remains a problem for surgeons (2). Therefore, intravesical chemotherapy
is widely used as an adjuvant treatment to prevent recurrence and
cancer progression following TURBt (3). However, the cytotoxicity and
incomplete efficacy of chemical agents limits their use as regular
intravesical drugs, even if they induce apoptosis of the cancer
cells (3). Thus, new intravesical
agents are urgently required for the clinical management of bladder
cancer.
Baicalein is a flavonoid derived from the root of
Scutellaria baicalensis, a plant widely used in Chinese
herbal medicine. Baicalein is a well-known inhibitor of
12-lipoxygenase (12-LOX) (4), the
expression of which correlates with tumorigenicity and tumor
progression (5,6). Previous studies have shown that
baicalein exerts numerous biological activities, including
anti-inflammatory, anti-viral and antioxidant activities (7–9).
Furthermore, other studies have indicated that this flavonoid also
inhibits cancer cell growth and induces apoptosis in human breast,
prostate, hepatocellular and myeloma cancer cells (10–13).
However, its antitumor role in bladder cancer is still unclear.
In the current study, we report that baicalein
suppresses the growth and clone-forming ability of T24 bladder
cancer cells in vitro. Additionally, T24 cells are arrested
at the G1/S phase and undergo apoptosis following baicalain
treatment. Baicalein inhibits Akt phosphorylation, activates
caspase-9 and caspase-3 and downregulates Bcl-2 expression.
Materials and methods
Cell culture and reagents
T24 human bladder cancer cells were obtained from
American Type Culture Collection (Manassas, VA, USA) and maintained
in DMEM (Gibco, Grand Island, NY, USA) supplemented with 10% fetal
bovine serum (FBS; Sijiqing, Hangzhou, China) at 3°C with 5%
CO2 in a humidified incubator. Baicalein (purity
>98%) and mytomycin were purchased from Sigma-Aldrich (St.
Louis, MO, USA) and dissolved in dimethyl sulfoxide (DMSO). Primary
antibodies for Akt, phosphorylated Akt, Bcl-2 and Bax were
purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA,
USA). The study was approved by the Ethical Review Board (ERB)
committee (The Second Affiliated Hospital of Medical College, Xi’an
Jiaotong University, Xi’an, China)
Cell viability assay
The T24 cells were seeded in 96-well plates at a
density of 1×104 cells per well and exposed to 0–120
μmol/l baicalein for 24 h. The cells were then washed with PBS
twice and recultured for 3 days. MTT (final concentration 0.5
mg/ml; Sigma-Aldrich) was then added, the cells were incubated for
a further 4 h, and finally the formazan crystals was dissolved in
DMSO. The optical density (OD) value was measured at 490 nm using a
microplate autoreader (BioTek Instruments, Inc., Winooski, VT, USA)
and the relative cell viability was calculated as a percentage.
Independent experiments were repeated in triplicate.
Colony formation assay
After pre-treatment with baicalein (0–80 μmol/l) for
24 h, 1,000 cells per well of a T24 single-cell suspension were
seeded in 6-well plates and incubated at 37°C with 5%
CO2 in a humidified incubator for 14 days; fresh medium
was added every 4 days. The plates were then washed with ice-cold
PBS, fixed with 4% paraformaldehyde, stained with crystal violet
solution for 15 min at room temperature and washed with distilled
water until no color was evident in the rinse. Plates were dried in
air and the colony numbers were counted.
Cell cycle assay
T24 bladder cancer cells were plated in 60-mm dishes
and treated with 0–80 μmol/l baicalein for 24 h. The cells were
then harvested, fixed with ice-cold 70% ethanol and incubated with
4 μg/ml propidium iodide (PI) solution in the presence of 100 μg/ml
RNase for 30 min. The samples were analyzed using a flow cytometer
(FACScan; Becton-Dickinson, San Jose, CA, USA). Three independent
experiments were performed.
Apoptosis and mitochondrial transmembrane
potential (ΔΨm) assay
Following treatment with 0–80 μmol/l baicalein for
24 h, the T24 cells were harvested and washed with PBS. An
apoptosis detection kit (Invitrogen, Carlsbad, CA, USA) containing
annexin V-FITC and PI was used to identify apoptotic cells
according to the manufacturer’s instructions. The ΔΨm was
determined by JC-1 staining. Data were collected by flow cytometric
analysis using a FACSCalibur flow cytometer (Becton-Dickinson). For
each assay, independent experiments were repeated in
triplicate.
Caspase-9 and -3 activity assay
T24 cells were incubated with 0–80 μmol/l baicalein
for 24 h and cellular extracts were then obtained. Caspase-9 and -3
activities were determined using a human caspase-9 and -3 (active)
ELISA kit (Invitrogen) according to the manufacturer’s
instructions. The OD value was then measured at 450 nm using a
Microplate Autoreader. Three independent experiments were
performed.
Western blot assay
T24 cells were incubated with 0–80 μmol/l baicalein
for 24 h. Total cellular extracts were then prepared using RIPA
buffer containing proteinase inhibitors. Cytosol/mitochondria
fractionation was carried out using a mitochondria extraction kit
(Runtai Biotech, Tianjin, China). Equal amounts of lysates (30 μg)
were separated by 12% SDS-PAGE and transferred to nitrocellulose
membranes. The membranes were initially blocked with 5% skimmed
milk in TBS for 1 h at room temperature and then incubated with
primary antibodies at 4°C overnight followed by secondary
antibodies coupled with horseradish peroxidase for 1 h at room
temperature. Protein signals were then detected using an ECL
chemiluminescent detection system (Amersham, Piscataway, NJ, USA).
GAPDH was used as a loading control.
Statistical analysis
All data analyses were performed using SPSS 13.0
software for Windows. P<0.05 was considered to indicate a
statistically significant result.
Results
Baicalein inhibits T24 bladder cancer
cell growth capability in vitro
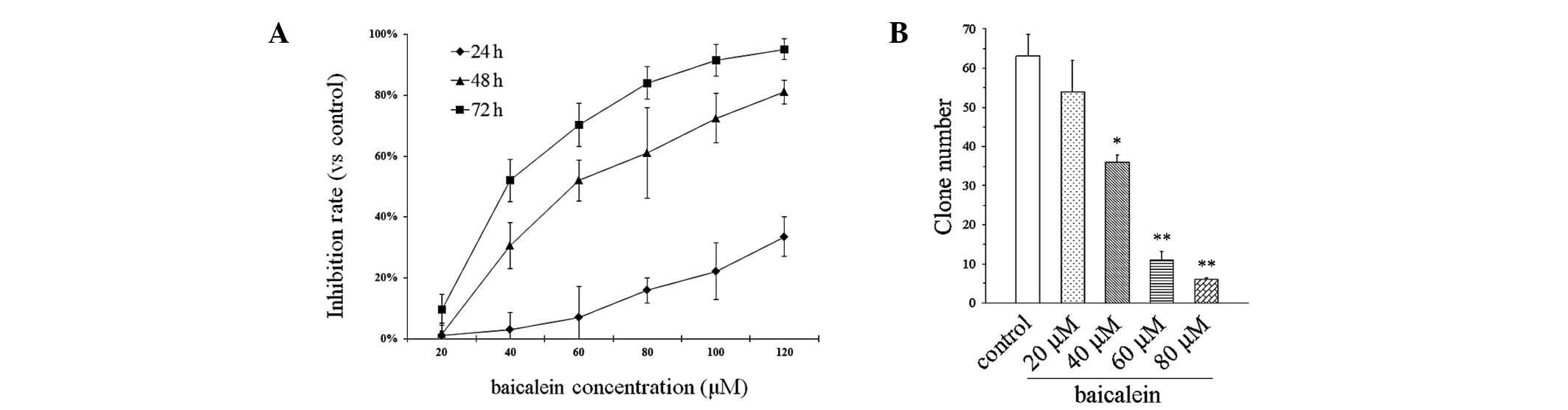
We investigated the effect of baicalein on T24 human
bladder cancer cell growth. As shown in Fig. 1A, the rate of cell growth was
reduced by treatment with baicalein in a concentration- and
time-dependent manner. High concentrations (80–120 μmol/l) almost
completely blocked cell proliferation. Colony formation following
baicalein treatment was also determined. The results revealed that
baicalein repressed the clonogenicity of the T24 cells in a
concentration-dependent manner (Fig.
1B).
Baicalein increases G1/S arrest and
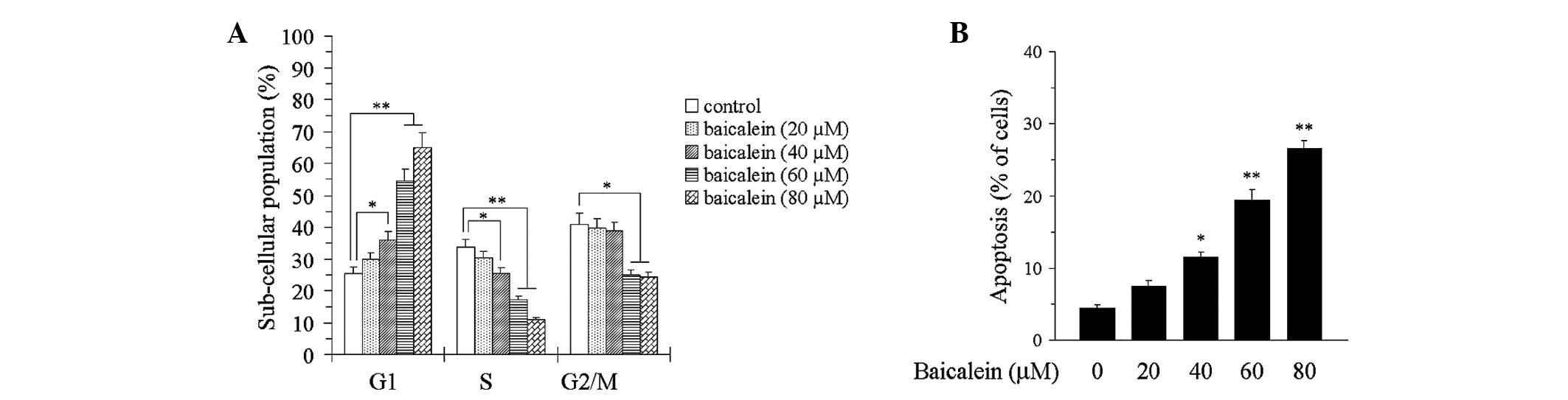
induces apoptosis
Since baicalein was able to suppress the
proliferation of the T24 cells, we next investigated its effect on
the cell cycle. As shown in Fig.
2A, the T24 cells were arrested at the G1/S phase following
baicalein treatment. In addition, the T24 cells underwent cellular
apoptosis following baicalein treatment. The levels of apoptosis
were significantly higher in the cells treated with 60 or 80 μmol/l
baicalein than in the control cells (P<0.05 and P<0.01,
respectively; Fig. 2B).
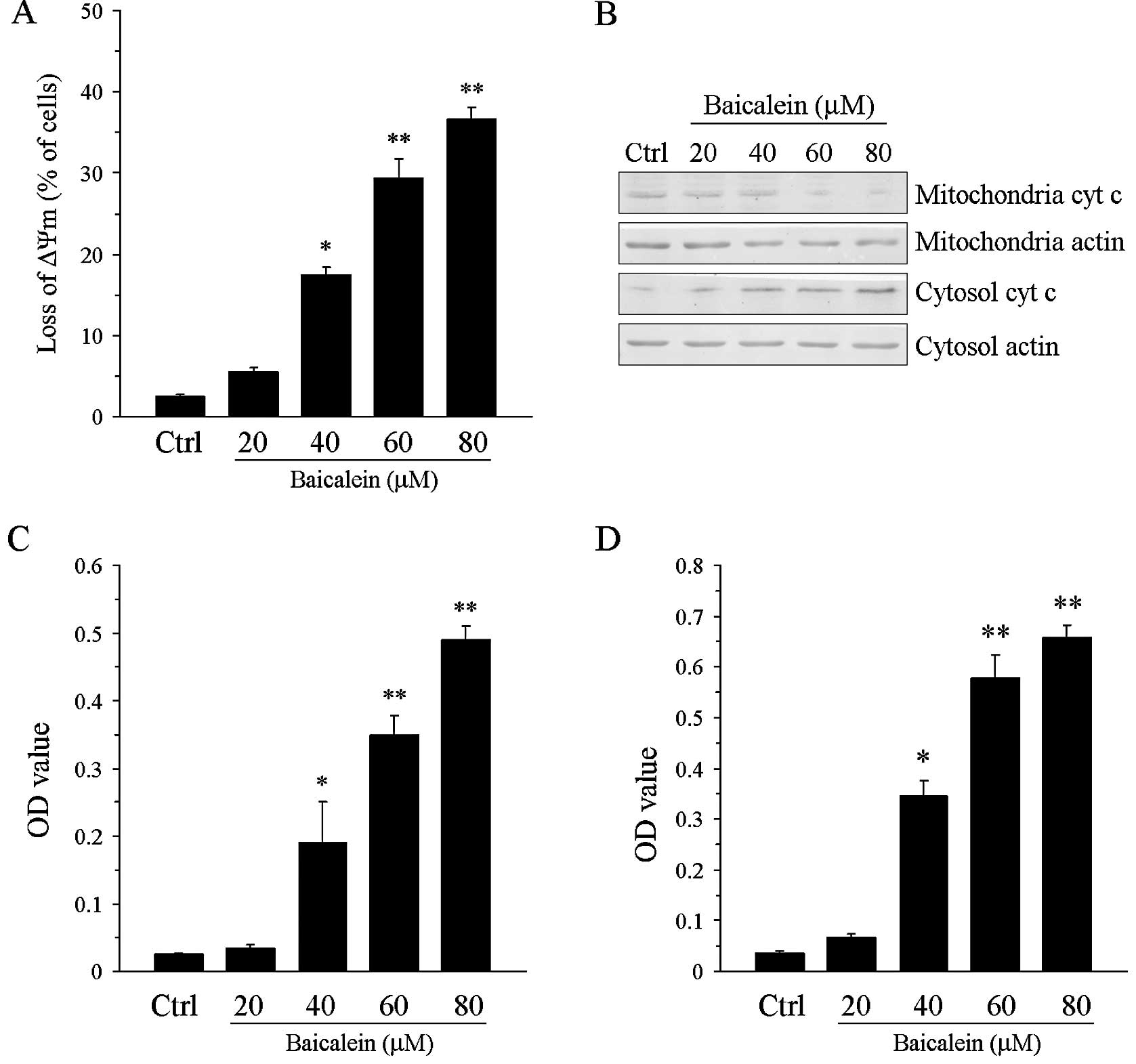
Baicalein induces ΔΨm collapse and
activates caspase-9 and -3
Since the loss of ΔΨm plays a critical role in
triggering apoptosis, we next examined the effect of baicalein
treatment on ΔΨm. Following treatment with baicalein for 24 h, a
significant concentration-dependent loss of ΔΨm was observed in the
treated cells compared with the control cells (P<0.05 or
P<0.01; Fig. 3A). The release
of cytochrome c from the mitochondria to the cytoplasm was also
observed (Fig. 3B). Furthermore,
we determined the activities of caspase-9 and -3 following
baicalein treatment. Consistent with the ΔΨm result, the
baicalein-treated T24 cells exhibited elevated caspase-9 and -3
activities compared with the control cells (P<0.05 or P<0.01;
Fig. 3C and D).
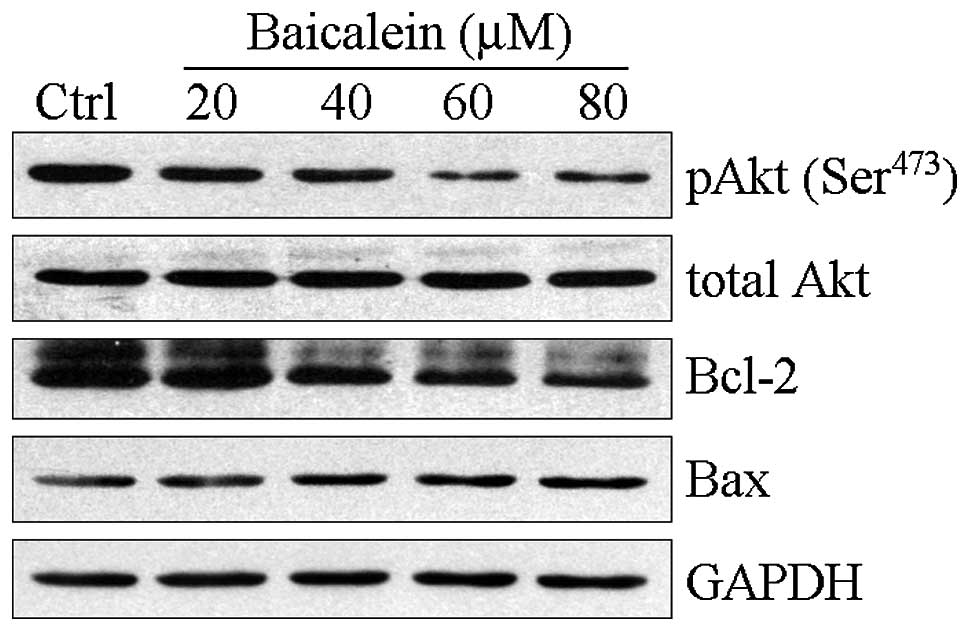
Baicalein inhibits Akt phosphorylation
and downregulates Bcl-2 expression
Since previous reports have indicated that the
PI3K/Akt pathway provides a survival signal to protect cells from
apoptosis (14), we determined the
effect of baicalein treatment on the activity of Akt. As shown in
Fig. 4, baicalein repressed Akt
(Ser473) phosphorylation in a concentration-dependent
manner. Bcl-2 and Bax belong to the Bcl-2 family. Bcl-2 is an
anti-apoptotic protein whereas Bax is a pro-apoptotic protein. The
Bax/Bcl-2 ratio is significant in apoptosis (15,16).
Therefore, we examined the alterations in the levels of these
proteins in response to baicalein exposure. The expression levels
of Bcl-2 were significantly decreased following baicalein
treatment, whereas those of Bax were upregulated.
Discussion
The high recurrence of bladder cancer following
TURBt is an obstacle in its clinical treatment. Intravesical
chemotherapy is used to prevent recurrence; however, tumor cells
often re-emerge, even in those patients undergoing regular
intravesical chemotherapy (3).
Therefore, an effective therapeutic strategy is urgently required.
In the current study, we report the ability of a Chinese herbal
medicine, baicalein, to inhibit growth and induce apoptosis in T24
bladder cancer cells, which indicates its potential as an antitumor
agent for the treatment of bladder cancer.
In an MTT assay, after incubating T24 cells with
baicalein, the tumor cells displayed a decreased growth rate
compared with the control cells. Moreover, high concentrations of
baicalein almost completely blocked cell growth. These data
indicate that baicalein is able to inhibit the growth of T24
bladder cancer cells. In addition, clonogenicity was also
suppressed following baicalein treatment. These data suggest that
baicalein is able to retard the growth of T24 cells in
vitro.
Tumor progression is dependent on the balance
between proliferating and apoptotic cells (17). Chemotherapy may induce cell cycle
arrest and cellular apoptosis in tumor cells, thereby altering the
ratio of proliferating to apoptotic cells and leading to repression
of the tumor. In the present study, T24 bladder cancer cells
arrested at the G1/S phase following incubation with baicalein for
24 h. Furthermore, the T24 cells underwent apoptosis in response to
baicalein treatment. Notably, baicalein induces cell cycle arrest
and apoptosis in a concentration-dependent manner. Previous reports
have shown that ΔΨm plays an initial role in the apoptotic cascade
(18). In this study, we
investigated the effect of baicalein on ΔΨm. As we expected, the
T24 cells exhibited a significant ΔΨm collapse following baicalein
treatment. Correspondingly, elevated activities of caspase-9 and -3
were observed in the T24 cells following incubation with baicalein.
Since activation of caspase-9 is a marker of intrinsic apoptosis
(19), we propose that baicalein
is capable of inducing intrinsic apoptosis in T24 bladder cancer
cells.
Consistent activation of the PI3K/Akt pathway has
been detected in numerous human tumor cells, including bladder
cancer (20,21). This signal pathway promotes tumor
survival, progression and metastasis (22,23).
Akt regulates several cellular activities, including proliferation,
the cell cycle and apoptosis (22,23).
In the current study, we demonstrated that baicalein repressed Akt
phosphorylation in a concentration- and time-dependent manner. The
Bcl-2 family consists of several proteins that execute pro- and
anti-apoptotic functions (24,25).
Bcl-2 is a member of this family and serves as an anti-apoptotic
protein (25). Previous studies
have indicated that Bcl-2 plays a critical role in the survival,
anti-apoptotic activity and chemoresistance of tumor cells
(26,27). Another Bcl-2 family member, Bax, is
a pro-apoptotic protein, which forms a heterodimer with Bcl-2 and
thus represses its anti-apoptotic function (28,29).
Therefore, the Bcl-2/Bax ratio is a key factor in apoptosis. In the
present study, we found that Bcl-2 expression was suppressed
whereas that of Bax was slightly upregulated following baicalein
treatment. Based on these results, we propose that baicalein
induces cell cycle arrest and apoptosis through the inhibition of
Akt and Bcl-2 in T24 bladder cancer cells.
In conclusion, we have shown that baicalein leads to
growth inhibition due to cell cycle arrest and apoptosis via the
loss of ΔΨm and activation of caspase-9 and -3 in T24 bladder
cancer cells. Moreover, baicalein treatment is able to inhibit Akt
phosphorylation and downregulate Bcl-2 expression. These results
indicate that baicalein may be an effective agent in the clinical
management of bladder cancer and expand our understanding of the
potential clinical applications of baicalein.
Acknowledgements
This study received financial support from the
Program for Changjiang Scholars and Innovative Research Team in
University (PCSIRT:1171) and Shaanxi Province Science and
Technology Research and Development Program (No. 2009K12-01).
References
|
1
|
Bischoff CJ and Clark PE: Bladder cancer.
Curr Opin Oncol. 21:272–277. 2009. View Article : Google Scholar
|
|
2
|
Schenk-Braat EA and Bangma CH:
Immunotherapy for superficial bladder cancer. Cancer Immunol
Immunother. 54:414–423. 2005. View Article : Google Scholar
|
|
3
|
Shen Z, Shen T, Wientjes MG, O’Donnell MA
and Au JL: Intravesical treatments of bladder cancer: review. Pharm
Res. 25:1500–1510. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sekiya K and Okuda H: Selective inhibition
of platelet lipoxygenase by baicalein. Biochem Biophys Res Commun.
105:1090–1095. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Pidgeon GP, Kandouz M, Meram A and Honn
KV: Mechanisms controlling cell cycle arrest and induction of
apoptosis after 12-lipoxygenase inhibition in prostate cancer
cells. Cancer Res. 62:2721–2727. 2002.PubMed/NCBI
|
|
6
|
Wong BC, Wang WP, Cho CH, Fan XM, Lin MC,
Kung HF and Lam SK: 12-Lipoxygenase inhibition induced apoptosis in
human gastric cancer cells. Carcinogenesis. 22:1349–1354. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Chen SF, Hsu CW, Huang WH and Wang JY:
Post-injury baicalein improves histological and functional outcomes
and reduces inflammatory cytokines after experimental traumatic
brain injury. Br J Pharmacol. 155:1279–1296. 2008. View Article : Google Scholar
|
|
8
|
Boyle SP, Doolan PJ, Andrews CE and Reid
RG: Evaluation of quality control strategies in Scutellaria
herbal medicines. J Pharm Biomed Anal. 54:951–957. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
He XL, Wang YH, Gao M, Li XX, Zhang TT and
Du GH: Baicalein protects rat brain mitochondria against chronic
cerebral hypoperfusion-induced oxidative damage. Brain Res.
1249:212–221. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wang L, Ling Y, Chen Y, et al: Flavonoid
baicalein suppresses adhesion, migration and invasion of MDA-MB-231
human breast cancer cells. Cancer Lett. 297:42–48. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Taniguchi H, Yoshida T, Horinaka M, et al:
Baicalein overcomes tumor necrosis factor-related
apoptosis-inducing ligand resistance via two different
cell-specific pathways in cancer cells but not in normal cells.
Cancer Res. 68:8918–8927. 2008. View Article : Google Scholar
|
|
12
|
Chen CH, Huang LL, Huang CC, Lin CC, Lee Y
and Lu FJ: Baicalein, a novel apoptotic agent for hepatoma cell
lines: a potential medicine for hepatoma. Nutr Cancer. 38:287–295.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ma Z, Otsuyama K, Liu S, et al: Baicalein,
a component of Scutellaria radix from Huang-Lian-Jie-Du-Tang
(HLJDT), leads to suppression of proliferation and induction of
apoptosis in human myeloma cells. Blood. 105:3312–3318.
2005.PubMed/NCBI
|
|
14
|
Carnero A: The PKB/AKT pathway in cancer.
Curr Pharm Des. 16:34–44. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Czabotar PE and Lessene G: Bcl-2 family
proteins as therapeutic targets. Curr Pharm Des. 16:3132–3148.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kirkin V, Joos S and Zörnig M: The role of
Bcl-2 family members in tumorigenesis. Biochim Biophys Acta.
1644:229–249. 2004. View Article : Google Scholar
|
|
17
|
Malaguarnera L: Implications of apoptosis
regulators in tumorigenesis. Cancer Metastasis Rev. 23:367–387.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Tsujimoto Y and Shimizu S: Role of the
mitochondrial membrane permeability transition in cell death.
Apoptosis. 12:835–840. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hengartner MO: The biochemistry of
apoptosis. Nature. 407:770–776. 2000. View
Article : Google Scholar : PubMed/NCBI
|
|
20
|
Goswami A, Ranganathan P and Rangnekar VM:
The phosphoinositide 3-kinase/Akt1/Par-4 axis: a cancer-selective
therapeutic target. Cancer Res. 66:2889–2892. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Chen M, Gu J, Delclos GL, Killary AM, et
al: Genetic variations of the PI3K-AKT-mTOR pathway and clinical
outcome in muscle invasive and metastatic bladder cancer patients.
Carcinogenesis. 31:1387–1391. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Jiang BH and Liu LZ: PI3K/PTEN signaling
in angiogenesis and tumorigenesis. Adv Cancer Res. 102:19–65. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hartmann W, Küchler J, Koch A, et al:
Activation of phosphatidylinositol-3′-kinase/AKT signaling is
essential in hepatoblastoma survival. Clin Cancer Res.
15:4538–4545. 2009.
|
|
24
|
Danial NN: BCL-2 family proteins: critical
checkpoints of apoptotic cell death. Clin Cancer Res. 13:7254–7263.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Yang J, Liu X, Bhalla K, et al: Prevention
of apoptosis by Bcl-2: Release of cytochrome c from mitochondria
blocked. Science. 275:1129–1132. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Skommer J, Brittain T and Raychaudhuri S:
Bcl-2 inhibits apoptosis by increasing the time-to-death and
intrinsic cell-to-cell variations in the mitochondrial pathway of
cell death. Apoptosis. 15:1223–1233. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhao Y, Zhang CL, Zeng BF, Wu XS, Gao TT
and Oda Y: Enhanced chemosensitivity of drug-resistant osteosarcoma
cells by lentivirus-mediated Bcl-2 silencing. Biochem Biophys Res
Commun. 390:642–647. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Reagan-Shaw S, Nihal M, Ahsan H, Mukhtar H
and Ahmad N: Combination of vitamin E and selenium causes an
induction of apoptosis of human prostate cancer cells by enhancing
Bax/Bcl-2 ratio. Prostate. 68:1624–1634. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Katiyar SK, Roy AM and Baliga MS:
Silymarin induces apoptosis primarily through a p53-dependent
pathway involving Bcl-2/Bax, cytochrome c release, and caspase
activation. Mol Cancer Ther. 4:207–216. 2005.PubMed/NCBI
|