Introduction
Nasopharyngeal carcinoma (NPC) is one of the most
common types of head and neck cancer in Asia, particularly in
Southeast Asia and China (1).
According to standards outlined by the American Joint Committee on
Cancer Staging, more than 50% of NPC cases are advanced (2). Radiotherapy is a standard treatment
utilized to improve patient survival rates (3). However, despite the majority of cases
responding well to initial radiotherapy or concurrent chemotherapy
(4–6), the development of drug resistance is
known to occur, resulting in failure to cure the disease.
Multidrug resistance (MDR) is a phenomenon in which
tumor cells acquire simultaneous resistance to a diverse group of
cytotoxic drugs. MDR remains a major obstacle in cancer
chemotherapy, and is observed in patients who have received prior
radiotherapy, particularly those that have undergone repeated
courses of chemotherapy (7,8).
Overexpression of P-glycoprotein (P-gp) is associated with drug
resistance and has been observed in cells of murine and human cell
lines following irradiation (9,10).
However, there is little information on whether radiation promotes
the development of drug resistance in NPC cells. The molecular
basis of radiotherapy-related MDR remains unclear.
The majority of in vitro studies of
radiation-drug interactions have involved exposure of cells to
single-dose radiation, despite the common use of fractionated
irradiation in clinical radiotherapy. In the present study, the
effect of various doses of fractionated irradiation on the the
human nasopharyngeal cell line CNE1 was investigated. Expression of
the membrane-associated protein P-gp and its corresponding gene,
MDR1, was analyzed over various time points, and functional
resistance to the cytotoxic drug cisplatin was also investigated.
Following this, gene chips were used to gain insight into the
molecular mechanisms associated with MDR induction by fractionated
irradiation.
Materials and methods
Cells and reagents
The human NPC cell line CNE1 was purchased from the
Chinese Academy of Science Cell Bank (Beijing, China). Cells were
maintained in RPMI-1640 medium with 10% fetal bovine serum (Thermo
Scientific Hyclone, Logan, UT, USA) and were incubated at 37°C in a
humidified atmosphere of 95% air and 5% CO2.
Radiation and drug cytotoxicity
assays
Cells growing exponentially in flasks were
irradiated with a total dose of 10, 20 or 50 Gy. For the 10 and 20
Gy groups, five fractions of 2.0 Gy/week were administered. For the
50 Gy group, 2.0 Gy two days/week was administered. Irradiation was
performed with a Varian 2300C/D linear accelerator (Varian,
Darmstadt, Germany) using 6 MV X-rays. Following irradiation,
culture medium was removed and the cells were rinsed to remove
floating dead cells. Cells were harvested each week from the
beginning of irradiation until week 5 for the 10 and 20 Gy groups.
For the 50 Gy group, cells were harvested each week following
completion of irradiation until week 7. Cells were collected when
the expression levels of MDR1 and P-gp were significantly increased
compared with the control. Drug sensitivity assays were performed
(11). A total of 1×105
cells in 0.2 ml medium/well were grown in 96-well culture plates
and incubated with increasing concentrations of cisplatin for 3
days in culture (final concentration 50 μg/ml). Cell viability
relative to untreated control cells was determined using the MTT
assay (12). The 50% inhibitory
concentration (IC50), defined as the drug concentration
causing 50% reduction in cell viability, was calculated, and the
relative drug resistance was calculated by dividing the
IC50 for the irradiated CNE1 cells by the
IC50 for the non-irradiated CNE1 cells. Experiments were
run in triplicate.
RNA preparation and reverse transcription
(RT)-PCR
Viable cells were harvested and resuspended in
guanidium thiocyanate buffer and stored at −80°C. Total RNA
extraction was performed using TRIzol reagent (Invitrogen, Grand
Island, NY, USA) according to the manufacturer’s instructions.
Concentration and purity of RNA was assessed electrophoretically
and quantified spectrophotometrically. cDNA was generated from 1 μg
total RNA by reverse transcription using a Reverse Transcription
System kit (Takara, Tokyo, Japan). RT-PCR was performed as
previously described (13), using
the primer sequences listed in Table
I. PCR products were size-fractioned and subjected to
electrophoresis on 2% agarose gels. Products were visualized by
staining with ethidium bromide. RNA integrity was confirmed by
performing PCR amplification of each cDNA with primers for the
reference gene glyceraldehyde 3-phosphate dehydrogenase (GAPDH;
Life Technologies, Carlsbad, CA, USA). Preliminary experiments were
carried out to determine the linear range of PCR amplification
using representative cases. The PCR conditions in this study were
as follows: MDR1, GAPDH, 2 min at 94°C, followed by 30 sec at 94°C,
30 sec at 53°C and 1 min at 72°C for 35 cycles, followed by 72°C
for 10 min; Bcl-2, 3 min at 94°C, followed by 30 sec at 94°C, 30
sec at 55°C and 30 sec at 72°C for 30 cycles, followed by 72°C for
10 min; MMP-7, 4 min at 94°C, followed by 30 sec at 94°C, 30 sec at
59°C and 30 sec at 72°C for 33 cycles, followed by 72°C for 10 min.
PCR fragments were quantified using the ImageMaster gel analysis
equipment (Amersham Pharmacia Biotech, Amersham, UK). Densitometric
analysis was performed to determine to the relative mRNA expression
levels of genes outlined in Table
I.
 | Table IPrimer sequences in RT-PCR. |
Table I
Primer sequences in RT-PCR.
| Gene | Primers/probe
sequences | Amplicon (bp) |
|---|
| MDR1 |
5′-CCCATCATTGCAATAGCAGG-3′
(forward)
5′-GTTCAAACTTCTGCTCCTGA-3′ (reverse) | 157 |
| BCL-2 |
5′-GGATTGTGGCCTTCTTGAG-3′
(forward)
5′-CCAAACTGAGCAGAGTCTTC-3′ (reverse) | 103 |
| MMP-7 |
5′-AAAATGCCAACAGTTTAGAAGC-3′
(forward)
5′-CGTCCAGCGTTCATCCTC-3′ (reverse) | 239 |
| GADPH |
5′-CGGGAAGCTTGTGTGATCAATGC-3′
(forward)
5′-GGCAGTGATGGCATGGACTG-3′ (reverse) | 125 |
Western blot analysis
Cells (1×107) were washed in
phosphate-buffered saline (PBS; pH 7.2) and resuspended in 200 ml
lysis buffer (50 mM Tris, 150 mmol/l NaCl, 5 mmol/l EDTA, 1% Triton
X-100, 1 mM PMSF, pH 8.0) and sonicated on ice. Membrane fractions
for P-gp were prepared as previously described (14). The membrane pellet was resuspended
in PBS, and the protein content was determined using the DC protein
assay kit (Bio-Rad, Hercules, CA, USA). Membrane-enriched fractions
containing 100 μg protein were separated on SDS-PAGE, followed by
western blot analysis. Blots were probed using an antibody against
P-gp (C219; Centocor, Malvern, PA, USA; 1:200). β-actin (sc-8432;
Santa Cruz Biotechnology Inc., Santa Cruz, CA, USA) was used as an
internal loading control. Proteins were detected using the
chemiluminescent method and quantified using the Amersham ECL gel
system (Amersham Pharmacia Biotech). All experiments were repeated
at least once.
Analysis of mRNA expression using cDNA
arrays
Relative mRNA expression levels of specific
apoptosis- and proliferation-related genes were analyzed with a
nonradioactively labeled cRNA on oligo GEArray series (Superarray,
Warren, OR, USA), according to the manufacturer’s instructions.
Using the TrueLabeling-AMP Linear RNA amplification kit (Takara),
mRNA was reverse transcribed to obtain cDNA and converted into
biotin-labeled cRNA using biotin-16-UTP (Roche Diagnostics,
Mannheim, Germany) by in vitro transcription. Prior to
hybridization, the cRNA probes were purified with the ArrayGrade
cRNA cleanup kit (Superarray). Purified cRNA probes were hybridized
to the pretreated Oligo GEArray Human Cancer Microarrays OHS-044
(Superarray), containing 128 genes associated with 15 signaling
pathways. Following several washing steps, array spots binding cRNA
were detected using alkaline phosphatase-conjugated streptavidin
and CDP-Star as the chemiluminescent substrate. The image data were
analyzed with the GEArray Expression Analysis Suite (Superarray).
Following normalization to the signal of the housekeeping gene, the
relative expression level of each gene was determined by comparing
the signal intensity of each gene in the array. Arbitrary units
were calculated as follows: (target gene signal - background
signal)/(housekeeping gene - background signal). Data filtering
criteria were as follows: at least one of the spot intensities to
be compared had to be more than twice the background intensity, and
the spot intensity ratios had to be >2 (for upregulation) or
<-2 (for downregulation). Validation of array results was
performed by RT-PCR.
Statistical analysis
Data were presented as the mean values ± SD.
Multiple comparisons were initially subjected to one-way analysis
of variance (ANOVA). Pairwise comparison between groups was
analyzed by LSD test. The statistical analysis was performed using
SPSS software 14.0. P<0.05 was considered to indicate a
statistically significant difference.
Results
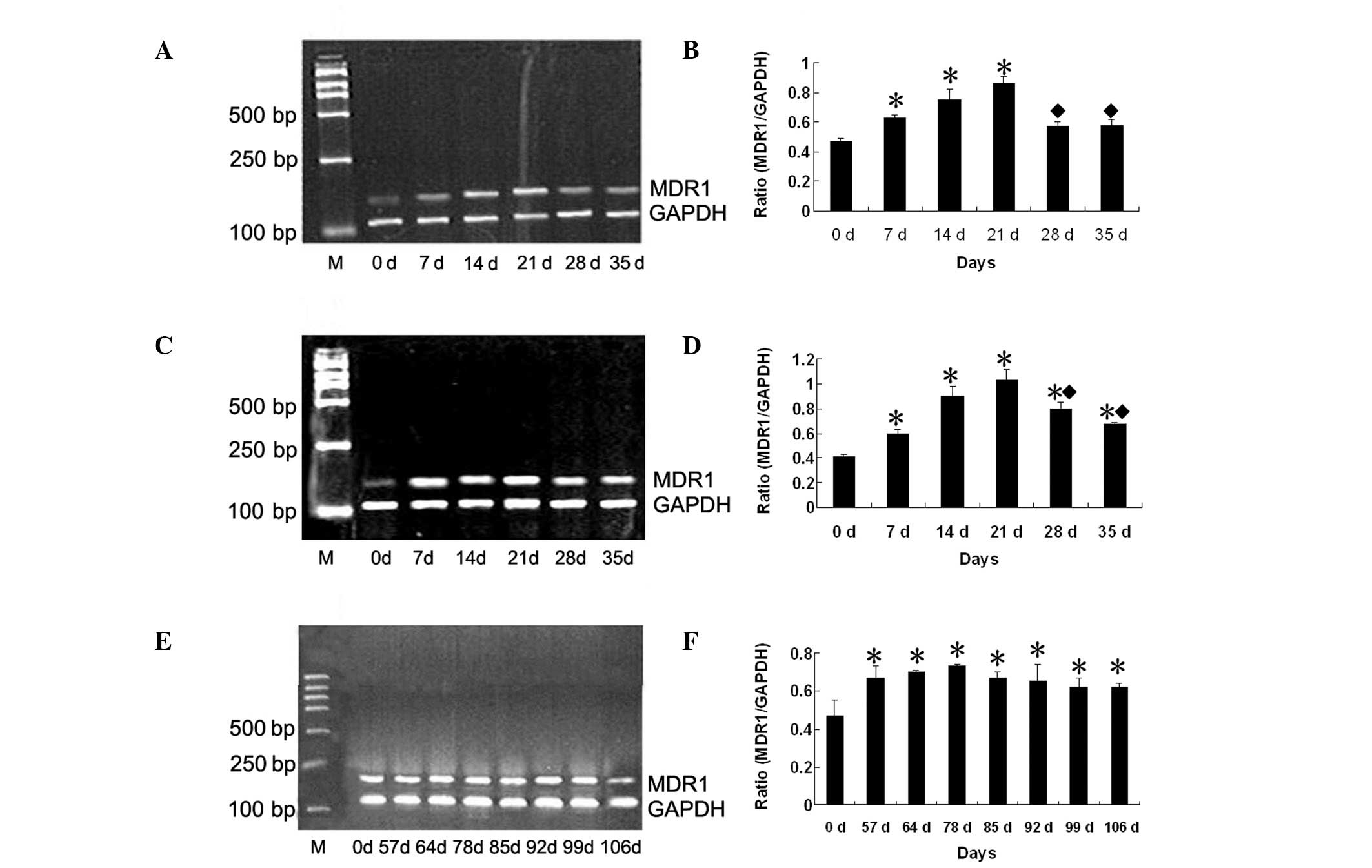
Expression of MDR1 mRNA following
fractionated irradiation
CNE1 cells were treated with fractionated radiation
with a cumulative dose of 10, 20 or 50 Gy, five fractions of 2
Gy/week for 10 and 20 Gy, 2 Gy every two days/week for 50 Gy.
RT-PCR was performed to examine the correlation between radiation
conditions and expression of the MDR gene, MDR1 (Fig. 1A, C and E). Low basal levels of
MDR1 mRNA expression were identified in non-irradiated CNE1 cells.
Compared with non-irradiated cells, the expression of MDR1 mRNA was
gradually increased following fractionated irradiation. The largest
increase in MDR1 expression levels was identified at day 21 for
treatment with 10 and 20 Gy (1.59- and 2.19-fold, respectively,
P<0.05; Fig. 1B and D). On days
28 and 35, MDR1 mRNA was decreased, but remained significantly
higher than that in non-irradiated CNE1 cells. Treatment with 20 Gy
yielded a 1.37-, 1.40- and 1.15-fold increase on days 21, 28 and
35, respectively (all P<0.05) compared with treatment with 10
Gy. MDR1 mRNA was studied following irradiation with a high dose of
50 Gy. Expression of MDR1 mRNA was significantly increased, and was
detected until day 106. Expression of MDR1 mRNA was highest on day
78 (1.55-fold, P<0.05; Fig.
1F).
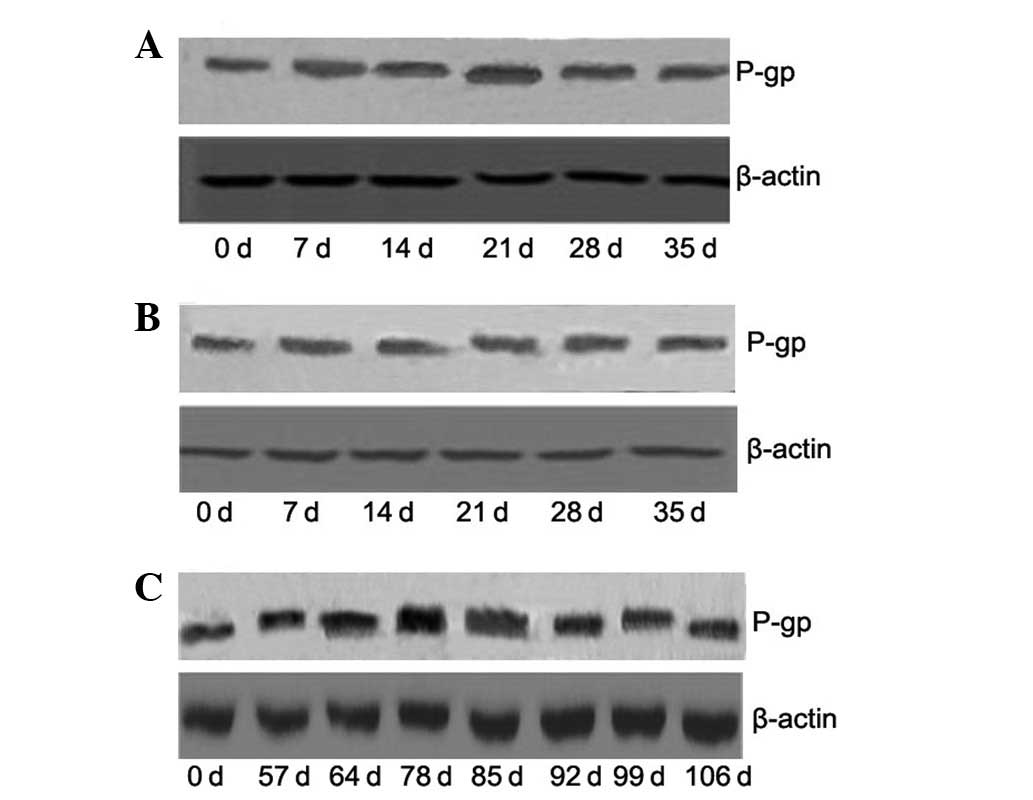
Expression of P-gp following fractionated
irradiation
Expression levels of the MDR1 gene protein product,
P-gp, were assessed by western blot analysis. As shown in Fig. 2, the protein expression of P-gp was
consistent with that of MDR1 mRNA expression. On day 21, protein
levels of P-gp were significantly increased by 1.86- and 2.25-fold
compared with control, following treatment with 10 and 20 Gy
radiation, respectively (all P<0.05). Treatment with 20 Gy
produced a 1.21-, 1.47- and 1.25-fold increase, compared with
treatment with 10 Gy radiation, on days 21, 28 and 35, respectively
(all P<0.05). On day 78, the protein expression of P-gp was
increased 1.74-fold (P<0.05) compared with the control,
following treatment with a high dose of 50 Gy radiation.
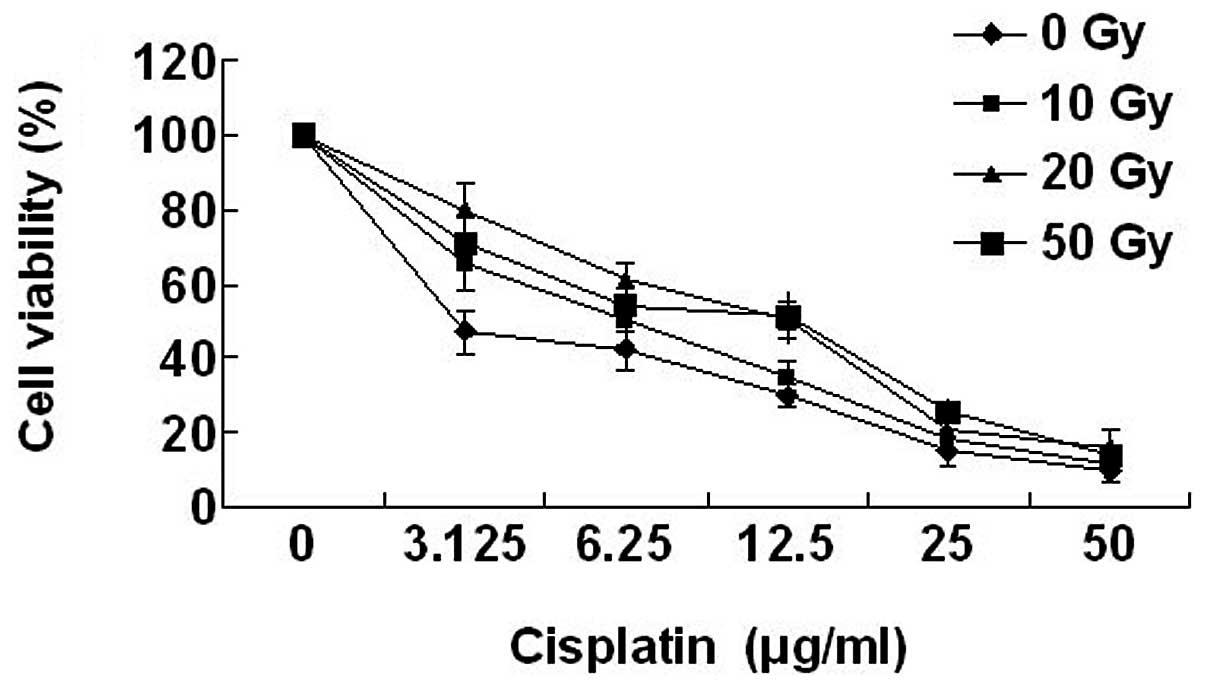
Drug sensitivity of the CNE1 cell line
following irradiation
Drug cytotoxicity curves obtained in the irradiated
and non-irradiated CNE1 cells are presented in Fig. 3. Cisplatin was selected for this
study on the basis of sensitivity of NPC to the cytotoxic agent.
Sensitivity of CNE1 cells to cisplatin was reduced following
irradiation, compared with the control. Compared with the median
viability of non-irradiated cells, a significant increase in the
median viability of the irradiated cells for 10 Gy (2.08-fold), 20
Gy (4.15-fold) and 50 Gy (4-fold; all P<0.05) was
identified.
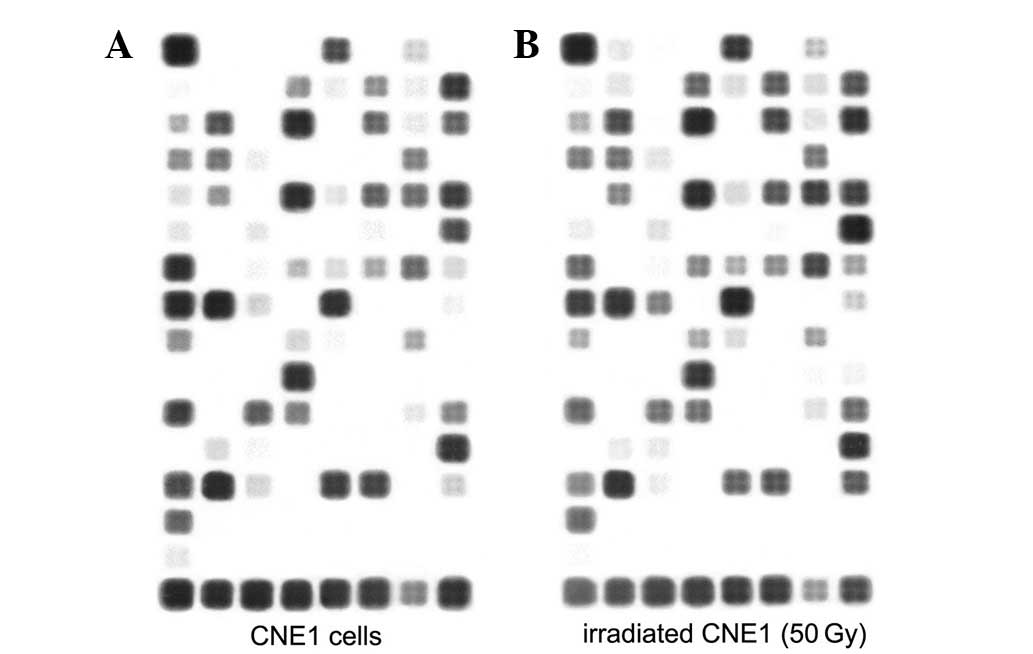
Irradiation regulates the expression of
multiple genes at the mRNA level
In order to test the hypothesis that exposure to
irradiation induces gene expression changes in signaling pathways
associated with cancer, CNE1 cells treated with 50 Gy irradiation
at day 78 were analyzed by microarray. Following irradiation of the
128 genes contained on the Oligo GEArray Human Cancer Microarray
OHS-044 (Fig. 4), 26 genes were
upregulated and 8 were downregulated according to the selected
filter criteria (Tables II and
III). These genes comprised a
variety of oncological groups associated with cellular functions,
including cell stress, inflammation, adhesion and cell death,
suggesting a functional role of irradiation in these processes. To
further quantify gene expression levels in cells used in the
microarray, we selected genes associated with proliferation and
apoptosis (BCL-2 and MMP-7) and performed further analysis by
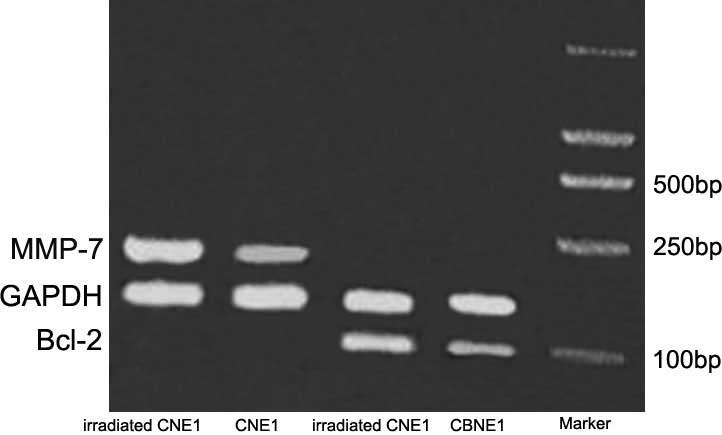
RT-PCR (Fig. 5).
 | Table IIList of the 26 genes with upregulated
expression in irradiated CNE1 cells compared with non-irradiated
CNE1 cells. |
Table II
List of the 26 genes with upregulated
expression in irradiated CNE1 cells compared with non-irradiated
CNE1 cells.
| Gene Bank accession
number | Symbol | Description | Irradiated
CNE1/CNE1 |
|---|
| NM_000014 | A2M |
α-2-macroglobulin | 7.6606850 |
| NM_000927 | ABCB1 | ATP-binding
cassette | 5.9007979 |
| NM_000633 | BCL2 | B-cell CLL/lymphoma
2 | 3.3802493 |
| NM_199173 | BGLAP | Bone
γ-carboxyglutamate (gla) protein (osteocalcin) | 2.4023970 |
| NM_001165 | BIRC3 | Baculoviral IAP
repeat-containing 3 | 2.3226417 |
| NM_000089 | COL1A2 | Collagen, type I, α
2 | 2.2922554 |
| NM_000799 | EPO | Erythropoietin | 2.6616851 |
| NM_000043 | FAS | Fas (TNF receptor
superfamily, 6) | 2.4901347 |
| NM_004476 | FOLH1 | Folate hydrolase
1 | 2.6467973 |
| NM_022475 | HMOX1 | Heme oxygenase
(decycling) 1 | 2.3548339 |
| NM_002133 | HSPA4 | Heat shock 70-kDa
protein 4 | 2.9072737 |
| NM_002154 | HSPA5 | Heat shock 70-kDa
protein 5 | 3.9989408 |
| NM_005347 | ICAM1 | Intercellular
adhesion molecule 1 | 3.6120906 |
| NM_000201 | ID2 | Inhibitor of DNA
binding 2, dominant negative helix-loop-helix protein | 2.3307183 |
| NM_002166 | IL4 | Interleukin 4 | 6.1478924 |
| NM_000589 | IRF1 | Interferon
regulatory factor 1 | 2.242845 |
| NM_002198 | KLK2 | Kallikrein-related
peptidase 2 | 11.837389 |
| NM_005551 | MDM2 | Mdm2, transformed
3T3 cell double minute 2, p53 binding protein | 4.7794018 |
| NM_002392 | MMP7 | Matrix
metallopeptidase 7 | 9.8154842 |
| NM_002423 | MT3 | Metallothionein
3 | 6.4771969 |
| NM_005954 | NOS2A | Nitric oxide
synthase 2A | 2.8604777 |
| NM_000625 | PECAM1 |
Platelet/endothelial cell adhesion 1 | 23.512991 |
| NM_000442 | TCF7 | Transcription
factor 7 | 2.6215746 |
| NM_003202 | TF | Transferrin | 2.0762113 |
| NM_001063 | TNFSF10 | Tumor necrosis
factor (ligand) superfamily, member 10 | 2.2066475 |
| NM_003810 | TP53 | Tumor protein
p53 | 3.4717765 |
 | Table IIIList of the 8 genes with
downregulated expression in irradiated CNE1 cells compared with
non-irradiated CNE1 cells. |
Table III
List of the 8 genes with
downregulated expression in irradiated CNE1 cells compared with
non-irradiated CNE1 cells.
| Gene Bank accession
number | Symbol | Description | Irradiated
CNE1/CNE1 |
|---|
| NM_001904 | CTNNB1 | Catenin, β 1, 88
kDa | 0.237207 |
| NM_001648 | KLK3 | Kallikrein-related
peptidase 3 | 0.215753 |
| NM_000595 | LTA | Lymphotoxin α | 0.178995 |
| NM_004536 | NAIP | NLR family,
apoptosis inhibitory protein | 0.02121 |
| NM_000264 | PTCH1 | Patched homolog 1
(Drosophila) | 0.424597 |
| NM_020182 | TMEPAI | Transmembrane,
prostate androgen induced RNA | 0.487702 |
| NM_000594 | TNF | Tumor necrosis
factor | 0.265229 |
| NM_015626 | WSB1 | WD repeat and SOCS
box-containing | 0.4685042 |
Discussion
In the present study, our first objective was to
confirm whether irradiation induces the upregulation of MDR1 mRNA
and P-gp in CNE1 cells. Unlike previous studies, the aim of the
present study was to detect changes in the expression levels of
MDR1 and P-gp at higher accumulative doses of irradiation, which
are more relevant to clinical protocols. In addition, the molecular
mechanisms of drug resistance were studied, using microarray
experiments.
We identified a limited expression of MDR1 mRNA and
P-gp in untreated CNE1 cells, supporting previous studies which
reported that the expression of MDR1 mRNA and P-gp was of minor
relevance to intrinsic therapy resistance of cancer (15). Expression of MDR1 mRNA and P-gp
initially increased and then decreased following irradiation in a
time-dependent manner. However, expression levels in irradiated
CNE1 cells were always higher than that of non-irradiated cells.
CNE1 cells treated by fractionated radiation with 10, 20 or 50 Gy
became resistant to cisplatin, the main drug used to treat NPC.
Development of resistance was correlated with the expression of
MDR1 mRNA and protein. Overexpression of MDR1 has been previously
reported to be associated with protection of normal tissues from
radiation- or chemotherapy-induced damage during tumor treatment,
by upregulation of the anti-apoptotic gene AKT3 (16). In addition, resistance of
hematopoietic progenitors to cyclophosphamide was conferred by a
high expression of MDR1, linking with increased transcription of
ALDH1 (17).
Previous studies have reported that P-gp inhibits
apoptosis by suppression of caspase activity. However, the nature
of this interaction has not been identified (18). Furthermore, addition of a general
caspase inhibitor has been demonstrated to increase clonogenic
survival rate of TK6 cells following irradiation (19). An effect of P-gp on caspases has
also been identified at the transcription level (16). Fractionated radiation treatment has
also been reported to cause drug resistance in ovarian carcinoma
cells (9,20), Chinese hamster ovary (CHO) cells
(20), ascites tumor cells
(10), SCLC cells (21), breast cancer cell lines and colon
cancer cell lines (22). In the
absence of further treatment, no marked change with continuous
culture for 56 days was identified, indicative of a stable
resistant phenotype. The stability and 4-fold resistance to
cisplatin exhibited by the CNE1 cells is indicative of the
resistance phenotype associated with NPC. Our results suggest that
fractionated radiation treatment alone may induce the development
of drug resistance. Other reports of radiation treatment causing
stable resistance to chemotherapeutic drugs in cancer cells have
been published. These include a study in colon cancer cells, where
durability of resistance was present for 18 days, following an
irradiation dose of 27 Gy (23).
In SCLC, a total dose of 37.5 Gy has been demonstrated to sustain
durability of resistance for 6 months (23,24).
Our studies suggest a possible mechanism of P-gp enhancement,
through an increase in protein half-life and stability or by
upregulation of MDR1 gene expression.
However, radiation in MDR cells has also been
demonstrated to mediate loss of extrachromosomally amplified MDR1
genes, concomitant with a reduction in P-gp levels (25). Fractionated irradiation led to the
restoration of drug sensitivity by the downregulation of P-gp in
MDR cells (26). Possible reasons
for these observations are the heterogeneity of cells or low does
of fractionated radiation (27).
Irradiation-induced stress response may affect cell
cycle regulation, cellular differentiation, oncogenic
transformation, cell survival and apoptosis (28). In order to understand the possible
molecular mechanism of the radiation-induced MDR, a cancer
microarray gene chip was utilized. Following irradiation, 26 genes
were upregulated, indicating that drug resistance is associated
with increased expression levels of a number of anti-apoptotic
genes, including PECAM-1, ICAM-1, BCL-2, MMP-7, HSP70 and IL-4. For
example, it is well known that overexpression of BCL-2 inhibits
apoptosis and exhibits radioprotective properties (29,30).
The largest increase in gene expression in
irradiated cells compared with non-irradiated cells was identified
in the gene PECAM-1 (23.5-fold). Another adhesion molecule, ICAM-1,
was also upregulated. Endothelial cells express the heterotypic
ligand (αvβ3) for PECAM1-CD31. Buckley et al speculated that
CD31 homophilic and heterophilic adhesion could be involved in a
signaling mechanism that regulated endothelial cell proliferation
and differentiation (31).
Therefore, as a general response to irradiation, CD31 upregulation
may disrupt this regulatory balance and hinder the ability of the
vasculature to recover from radiation damage, potentially causing
abnormal cell proliferation. Further studies are required to
determine whether CD31 could function as a promising therapeutic
target (32,33). Additional studies have demonstrated
the upregulation of ICAM-1 in rat liver following irradiation
(34), indicative of a significant
role in radiation-induced lung injury in patients with lung cancer
(35).
MMP-7, another upregulated gene, has been linked
with tumor invasion and metastasis. MMP-7 expression in tumor cell
lines resulted in loss of responsiveness to chemotherapeutic agents
(36,37). Chronic exposure to MMP-7 acted as a
selective pressure for apoptotic resistance, through the generation
of a chronic Fas-activating signal. This resulted in alteration of
the apoptosis signaling pathway, leading to loss of sensitivity to
various death stimuli (38–40).
Mitsiades et al(37)
demonstrated one mechanism whereby MMP-7 promotes tumor survival
rate and resistance to doxorubicin through cleavage of FasL.
Proteolysis of the ligand was found to reduce its effectiveness in
triggering Fas-mediated apoptosis (41). Insulin-like growth factor-1 (IGF-I)
bioavailability has been demonstrated to be regulated by MMP-7
proteolysis of IGFBP-3, leading to the promotion of cell survival
(42).
Previously, IL-4 was associated with survivin
expression and localization, through activation of the STAT6
signaling pathway (43), and
decrease of apoptosis in HCT116 colon cancer cells (44).These results have shed more light on
the molecular mechanisms involved in IL-4-mediated
chemoresistance.
Heat shock proteins (HSPs) are a super family of
highly conserved molecular chaperone proteins, which are induced in
response to stress. HSP70 has been demonstrated to inhibit
apoptosis induced by a number of chemotherapeutic agents. HSP70
inhibitors enhanced melphalan-induced apoptosis and reversed
melphalan-induced cell adhesion-mediated drug resistance phenotype
(45). High-dose radiation
upregulated the expression of HSPB1 and particularly HSP70. These
results suggest that HSP70 is a key molecule in the induction of
the adaptive response (46).
Although Fas and p53 were upregulated, a balance
between the expression of these functionally antagonistic proteins
is thought to determine whether a cell survives or undergoes
apoptosis. Radiation activated nitric oxide synthase production,
which plays a role in the regulation of apoptosis. However, a
cytoprotective role remains controversial (47). Other differentially expressed genes
identified in the microarray, included α-2-macroglobulin, folate
hydrolase 1 and kallikrein-related peptidase 2. These genes need to
be studied further to confirm their correlation with MDR.
In conclusion, our study extended prior reports of
radiation-induced MDR. Overexpression of MDR1 in cancer cells has
been shown to protect against radiation-induced apoptosis and
clonogenic cell death. Our results suggest that optimization of
fractionated irradiation could lead to important considerations in
the design of chemoradiation strategies against MDR cells. This
study provides a good foundation for the confirmation of the
specific molecular mechanism of radiation-induced MDR.
Acknowledgements
This study was supported in part by a grant from the
National Natural and Scientific Foundation of China, no.
30970869.
References
|
1
|
Smith C, Tsang J, Beagley L, et al:
Effective treatment of metastatic forms of Epstein-Barr
virus-associated nasopharyngeal carcinoma with a novel
adenovirus-based adoptive immunotherapy. Cancer Res. 72:1116–1125.
2012. View Article : Google Scholar
|
|
2
|
Hughes J, Alusi G and Wang Y: Gene therapy
and nasopharyngeal carcinoma. Rhinology. 50:115–121. 2012.
|
|
3
|
Sun Y, Tang LL, Chen L, et al: Promising
treatment outcomes of intensity-modulated radiation therapy for
nasopharyngeal carcinoma patients with N0 disease according to the
seventh edition of the AJCC staging system. BMC Cancer. 12:682012.
View Article : Google Scholar
|
|
4
|
Zhang X and Li W: 5-Fluorouracil in
combination with cisplatin alters the microRNA expression profile
in the CNE nasopharyngeal carcinoma cell line. Mol Med Rep.
6:303–308. 2012.PubMed/NCBI
|
|
5
|
Ma BB and Chan AT: Recent perspectives in
the role of chemotherapy in the management of advanced
nasopharyngeal carcinoma. Cancer. 103:22–31. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Komatsu M, Tsukuda M, Matsuda H, et al:
Comparison of concurrent chemoradiotherapy versus induction
chemotherapy followed by radiation in patients with nasopharyngeal
carcinoma. Anticancer Res. 32:681–686. 2012.
|
|
7
|
Bansal T, Jaggi M, Khar RK and Talegaonkar
S: Emerging significance of flavonoids as P-glycoprotein inhibitors
in cancer chemotherapy. J Pharm Pharm Sci. 12:46–78.
2009.PubMed/NCBI
|
|
8
|
Chang JW, Yu YS, Kim JY, et al: The
clinical outcomes of proton beam radiation therapy for
retinoblastomas that were resistant to chemotherapy and focal
treatment. Korean J Ophthalmol. 25:387–393. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Delou JM, Capella MA and Gattass CR:
Betulinic acid does not modulate the activity of P-gp/ABCB1 or
MRP1/ABCC1 in a non-tumoral renal cell line: Possible utility in
multidrug resistance cancer chemotherapy. Mol Med Rep. 2:271–275.
2009.PubMed/NCBI
|
|
10
|
Nielsen D, Maare C, Eriksen J, et al:
Expression of P-glycoprotein and multidrug resistance associated
protein in Ehrlich ascites tumor cells after fractionated
irradiation. Int J Radiat Oncol Biol Phys. 51:1050–1057. 2001.
View Article : Google Scholar
|
|
11
|
Hofstetter B, Vuong V, Broggini-Tenzer A,
et al: Patupilone acts as radiosensitizing agent in
multidrug-resistant cancer cells in vitro and in vivo. Clin Cancer
Res. 11:1588–1596. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhu H, Yu WJ, Le Y, et al: High glucose
levels increase the expression of neurotrophic factors associated
with p-p42/p44 MAPK in Schwann cells in vitro. Mol Med Rep.
6:179–184. 2012.PubMed/NCBI
|
|
13
|
Okumura N, Yoshida H, Nishimura Y, et al:
Clobetasol synergistically diminishes Ciz1 expression with
genistein in U937 cells. Mol Med Report. 5:567–569. 2012.PubMed/NCBI
|
|
14
|
Chen M, Huang SL, Zhang XQ, et al:
Reversal effects of pantoprazole on multidrug resistance in human
gastric adenocarcinoma cells by down-regulating the
V-ATPases/mTOR/HIF-1α/P-gp and MRP1 signaling pathway in vitro and
in vivo. J Cell Biochem. 113:2474–2487. 2012.PubMed/NCBI
|
|
15
|
Berger W, Setinek U, Hollaus P, et al:
Multidrug resistance markers P-glycoprotein, multidrug resistance
protein 1, and lung resistance protein in non-small cell lung
cancer: prognostic implications. J Cancer Res Clin Oncol.
131:355–363. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Maier P, Fleckenstein K, Li L, et al:
Overexpression of MDR1 using a retroviral vector differentially
regulates genes involved in detoxification and apoptosis and
confers radioprotection. Radiat Res. 166:463–473. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kohn FR and Sladek NE: Ex vivo treatment
of murine splenocyte-supplemented bone marrow inocula with
mafosfamide prior to allogeneic transplantation in an attempt to
prevent lethal graft-versus-host disease without compromising
engraftment. Immunopharmacol Immunotoxicol. 10:387–398. 1988.
View Article : Google Scholar
|
|
18
|
Tainton KM, Smyth MJ, Jackson JT, et al:
Mutational analysis of P-glycoprotein: suppression of caspase
activation in the absence of ATP-dependent drug efflux. Cell Death
Differ. 11:1028–1037. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Schäfer J, Bachtler J, Engling A, et al:
Suppression of apoptosis and clonogenic survival in irradiated
human lymphoblasts with different TP53 status. Radiat Res.
158:699–706. 2002.PubMed/NCBI
|
|
20
|
Hill BT, Whelan RD, Hurst HC and McClean
S: Identification of a distinctive P-glycoprotein-mediated
resistance phenotype in human ovarian carcinoma cells after their
in vitro exposure to fractionated X-irradiation. Cancer.
73:2990–2999. 1994. View Article : Google Scholar
|
|
21
|
Henness S, Davey MW, Harvie RM, et al:
Changes in gene expression associated with stable drug and
radiation resistance in small cell lung cancer cells are similar to
those caused by a single X-ray dose. Radiat Res. 161:495–503. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Bottke D, Koychev D, Busse A, et al:
Fractionated irradiation can induce functionally relevant multidrug
resistance gene and protein expression in human tumor cell lines.
Radiat Res. 170:41–48. 2008. View
Article : Google Scholar
|
|
23
|
Park JJ, Yi JY, Jin YB, et al: Sialylation
of epidermal growth factor receptor regulates receptor activity and
chemosensitivity to gefitinib in colon cancer cells. Biochem
Pharmacol. 83:849–857. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Grandjean F, Brémaud L, Verdier M, et al:
Sequential gene expression of P-glycoprotein (P-gp), multidrug
resistance-associated protein (MRP) and lung resistance protein:
functional activity of P-gp and MRP present in the
doxorubicin-resistant human K562 cell lines. Anticancer Drugs.
12:247–258. 2001. View Article : Google Scholar
|
|
25
|
Trussardi-Regnier A, Millot JM, Gorisse
MC, et al: Detection of drug-resistance genes using single
bronchoscopy biopsy specimens. Oncol Rep. 18:703–708.
2007.PubMed/NCBI
|
|
26
|
Ryu JS, Um JH, Kang CD, et al:
Fractionated irradiation leads to restoration of drug sensitivity
in MDR cells that correlates with down-regulation of P-gp and
DNA-dependent protein kinase activity. Radiat Res. 162:527–535.
2004. View
Article : Google Scholar : PubMed/NCBI
|
|
27
|
Shareef MM, Brown B, Shajahan S, et al:
Lack of P-glycoprotein expression by low-dose fractionated
radiation results from loss of nuclear factor-kappaB and NF-Y
activation in oral carcinoma cells. Mol Cancer Res. 6:89–98. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
McAllister KA, Lorimore SA, Wright EG and
Coates PJ: In vivo interactions between ionizing radiation,
inflammation and chemical carcinogens identified by increased DNA
damage responses. Radiat Res. 177:584–593. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Chen Z, Chua CC, Ho YS, et al:
Overexpression of Bcl-2 attenuates apoptosis and protects against
myocardial I/R injury in transgenic mice. Am J Physiol Heart Circ
Physiol. 280:H2313–2320. 2001.PubMed/NCBI
|
|
30
|
Langenau DM, Jette C, Berghmans S, et al:
Suppression of apoptosis by bcl-2 overexpression in lymphoid cells
of transgenic zebrafish. Blood. 105:3278–3285. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Buckley CD, Doyonnas R, Newton JP, et al:
Identification of alpha v beta 3 as a heterotypic ligand for
CD31/PECAM-1. J Cell Sci. 109:437–445. 1996.PubMed/NCBI
|
|
32
|
Dangerfield JP, Wang S and Nourshargh S:
Blockade of alpha6 integrin inhibits IL-1beta- but not
TNF-alpha-induced neutrophil transmigration in vivo. J Leukoc Biol.
77:159–165. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Riederer I, Sievert W, Eissner G, et al:
Irradiation-induced up-regulation of HLA-E on macrovascular
endothelial cells confers protection against killing by activated
natural killer cells. PLoS One. 5:e153392010. View Article : Google Scholar
|
|
34
|
Moriconi F, Malik I, Ahmad G, et al:
Effect of irradiation on gene expression of rat liver adhesion
molecules: in vivo and in vitro studies. Strahlenther Onkol.
185:460–468. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Liu Y, Yu H, Zhang C, et al: Protective
effects of berberine on radiation-induced lung injury via
intercellular adhesion molecular-1 and transforming growth
factor-beta-1 in patients with lung cancer. Eur J Cancer.
44:2425–2432. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Hashimoto H, Takeuchi T, Komatsu K, et al:
Structural basis for matrix metalloproteinase-2 (MMP-2)-selective
inhibitory action of β-amyloid precursor protein-derived inhibitor.
J Biol Chem. 286:33236–33243. 2011.PubMed/NCBI
|
|
37
|
Mitsiades N, Yu WH, Poulaki V, et al:
Matrix metalloproteinase-7-mediated cleavage of Fas ligand protects
tumor cells from chemotherapeutic drug cytotoxicity. Cancer Res.
61:577–581. 2001.PubMed/NCBI
|
|
38
|
Ohno S, Inagawa H, Dhar DK, et al: Role of
tumor-associated macrophages (TAM) in advanced gastric carcinoma:
the impact on FasL-mediated counterattack. Anticancer Res.
25:463–470. 2005.PubMed/NCBI
|
|
39
|
Holler N, Tardivel A,
Kovacsovics-Bankowski M, et al: Two adjacent trimeric Fas ligands
are required for Fas signaling and formation of a death-inducing
signaling complex. Mol Cell Biol. 23:1428–1440. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Fingleton B, Vargo-Gogola T, Crawford HC
and Matrisian LM: Matrilysin [MMP-7] expression selects for cells
with reduced sensitivity to apoptosis. Neoplasia. 3:459–468.
2001.
|
|
41
|
Alla V, Kashyap A, Gregor S, et al: Human
leukocyte elastase counteracts matrix metalloproteinase-7 induced
apoptosis resistance of tumor cells. Cancer Lett. 268:331–339.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Miyamoto S, Yano K, Sugimoto S, et al:
Matrix metalloproteinase-7 facilitates insulin-like growth factor
bioavailability through its proteinase activity on insulin-like
growth factor binding protein 3. Cancer Res. 64:665–671. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Di Stefano AB, Iovino F, Lombardo Y, et
al: Survivin is regulated by interleukin-4 in colon cancer stem
cells. J Cell Physiol. 225:555–561. 2010.PubMed/NCBI
|
|
44
|
Koller FL, Hwang DG, Dozier EA and
Fingleton B: Epithelial interleukin-4 receptor expression promotes
colon tumor growth. Carcinogenesis. 31:1010–1027. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Nimmanapalli R, Gerbino E, Dalton WS, et
al: HSP70 inhibition reverses cell adhesion mediated and acquired
drug resistance in multiple myeloma. Br J Haematol. 142:551–561.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Kang CM, Park KP, Cho CK, et al: Hspa4
(HSP70) is involved in the radioadaptive response: results from
mouse splenocytes. Radiat Res. 157:650–655. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Liu W and Wu S: Differential roles of
nitric oxide synthases in regulation of ultraviolet B light-induced
apoptosis. Nitric Oxide. 23:199–205. 2010. View Article : Google Scholar : PubMed/NCBI
|