Introduction
Melanoma ranks as one of the top 10 new types of
cancer diagnoses in American males and females (1). The worldwide incidence rate of
melanoma is higher than that of other common cancers and doubles
approximately every 10–20 years in the Caucasian populations, which
has resulted in the greatest ever number of skin cancer-related
mortalities (1–3). Furthermore, melanoma with
dissemination to distant sites and visceral organs is almost
invariably incurable, with a median survival time of 8–9 months and
a 3-year survival rate of 10–15% (4). Therefore, malignant melanoma is
considered to be one of the most aggressive cancers and has thus
become a substantial clinical challenge. It is imperative to
establish novel strategies for the treatment of malignant melanoma
that are able to improve the quality of life and survival of
patients.
Heat shock proteins (HSPs) are widely distributed in
nature and are among the most highly conserved molecules of the
biosphere. The concentrations of HSPs in non-stressed cells are
low, however they are high in stressed cells. Tumors are
highly-stressed collections of cells that are able to display or
express HSPs on the surface, while normal cells do not (5–8).
Recent research has revealed the role of HSPs in cancer (6,9–13).
In the presence of HSPs, tumors not only cope with stresses via the
cytoprotective activities of chaperones, but thrive with
upregulated chaperone expression and activity (13–15).
It has been reported that overexpression of HSP60 could be utilized
as a clinical assessment index (9–11,14–18).
As a member of the HSP60 family, HSP65 is able to
induce humoral and cellular immune responses (16) and plays important roles in antigen
presentation and cross-presentation (6,17–19).
In this study, we investigated the effects of a HSP65 vaccine on
the growth, angiogenesis and metastasis of B16-F10 melanoma cells
in tumor-bearing mice. The results demonstrated that the HSP65
vaccine was able to markedly inhibit the growth of B16-F10 melanoma
in vivo and prolong the survival of tumor-bearing mice,
suggesting that HSP65 has potential clinical significance.
Materials and methods
Preparation of protein and animals
The HSP65 protein was prepared using the same method
as previously described (20).Male
C57BL/6 mice, 5–6 weeks of age, were purchased from the Laboratory
Animal Center of Yangzhou University, China, and housed in plastic
cages under pathogen-free conditions on a 12-h light/12-h dark
schedule. All experiments were conducted according to the National
Institutes of Health Guide for the Care and Use of Laboratory
Animals and approved by the Institutional Animal Care and Use
Committee of China Pharmaceutical University (IACUC).
Tumor cell lines
The B16-F10 cell line was maintained in our
laboratory. Tumor cells were cultured in growth medium containing
Dulbecco’s modified Eagle’s medium (Gibco, Carlsbad, CA, USA)
supplemented with 10% heat-inactivated fetal bovine serum (FBS;
Gibco), 2 mmol/l glutamine, 100 U/ml penicillin (Gibco) and 100
μg/ml streptomycin at 37°C in a humidified atmosphere of 95% air/5%
CO2.
Immunization and tumor challenge
Male C57BL/6 mice, age 5–6 weeks, were randomly
divided into a phosphate-buffered saline (PBS) group and a HSP65
group (20 in each group). The HSP65 group were subcutaneously
injected into the left flank with purified protein HSP65 (50 μg in
a final volume of 100 μl PBS) 6 times at biweekly intervals.
Control mice were administered an equal volume of blank PBS. Sera
were collected weekly for immunoassay following the initial
immunization. Tumor challenge was performed by the subcutaneous
injection of murine melanoma cells (5×105 cells in 100
μl PBS) into the right flank of all mice on the 14th day following
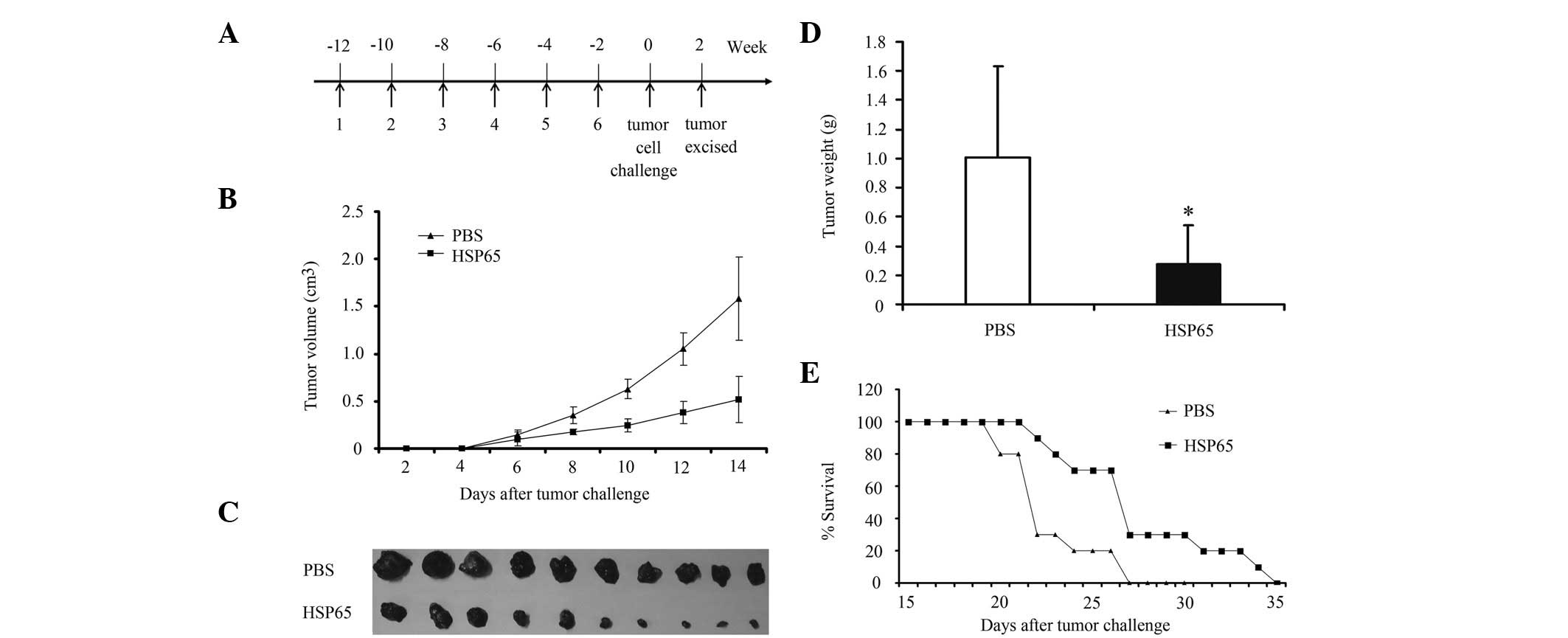
the last immunization (Fig. 1A).
Tumors were measured in two dimensions using a caliper every 2 days
following the tumor challenge (Fig.
1B). The tumor volume was calculated as follows: length ×
width2 × 0.4 (21). On
day 14 following the challenge of tumor cells, half of the mice (10
per group) were sacrificed for tumor weight analysis (Fig. 1C and D). Subsequently, formalin
fixation was performed to prepare for hematoxylin and eosin
(H&E) staining and immunohistochemical analysis. The other half
of the mice were used for the survival monitoring experiment
(Fig. 1E).
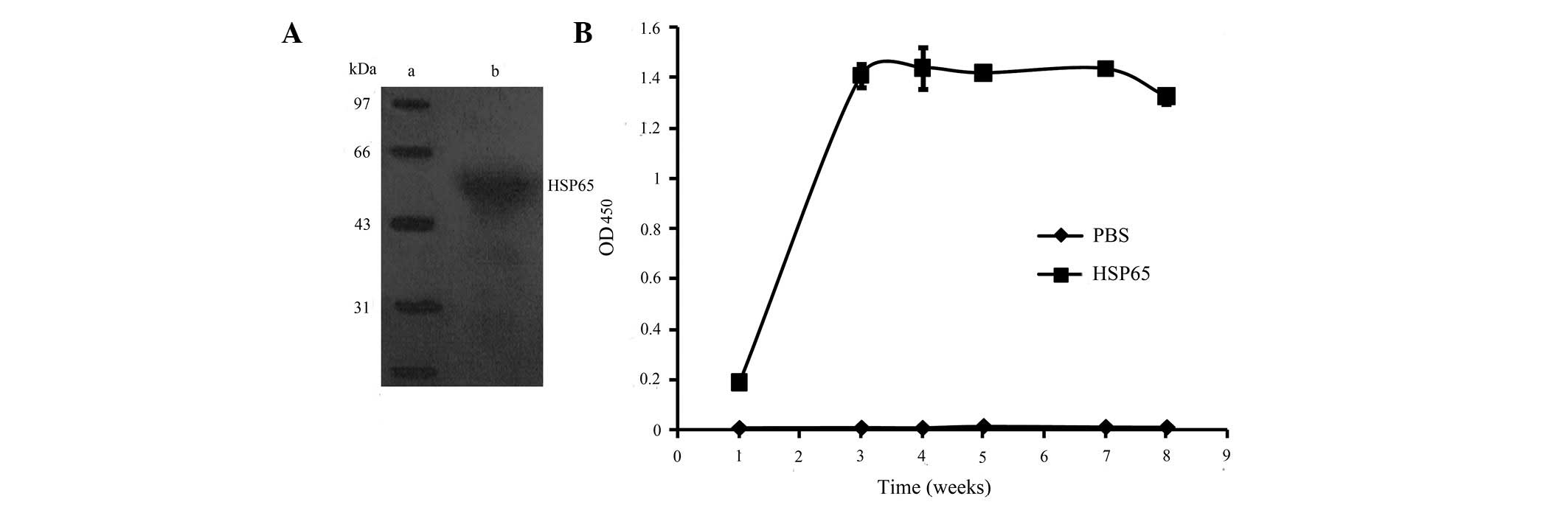
Western blot analysis for specificity of
anti-HSP65 antibodies
Western blot analysis was used to analyze the
specificity of the anti-HSP65 antibodies from the mice immunized
with HSP65. The purified protein HSP65 (in the presence of DTT) was
electrophoresed on a 12% sodium dodecyl sulfate polyacrylamide gel
electropheresis (SDS-PAGE) gel under denaturing conditions, and
then transferred to a nitrocellulose membrane (Millipore,
Billerica, MA, USA). The membrane was blocked with 5% bovine serum
albumin (BSA; Sigma, St. Louis, MO, USA), washed and probed with
mice sera at 1:50 for 1 h at 37°C, followed by utilization of
horseradish peroxidase (HRP)-conjugated goat anti-mouse IgG (Sigma)
(Fig. 2A). The reaction was
completed with 0.05% 3,3′-diaminobenzidine and 0.012%
H2O2 for 15 min at 37°C.
Enzyme-linked immunosorbent assay (ELISA)
for anti-HSP65 IgG
Humoral immune responses to HSP65 were measured
using an indirect ELISA as previously described (22). Briefly, 96-well flat-bottomed ELISA
plates (Corning Costar, Acton, MA, USA) were coated with 100
μl/well of HSP65 protein (50 μg/well) conjugated with 5% BSA in 0.1
mM carbonate-bicarbonate buffer and stored overnight at 4°C. Plates
were blocked with PBS containing 5% (w/v) BSA for 1 h and then
incubated with 100 μl/well 1:100 dilution of serum collected from
the immunized animals in PBS containing 2% BSA. Following
incubation for 1 h at 37°C, wells were washed with PBST (PBS
containing 0.1% Tween-20) three times, and then incubated with 100
μl/well of HRP-conjugated goat anti-mouse IgG (Sigma) diluted at
1:20,000 in PBS containing 1% BSA for 1 h at 37°C. Wells were
intensively washed 6 times in PBST and then incubated with 100
μl/well peroxidase substrate 0.01% 3,3′,5,5′-tetramethylbenzidine
(TMB) and 0.24% (w/v) H2O2-urea solubilized
in 0.2 M Na2HPO4 to 0.1 M citrate buffer (pH
5.5) for 20 min at 37°C. The reaction was terminated with 50
μl/well of 2 M H2SO4 and finally the optical
density 450 (OD450) value was measured using an ELISA reader
(Bio-Rad, Hercules, CA, USA) (Fig.
2B).
Tumor therapy efficacy of HSP65 protein
in C57BL/6 mice
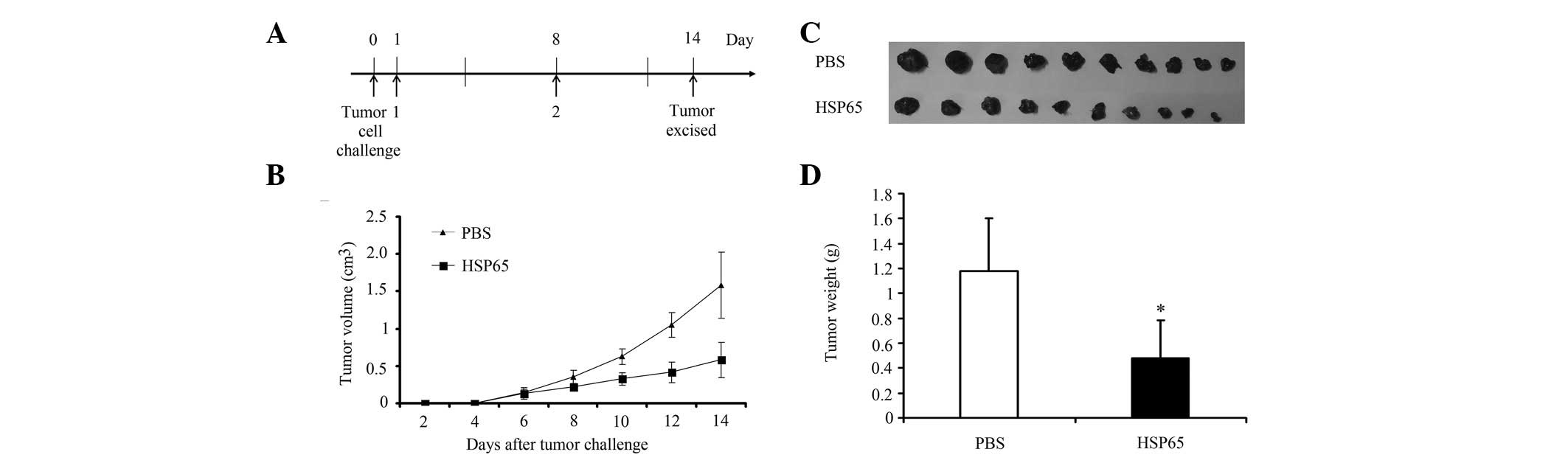
For tumor therapy experiments, two groups of C57BL/6
mice (10 per group) were injected subcutaneously into the right
flank with 5×105 of B16-F10 cells per mouse on day 0.
The HSP65 group of mice were immunized subcutaneously into the left
flank with the purified protein HSP65 (50 μg in a final volume of
100 μl PBS) on day 1. Booster injections were provided 1 week later
subcutaneously in the left flank with purified protein HSP65
(Fig. 3A). The control group was
administered an equal volume of blank PBS. Tumors were measured in
two dimensions using a caliper every 2 days following the tumor
challenge (Fig. 3B). All mice were
sacrificed for tumor weight analysis 1 week after the challenge of
tumor cells (Fig. 3C and D).
In vivo angiogenesis assay
To evaluate the effect of HSP65 on the
neovascularization of the B16-F10 tumor, an intradermal tumor model
was employed. In the model, neovasculature, discerned predominantly
at the tumor periphery, was quantified using the vessel counting
method as described by Kreisle (23). Briefly, 2 groups of mice (6 mice
per group) were vaccinated with PBS or HSP65 6 times at biweekly
intervals, respectively. An intracutaneous transplantation
experiment was then performed by injecting 5×105 B16-F10
cells in 50 μl of PBS into the mice at two sites in the abdominal
region 2 weeks after the last immunization (Fig. 4A). On day 7 after the tumor
implantation, all mice were sacrificed and the tumors were isolated
for neovascularization analysis (Fig.
4B). For vessel counting, sections containing tumors were
visualized using light microscopy (magnification, ×10), and the
total number of blood vessels (major vessels and branching points)
within a 1-cm2 area around each implant site was
determined.
Experimental lung metastasis model
To evaluate the influence of HSP65 on the pulmonary
metastasis of B16-F10 tumor, the tail intravenously-injected tumor
model was used. Two groups of mice (10 each group) were vaccinated
with PBS or HSP65 6 times at biweekly intervals, respectively.
B16-F10 melanoma cells (5×105 cells/mouse) were injected
into the tail veins of these mice 2 weeks after the last
immunization (Fig. 4C). On day 21
after the challenge of tumor cells, all the mice were sacrificed
for lung tumor analysis (Fig.
4D).
Statistical analysis
Data are expressed as the mean ± standard deviation
(SD). A Student’s t-test was used to calculate the significance
among the groups. P<0.05 was considered to indicate a
statistically significant difference.
Results
Western blot analysis of anti-HSP65
antibody
To determine the specificity of the antibodies from
the mice immunized with HSP65, HSP65 was processed for western blot
analysis using the antisera from mice vaccinated with HSP65.
Antibodies representing HSP65 (lane 2) suggested that the
antibodies specifically recognized the HSP65 antigen (Fig. 2A).
ELISA for anti-HSP65 IgG
In order to investigate whether the HSP65 protein
vaccine was able to enhance the immunogenicity of the anti-HSP65
vaccine, an ELISA assay was conducted to determine the
concentrations of anti-HSP65 antibodies in the sera collected from
the mice immunized with HSP65 and PBS, respectively. HSP65-specific
IgG antibody responses were evident in the mice immunized with
HSP65, exhibiting a peak 3 weeks after the vaccination (Fig. 2B). In contrast, no antibody was
elicited in the mice vaccinated with PBS. These observations
indicate that HSP65 was capable of inducing an intense immune
response.
Tumor protection efficacy of HSP65
To investigate whether HSP65 immunization was able
to inhibit the growth of B16-F10 tumors in mice, a B16-F10 tumor
model was utilized. Four weeks after the last immunization, half of
the mice (10 per group) were sacrificed and the tumors were removed
for tumor weight analysis. The growth of the tumor was monitored
for 2 weeks (Fig. 1A). HSP65
immunization significantly inhibited the growth of B16-F10 tumor in
mice (Fig. 1B). The mean tumor
weight of the mice immunized with HSP65 was significantly lower
than that of the PBS group (P<0.05; Fig. 1C and D). In the survival monitoring
experiment, all mice in the PBS group died, while 33.33% of the
mice remained alive in the group immunized with HSP65 day 27
post-tumor implantation, displaying a prolonged survival. These
results demonstrate that HSP65 effectively inhibited the growth of
B16-F10 cells and prolonged the survival of mice (Fig. 1E).
Therapeutic efficacy of HSP65 on
tumors
In order to determine the therapeutic effect of
HSP65, two groups of C57BL/6 mice (10 mice per group) were
inoculated with B16-F10 tumor cells (5×105/mouse) on day
0 (Fig. 3A). The growth of the
tumor was monitored for 2 weeks. In comparison with the PBS control
group, tumor growth was significantly inhibited in the mice
immunized with HSP65 (Fig. 3B).
The mean tumor weight of the mice immunized with HSP65 was
significantly lower than that of the PBS group (P<0.05; Fig. 3C and D).
Neovascularization inhibition
In order to evaluate the effect of HSP65
immunization on angiogenesis, a B16-F10 tumor model was applied. On
day 7 after the challenge of tumor cells, all the mice were
sacrificed for tumor analysis (Fig.
4A). For angiogenesis, the total number of blood vessels around
each implant site from the mice immunized with HSP65 was
significantly lower than that from the PBS group mice (Fig. 4B).
Pulmonary metastasis inhibition
The anti-metastatic effects of HSP65 were evaluated
in the tail intravenously-injected model of mice inoculated with
B16-F10 melanoma cells. On day 21 after the tumor cell challenge,
all mice were sacrificed for lung tumor analysis (Fig. 4C). In the PBS group, three of the
mice exhibited serious invasions of tumor cells in their lungs,
which were completely surrounded by black tumors (data not shown).
However, none of the HSP65 pretreated mice demonstrated the same
phenomena, and the lung weights of the HSP65 group were much lower
than those of the PBS group (P<0.05; Fig. 4D).
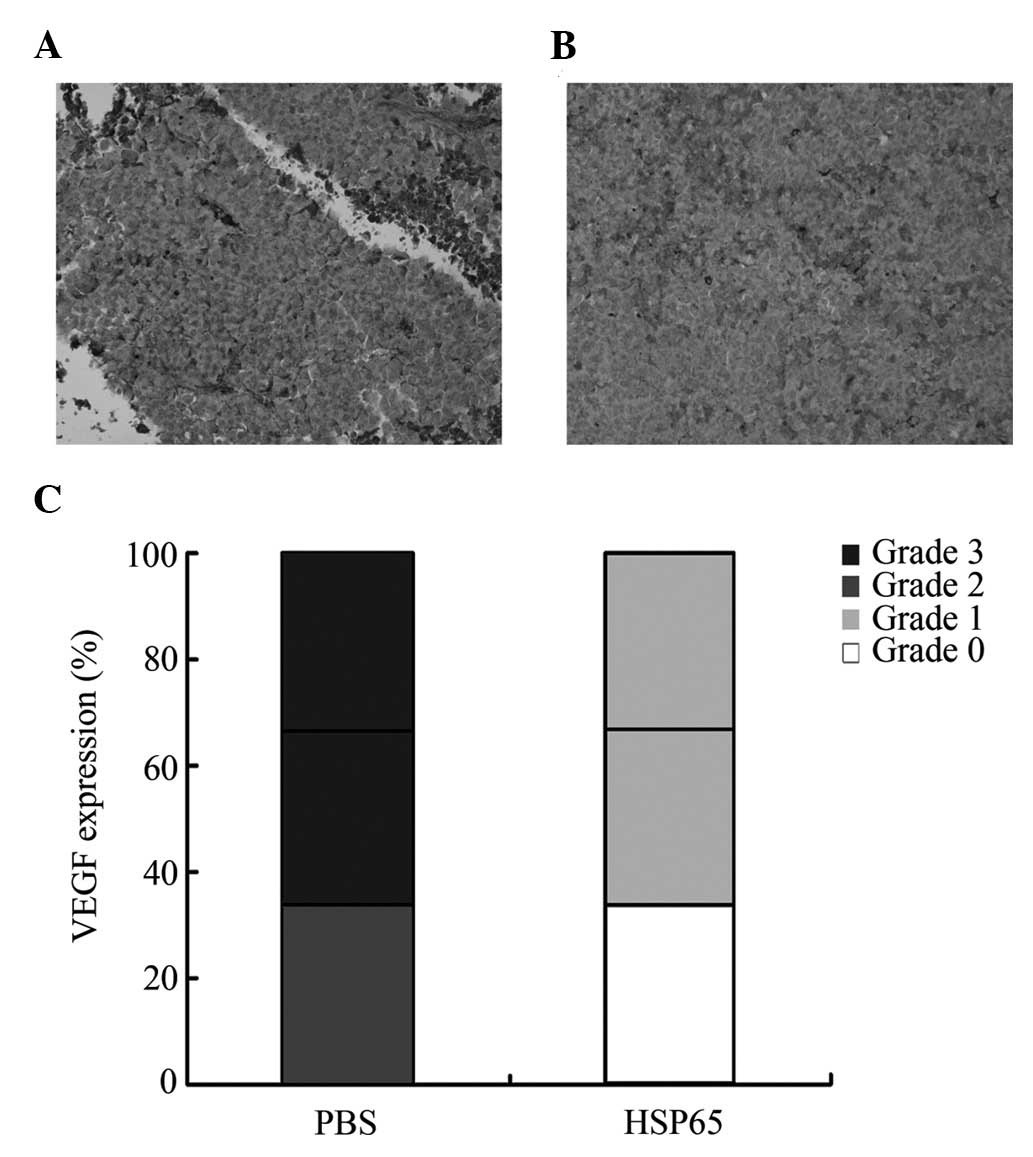
Photomicrographs of the pathological
slices of tumors
H&E staining of the liver, lungs, heart, kidneys
and spleen of the mice revealed no significant pathological changes
between the HSP65 group and the PBS group (data not shown).
Immunohistochemical analysis demonstrated that HSP65 immunization
downregulated vascular endothelial growth factor (VEGF) expression
in tumor tissues (Fig. 5).
Discussion
In this study, the antitumor effects and possible
antitumor mechanism of HSP65 were explored. Mice were immunized
with HSP65, and HSP65-specific antibodies were detected from the
immunized mice sera. The growth, angiogenesis and metastasis of
B16-F10 melanoma cells were significantly inhibited in vivo
by the administration of HSP65, and the survival of vaccinated mice
were prolonged as a result. VEGF expression was downregulated by
HSP65 vaccination. To our knowledge, this is the first study to
demonstrate that vaccination with the HSP65 protein inhibits the
growth of B16-F10 murine melanoma cells in mice.
HSP60 is overexpressed in the majority of tumor
cells (9–11,14–18).
It has been demonstrated that HSP60 accumulates in cancer cells and
is involved in their survival (13,14).
However, extracellular HSP60 mediates immunological functions to
induce a specific antitumor immune response that is able to reject
the tumor (19). HSPs have
remained almost unchanged throughout evolution, and greater than
50% of HSP65 is identical to HSP60 (19,24–27).
Owing to this homology, antibodies elicited against HSP65 are able
to cross-react with HSP60 (28).
Results from ELISA and western blot analysis verified that HSP65
was able to trigger effective immune responses, resulting in
specific anti-HSP65 IgG antibodies (Fig. 2). The possible antitumor mechanism
of HSP65 may be that the antibodies neutralized the elevated levels
of HSP60 issued from the tumor cells, thereby blocking the
activation of HSP60 in malignant melanoma cells.
Protein vaccination provides an attractive and
acceptable strategy for cancer immunotherapy, accompanied by
vaccine safety, cost-effectiveness and acceptance. A vaccination
strategy against tumors may be considered successful after the
demonstration of the inhibition of angiogenesis and metastasis of
tumors.
Metastasis of tumor cells is difficult to approach
in clinical therapeutics, as it induces cancer recurrence following
surgery and may lead to the mortality of cancer patients. Malignant
melanoma is among the most common causes accounting for ‘metastatic
cancer of unknown primary’ (29),
and has been well-documented as an angiogenic tumor (30,31).
Angiogenesis is critical for tumor growth and metastasis (2,2–34),
and the use of anti-angiogenic agents is becoming feasible as the
fourth cancer treatment following surgery, chemotherapy and
radiotherapy (2).
Angiogenic signaling, which plays a critical role in
melanoma, is mediated by pathways of growth factor receptors,
including VEGF (35). VEGF is a
glycosylated, multifunctional cytokine that is abundantly expressed
and secreted by the majority of human and animal tumors. It is
considered to play essential roles in the migration, proliferation
and differentiation of endothelial cells in the tumor environment
(32,36,37),
and in the stimulation of angiogenesis and tumor growth (37). VEGF has been revealed to be
stimulated by intracellular HSP60 (38), and thus could be inhibited by
extracellular HSP65/60 immunointervention, as observed in this
study. Photomicrographs of pathological sections from tumors in
this study demonstrate that VEGF expression in the tumors of
HSP65-preimmunized mice were downregulated (Fig. 5), revealing that vaccination with
HSP65 participated in the inhibition of tumor-induced angiogenesis
and tumor growth (Fig. 4). As a
result, the growth of the solid tumor of B16-F10 was remarkably
inhibited by HSP65 vaccination (Figs.
1 and 3), which was
demonstrated to be highly efficacious against B16-F10 tumors in
vivo.
In conclusion, it has been preliminarily
demonstrated that administration of the HSP65 protein is able to
effectively inhibit the growth of B16-F10 melanoma in vivo.
Further studies are required to investigate the anti-angiogenic
mechanism of HSP65 and elucidate the underlying mechanism of HSP65
against melanoma.
Acknowledgements
This study was supported by the China National
Natural Science Fund Committee (Grant nos. 30772570, 30672464,
30701023, 30500458 and 30872393), the Natural Science Foundation of
Jiangsu Province (No. BK 2007170), and the Fundamental Research
Funds for the Central Universities (Program nos. JKY2009021 and
JKQ2009022).
References
|
1
|
Jemal A, Siegel R, Xu J and Ward E: Cancer
statistics, 2010. CA Cancer J Clin. 60:277–300. 2010. View Article : Google Scholar
|
|
2
|
Emmett MS, Dewing D and Pritchard-Jones
RO: Angiogenesis and melanoma - from basic science to clinical
trials. Am J Cancer Res. 1:852–868. 2011.PubMed/NCBI
|
|
3
|
Carlson JA, Slominski A, Linette GP, et
al: Malignant melanoma 2003: predisposition, diagnosis, prognosis,
and staging. Am J Clin Pathol. 120(Suppl): S101–S127.
2003.PubMed/NCBI
|
|
4
|
Eggermont AM: Advances in systemic
treatment of melanoma. Ann Oncol. 21(Suppl 7): vii339–344. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Griguer CE, Oliva CR, Kelley EE, Giles GI,
Lancaster JR Jr and Gillespie GY: Xanthine oxidase-dependent
regulation of hypoxia-inducible factor in cancer cells. Cancer Res.
66:2257–2263. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Schmitt E, Gehrmann M, Brunet M, Multhoff
G and Garrido C: Intracellular and extracellular functions of heat
shock proteins: repercussions in cancer therapy. J Leukoc Biol.
81:15–27. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Graner MW, Cumming RI and Bigner DD: The
heat shock response and chaperones/heat shock proteins in brain
tumors: surface expression, release, and possible immune
consequences. J Neurosci. 27:11214–11227. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Eustace BK and Jay DG: Extracellular roles
for the molecular chaperone, hsp90. Cell Cycle. 3:1098–1100. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Xu X, Wang W, Shao W, et al: Heat shock
protein-60 expression was significantly correlated with the
prognosis of lung adenocarcinoma. J Surg Oncol. 104:598–603. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lin C-S, He P-J, Tsai N-M, et al: A
potential role for Helicobacter pylori heat shock protein 60
in gastric tumorigenesis. Biochem Biophys Res Commun. 392:183–189.
2010.
|
|
11
|
Hwang YJ, Lee SP, Kim SY, et al:
Expression of heat shock protein 60 kDa is upregulated in cervical
cancer. Yonsei Med J. 50:399–406. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Cappello F, David S, Rappa F, et al: The
expression of HSP60 and HSP10 in large bowel carcinomas with lymph
node metastase. BMC Cancer. 5:1392005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kamal A, Boehm MF and Burrows FJ:
Therapeutic and diagnostic implications of Hsp90 activation. Trends
Mol Med. 10:283–290. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Garrido C, Schmitt E, Cande C, Vahsen N,
Parcellier A and Kroemer G: HSP27 and HSP70: potentially oncogenic
apoptosis inhibitors. Cell Cycle. 2:579–584. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Sedlackova L, Spacek M, Holler E,
Imryskova Z and Hromadnikova I: Heat-shock protein expression in
leukemia. Tumour Biol. 32:33–44. 2010. View Article : Google Scholar
|
|
16
|
Xiong Q, Jin L, Li J, et al: A Th2 immune
shift to heat shock protein 65 fails to arrest atherosclerosis:
proatherogenic role of Th2-deviated autoantibodies. Autoimmunity.
42:475–483. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ausiello CM, Fedele G, Palazzo R,
Spensieri F, Ciervo A and Cassone A: 60-kDa heat shock protein of
Chlamydia pneumoniae promotes a T helper type 1 immune
response through IL-12/IL-23 production in monocyte-derived
dendritic cells. Microbes Infect. 8:714–720. 2006.PubMed/NCBI
|
|
18
|
Chen K, Lu J, Wang L and Gan YH:
Mycobacterial heat shock protein 65 enhances antigen
cross-presentation in dendritic cells independent of Toll-like
receptor 4 signaling. J Leukoc Biol. 75:260–266. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhao Y, Yokota K, Ayada K, et al:
Helicobacter pylori heat-shock protein 60 induces
interleukin-8 via a Toll-like receptor (TLR)2 and mitogen-activated
protein (MAP) kinase pathway in human monocytes. J Med Microbiol.
56:154–164. 2007. View Article : Google Scholar
|
|
20
|
Jin L, Wang Y, Xiong Q, et al:
Long-lasting specific antibodies against P277 induced by mucosal
administration of P277 repeat sequences carried by Hsp65 in the
absence of adjuvants. Vaccine. 25:2043–2050. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lu Y, Ouyang K, Fang J, et al: Improved
efficacy of DNA vaccination against prostate carcinoma by boosting
with recombinant protein vaccine and by introduction of a novel
adjuvant epitope. Vaccine. 27:5411–5418. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Guojun W, Wei G, Kedong O, et al: A novel
vaccine targeting gastrin-releasing peptide: efficient inhibition
of breast cancer growth in vivo. Endocr Relat Cancer. 15:149–159.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kreisle RA and Ershler WB: Investigation
of tumor angiogenesis in an id mouse model: role of host-tumor
interactions. J Natl Cancer Inst. 80:849–854. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Xiong Q, Li J, Jin L, Liu J and Li T:
Nasal immunization with heat shock protein 65 attenuates
atherosclerosis and reduces serum lipids in cholesterol-fed
wild-type rabbits probably through different mechanisms. Immunol
Lett. 125:40–45. 2009. View Article : Google Scholar
|
|
25
|
Marcatili A, Cipollaro de l’Ero G,
Galdiero M, Folgore A and Petrillo G: TNF-alpha, IL-1 alpha, IL-6
and ICAM-1 expression in human keratinocytes stimulated in vitro
with Escherichia coli heat-shock proteins. Microbiology.
143(Pt 1): 45–53. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lillicrap MS, Duggleby RC, Goodall JC and
Gaston JS: T cell recognition of a highly conserved epitope in heat
shock protein 60: self-tolerance maintained by TCR distinguishing
between asparagine and aspartic acid. Int Immunol. 16:405–414.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Hartl FU and Hayer-Hartl M: Molecular
chaperones in the cytosol: from nascent chain to folded protein.
Science. 295:1852–1858. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Verdegaal ME, Zegveld ST and van Furth R:
Heat shock protein 65 induces CD62e, CD106, and CD54 on cultured
human endothelial cells and increases their adhesiveness for
monocytes and granulocytes. J Immunol. 157:369–376. 1996.PubMed/NCBI
|
|
29
|
Chin L, Garraway LA and Fisher DE:
Malignant melanoma: genetics and therapeutics in the genomic era.
Genes Dev. 20:2149–2182. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ferrara N: Role of myeloid cells in
vascular endothelial growth factor-independent tumor angiogenesis.
Curr Opin Hematol. 17:219–224. 2010.PubMed/NCBI
|
|
31
|
Shojaei F, Zhong C, Wu X, Yu L and Ferrara
N: Role of myeloid cells in tumor angiogenesis and growth. Trends
Cell Biol. 18:372–378. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Yang L, Chen G, Mohanty S, et al: GPR56
regulates VEGF production and angiogenesis during melanoma
progression. Cancer Res. 71:5558–5568. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Folkman J: What is the evidence that
tumors are angiogenesis dependent? J Natl Cancer Inst. 82:4–6.
1990. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Carmeliet P and Jain RK: Angiogenesis in
cancer and other diseases. Nature. 407:249–257. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Sekulic A, Haluska P Jr, Miller AJ, et al:
Malignant melanoma in the 21st century: the emerging molecular
landscape. Mayo Clin Proc. 83:825–846. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Carmeliet P: VEGF as a key mediator of
angiogenesis in cancer. Oncology. 69(Suppl 3): 4–10. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Verheul HM, Hammers H, van Erp K, et al:
Vascular endothelial growth factor trap blocks tumor growth,
metastasis formation, and vascular leakage in an orthotopic murine
renal cell cancer model. Clin Cancer Res. 13:4201–4208. 2007.
View Article : Google Scholar
|
|
38
|
Pugh CW and Ratcliffe PJ: Regulation of
angiogenesis by hypoxia: role of the HIF system. Nat Med.
9:677–684. 2003. View Article : Google Scholar : PubMed/NCBI
|