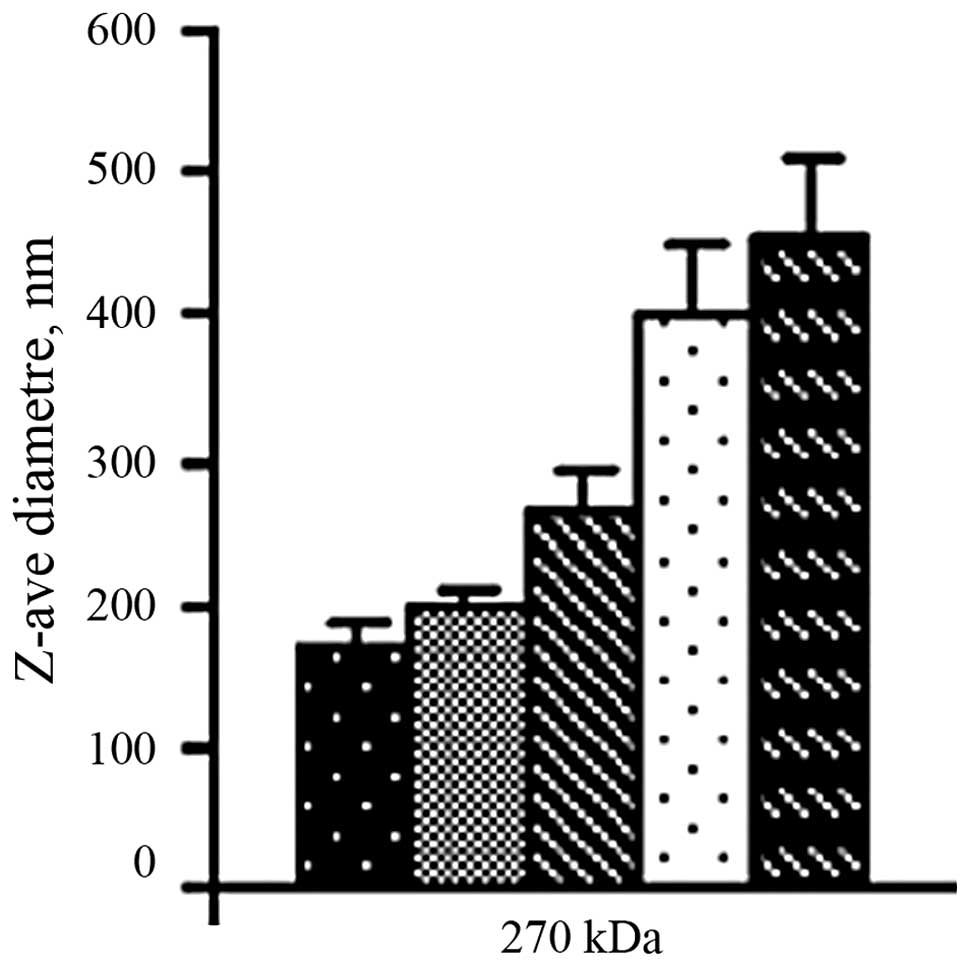
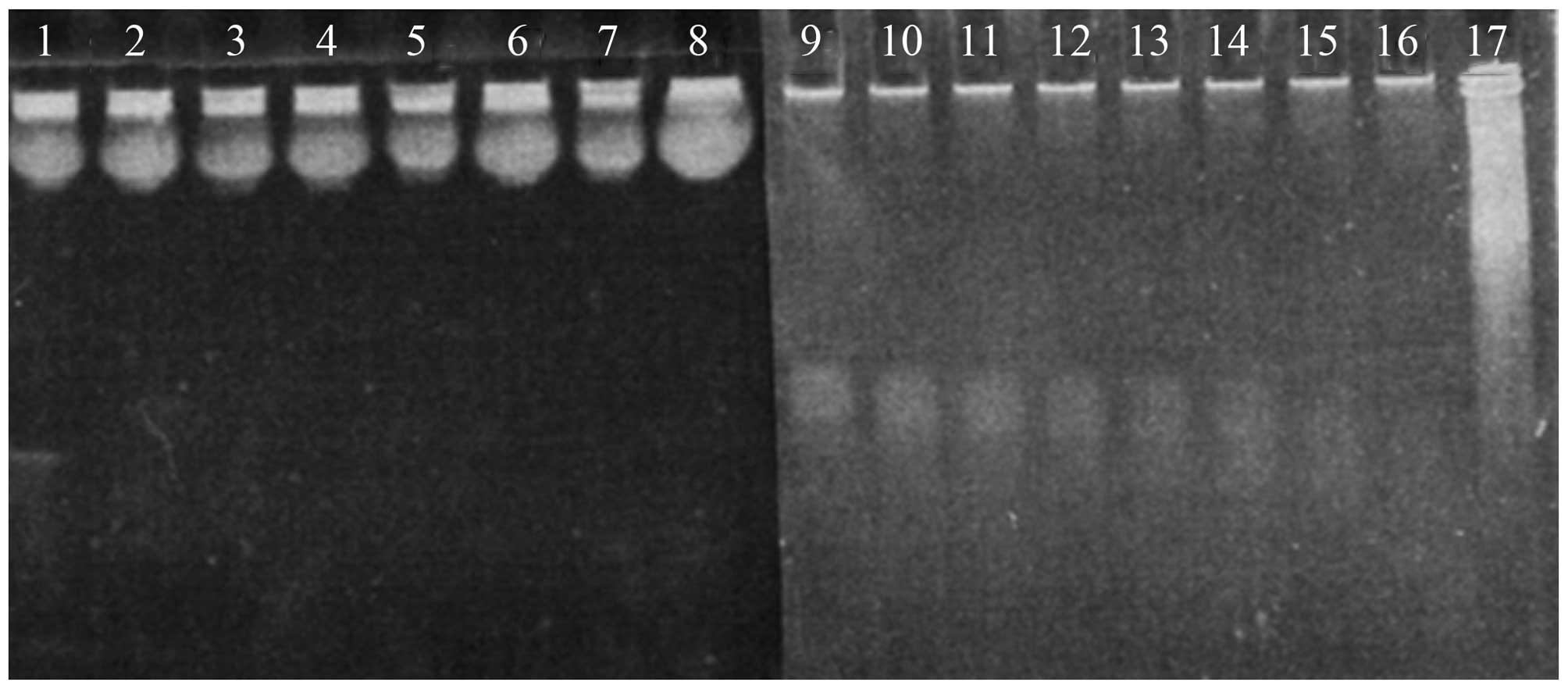
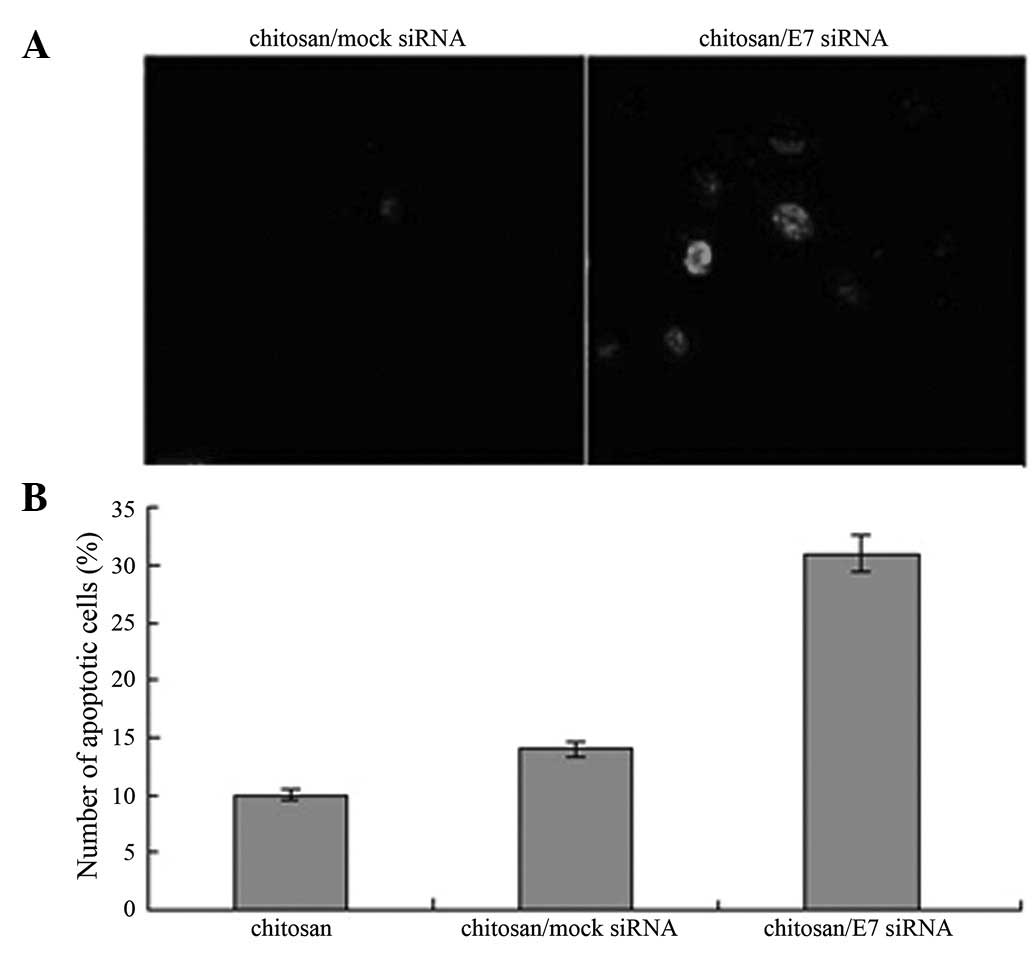
|
1
|
Ma B, Roden R and Wu TC: Current status of
human papillomavirus vaccines. J Formos Med Assoc. 109:481–483.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Shao J, Chen ZY, Wang J and Ma R:
Quantification of human papilloma virus DNA in the plasma of
patients with cervical cancer. Clin J Surg Oncol. 2:288–291.
2010.
|
|
3
|
Qiao YL, Zhang WH, Li L, et al: The
cross-sectional comparative research of cervical cancer gene
screening method. Acta Acad Med Sin. 24:50–53. 2002.
|
|
4
|
Liu X, Roberts J, Dakic A, Zhang Y and
Scheleqel R: HPV E7 contributes to the telomerase activity of
immortalized and tumorigenic cells and augments E6-induced hTERT
promoter function. Virology. 375:611–623. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Chu NR, Wu HB, Wu T, Boux LJ, Sieqel MI
and Mizzen LA: Immunotherapy of a human papillomavirus (HPV) type
16 E7-expressing tumour by administration of fusion protein
comprising mycobacterium bovis bacille calmette-guerin (BCG) hsp65
and HPV16 E7. Clin Exp Immunol. 121:216–225. 2000. View Article : Google Scholar
|
|
6
|
Kaufmann AM, Stern PL, Rankin EM, et al:
Safety and immunogenicity of TA-HPV, a recombinant vaccinia virus
expressing modified human papillomavirus (HPV)-16 and HPV-18 E6 and
E7 genes, in women with progressive cervical cancer. Clin Cancer
Res. 8:3676–3685. 2002.
|
|
7
|
Veldman T, Horikawa I, Barrett JC and
Schleqel R: Transcriptional activation of the telomerase hTERT gene
by human papillomavirus type 16 E6 oncoprotein. J Virol.
75:4467–4472. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Nishimura A, Nakahara T, Ueno T, et al:
Requirement of E7 oncoprotein for viability of HeLa cells. Microbes
Infect. 8:984–993. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Dyson N, Howley PM, Münger K and Harlow E:
The human papilloma virus-16 E7 oncoprotein is able to bind to the
retinoblastoma gene product. Science. 243:934–937. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Boyer SN, Wazer DE and Band V: E7: protein
of human papilloma virus-16 induces degradation of retinoblastoma
protein through the ubiquitin-proteasome pathway. Cancer Res.
56:4620–4624. 1996.PubMed/NCBI
|
|
11
|
Gonzalez SL, Stremlau M, He X, Basile JR
and Münqer K: Degradation of the retinoblastoma tumor suppressor by
the human papillomavirus type 16 E7 oncoprotein is important for
functional inactivation and is separable from proteasomal
degradation of E7. J Virol. 75:7583–7591. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Gammoh N, Grm HS, Massimi P and Banks L:
Regulation of human papillomavirus type 16 E7 activity through
direct protein interaction with the E2 transcriptional activator. J
Virol. 80:1787–1797. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhang B, Chen W and Roman A: The E7
proteins of low- and high-risk human papillomaviruses share the
ability to target the pRB family member p130 for degradation. Proc
Natl Acad Sci USA. 103:437–442. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Caldeira S, Dong W and Tommasino M:
Analysis of E7/Rb associations. Methods Mol Med. 119:363–379.
2005.PubMed/NCBI
|
|
15
|
Wu EW, Clemens KE, Heck DV and Münger K:
The human papillomavirus E7 oncoprotein and the cellular
transcription factor E2F bind to separate sites on the
retinoblastoma tumor suppressor protein. J Virol. 67:2402–2407.
1993.
|
|
16
|
Alani RM and Münger K: Human
papillomaviruses and associated malignancies. J Clin Oncol.
116:330–337. 1998.
|
|
17
|
Cobrinik D: Pocket proteins and cell cycle
control. Oncogene. 24:2796–2809. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Dimova DK and Dyson NJ: The E2F
transcriptional network: old acquaintances with new faces.
Oncogene. 24:2810–2826. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Helt AM and Galloway DA: Destabilization
of the retinoblastoma tumor suppressor by human papillomavirus type
16 E7 is not sufficient to overcome cell cycle arrest in human
keratinocytes. J Virol. 75:6737–6747. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wang J, Sampath A, Raychaudhuri P and
Baqchi S: Both Rb and E7 are regulated by the ubiquitin proteasome
pathway in HPV-containing cervical tumor cells. Oncogene.
20:4740–4749. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Chen M, Gao S, Dong M, et al:
Chitosan/siRNA nanoparticles encapsulated in PLGA nanofibers for
siRNA delivery. ACS Nano. 6:4835–4844. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Holzerny P, Ajdini B, Heusermann W, Bruno
K, Schuleit M, Meinel L and Keller M: Biophysical properties of
chitosan/siRNA polyplexes: profiling the polymer/siRNA interactions
and bioactivity. J Control Release. 157:297–304. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Guo C, Liu K, Zheng Y, Luo H, Chen H and
Huang L: Apoptosis induced by an antagonist peptide against HPV16
E7 in vitro and in vivo via restoration of p53. Apoptosis.
16:606–618. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Shu XZ and Zhu KJ: The influence of
multivalent phosphate structure on the properties of ionically
cross-linked chitosan films for controlled drug release. Eur J
Pharm Biopharm. 54:235–243. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Gref R, Domb A, Quellec P, Blunkc T,
Müllerd RH, Verbavatze JM and Langerf R: The controlled intravenous
delivery of drugs using PEG-coated sterically stabilized
nanospheres. Adv Drug Deliv Rev. 16:215–233. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Bivas-Benita M, Romeijn S, Junginger HE
and Borchard G: PLGA-PEI nanoparticles for gene delivery to
pulmonary epithelium. Eur J Pharm Biopharm. 58:1–6. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Panyam J and Labhasetwar V: Biodegradable
nanoparticles for drug and gene delivery to cells and tissue. Adv
Drug Deliv Rev. 55:329–347. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zauner W, Farrow NA and Haines AM: In
vitro uptake of polystyrene microspheres: effect of particle size,
cell line and cell density. J Control Release. 71:39–51. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Huang M, Fong CW, Khor E and Lim LY:
Transfection efficiency of chitosan vectors: effect of polymer
molecular weight and degree of deacetylation. J Control Release.
106:391–406. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Chan V, Mao HQ and Leong KW: Effect of
chitosan molecular weight in gene delivery process. Abs Pap Am Chem
Soc. 221:U347. 2001.
|
|
31
|
Rozema DB and Lewis DL: siRNA delivery
technologies for mammalian systems. Targets. 2:253–260. 2003.
View Article : Google Scholar
|
|
32
|
Keller M: Lipidic carriers of RNA/DNA
oligonucleotides and polynucleotides: what a difference a
formulation makes. J Control Release. 103:537–540. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Tam P, Monck M, Lee D, et al: Stabilized
plasmid-lipid particles for systemic gene therapy. Gene Ther.
7:1867–1874. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Bloomfield VA, He S, Li AZ and Arscott PB:
Light scattering studies on DNA condensation. Biochem Soc Trans.
19:4961991.PubMed/NCBI
|
|
35
|
Bloomfield VA: Condensation of DNA by
multivalent cations: considerations on mechanism. Biopolymers.
31:1471–1481. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Bloomfield VA: DNA condensation. Curr Opin
Struct Biol. 6:334–341. 1996. View Article : Google Scholar
|
|
37
|
Chang JT, Kuo TF, Chen YJ, et al: Highly
potent and specific siRNAs against E6 or E7 genes of HPV16- or
HPV18-infected cervical cancers. Cancer Gene Ther. 17:827–836.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Sima N, Wang W, Kong D, et al: RNA
interference against HPV16 E7 oncogene leads to viral E6 and E7
suppression in cervical cancer cells and apoptosis via upregulation
of Rb and p53. Apoptosis. 13:273–281. 2008. View Article : Google Scholar : PubMed/NCBI
|