Introduction
Previous studies have shown that radiation therapy
can cause unavoidable post-radiation impairments, such as
osteopenia and osteoporosis. Skeletal complications of radiation
therapy have been described in breast, brain and pelvic cancer as
well as in leukemia (1–4). Osteoclast precursors have the
potential to differentiate into osteoclasts and are hypersensitive
to radiation. We hypothesized that irradiated osteoclast precursors
are associated with bone loss caused by radiation. To date, little
information is available on the effects of irradiated osteoclast
precursors on osteoclast dysfunction.
RAW264.7 cells are mouse monocyte/macrophage cells;
they are regarded as osteoclast precursors (5) and differentiate into
tartrate-resistant acid phosphatase (TRAP)-positive multinuclear
osteoclasts following treatment with the nuclear factor (NF)-κB
ligand (RANKL) (6–8).
To investigate the role of irradiated osteoclast
precursors in the formation of abnormal osteoclasts, RAW264.7 cells
were irradiated and differentiated into osteoclasts in
vitro. In the present study, quantitative real-time polymerase
chain reaction (QRT-PCR) was used to assess the expression of a
panel of osteoclast marker genes.
Materials and methods
Cell culture
RAW264.7 cells were obtained from the Cell Bank of
the Institute of Basic Medicine at the Chinese Academy of Medical
Science (Beijing, China) and maintained in Dulbecco's modified
Eagle's medium (DMEM; Invitrogen), which was supplemented with 10%
heat-inactivated fetal bovine serum (FBS; Gibco) and 100 μg/ml of
penicillin/streptomycin, in a humidified atmosphere of 5%
CO2 at 37˚C, until reaching 80% confluency. The medium
was changed every 3 days.
RAW264.7 cells were divided into 4 groups: Group A,
normal RAW264.7 cells used as the control group; group B, RAW264.7
cells cultivated in the presence of 50 ng/ml of RANKL (PeproTech)
for osteoclast formation (9);
group C, RAW264.7 cells exposed to 2-Gy γ-rays to irradiate the
osteoclast precursor cells; and group D, RAW264.7 cells treated
with both 2-Gy γ-rays and 50 ng/ml of RANKL to induce osteoclast
formation with radiation damage. All groups were maintained for 7
days. As a radiation source, 137Cs was used.
Assessment of TRAP-positive cells
The TRAP kit was purchased from the Science and
Technology Company of the Institute of Hematology at the Chinese
Academy of Medical Sciences (Tianjin, China). After the cells were
fixed with paraformaldehyde and incubated with the TRAP solution at
37˚C for 1 h, the cells were washed in distilled water and
counterstained with hematoxylin. Multinucleated TRAP-positive cells
were observed using an inverted phase contrast microscope, and
their images were captured.
RNA extraction, reverse transcription and
QRT-PCR analysis
Total RNA was extracted using the TRIzol reagent
(Invitrogen). First-strand cDNA was synthesized using the reverse
transcription kit (Takara) with total RNA (4 μg). QRT-PCR analyses
for TRAP, calcitonin receptor (CTR), integrin β3 and receptor
activator of NF-κB (RANK) were performed using the ABI Prism 7000
sequence detection system (Applied Biosystems) with
Platinum® SYBR-Green qPCR SuperMix-UDG (Invitrogen). The
reaction conditions were: 50˚C for 2 min and 95˚C for 10 min,
followed by 40 cycles of 95˚C for 30 sec and 60˚C for 45 sec. The
levels of β-actin mRNA were used as the internal control, and the
gene-specific mRNA expression was normalized against β-actin
expression. The sequences of the primers used in these analyses are
listed in Table I.
 | Table ISequences of primers for quantitative
real-time polymerase chain reaction. |
Table I
Sequences of primers for quantitative
real-time polymerase chain reaction.
| Target | Primers
(5′–3′) |
|---|
| TRAP | Forward:
AGGACGTGTTCTCTGACCG
Reverse: CGCAAACGGTAGTAAGGG |
| CTR | Forward:
TAGGAGGTGGAGGATAGC
Reverse: TGACTTGGTGTTGAGGAC |
| Integrin β3 | Forward:
CCTTCGGATTGGCTTTGG
Reverse: TCATTGAAGCGGGACACC |
| RANK | Forward:
GTCTGCAGCTCTTCCATG
Reverse: TCCCTTCCTGTAGTAAACG |
| β-actin | Forward:
GGGTGTGATGGTGGGAATG
Reverse: CTCATTGTAGAAGGTGTGGTGC |
Statistical analysis
Data are presented as the means ± SD. The data were
compared using two-tailed unpaired Student's t-test (SPSS 13.0 for
Windows). P<0.05 was indicative of a statistically significant
difference.
Results
Morphological features of osteoclasts
derived from RAW264.7 cells
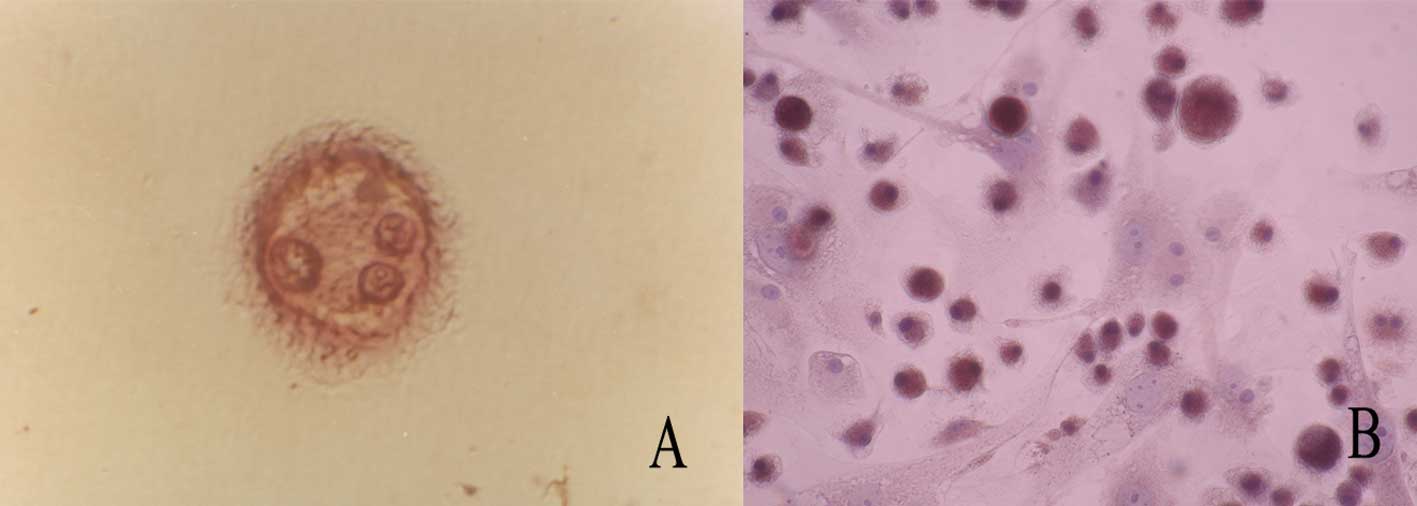
After RAW264.7 cells were plated in flasks and
treated with RANKL for 7 days, they were stained with TRAP and
counterstained with hematoxylin. The adherent osteoclasts displayed
a ruffled membrane (Fig. 1A),
pseudopodia and a multinuclei phenotype (Fig. 1B).
Expression of TRAP, CTR, integrin β3 and
RANK mRNA
To examine the effect of RANKL on the initial
proliferation of osteoclast precursor cells, RAW264.7 cells were
grown in the presence of 50 ng/ml of RANKL for up to 7 days.
RANKL-induced osteoclast precursor cells had increased TRAP and
RANK expression and decreased CTR expression compared to the
control cells not treated with RANKL (Fig. 2).
To investigate the effect of radiation on osteoclast
precursor cells, RAW264.7 cells were exposed to 2-Gy γ-rays and
maintained without RANKL for up to 7 days. These osteoclast
precursor cells had upregulated integrin β3 and RANK expression and
downregulated CTR expression compared to the control cells
(Fig. 2).
To evaluate the effect of radiation on RANKL-induced
osteoclast differentiation, we compared an expression panel of
osteoclast marker genes in RAW264.7 cells that were treated with
RANKL alone or in combination with radiation. The irradiation of
RANKL-induced osteoclasts led to an increased expression of CTR and
a decreased expression of RANK and TRAP compared to the
non-irradiated RANKL-induced osteoclasts (Fig. 2).
Discussion
Bone metabolism is a dynamic and continuous
remodeling process that is normally maintained in a tightly coupled
balance between resorption of old or injured bone and the formation
of new bone. This coordinated regulation of bone-forming cells
(osteoblasts) and bone-resorbing cells (osteoclasts) is regulated
by a complex network of cytokines, cell surface receptors and
various signaling pathways (10).
Osteoclasts are derived from hematopoietic progenitor cells of a
monocyte/macrophage lineage and multinucleated cells that degrade
the mineralized bone matrix (11).
Recent studies have identified activated osteoclasts as a
pathological feature of osteopenia and osteoporosis (12). Osteoclast precursors synthesize DNA
and proliferate. Therefore, osteoclast precursors are more
vulnerable to radiation injury than osteoclasts. Little attention
has been given to evaluating the effects of radiation on osteoclast
precursor function.
TRAP, a metallophosphatase that is highly expressed
in osteoclasts, is secreted into the resorption lacuna and is
associated with the resorbing matrix (13–16).
TRAP expression is dramatically upregulated during osteoclast
differentiation. Hence, TRAP activity is commonly used as a
histochemical marker of osteoclasts (17). When activated by proteolytic
processing, TRAP exhibits protein phosphatase activity toward
several bone matrix proteins, including osteopontin (15,16).
In accordance with previous reports (18), our results show that RANKL-induced
RAW264.7 cells had increased TRAP expression (group B). We
demonstrated that the expression of TRAP was not significantly
different between groups A and C, suggesting that radiation may
have little impact on TRAP expression in osteoclast precursors. We
initially hypothesized that TRAP expression in irradiated mature
osteoclasts may be higher compared to non-irradiated counterparts
according to skeletal complications in patients receiving
radiotherapy. Notably, group D, which comprised RANKL-induced
RAW264.7 cells that were irradiated with 2-Gy 137Cs
γ-rays, showed lower levels of TRAP than group B, in which the
cells were cultivated in the presence of 50 ng/ml of RANKL for
osteoclast formation. These results indicate that radiation may
have a negative effect on TRAP expression, although the underlying
mechanism remains unknown.
As part of bone remodeling, osteoclasts bind to the
bone matrix, form an actin ring-mediated sealing zone, secrete
enzymes and acid to degrade the bone and then migrate to a new
site. Each of these functions is regulated in part by integrins
that are located on the membrane surface of the osteoclast and
interact with neighboring cells and the extracellular matrix
(19). The predominant integrin in
osteoclasts is αvβ3. Antibody inhibition of αvβ3 inhibits
osteoclast attachment to the bone matrix and osteoclast-mediated
bone resorption (20). The β3
subunit, which is a component of αIIβ3 and αvβ3 integrins, plays an
important role during early fracture healing (21). Our findings indicate that
irradiated RAW264.7 cells expressed significantly higher levels of
integrin β3 than the control group. However, the expression levels
of integrin β3 between groups B and D showed no significant
differences. These results suggest that irradiation promotes the
activity of osteoclast precursor cells, but not that of
osteoclasts.
In the bone, CTR is a specific marker of osteoclasts
(22–25), particularly osteoclast
differentiation (26), and
osteoclasts are normally associated with osteolysis. The binding of
calcitonin to its receptor is known to dampen osteoclast activation
(27). We found that cells in the
RANKL-induced group and the irradiated group expressed lower levels
of CTR than those in the control group, suggesting that mature
osteoclasts express lower levels of CTR than osteoclast precursors.
However, cells in the group receiving RANKL in combination with
radiation treatment expressed higher levels of CTR compared to
those in the control group. Our results suggest that radiation
exposure may increase the activity of osteoclast precursors, but
may damage the resorption ability of osteoclasts.
RANK expression on hematopoietic precursor cells is
required in the murine model for osteoclast differentiation and
activation, the resorption of bone and the regulation of calcium
homeostasis by calcitropic hormones (28,29).
Our results showed that cells in the RANKL-induced group (group B)
and the irradiated group (group C) expressed higher levels of RANK.
The RANK mRNA expression did not significantly differ between
groups C and D. The irradiation of RANKL-induced osteoclasts (group
D) led to a decreased RANK expression compared to the cells in
group B. These results suggest that radiation exposure may promote
RANK expression in osteoclast precursors, but not in
osteoclasts.
In conclusion, our experiments revealed that
RAW264.7 cells differentiated into functional osteoclasts in the
presence of RANKL. Radiation damage may promote the activities of
osteoclast precursors, but it decreases those of osteoclasts. We
inferred that radiation impairs the function of osteoclasts and
stimulates the differentiation of osteoclast precursors. Therefore,
irradiated osteoclast precursors may play a significant role in
bone damage and may mediate skeletal complications in patients
receiving radiotherapy.
Acknowledgements
This study was supported by grants from the National
Nature Science Foundation of China (no. 30970867) (http://www.nsfc.gov.cn).
References
|
1
|
A BanfiG BianchiM GalottoR CanceddaR
QuartoBone marrow stromal damage after chemo/radiotherapy:
occurrence, consequences and possibilities of treatmentLeuk
Lymphoma42863870200110.3109/1042819010909770511697641
|
|
2
|
NN BaxterEB HabermannJE TepperSB DurhamBA
VirnigRisk of pelvic fractures in older women following pelvic
irradiationJAMA29425872593200510.1001/jama.294.20.258716304072
|
|
3
|
KH DarzySM ShaletHypopituitarism after
cranial irradiationJ Endocrinol Invest287887200516114281
|
|
4
|
AO LanglandsWA SouterE SamuelAT
RedpathRadiation osteitis following irradiation for breast
cancerClin Radiol289396197710.1016/S0009-9260(77)80134-7852230
|
|
5
|
BL CuetaraTN CrottiAJ O'DonoghueKP
McHughCloning and characterization of osteoclast precursors from
the RAW264.7 cellineIn Vitro Cell Dev Biol
Anim42182188200610.1290/0510075.1
|
|
6
|
M ItoN MatsukaM IzukaCharacterization of
inorganic phosphate transport in osteoclast-like cellsAm J Physiol
Cell Physiol288C921C931200510.1152/ajpcell.00412.200415601753
|
|
7
|
H HsuDL LaceyCR DunstanTumor necrosis
factor receptor family member RANK mediates osteoclast
differentiation and activation induced by osteoprotegerin
ligandProc Natl Acad Sci
USA9635403545199910.1073/pnas.96.7.3540
|
|
8
|
TL BurgessY QianS KaufmanThe ligand for
osteoprotegerin (OPGL) directly activates mature osteoclastsJ Cell
Biol145527538199910.1083/jcb.145.3.52710225954
|
|
9
|
S MakihiraY MineE KosakaH NikawaTitanium
surface roughness accelerates RANKL-dependent differentiation in
the osteoclast precursor cell line, RAW264.7Dent Mater
J26739745200710.4012/dmj.26.73918203477
|
|
10
|
DR HaynesTN CrottiH ZreiqatRegulation of
osteoclast activity in peri-implant
tissuesBiomaterials2548774885200410.1016/j.biomaterials.2004.01.00315109848
|
|
11
|
M AsagiriH TakayanagiThe molecular
understanding of osteoclast
differentiationBone40251264200710.1016/j.bone.2006.09.02317098490
|
|
12
|
JS WilleyEW LivingstonME RobbinsJD
BourlandL Tirado-LeeH Smith-SielickiTA BatemanRisedronate prevents
early radiation-induced osteoporosis in mice at multiple skeletal
locationsBone46101111201010.1016/j.bone.2009.09.00219747571
|
|
13
|
K HollbergJ NordahlK HultenbyS
Mengarelli-WidholmG AnderssonFP ReinholtPolarization and secretion
of cathepsin K precede tartrate-resistant acid phosphatase
secretion to the ruffled border area during the activation of
matrix-resorbing clastsJ Bone Miner
Metab23441449200510.1007/s00774-005-0626-316261450
|
|
14
|
B KirsteinTJ ChambersK FullerSecretion of
tartrate-resistant acid phosphatase by osteoclasts correlates with
resorptive behaviorJ Cell
Biochem9810851094200610.1002/jcb.2083516475168
|
|
15
|
G AnderssonB Ek-RylanderK HollbergTRACP as
an osteopontin phosphataseJ Bone Miner
Res1819121915200310.1359/jbmr.2003.18.10.191214584906
|
|
16
|
A SuterV EvertsA BoydeOverlapping
functions of lysosomal acid phosphatase (LAP) and
tartrate-resistant acid phosphatase (Acp5) revealed by doubly
deficient miceDevelopment12848994910200111731469
|
|
17
|
NC WalshM CahillP CarninciMultiple
tissue-specific promoters control expression of the murine
tartrate-resistant acid phosphatase
geneGene307111123200310.1016/S0378-1119(03)00449-912706893
|
|
18
|
R BattaglinoD KimJ FuB VaageXY FuP
Stashenkoc-myc is required for osteoclast differentiationJ Bone
Miner Res17763773200210.1359/jbmr.2002.17.5.76312009006
|
|
19
|
SL TeitelbaumFP RossGenetic regulation of
osteoclast development and functionNat Rev
Genet4638649200310.1038/nrg112212897775
|
|
20
|
FP RossJ ChappelJI AlvarezInteractions
between the bone matrix proteins osteopontin and bone sialoprotein
and the osteoclast integrin alpha v beta 3 potentiate bone
resorptionJ Biol Chem2689901990719938486670
|
|
21
|
D HuC LuA SapozhnikovaM BarnettC SparreyT
MiclauRS MarcucioThe absence of beta-3 integrin accelerates early
skeletal repairJ Orthop Res283237201019637214
|
|
22
|
GC NicholsonJM MoseleyPM SextonFA
MendelsohnTJ MartinAbundant calcitonin receptors in isolated rat
osteoclasts. Biochemical and autoradiographic characterizationJ
Clin Invest78355360198610.1172/JCI1125843016026
|
|
23
|
M ZaidiM PazianasVS ShankarOsteoclast
function and its controlExp
Physiol78721739199310.1113/expphysiol.1993.sp0037218311941
|
|
24
|
JM QuinnM MorfisMH LamCalcitonin receptor
antibodies in the identification of
osteoclastsBone2518199910.1016/S8756-3282(99)00094-010423015
|
|
25
|
J CornishKE CallonU BavaSA KamonaGJ
CooperIR ReidEffects of calcitonin, amylin, and calcitonin
gene-related peptide on osteoclast
developmentBone29162168200110.1016/S8756-3282(01)00494-X11502478
|
|
26
|
SK LeeSR GoldringJA LorenzoExpression of
the calcitonin receptor in bone marrow cell cultures and in bone: a
specific marker of the differentiated osteoclast that is regulated
by calcitoninEndocrinology1364572458119957664679
|
|
27
|
WJ BoyleWS SimonetDL LaceyOsteoclast
differentiation and
activationNature423337342200310.1038/nature0165812748652
|
|
28
|
J LiI SarosiXQ YanRANK is the intrinsic
hematopoietic cell surface receptor that controls
osteoclastogenesis and regulation of bone mass and calcium
metabolismProc Natl Acad Sci
USA9715661571200010.1073/pnas.97.4.156610677500
|
|
29
|
WC DougallM GlaccumK CharrierRANK is
essential for osteoclast and lymph node developmentGenes
Dev1324122424199910.1101/gad.13.18.241210500098
|