Introduction
Pulmonary radiotherapy is an effective curative and
palliative option for patients with malignant thoracic cancer.
However, radiation-induced lung injury (RILI) remains a significant
clinical toxicity from thoracic radiation with poor prognosis
(1–3), thus limiting the dose of radiation
delivered to tumors. Histopathologically, damage to endothelial or
epithelial cells is assumed to be the initial step of RILI, which
can result in pneumonitis and ultimately pulmonary fibrosis
(4). Irradiation pneumonitis is
characterized by edema of the interstitium and exudation into air
spaces, infiltration of inflammatory cells, the loss of type I
pneumocytes, increase in capillary permeability, and thickening of
the alveolar septa (5,6). Pulmonary fibrosis is characterized by
the progressive fibrosis of alveolar septa and capillaries causing
spread and obliteration of the residual alveoli and the lumina of
the capillaries, which is considered an outcome of the repair
mechanisms triggered by radiation pneumonitis. However, despite an
extensive research effort in the past decades, the molecular events
underlying the development of RILI remain unclear.
A number of pro-inflammatory cytokines and growth
factors have been demonstrated to contribute to the pathogenesis of
RILI (7–10,11).
Among these, transforming growth factor-β1 (TGF-β1) is thought to
play a crucial role (10,12,13).
TGF-β1 is usually released from platelets at the site of a wound,
where it recruits monocytes and macrophages, inhibits the
proliferation of epithelial cells, enhances the maturation of
fibroblasts into postmitotic fibrocytes resulting in production of
fibrous tissue, promotes angiogenesis, and inhibits degradation of
the extracellular matrix (14). In
addition to its role in wound healing, TGF-β1 has been detected in
elevated levels in the lung following irradiation (8).
Agents, including glucocorticoid,
angiotensin-1-converting enzyme inhibitors and non-steroidal
anti-inflammatory drugs, are commonly applied for the treatment of
RILI, however, their effectiveness has not met satisfactory levels
and the side effects are significant (15). Endostatin, a proteolytic fragment
of collagen VIII, is a naturally occurring endogenous potent
inhibitor of angiogenesis (16).
It has been proven that endostatin can effectively suppress
endothelial cell proliferation and migration in vitro
(17), and inhibit tumor growth in
various types of animal models in vivo (18). However, purification of endogenous
endostatin is time-consuming, and production of the soluble type of
recombinant endostatin in a yeast system has a low yield and high
cost, therefore significantly limiting extensive clinical use of
this anti-angiogenic agent (19).
Endostar is a novel recombinant analogue of human
endostatin, which is expressed and purified in E. coli, and
has an additional nine-amino acid sequence (MGGSHHHHH) at the
N-terminal and a poly-histidine (six) tag. The modified protein has
a longer half-life and higher affinity than endostatin (19,20).
It was first approved by the State Food and Drug Administration in
2005 for the treatment of non-small cell lung cancer in China
(21). Pre-clinical studies have
demonstrated that Endostar is capable of enhancing the response of
human nasopharyngeal carcinoma and lung adenocarcinoma xenografts
to radiotherapy in nude mice (22,23).
Given the involvement of TGF-β1 in the pathogenesis
of RILI and the synergy between Endostar and radiation in treating
malignant tumors, we hypothesize that Endostar may possibly protect
normal lung tissue against thoracic radiation-induced injury
through modulating the expression of TGF-β1. This study aimed to
test this hypothesis in a mouse model of thoracic irradiation.
Materials and methods
Animals
Female C57BL/6 mice, 8 weeks old and weighing 20±2
g, were purchased from the Research Animal Facility of Tongji
Medical College (Wuhan, China). They were housed in groups of 4–6
per cage in laminar flow hoods in a pathogen-free environment
(22±2°C, 55±10% humidity and 12-12 h/light-dark cycle) with free
access to a standard laboratory diet and water. The animals were
acclimatized for 1 week prior to the experiments. The study
protocol was reviewed and approved by the Medical Sciences Animal
Care and Use Committee of the Huazhong University of Science and
Technology.
Treatment allocations
Animals were randomized into: i) irradiation group,
animals received a single dose of irradiation and multiple doses of
normal saline (7.75 ml/kg per day) through intraperitoneal
injection; ii) Endostar group, animals received sham irradiation
once and multiple doses of Endostar (7.75 ml/kg per day, 0.3 mg/ml;
Simcere Pharmaceutical Research, Nanjing, China) through
intraperitoneal injection; iii) irradiation plus Endostar group,
animals received a single dose of irradiation as in group 1 and
Endostar as in group 2; iv) control group, animals received sham
irradiation as in group 2 and normal saline as in group 1. The
initial administration of Endostar and normal saline was performed
2 h prior to irradiation or sham irradiation. A total of 3 animals
from each of the 4 groups were sacrificed by cervical dislocation
at each of the times (1, 6, 24, 72 h and 2, 4, 8 and 24 weeks)
following irradiation or sham irradiation for analyses described
below. The drug (Endostar) or sham (normal saline) treatment were
administered daily until sacrifice at the corresponding times.
Irradiation was performed as previously described
(5). The animal was briefly
restrained with a plastic jig, and a single dose of 12 Gy (10 MV
photons of beam energy at 2.4 Gy/min) was administered to the lung
area (18 cm × 10 cm) at a source surface distance (SSD) of 1 m
while the head and abdomen were shielded with lead strips. The
relative dose, location and area size of the thoracic irradiation
were determined based on the ADAC Pinnacle Three-dimensional
Treatment Planning System. Similar procedures were applied to
animals designated to receive sham irradiation, but the irradiation
area in the lung was completely shielded with lead blocks. Both
irradiation and sham irradiation were performed under ketamine
(67.5 mg/kg) and xylazine (4.5 mg/kg) anesthesia.
Tissue collection and initial
processing
After sacrifice of the animals at the specific
times, intact lungs were obtained. The left lobes were fixed in 10%
neutral-buffered formaldehyde, embedded in paraffin and cut into
4-μm sections. The tissue sections were mounted onto slides and
stained for histological and immunohistochemical analyses. The
right lung lobes were snap-frozen in liquid nitrogen for RNA
isolation and subsequent real-time quantitative polymerase chain
reaction (RT-QPCR).
Hematoxylin and eosin staining
Tissue sections were deparaffinized in xylene and
re-hydrated in a series of alcohol solutions. After a brief wash in
distilled water, the sections were stained in Harris hematoxylin
solution for 5 min, washed in tap water and counterstained in
eosin-phloxine solution for 2 min.
Masson staining
Paraffin-embedded tranverse sections were cut (4 μm)
and stained using Masson Trichrome. Lung injury was assessed by the
manifestation of alveolitis and fibrosis. The severity of
alveolitis and fibrosis was semi-quantitated using the score-grade
system as described by Szapiel et al (24): absence of alveolitis (-, score 0,
grade 0); mild alveolitis with widened alveolar septum due to cell
infiltration and the lesioned tissues accounted for <20% of
total lung (+, 1, 1); moderate alveolitis with lesions in 20–50% of
the pulmonary tissue (++, 2, 2); severe alveolitis with lesions in
>50% of the pulmonary tissue (+++, 3, 3).
RT-QPCR analysis
Total RNA was extracted from frozen pulmonary tissue
using TRIzol reagent (Invitrogen, Shanghai, China) according to the
manufacturer’s instructions. Oligo (dT18)-primed first-strand cDNA
was synthesized using a First-Strand cDNA Synthesis kit
(Invitrogen) according to the manufacturer’s instructions. TGF-β1
was amplified from the RT reaction mixture by 30 cycles of PCR (30
sec at 94°C, 40 sec at 57°C, 40 sec at 72°C and, finally, 5 min at
72°C), followed by one cycle of denaturation of 4 min at 94°C. A
β-actin fragment was simultaneously amplified in the same reaction
as an internal control. The primer sequences were as follows:
TGF-β1 sense, 5′-ATC CTG TCC AAA CTA AGG CTC G-3′ and anti-sense,
5′-ACC TCT TTA GCA TAG TAG TCC GC-3′; β-actin sense, 5′-AGA GGG AAA
TCG TGC GTG AC-3′ and anti-sense, 5′-CAA TAG TGA TGA CCT GGC
CGT-3′. PCR products (TGF-β1, 167 bp and β-actin fragments, 138 bp)
were visualized by ethidium bromide following electrophoresis on a
1% agarose gel, and quantitated by densitometry using a
dual-intensity transilluminator equipped with Gel-Pro Analyzer
version 3.1 (Media Cybernetics, Bethesda, MD, USA). The relative
level of TGF-β1 mRNA was expressed as the ratio of the
densitometric value of the TGF-β1 band over that of the β-actin
band.
Immunohistochemistry
Tissue sections were blocked in 3%
H2O2 at 37°C for 10 min and incubated with
the rabbit anti-mouse TGF-β1 antibody (1:100, R&D Systems,
Minneapolis, MN, USA) at 4°C overnight, following
deparaffinization, rehydration and dehydration. The following day,
the sections were incubated with biotinylated anti-rabbit antibody
(1:200, Invitrogen, CA, USA) at 37°C for 1 h, and then with the
avidin-biotin-peroxidase complex (ABC Complex; Dako, Glostrup,
Denmark) for 30 min at room temperature. Following a final washing
in PBS, TGF-β1 signals were visualized with a DAB kit (Sigma, St.
Louis, MO, USA), counterstained with haematoxylin and mounted in
Kayser’s glycerine gelatine. PBS replaced the primary antibody to
serve as a negative control. TGF-β1 immunoreactivity was quantified
under a microscope using a semi-quantitative scoring method as
previously described (26): 0, no
immunostaining; 1, weak (light yellow); 2, moderate (yellow-brown);
and 3, strong (brown). A score >3 was defined as a positive
immune response. A total of 5 random fields were observed for each
specimen and the scores were averaged for each time and treatment
combinations. This analysis was repeated twice by an investigator
blinded to the specimen identification.
Statistical analysis
The TGF-β1 mRNA levels and TGF-β1 protein expression
(immunoreactivity in the lung parenchyma, expressed as positive
cell counts) were analyzed by one-way analysis of variance (ANOVA)
followed by Bonferroni test for multiple comparisons. SPSS 17.0
statistical software was used. P<0.05 was considered to indicate
a statistically significant difference.
Results
Histological changes
Pulmonary alveolitis was not detected in mice in the
control and Endostar treatment groups, but was evident in mice in
the irradiation and irradiation plus Endostar treatment groups at
various times, as demonstrated by hematoxylin and eosin staining
(Fig. 1). Alveolitis was
characterized by macrophage infiltration into the air spaces, edema
of the alveolar wall, desquamation of epithelial cells from the
alveolar walls, hyperemia, thickening of the alveolar septa by
infiltration of inflammatory cells, collagen deposition,
progressive fibrosis and obliteration of the alveoli. Focal
fibrosis was clearly observed within the inflammation region 24
weeks after irradiation. The mean grade of alveolitis was 1–2 in
the irradiation plus Endostar treatment group and was 2–3 in the
irradiation group; the difference was significant (P<0.05).
To further confirm fibrosis, Masson staining was
performed. The results demonstrated that irradiation induced
fibrosis, with accumulation of fibroblasts and deposition of
collagen around blood vessels and in the interstitial region in the
lung, and Endostar partially ameliorated the irradiation-induced
fibrosis (Fig. 2).
Changes in TGF-β1 mRNA level
The data on the relative levels of TGF-β1 mRNA in
the lungs of mice in the different groups are presented in Fig. 3. Compared with the control,
Endostar alone did not alter the TGF-β1 mRNA abundance at any of
the times studied (P>0.05), but thoracic irradiation elevated
TGF-β1 mRNA level in a time-dependent manner. Treatment of the
irradiated animals with Endostar significantly attenuated the
irradiation-induced elevation of TGF-β1 mRNA level at 12, 24 h and
2 weeks after irradiation (P<0.05), but not at 1, 6 and 72 h and
4, 8, 24 weeks after irradiation; P>0.05).
Changes in TGF-β1 immunoreactivity
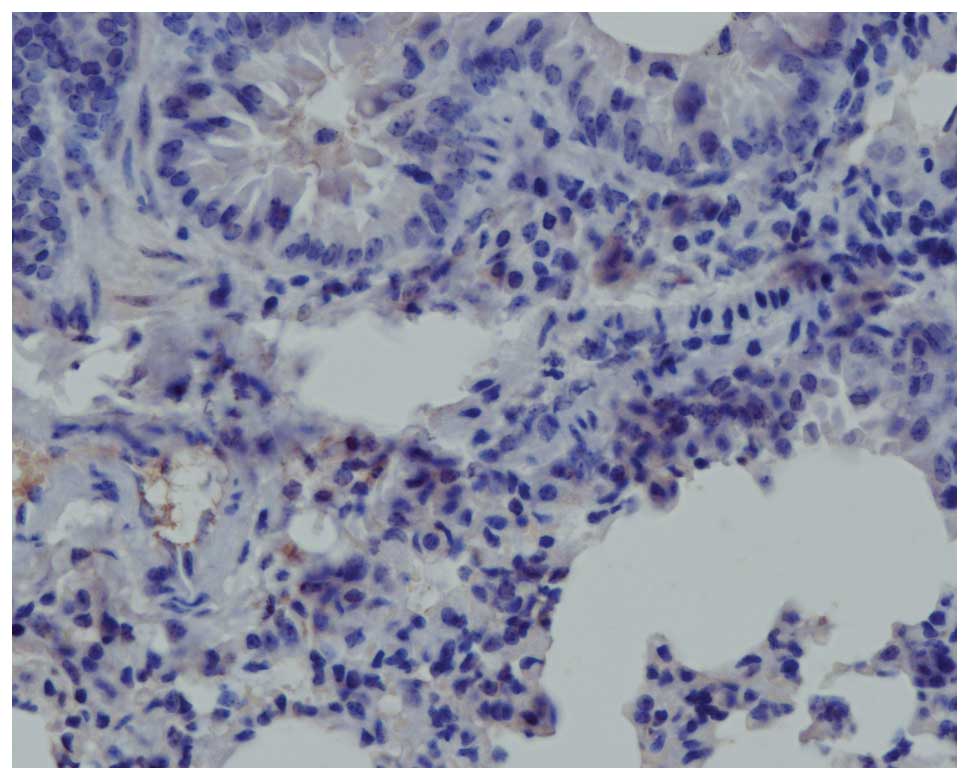
Fig. 4 shows
digital images demonstrating TGF-β1 histoimmunochemical staining
signals on the pulmonary tissue sections prepared from animals in
the different experimental groups at various times. Significant
increases in TGF-β1 expression were observed at every time
post-irradiation for all radiation groups compared to the control
and Endostar groups. In the control group, positive staining was
restricted to the bronchiolar epithelium, while TGF-β1 staining
obtained 4 weeks after irradiation demonstrated strong
immunostaining of the bronchial epithelial cells and interstitial
inflammatory cells. Treatment with Endostar attenuated the
expression of TGF-β1 in the lung tissue post-irradiation. The
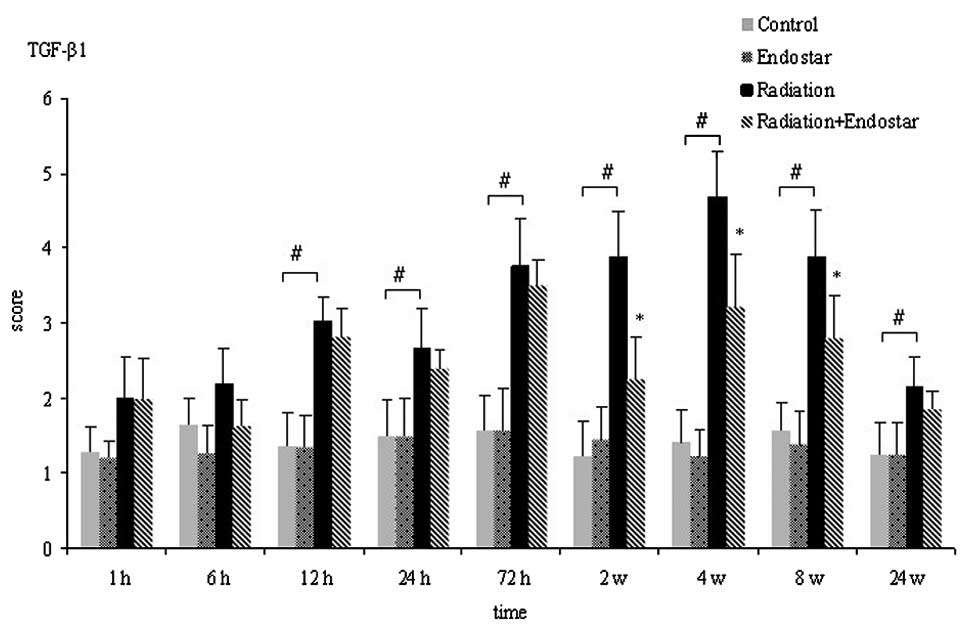
immunohistochemical scores of TGF-β1 varied with time (Fig. 5). The TGF-β1 score peaked at 4
weeks post-irradiation. Although the changes in TGF-β1 expression
followed the same trend in the radiation plus Endostar and
radiation groups, the former had significantly lower levels of
TGF-β1 expression than the latter at 2, 4 and 8 weeks after
irradiation (Fig. 5;
P<0.05).
Discussion
The human lung is among the most sensitive and
critical tissue of concern in local and systemic radiation
exposure. Lung injuries are frequently observed in patients who
have undergone thoracic irradiation for the treatment of lung,
breast or hematologic malignancies. Radiation-induced damage to
normal lung tissue remains the dose-limiting factor in chest
radiotherapy. Therefore, preclinical research in animal models,
aimed to uncover the underlying mechanism of RILI and evaluate
mitigating therapies, is urgently required (25). In the present study, using mice as
a model, we investigated the involvement of TGF-β1 in the
pathogenesis of RILI, and the novel role of Endostar in preventing
RILI.
We first assessed morphopathological changes in the
lung by histochemical and Masson staining. The results clearly
demonstrated pulmonary lesions characterized by alveolitis and
pneumonitis with inflammatory cell accumulation and collagen
deposition in the irradiated mice (Fig. 1). Cellular interactions between
lung parenchymal cells and circulating immune cells have been
suggested as a mechanism underlying the pathogenesis of RILI
(6,26–28).
Results from our morphopathological analysis support this
mechanism.
We next assessed the changes in TGF-β1 expression in
the lung, in order to attempt to understand the role of this
cytokine in the pathogenesis and recovery of RILI. There is
increasing evidence suggesting that the development of certain
types of radiation injury may be mediated through the
Smad-independent TGF-β1 signaling pathway (29,30).
It has been identified that binding of TGF-β1 to its type II
transmembrane receptor results in the subsequent formation of a
complex with the type II receptor. Upon phosphorylation, this
complex activates the signaling proteins Smad2 and Smad3. The
activated Smad2 and 3 proteins then bind to Smad4 and translocate
to the cell nucleus, where they modulate downstream target gene
transcription through binding to the response elements on the
promoters of target genes (14).
In the present study, changes in the expression of TGF-β1 in the
lung were analyzed at mRNA and protein levels through real-time PCR
and immunostaining, respectively. The results demonstrated that
TGF-β1 mRNA levels and immunostaining intensity in the lung
increased in a time-dependent manner; detectable event 1 h after
thoracic irradiation, significant from post-irradiation hour 6
onwards and sustained to the end of the experiment (24 weeks). This
pattern of change in TGF-β1 expression in the lung, associatied
with the development of RILI, is consistent with the pattern
observed in previous in vivo studies in mice and rats
(5,31,32).
Endostar is an analogue of endostatin, a naturally
occurring endogenous inhibitor of neovascularization, which has
been widely used in anti-angiogenesis therapy for cancer (21,33).
Recently, it has been demonstrated that endostatin can
synergistically increase the sensitivity of tumors to radiotherapy
(34). These findings advocated
the evaluation of the value of Endostar in protection against
thoracic irradiation-induced adverse effects. Through histological,
RT-QPCR and immunohistochemical analyses, we demonstrated that the
pulmonary morphology and TGF-β1 mRNA abundance and immunoreactivity
in the pulmonary tissue in animals of the Endostar treatment group
were similar to those in the normal control animals. It was also
revealed that pulmonary alveolitis, edema, inflammation and
fibrosis were less severe with decreased expression of TGF-β1 in
the irradiated animals that received Endostar treatment compared to
those irradiated animals that did not receive Endostar treatment.
These observations clearly suggest that Endostar neither adversely
affected the pulmonary morphology nor significantly changed the
pulmonary expression of TGF-β1, but effectively protected the lung
from severe irradiation-induced injury. Although the mechanism
underlying the protection of Endostar against RILI require further
investigation, our findings suggest that protection may involve
molecular cascades mediated by profibrogenic agents and
proangiogenic cytokines. In accordance with this hypothesis, Tanabe
et al (35) reported that
administration of endostatin significantly increased expression of
VEGF-A, α-SMA and profibrotic TGF-β1, and supressed peritoneal
sclerosis in a dose-dependent manner in a mouse model. Vujaskovic
et al (36) and
Gauter-Fleckenstein et al (37) further demonstrated that
profibrinogenic agents, including TGF-β1 and proangiogenic
cytokines, were involved in the pathogenesis of RILI, while Jackson
et al (38) demonstrated
that radiation injury was associated with hypoxia-induced increases
in macrophage infiltration/activation and TGF-β and VEGF
production.
In summary, the present mouse study demonstrated
that irradiation-induced TGF-β upregulation-associated pulmonary
lesions and the anti-angiogenic agent, Endostar, was able to
effectively alleviate the severity of irradiation-induced pulmonary
lesions. These novel findings suggest that Endostar has great
potential to be used in treating malignant cancer patients to
protect from radiotherapy-induced lung injury in clinical
settings.
References
|
1
|
Marks LB, Yu X, Vujaskovic Z, Small W Jr,
Folz R and Anscher MS: Radiation-induced lung injury. Semin Radiat
Oncol. 13:333–345. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Mehta V: Radiation pneumonitis and
pulmonary fibrosis in non-small-cell lung cancer: pulmonary
function, prediction, and prevention. Int J Radiat Oncol Biol Phys.
63:5–24. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kong FM, Hayman JA, Griffith KA,
Kalemkerian GP, Arenberg D, Lyons S, Turrisi A, Lichter A, Fraass
B, Eisbruch A, Lawrence TS and Ten Haken RK: Final toxicity results
of a radiation-dose escalation study in patients with
non-small-cell lung cancer (NSCLC): predictors for radiation
pneumonitis and fibrosis. Int J Radiat Oncol Biol Phys.
65:1075–1086. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Rübe CE, Wilfert F, Uthe D, Schmid KW,
Knoop R, Willich N, Schuck A and Rübe C: Modulation of
radiation-induced tumor necrosis factor alpha (TNF-alpha)
expression in the lung tissue by pentoxifylline. Radiother Oncol.
64:177–187. 2002.PubMed/NCBI
|
|
5
|
Rübe CE, Uthe D, Schmid KW, Richter KD,
Wessel J, Schuck A, Willich N and Rübe C: Dose-dependent induction
of transforming growth factor beta (TGF-beta) in the lung tissue of
fibrosis-prone mice after thoracic irradiation. Int J Radiat Oncol
Biol Phys. 47:1033–1042. 2000.PubMed/NCBI
|
|
6
|
Dong XR, Wang JN, Liu L, Chen X, Chen MS,
Chen J, Ren JH, Li Q and Han J: Modulation of radiation-induced
tumor necrosis factor-α and transforming growth factor β1
expression in the lung tissue by Shengqi Fuzheng injection. Mol Med
Report. 3:621–627. 2010.
|
|
7
|
Brush J, Lipnick SL, Phillips T, Sitko J,
McDonald JT and McBride WH: Molecular mechanisms of late normal
tissue injury. Semin Radiat Oncol. 17:121–130. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Rübe CE, Uthe D, Wilfert F, Ludwig D, Yang
K, König J, Palm J, Schuck A, Willich N, Remberger K and Rübe C:
The bronchiolar epithelium as a prominent source of
pro-inflammatory cytokines after lung irradiation. Int J Radiat
Oncol Biol Phys. 61:1482–1492. 2005.PubMed/NCBI
|
|
9
|
Kong FM, Ao XP, Wang L and Lawrence TS:
The use of blood biomarkers to predict radiation lung toxicity: a
potential strategy to individualize thoracic radiation therapy.
Cancer Control. 15:140–150. 2008.PubMed/NCBI
|
|
10
|
Novakova-Jiresova A, Van Gameren MM,
Coppes RP, Kampinga HH and Groen HJM: Transforming growth
factor-beta plasma dynamics and post-irradiation lung injury in
lung cancer patients. Radiother Oncol. 71:183–189. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ao XP, Zhao LJ, Davis MA, Lubman DM,
Lawrence TS and Kong FM: Radiation produces differential changes in
cytokine profiles in radiation lung fibrosis sensitive and
resistant mice. J Hematol Oncol. 2:62009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Munger JS, Huang X, Kawakatsu H, Griffiths
MJ, Dalton SL, Wu J, Pittet JF, Kaminski N, Garat C, Matthay MA,
Rifkin DB and Sheppard D: The integrin alpha v beta 6 binds and
activates latent TGF beta 1: a mechanism for regulating pulmonary
inflammation and fibrosis. Cell. 96:319–328. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Anscher MS, Thrasher B, Rabbani Z, Teicher
B and Vujaskovic Z: Antitransforming growth factor-beta antibody
1D11 ameliorates normal tissue damage caused by high-dose
radiation. Int J Radiat Oncol Biol Phys. 65:876–881. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Anscher MS: Targeting the TGF-beta1
pathway to prevent normal tissue injury after cancer therapy.
Oncologist. 15:350–359. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Sekine I, Sumi M, Ito Y, Nokihara H,
Yamamoto N, Kunitoh H, Ohe Y, Kodama T, Saijo N and Tamura T:
Retrospective analysis of steroid therapy for radiation-induced
lung injury in lung cancer patients. Radiother Oncol. 80:93–97.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
O’Reilly MS, Boehm T, Shing Y, Fukai N,
Vasios G, Lane WS, Flynn E, Birkhead JR, Olsen BR and Folkman J:
Endostatin: an endogenous inhibitor of angiogenesis and tumor
growth. Cell. 88:277–285. 1997.
|
|
17
|
Wang YS, Eichler W, Friedrichs U, Yafai Y,
Hoffmann S, Yasukawa T, Hui YN and Wiedemann P: Impact of
endostatin on bFGF-induced proliferation, migration, and matrix
metalloproteinase-2 expression/secretion of bovine choroidal
endothelial cells. Curr Eye Res. 30:479–489. 2005. View Article : Google Scholar
|
|
18
|
Ling Y, Lu N, Gao Y, Chen Y, Wang S, Yang
Y and Guo QL: Endostar induces apoptotic effects in HUVECs through
activation of caspase-3 and decrease of Bcl-2. Anticancer Res.
29:411–417. 2009.PubMed/NCBI
|
|
19
|
Zhang L, Ge W, Hu K, Zhang YY, Li CH, Xu
XM, He D, Zhao ZY, Zhang JZ, Jie FF, Chen Y and Zheng YF: Endostar
down-regulates HIF-1 and VEGF expression and enhances the
radioresponse to human lung adenocarcinoma cancer cells. Mol Bio
Rep. 39:89–95. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Jia H and Kling J: China offers
alternative gateway for experimental drugs. Nat Biotechnol.
24:117–118. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ling Y, Yang Y, Lu N, You QD, Wang S, Gao
Y, Chen Y and Guo QL: Endostar, a novel recombinant human
endostatin, exerts antiangiogenic blocking VEGF-induced tyrosine
phosphorylation of KDR/Flk-1 of endothelial cells. Biochem Biophys
Res Commun. 361:79–84. 2007. View Article : Google Scholar
|
|
22
|
Jiang XD, Dai P, Wu J, Song DA and Yu JM:
Inhibitory effect of radiotherapy combined with weekly recombinant
human endostatin on the human pulmonary adenocarcinoma A549
xenografts in nude mice. Lung Cancer. 72:165–171. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wen QL, Meng MB, Yang B, Tu LL, Jia L,
Zhou L, Xu Y and Lu Y: Endostar, a recombined humanized endostatin,
enhances the radioresponse for human nasopharyngeal carcinoma and
human lung adenocarcinoma xenografts in mice. Cancer Sci.
100:1510–1519. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Szapiel SV, Elson NA, Fulmer JD,
Hunninghake GW and Crystal RG: Bleomycin-induced interstitial
pulmonary disease in the nude, athymic mouse. Am Rev Respir Dis.
120:839–899. 1979.PubMed/NCBI
|
|
25
|
Friedrichs K, Gluba S, Eidtmann H and
Jonat W: Overexpression of p53 and prognosis in breast cancer.
Cancer. 72:3641–3647. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Jackson IL, Vujaskovic Z and Down JD: A
further comparison of pathologies after thoracic irradiation among
different mouse strains: finding the best preclinical model for
evaluating therapies directed against radiation-induced lung
damage. Radiat Res. 175:510–518. 2011. View
Article : Google Scholar
|
|
27
|
Tsoutsou PG and Koukourakis MI: Radiation
pneumonitis and fibrosis: mechanisms underlying its pathogenesis
and implications for future research. Int J Radiat Oncol Biol Phys.
66:1281–1293. 2006. View Article : Google Scholar
|
|
28
|
Liu L, Ding Q, Dai XF, Zhao YX, Ke Y and
Wu G: Study on the controlling effect of Shengqi Fuzheng injection
on plasma cytokine network in patients with thoracic tumor
undergoing radiotherapy. Zhongguo Zhong Xi Yi Jie He Za Zhi.
27:1082–1085. 2007.(In Chinese).
|
|
29
|
Haydont V, Mathé D, Bourgier C, Abdelali
J, Aigueperse J, Bourhis J and Vozenin-Brotons MC: Induction of
CTGF by TGF-beta1 in normal and radiation enteritis human smooth
muscle cells: Smad/Rho balance and therapeutic perspectives.
Radiother Oncol. 76:219–225. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Haydont V, Riser BL, Aigueperse J and
Vozenin-Brotons MC: Specific signals involved in the long-term
maintenance of radiation-induced fibrogenic differentiation: a role
for CCN2 and low concentration of TGF-beta1. Am J Physiol Cell
Physiol. 294:C1332–C1341. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Franko AJ, Sharplin J, Ghahary A and
Barcellos-Hoff MH: Immunohistochemical localization of transforming
growth factor beta and tumor necrosis factor alpha in the lungs of
fibrosis-prone and ‘non-fibrosing’ mice during the latent period
and early phase after irradiation. Radiat Res. 147:245–256.
1997.
|
|
32
|
Chen L, Brizel DM, Rabbani ZN, Samulski
TV, Farrell CL, Larrier N, Anscher MS and Vujaskovic Z: The
protective effect of recombinant human keratinocyte growth factor
on radiation-induced pulmonary toxicity in rats. Int J Radiat Oncol
Biol Phys. 60:1520–1529. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Folkman J: Antiangiogenesis in cancer
therapy-endostatin and its mechanisms of action. Exp Cell Res.
312:594–607. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Itasaka S, Komaki R, Herbst RS, Shibuya K,
Shintani T, Hunter NR, Onn A, Bucana CD, Milas L, Ang KK and
O’Reilly MS: Endostatin improves radioresponse and blocks tumor
revascularization after radiation therapy for A431 xenografts in
mice. Int J Radiat Oncol Biol Phys. 67:870–878. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Tanabe K, Maeshima Y, Ichinose K, Kitayama
H, Takazawa Y, Hirokoshi K, Kinomura M, Sugiyama H and Makino H:
Endostatin peptide, an inhibitor of angiogenesis, prevents the
progression of peritoneal sclerosis in a mouse experimental model.
Kidney Int. 71:227–238. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Vujaskovic Z, Anscher MS, Feng QF, Rabbani
ZN, Amin K, Samulski TS, Dewhirst MW and Haroon ZA:
Radiation-induced hypoxia may perpetuate late normal tissue injury.
Int J Radiat Oncol Biol Phys. 50:851–855. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Gauter-Fleckenstein B, Fleckenstein K,
Owzar K, Jiang C, Rebouças JS, Batinic-Haberle I and Vujaskovic Z:
Early and late administration of MnTE-2-PyP5+ in
mitigation and treatment of radiation-induced lung damage. Free
Radic Biol Med. 48:1034–1043. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Jackson IL, Chen L, Batinic-Haberle I and
Vujaskovic Z: Superoxide dismutase mimetic reduces hypoxia-induced
O2*−, TGF-beta, and VEGF production by
macrophages. Free Radic Res. 41:8–14. 2007. View Article : Google Scholar : PubMed/NCBI
|