Introduction
Gastric cancer (GC) is the fourth most common type
of cancer in the world and the second leading cause of death due to
cancer. In general, the incidence of GC is two to three times
higher in developing countries and the disease is more frequent
among men than women (1–4). Gastric carcinogenesis is a
multifactorial process involving endogenous and exogenous factors
that cause genetic mutations. GC comprises different stages, such
as superficial gastritis, atrophic gastritis, intestinal
metaplasia, dysplasia and, finally, carcinoma (5). In addition to infection with
Helicobacter pylori, epidemiological studies have
demonstrated the importance of environmental factors, such as
consumption of salty, canned and smoked foods for the genesis of GC
(6).
P-glycoprotein (P-gp) is encoded by the multidrug
resistance 1 (MDR1) gene and confers resistance to
multiple antineoplastic agents. In addition, this protein affects
the kinetic disposition of certain drugs and carcinogens (7). Various isoforms of P-gp are known,
which are classified into classes I, II and III. Classes I and II
are related to multidrug resistance, whereas class III is involved
in the transport of phospholipids (8,9).
Although first detected in tumor cells, P-gp is also expressed in
normal tissues. In the gastrointestinal tract, P-gp is the first
defense of the body against oral exposure to drugs and toxins
(10). P-gp is a membrane-bound
transporter that was first identified to be responsible for the
development of multidrug resistance (7). This glycoprotein acts as an
ATP-dependent efflux pump that exports drugs, reducing the
intercellular concentrations of different chemotherapeutic agents.
P-gp shows an expression gradient in gastrointestinal epithelial
cells, with this protein being more expressed in the apical region.
However, it is also found in hepatic canaliculi and in the proximal
tubules of the kidneys, where it contributes to the excretion of
biliary and urinary products, respectively (10–13).
There is growing evidence that changes in the
function and/or expression of the MDR1 gene contribute to
the pathogenesis of inflammatory diseases of the gastrointestinal
tract (14). Researchers have
demonstrated that a polymorphism in the C3435T region of the
MDR1 gene influences the development of different cancers,
since it is directly related to the transport of potentially
carcinogenic substances (15–19).
Although there are several reports on the pharmacological and
enzymatic action of P-gp, studies investigating the association of
the MDR1 gene polymorphism and GC are scarce. Therefore, the
aim of the present study was to characterize the genotypic profile
of this genetic polymorphism and to correlate this polymorphism
with the risk of GC and tumor aggressiveness.
Patients and methods
Sample population
A case-control study was conducted. The case group
consisted of patients with GC seen at the outpatient clinic of the
Discipline of Clinical Gastroenterology, Federal University of Sao
Paulo-Escola Paulista de Medicina, Brazil. Patients with a
confirmed histological diagnosis of non-cardia adenocarcinoma were
invited to participate in the study. The control group consisted of
healthy subjects who attended the blood collection service of the
Central Laboratory of the Sao Paulo Hospital. The study was
approved by the Ethics Committee of the institution, and all
patients signed an informed consent form.
Assay methods
An ELISA kit (R-Biopharm GmbH, Germany) was used for
the detection of serum anti-H. pylori IgG antibodies. DNA
was extracted from peripheral venous blood leukocytes collected
with EDTA as an anticoagulant using the Blood Spin Mini kit
(Invisorb, Germany). The samples were submitted to polymerase chain
reaction (PCR) and then genotyped using the PCR-restriction
fragment length polymorphism (RFLP) technique. The following
primers were used: forward 5′-TGC TGG TCC TGA AGT TGA TCT GTG
AAC-3′ and reverse 5′-ACA TTA GGC AGT GAC TCG ATG AAG GCA-3′. The
reaction mixture for PCR contained 40 ng DNA, 1X reagent buffer,
0.125 mmol/l of each deoxynucleotide triphosphate (dNTP), 1.5
mmol/l MgCl2, 0.75 μmol/l of each primer and 0.5 units
Platinum Taq DNA polymerase in a final volume of 10 μl. DNA
was denatured at 94°C for 2 min, followed by 35 cycles at 94°C for
30 sec, 60°C for 30 sec and 72°C for 30 sec, with a final extension
at 72°C for 4 min. After amplification, the products were digested
with 5 units of the restriction enzyme MboI (New England
Biolabs) at 37°C for 3 h and the fragments were visualized on 3%
agarose gel stained with ethidium bromide. Genome sequencing was
used to confirm the PCR and RFLP techniques using random samples of
the two groups. The PCR product of the MDR1 gene was
purified using the Big Dye XTerminator kit (Applied Biosystems) and
sequenced in an ABI Prism 3100 sequencer (Applied Biosystems). The
reverse primer was used for sequencing. The electropherogram was
analyzed with the Sequence Scanner v1.0 program.
Statistical analyses
The Statistical Package for the Social Sciences
v19.0 was used for statistical analysis. Age was compared between
groups by the Student's t-test. The Chi-square test was used to
determine differences in genotypes and alleles between the two
groups. Survival was estimated by the Kaplan-Meier method and
survival curves were compared by the log-rank test.
Results
The case group consisted of 98 patients with GC,
including 43 (43.9%) women. The control group consisted of 203
subjects, including 110 (54.2%) women. The patients of the case and
control groups were admitted during the same period. There was no
difference in gender (p=0.12) or age (p=0.125) between the two
groups (Table I).
 | Table ICharacteristics of the patients with
gastric cancer and controls. |
Table I
Characteristics of the patients with
gastric cancer and controls.
| Control n=203
(%) | Cancer n=98 (%) | p-valuea |
|---|
| Gender | | | 0.120 |
| Female | 110 (54.2) | 43 (43.9) | |
| Male | 93 (45.8) | 55 (56.1) | |
| Age (years) | | | 0.125 |
| Mean ± SD | 63.2±12.2 | 60.4±12.4 | |
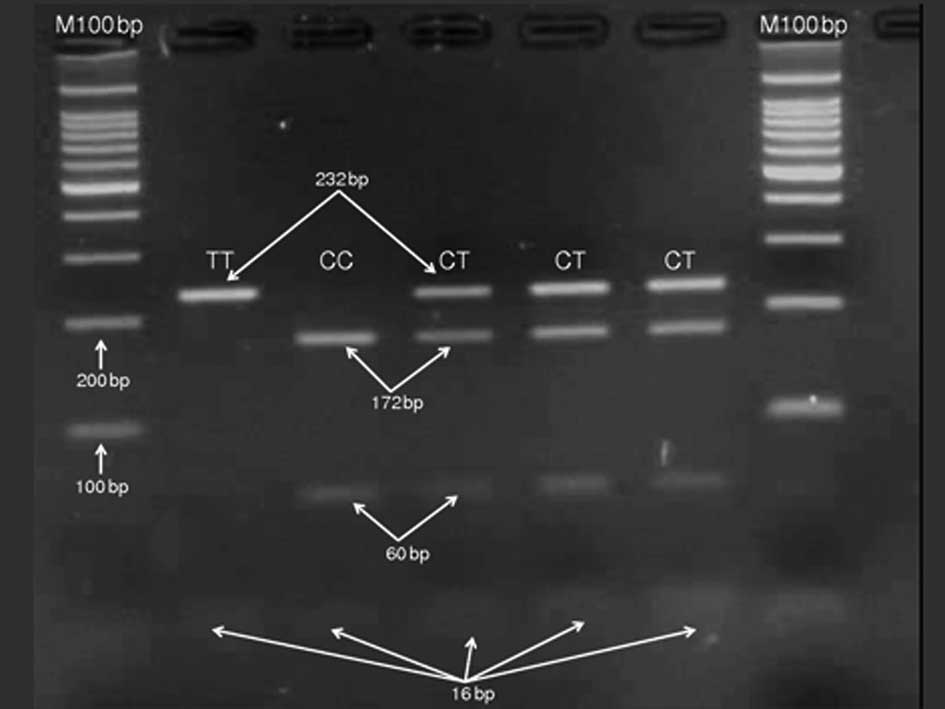
Specific primers were used for the amplification of
a DNA fragment of the MDR1 gene polymorphism, which resulted
in a product of 248 bp. Digestion of the amplicon with MboI
produced four bands of 232, 172, 60 and 16 bp in patients carrying
the heterozygous CT genotype, two bands of 232 and 16 bp in
patients with the homozygous mutant TT genotype and three bands of
232, 172 and 16 bp in patients carrying the homozygous CC genotype
(Fig. 1).
In the control population, the genotypes were in
Hardy-Weinberg equilibrium and the frequency of TT, CC and CT
genotypes was 19.2, 33.0 and 47.8%, respectively. In patients with
GC, the frequency of TT, CC and CT genotypes was 15.3, 32.7 and
52.0%, respectively. Genotype analysis showed no difference in
genotype frequencies between patients with GC and controls
(p=0.668). Similarly, no significant difference in the frequency of
the C and T alleles was observed between patients with GC and
healthy controls (p=0.745) (Table
II). Comparison of genotypes between patients with metastatic
(stage IV) and non-metastatic GC (stages I, II and III) showed no
significant difference (p=0.257).
 | Table IIGenotype and allele frequency in
patients with gastric cancer and controls. |
Table II
Genotype and allele frequency in
patients with gastric cancer and controls.
| Control n=203
(%) | Cancer n=98 (%) | p-valuea |
|---|
| Genotype | | | 0.668 |
| TT | 39 (19.2) | 15 (15.3) | |
| CC | 67 (33.0) | 32 (32.7) | |
| CT | 97 (47.8) | 51 (52.0) | |
| Allele | | | 0.745 |
| T | 175 (43.1) | 81 (41.3) | |
| C | 231 (56.9) | 115 (58.7) | |
The number of subjects infected with H.
pylori was similar in the two groups (GC: n=43, 43.9%; control:
n=111, 54.7%) (p=0.160). No significant difference in CC, TT and CT
genotype frequencies was observed between patients with GC
(p=0.662) or controls (p=0.399) infected or not with H.
pylori (Table III). There
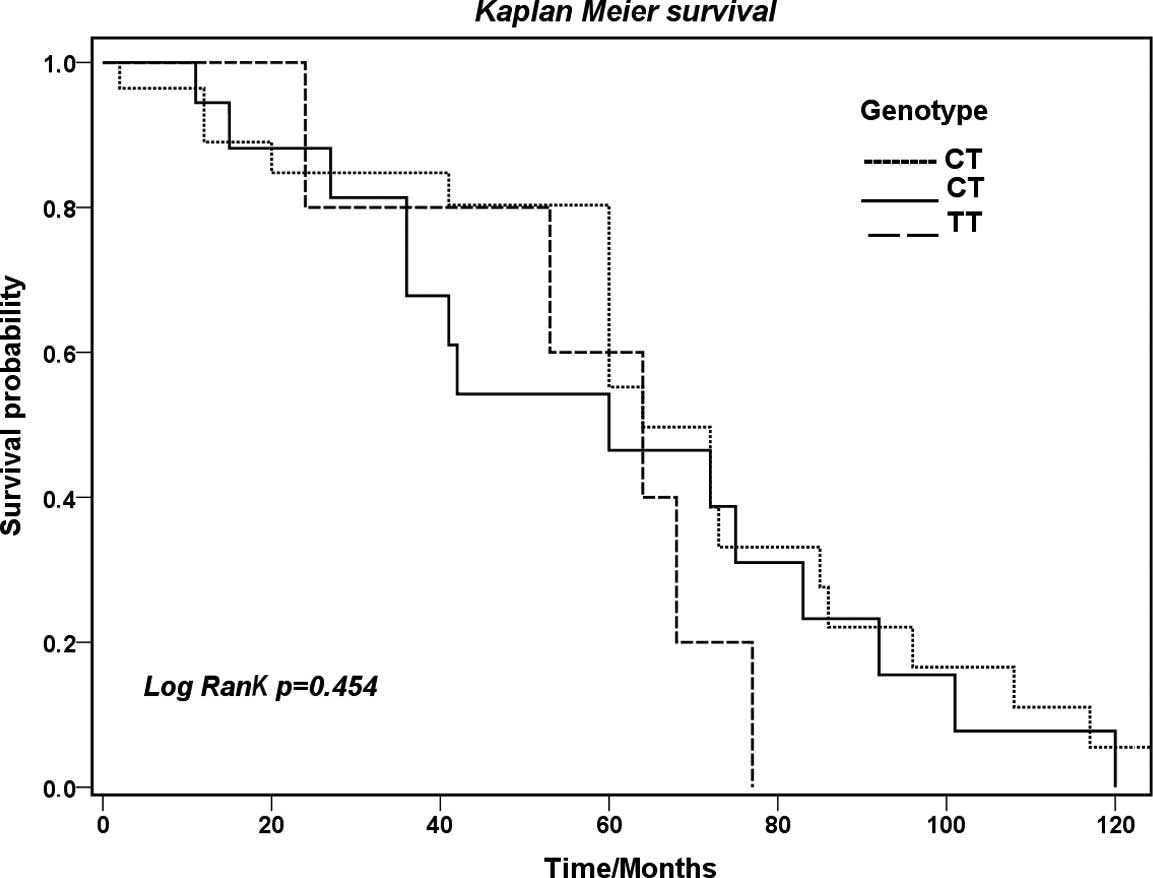
was no difference in survival between the genotypes studied
(p=0.454) (Fig. 2).
 | Table IIIFrequency of the MDR1
polymorphism in patients with gastric cancer and controls infected
or not with Helicobacter pylori. |
Table III
Frequency of the MDR1
polymorphism in patients with gastric cancer and controls infected
or not with Helicobacter pylori.
| CC n (%) | TT n (%) | CT n (%) | p-valuea |
|---|
| Cancer | | | | 0.662 |
| H.
pylori-positive | 5 (5.1) | 15 (15.3) | 23 (23.5) | |
| H.
pylori-negative | 10 (10.2) | 17 (17.2) | 28 (28.6) | |
| Control | | | | 0.399 |
| H.
pylori-positive | 21 (10.3) | 41 (20.2) | 49 (24.1) | |
| H.
pylori-negative | 18 (8.9) | 26 (12.8) | 48 (23.6) | |
Discussion
According to the Brazilian National Cancer Institute
(INCA) (1), GC was the most
frequently diagnosed cancer among men (64.3%) in 2010, with a
higher incidence of the disease in men and women above the age of
50. A higher prevalence of GC among men (56.1%) was also observed
in the present study. The mean age of the patients was 60.2 years.
Studying cases of acute leukemia in India, Rao et al
(20) observed a higher percentage
of the TT genotype (51.7%) among patients with acute lymphoid
leukemia (ALL) when compared to the control group (28.9%). However,
no association was observed for patients with acute myeloid
leukemia. In that study, the CC genotype was associated with a
poorer prognosis of ALL. By contrast, Jamroziak et al
(21) found a higher frequency of
the CT genotype (48%) in Polish patients with ALL, but the
difference was not significant. A higher frequency of the T allele
and CT genotype has been observed in Iranian breast cancer patients
when compared to a group without cancer, but the difference was not
significant (18). The C3435T
polymorphism of the MDR1 gene has been investigated in
inflammatory diseases, such as peptic ulcer, and in cancer, but the
results are contradictory. In intestinal inflammatory disease,
Schwab et al (22) found a
higher frequency of the T allele and TT genotype of the MDR1
gene in German patients with ulcerative colitis, but not in
patients with Crohn's disease when compared to healthy subjects.
Tahara et al (17) showed
that the TT genotype of the MDR1 gene was associated with a
reduced risk of GC in the Japanese population. The authors also
investigated the effect of the MDR1 polymorphism on the risk
of GC in patients infected or not with H. pylori and found
that patients carrying the TT genotype had a lower risk of the
disease, irrespective of the presence or absence of H.
pylori infection (17). Also
in Japan, Sugimoto et al (16) demonstrated a higher incidence of
the CT genotype in patients with GC, gastric and duodenal ulcer and
gastritis, but the difference was not significant when compared to
the control group. The homozygous TT genotype was found to be
associated with a higher risk of GC in an Iranian population when
compared to a control group (23).
Comparison of the frequencies of the C and T alleles between
patients with GC and controls showed a higher prevalence of the C
allele in the two groups (58.7 and 56.9%, respectively), but the
difference was not significant. CT genotype was the most frequent
in both the control group and the case group (47.2 and 52%,
respectively), but the finding was again not statistically
significant. Balram et al (24) compared the frequency of the C
allele among Asian, European and African populations and found a
mean frequency of 43% in the Asian population, 52% in European
Caucasians and 78% in Africans. In the present study, the frequency
of the C allele was 56.9% in the control group and 58.7% in
patients with GC, values similar to those reported for European
Caucasians.
Gastric carcinogenesis is a multifactorial process
and infection with H. pylori is one of the risk factors
(5,6). In Japan, Sugimoto et al
(17) found no association between
the MDR1 C3435T polymorphism and the risk of GC in patients
infected with H. pylori or in patients with peptic ulcer. In
the present study, we also observed no difference between
genotypes, infection with H. pylori and GC risk. The results
suggest that H. pylori infection is not associated with the
MDR1 C3435T polymorphism. Patients with advanced-stage GC
had poor survival (p=0.003). However, no difference in survival was
observed between the genotypes studied. Although MDR1 T-55C
and BCRP C43A were reported to be functional SNPs that change their
protein functions (25,26), the C3435T SNPs analyzed in this
study had no association with overall survival. This suggests that
this gene is probably not the most important determinant in
survival analysis. However, it is also possible that the limited
number of SNPs selected for this study missed the most important
functional variants of this gene. Further investigation is required
to illustrate how these genes may affect the clinical outcome of
GC. The differences in the results of studies investigating the
MDR1 C3435T polymorphism may be related to differences in
the ethnic background of the populations studied and in the number
of patients included in each study. In addition, the MDR1
C3435T SNP is located in a non-coding region and therefore may not
interfere with the expression of P-gp (23), a fact explaining the lack of
association between this polymorphism and gastric
carcinogenesis.
In conclusion, the present results provide evidence
that the C3435T polymorphism of the MDR1 gene is not
associated with susceptibility to gastric cancer. No correlation
was observed between this polymorphism and infection with H.
pylori or survival.
Acknowledgements
This study was generously funded by The Sao Paulo
Research Foundation (FAPESP).
Abbreviations:
|
CG
|
gastric cancer
|
|
P-gp
|
P-glycoprotein
|
|
SNP
|
single-nucleotide polymorphism
|
|
MDR1
|
multidrug resistance 1
|
|
PCR
|
polymerase chain reaction
|
|
RFLP
|
restriction fragment length
polymorphism
|
|
dNTP
|
deoxynucleotide triphosphate
|
References
|
1
|
Instituto Nacional de Câncer - INCA.
Brasília: Ministério da Saúde; Estimativa 2012: Incidência de
Câncer no Brasil. Available at: http://www.inca.gov.br/estimativa/2012/estimative20122111.pdf.
|
|
2
|
Matysiak-Budnika T and Mégraudb F: H.
pylori infection and gastric cancer. Eur J Cancer. 42:708–716.
2006. View Article : Google Scholar
|
|
3
|
Wen S and Moss SF: H. pylori
virulence factors in gastric carcinogenesis. Cancer Lett. 282:1–8.
2009. View Article : Google Scholar
|
|
4
|
Ferlay J, Autier P, Boniol M, Heanue M,
Colombet M and Boyle P: Estimates of the cancer incidence and
mortality in Europe in 2006. Ann Oncol. 18:581–592. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Correa P and Houghton J: Carcinogenesis of
Helicobacter pylori. Gastroenterology. 133:659–672.
2007.
|
|
6
|
Shin CM, Kim N, Lee HS, Lee DH, Kim JS,
Jung HC and Song IS: Intrafamilial aggregation of gastric cancer: a
comprehensive approach including environmental factors,
Helicobacter pylori virulence, and genetic susceptibility.
Eur J Gastroenterol Hepatol. 23:411–417. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ueda K, Cornwell MM, Gottesman MM, Pastan
I, Roninson IB, Ling V and Riordan JR: The MDR1 gene, responsible
for multidrug-resistance, codes for P-glycoprotein. Biochem Biophys
Res Commun. 141:956–962. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
van Helvoort A, Smith AJ, Sprong H,
Fritzsche I, Schinkel AH, Borst P and van Meer G: MDR1
P-glycoprotein is a lipid translocase of broad specificity, while
MDR3 P-glycoprotein specifically translocates phosphatidylcholine.
Cell. 87:507–517. 1996.PubMed/NCBI
|
|
9
|
Dey S: Single nucleotide polymorphisms in
human P-glycoprotein: its impact on drug delivery and disposition.
Expert Opin Drug Deliv. 3:23–35. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Thörn M, Finnström N, Lundgren S, Rane A
and Lööf L: Cytochromes P450 and MDR1 mRNA expression along the
human gastrointestinal tract. Br J Clin Pharmacol. 60:54–60.
2005.PubMed/NCBI
|
|
11
|
Willingham MC, Cornwell MM, Cardarelli CO,
Gottesman MM and Pastan I: Single cell analysis of daunomycin
uptake and efflux in multidrug-resistant and -sensitive KB cells:
effects of verapamil and other drugs. Cancer Res. 46:5941–9546.
1986.PubMed/NCBI
|
|
12
|
Thiebaut F, Tsuruo T, Hamada H, Gottesman
MM, Pastan I and Willingham MC: Cellular localisation of the
multidrug-resistance gene product P-glycoprotein in normal human
tissues. Proc Natl Acad Sci USA. 84:7735–7738. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Jäeger W: Classical resistance mechanisms.
Int J Clin Pharmacol Ther. 47:46–48. 2009.PubMed/NCBI
|
|
14
|
Ho GT, Moodie FM and Satsangi J: Multidrug
resistance 1 gene (Pglycoprotein170): an important determinant in
gastrointestinal disease? Gut. 52:759–766. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Paule B, Castagne V, Picard V, Saffroy R,
Adam R, Guettier C, Farinotti R and Bonhomme-Faivre L: MDR1
polymorphism role in patients treated with cetuximab and irinotecan
in refractory colorectal cancer. Med Oncol. 27:1066–1072. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Sugimoto M, Furuta T, Shirai N, et al:
MDR1 C3435T polymorphism has no influence on developing
Helicobacter pylori infection-related gastric cancer and
peptic ulcer in Japanese. Life Sci. 83:301–304. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Tahara T, Arisawa T, Shibata T, Hirata I
and Nakano H: Multi-drug resistance 1 polymorphism is associated
with reduced risk of gastric cancer in the Japanese population. J
Gastroenterol Hepatol. 22:1678–1682. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Taheri M, Mahjoubi F and Omranipour R:
Effect of MDR1 polymorphism on multidrug resistance expression in
breast cancer patients. Genet Mol Res. 9:34–40. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Turgut S, Turgut G and Atalay EO: Genotype
and allele frequency of human multidrug resistance (MDR1) gene
C3435T polymorphism in Denizli province of Turkey. Mol Biol Rep.
33:295–300. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Rao DN, Anuradha C, Vishnupriya S, Sailaja
K, Surekha D, Raghunadharao D and Rajappa S: Association of an MDR1
gene (C3435T) polymorphism with acute leukemia in India. Asian Pac
J Cancer Prev. 11:1063–1066. 2010.PubMed/NCBI
|
|
21
|
Jamroziak K, Balcerczak E, Cebula B, et
al: Multi-drug transporter MDR1 gene polymorphism and prognosis in
adult acute lymphoblastic leukemia. Pharmacol Rep. 57:882–888.
2005.PubMed/NCBI
|
|
22
|
Schwab M, Schaeffeler E, Marx C, et al:
Association between the C3435T MDR1 gene polymorphism and
susceptibility for ulcerative colitis. Gastroenterology. 124:26–33.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Sabahi Z, Salek R, Heravi RE, et al:
Association of gastric cancer incidence with MDR1 gene polymorphism
in an ethnic Iranian population. Indian J Cancer. 47:317–321. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Balram C, Sharma A, Sivathasen C and Lee
EJ: Frequency of C3435T single nucleotide MDR1 genetic polymorphism
in an Asia population: phenotypic-genotypic correlates. Br J Clin
Pharmacol. 56:78–83. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kimchi-Sarfaty C, Oh JM, Kim IW, Sauna ZE,
Calcagno AM, Ambudkar SV and Gottesman MM: A ‘silent’ polymorphism
in the MDR1 gene changes substrate specificity. Science.
315:525–528. 2007.
|
|
26
|
Mizuarai S, Aozasa N and Kotani H: Single
nucleotide polymorphisms result in impaired membrane localization
and reduced atpase activity in multidrug transporter ABCG2. Int J
Cancer. 109:238–246. 2004. View Article : Google Scholar
|